Abstract
Recently, the highly invasive Asian tiger mosquito, Aedes albopictus, rapidly displaced resident populations of the yellow fever mosquito, Aedes aegypti in the southeastern United States and in Bermuda. Although multiple mechanisms of competitive displacement have been hypothesized, recent evidence of cross-insemination between these species in nature and the sterilizing effects of male accessory gland products asymmetrically favoring A. albopictus in interspecific matings support a role for satyrization (a form of reproductive interference) to explain the rapid displacements. Because of the drastic reproductive loss of A. aegypti females satyrized by A. albopictus males, we predicted selection for prezygotic isolation in populations of A. aegypti sympatric with A. albopictus. Exposures in cages demonstrated that female A. aegypti from populations in Florida sympatric with A. albopictus for the past 20 y were significantly less likely than nearby allopatric populations to mate with heterospecific males. Cross-inseminations of A. albopictus females by A. aegypti males were significantly less common, supporting the one-way direction of displacements observed in nature. Our results indicate rapid sexual selection leading to reproductive character displacement and the potential for satyr-resistant A. aegypti to recover from competitive displacements. These results have implications for increased risks of dengue transmission where these vector species meet worldwide.
Competitive displacement is based on the principle that two species cannot simultaneously occupy the same niche, leading to population reduction of one by interspecific competition (1). This phenomenon has been documented in nature, often in the context of biotic invasions or species introductions for biological control (2–4). Both exploitative and interference competition have been implicated in such displacements, which may be mediated by noncompetitive factors (3).
Among mosquitoes, a recent example of competitive displacement between vector species was the rapid reduction in range and abundance of Aedes aegypti (Linnaeus) (5, 6) following the invasion and spread of Aedes albopictus (Skuse) throughout most of the southeastern United States in the 1980s (7, 8). Despite the potential impacts for public health—A. aegypti being considered the primary vector of epidemic dengue (9), and A. albopictus recently emerging as the most important transmitter of chikungunya virus as well as frequently vectoring dengue (e.g., ref. 10)—our current understanding of the causative mechanisms involved in this species displacement has proven to be inadequate for explaining the patterns observed in nature. Among the possible mechanisms, the most widely cited is larval resource competition (11, 12). However, it is considered unlikely that larval competition alone could account for the rapid competitive reductions of A. aegypti within 1–3 y in the southeastern United States (7, 13, 14) or in Bermuda, where A. albopictus more recently displaced A. aegypti with comparable rapidity (15). In addition to larval competition, hypotheses to explain these displacements include greater reproductive efficiency in A. albopictus (16); apparent competition mediated by the intestinal gregarine protozoan Ascogregarina taiwanesis (12); and asymmetric reproductive interference between A. aegypti and A. albopictus (4, 13). Greater reproductive efficiency in A. albopictus, although possibly beneficial in the long term, does not adequately explain the rapid declines of A. aegypti. Furthermore, surveys of larval habitats (17) and manipulative field experiments (11) did not support a substantial role for apparent competition as an explanation for the outcome of these species interactions. In contrast, reproductive interference or “satyrization,” whereby males of one species mate with females of a related species, producing no viable offspring (18, 19), has been shown to be a strong possible mechanism for population suppression and under certain circumstances can lead to population extinctions (18). However, inconsistent results from cage experiments (13, 20) and the absence of evidence of cross-matings in nature led to waning confidence in satyrization as a plausible competitive displacement mechanism in this system.
Following recent findings that A. aegypti and A. albopictus mate bidirectionally in sites of sympatry in Florida (14) and that heterospecific male accessory gland products render A. aegypti but not A. albopictus females refractory to further insemination by conspecific males (14), we here provide evidence that satyrization has led to reproductive character displacement of A. aegypti by A. albopictus. In particular, we compare the frequency of interspecific mating between A. aegypti and A. albopictus from sympatric and allopatric populations, that is, populations where the two species either have been exposed to interspecific mating in the field or have not yet come in contact. Because the mistake of mating with an A. albopictus male is extremely costly for A. aegypti females [i.e., sterilization and loss of future reproductive potential (14)], evolution should favor females refractory to satyrization. A. aegypti populations that have been exposed to this pressure therefore are likely to show a certain amount of resistance, because avoidance mechanisms would be expected to evolve over time (e.g., refs. 21–26). Consequently, we hypothesized that rates of interspecific mating would be lower in A. aegypti females sympatric with A. albopictus than in geographically isolated populations of A. aegypti that would be more susceptible to cross-insemination.
Results
A. aegypti females from either allopatric or sympatric populations were exposed to A. albopictus males from allopatric or sympatric populations, and vice versa. Furthermore, intraspecific control crosses were conducted simultaneously for each species. After 3 wk of exposure to interspecific or intraspecific males, females were dissected for evidence of insemination.
Female survivorship did not differ significantly among treatments (F = 0.70, df = 15, P = 0.76) and averaged 76.11 ± 11.9% (throughout, data are expressed as SEM).
Among surviving females, intraspecific (control) crosses were 98–100% inseminated. On average, a significantly higher proportion (0.43 ± 0.29) of A. aegypti females was inseminated by A. albopictus males than in the reverse cross (A. albopictus females × A. aegypti males) (0.12 ± 0.16) (F = 32.31, df = 1, P < 0.001).
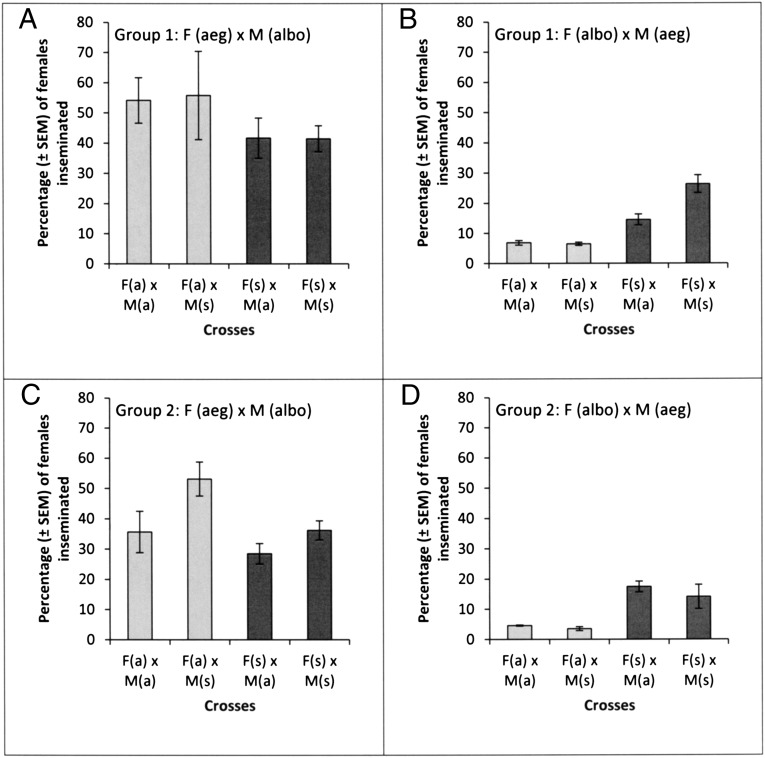
In crosses of A. aegypti females with A. albopictus males, the origin of the female had a significant effect [χ(1)2 = 42.29, P < 0.001] on the likelihood of insemination, with females from allopatric populations more likely to be inseminated than females from sympatric populations (Fig. 1 A and C). This trend was consistent between group 1 and 2 [χ(1)2 = 0.15, P = 0.70] of A. aegypti females (Key West, FL/Vero Beach, FL and Miami, FL/Fort Pierce, FL); however females of the second group (Miami/Fort Pierce) mated less readily overall (Fig. 1). The origin of the A. albopictus males had no effect on the insemination rates of females in the first group (Key West/Vero Beach) [χ(1)2 = 0.08, P = 0.77] but did significantly affect the frequency of insemination in females of the second group (Miami/Fort Pierce) [χ(1)2 = 24.88, P < 0.001], with sympatric males (Vero Beach) inseminating significantly more females than their allopatric (East St. Louis, IL) counterparts (Fig. 1 A and C). The significant group effect [χ(1)2 = 30.69, P < 0.001] therefore can be attributed mainly to the origin of the males. The three-way interaction (female origin, male origin, and group) was not significant [χ(1)2 = 0.56, P = 0.45].
Fig. 1.
Frequencies of insemination in interspecific exposures of A. aegypti (aeg) and A. albopictus (albo) mosquitoes from allopatric (a) and sympatric (s) populations. “F” (female) and “M” (male) denote the sex of the mosquitoes in a particular cross. Light gray columns represent exposures in which the female is from an allopatric population; dark gray columns represent crosses in which the female is from a sympatric population. (A and B) Group 1: A. aegypti strains are from Key West (a) and Vero Beach (s), and A. albopictus strains are from East St. Louis (a) and Vero Beach (s). (C and D) Group 2: A. aegypti strains are from Miami (a) and Fort Pierce (s), and A. albopictus strains are as in Group 1. See Table 1 for details of strain origins.
In the reverse crosses of A. albopictus females with A. aegypti males, the origin of the females also had a significant effect [χ(1)2 = 110.47, P < 0.001], with females from allopatric populations (East St. Louis) showing a lower insemination rate than those from sympatric populations (Vero Beach). This effect was consistent across groups [χ(1)2 = 0.80, P = 0.37], although females in the second group (crossed to Miami/Fort Pierce males) showed slightly lower insemination rates (Fig. 1 B and D).
The origin of the A. aegypti male had no influence on the likelihood a female would be inseminated [χ(1)2 = 0.03, P = 0.86]. The significant group effect [χ(1)2 = 7.32, P = 0.007] in this set of crosses therefore is attributable mainly to the response of the females (Fig. 1 B and D). Again, the three-way interaction (female origin, male origin, and group) was not significant [χ(1)2 = 2.06, P = 0.15].
Discussion
Species Origins and Competitive Displacements.
A. aegypti originated in Africa and was introduced to the Americas between the 15th and 18th centuries (27), in all probability on ships involved in the slave trade, leading to its establishment across the southeastern United States. A. albopictus is of Asian origin and arrived in the New World relatively recently. It was first established in Houston, TX, in the mid 1980s (28) and spread rapidly across the southeastern United States (29, 30) into areas occupied by A. aegypti. The range expansion of A. albopictus coincided with rapid declines and extinctions of A. aegypti populations (5, 6, 31–34). However, the mechanisms examined to date have not been adequate to account for the rapidity of A. aegypti extinctions.
We propose that asymmetric reproductive interference or satyrization has played an important role in these competitive displacements and could be key in explaining the rapid population declines of A. aegypti. Furthermore, our results provide an example of rapid directional evolution in nature which potentially could help explain the maintenance of distributions of A. aegypti and A. albopictus in places where they coexist around the world while shedding light on some of the discrepancies between results of earlier work that led to a decline in interest in satyrization as a driving mechanism for species displacement. We discuss our results with regard to their epidemiological implications as well as their significance for the evolutionary biology of A. aegypti.
Mating Behavior and Reproductive Character Displacement.
Both A. aegypti and A. albopictus belong to the subgenus Stegomyia and share similar life histories and mating behavior; aggregating at vertebrate hosts (often humans) during similar diurnal peak activity periods (35, 36), where mating is initiated in flight, most likely following both visual and auditory cues (37, 38). Although the two species cannot produce viable hybrids (39), these similarities in mating strategy may increase the likelihood of errant interspecific mating when the species first come in contact.
Cage populations of laboratory strains of A. aegypti typically achieve 100% insemination within 72 h after teneral females and males are placed together (40). Despite the longer exposure times needed to achieve interspecific inseminations in our experiments, results showed a significantly higher interspecific insemination rate in female A. aegypti from allopatric (44.37–54.96%) populations than in females from populations with a history of sympatry (32.33–41.54%) with A. albopictus. This decrease in the frequency of interspecific mating in sympatric populations suggests that selection to avoid errant mating, which causes effective sterilization, is high enough for the development of prezygotic mating-avoidance mechanisms in A. aegypti females. Development of similar mating barriers forming within mosquito species complexes has been documented, e.g., in the A. albopictus subgroup of Southeast Asia (26), and was studied extensively in Drosophila species by Dobzhansky and his successors, who reported that sympatric populations were more refractory to interspecific mating than allopatric populations of the same species (41, 42).
Asymmetric Satyrization and Mate Choice.
Therefore, acting in combination with previously documented mechanisms, such as larval competition, satyrization may be key to population reductions of A. aegypti, particularly when the two species first come in contact. A simulation model applicable to arthropod pests and vectors predicts reproductive interference to be much more powerful, even at low frequencies of interspecific mating, than Lotka–Volterra competition when the two mechanisms operate jointly to cause competitive displacement and extinction (18, 19).
Furthermore, A. albopictus females generally were far less likely to mate interspecifically in cages (mean = 11.74%) than A. aegypti females (mean = 43.30%), corroborating earlier reports of unequal bidirectional mating (13) and favoring the observed asymmetry of satyrization in this species pair.
In contrast, the origin of A. aegypti males had no influence on the likelihood that a female A. albopictus would be inseminated. This asymmetry in the response between the sexes is not surprising, because the pressure for female A. aegypti to protect their reproductive potential (against sterilization) will be stronger than the cost to males of an incompatible mating (time, energy, and gamete expenditure). Our results, however, do add to the growing evidence suggesting that the traditional assumption that the mating system of A. aegypti is driven predominantly by male scramble competition (i.e., the first male to seize a female also will mate successfully with that female) may be too simplistic. Instead, the relatively rapid development of mating barriers suggests a more complex system in which female choice is exercised. Recent work on acoustic courtship in this species, which assesses the harmonic convergence of wing beat frequencies in courting mosquitoes (43), also supports this view.
Rapid Evolution and Species Distributions.
The development of reproductive character displacement, evident from comparisons of allopatric strains of A. aegypti and strains that were colonized from sites of sympatry with A. albopictus, is an example of rapid evolutionary change, the sympatric strains in our study having first come in contact as recently as 20–22 y ago. Artificial selection experiments as well as numerous examples from the field (reviewed in refs. 44 and 45) demonstrate the wide-ranging potential for fast evolutionary change, and mathematical models predict that such rapid change in interspecific interactions significantly affects population structure and dynamics. As discussed by Thompson (45), many of the best-documented examples of such rapid directional evolution have involved introduced species and are informative examples of the rate at which populations can adapt to fluctuating environmental conditions and the speed at which evolution continually can reshape community structure. In the case of interactions between A. aegypti and A. albopictus, rapid rates of adaptation could affect the stability of distribution patterns of these two species in areas where they frequently co-occur around the world (4, 46). Satyrization may suppress A. aegypti populations, whereas the development of resistance to satyrization may allow recovery. In combination with other biotic and abiotic factors (47, 48), this interplay could account for the observed patchy distributions of these two species where they encounter one another (e.g., refs. 49 and 50).
Furthermore, the high variability in the rates of insemination may explain the discrepancies in past laboratory mating trials (13, 20). The conflicting results may be caused, in part, by the tested populations displaying varying levels of adaptation to satyrization pressure. However, differences in experimental protocol, such as cage size, exposure time, and sex ratio, also may have contributed to the variation among results.
Vector Displacements and Arbovirus Transmission.
The epidemiological implications of the competitive displacement of A. aegypti by A. albopictus should be considered. Assessing the speed at which mate-choice preferences or avoidance mechanisms develop may help predict future changes in the distribution and abundance of vector populations and, by extension, the risks of arbovirus transmission. If, for example, strong mating barriers and therefore satyrization-resistant populations are established, A. aegypti may be able in the future to recolonize areas from which it was displaced by A. albopictus.
Although a recent meta-analysis confirmed A. aegypti to be the primary outbreak vector of epidemics of severe dengue (51), A. albopictus has been documented as the principal vector of dengue where A. aegypti is rare or uncommon [e.g., in China (52–54), Bangladesh (55), and South India (56, 57)] as well as in recently invaded areas of Africa in the native range of A. aegypti (10).With regard to dengue fever, if A. albopictus is less of a public health threat than A. aegypti (51), then in dengue-endemic regions the displacement of A. aegypti by A. albopictus may lower transmission rates. In turn, a reduced prevalence of dengue may result in lower herd immunity in the resident human population. In this case, the reinvasion of satyrization-resistant A. aegypti, after a period of absence, could cause a resurgence of disease.
Methods
Sympatric and allopatric A. aegypti and A. albopictus were obtained in 2011 from field collections of aquatic immatures from artificial containers, such as discarded tires or cemetery vases, using a turkey baster (Table 1). Individuals were identified to species, sorted, and reared separately to adulthood in insectaries at the Florida Medical Entomology Laboratory. Each colony was established from no fewer than 100 individuals, except for the A. aegypti line collected from White City Cemetery, Fort Pierce, which was established from 30–40 individuals.
Table 1.
Laboratory strains established from field populations
| Group |
A. aegypti |
A. albopictus |
||
| Allopatric | Sympatric | Allopatric | Sympatric | |
| Group 1 | Old Town, Key West; population probably never exposed to A. albopictus (59) | M&K salvage yard, Vero Beach; populations of A. aegypti and A. albopictus have coexisted at this site for 20–22 y (60) | East St. Louis; populations never exposed, because the location lies outside the range of A. aegypti before the arrival of A. albopictus in the United States (61). | M&K salvage yard, Vero Beach; Populations of A. aegypti and A. albopictus have coexisted at this site for 20–22 y (60) |
| Group 2 | Woodlawn Cemetery, Miami; A. albopictus has not been detected in yearly surveillance in this cemetery since 1994–1995 (62) | White City Cemetery, Fort Pierce; populations of A. aegypti and A. albopictus have coexisted at this site for 20–22 y (60) | East St. Louis (see above) | M&K salvage yard, Vero Beach (see above) |
Adults used in the experiments were second generation (F2), except for the allopatric strain of A. albopictus, which was F5. Second-generation mosquitoes were used to avoid maternal effects (58). Experiments were carried out in screened, plastic BugDorm (BioQuip Products Inc.) cages (30 × 30 × 30 cm) in an insectary maintained at 27 ± 0.62 °C and 89 ± 5.28% relative humidity under a 14-h light:10-h dark photoperiod.
Larvae were reared from hatch to pupation in pans containing 1 L of tap water (100 larvae per pan) and were provided with 0.6 g of a 1:1 brewer’s yeast/egg albumin mix on day 1. Pupae were sexed according to morphological differences in their external genitalia and segregated by species and sex in small containers (10–20 pupae per container) for emergence. If a mistake in sexing was detected after emergence, the container was discarded. Three days after emergence, adults were transferred to BugDorm cages (150 females crossed with 150 males per cage) and left to cohabit for 3 wk.
Experiments were conducted in two sequential rounds (groups 1 and 2; Table 1). The groupings of the allopatric/sympatric lines of A. aegypti (Key West/Vero Beach and Miami/Fort Pierce) were arbitrary (based on strain availability) and therefore were treated as a blocking effect in the statistical analysis.
In each group (Table 1), A. aegypti females from either allopatric or sympatric populations were exposed to A. albopictus males from allopatric or sympatric populations. The reciprocal crosses between A. albopictus females and A. aegypti males also were performed, giving a total of eight combinations. Each cross combination was replicated in three sequential repeats. Additionally, three conspecific cages containing males and females of either A. aegypti or A. albopictus were set up as controls.
Females were dissected 3 wk after initial exposure, and the presence of sperm in one or more spermathecae was recorded as an insemination. Mosquitoes were provided a 10% sugar solution throughout the exposure periods.
Statistical Analyses.
Data were analyzed with JMP version 7.0 (www.jmpdiscovery.com).
To detect variations in insemination frequency between A. aegypti and A. albopictus females, the proportions of females inseminated were Arcsine transformed and analyzed by ANOVA. The effect of population origin (sympatric vs. allopatric) of males and females on the likelihood of cross-mating was analyzed with a nominal logistic model, including “group” as a blocking effect. Differences between crosses in adult survivorship were analyzed by ANOVA.
Acknowledgments
We thank George O’Meara and Michael Reiskind for comments; Steve Juliano for providing eggs of allopatric A. albopictus; Barry Alto for providing A. aegypti eggs from Key West; and Karen Garrett-Kraus and Naoya Nishimura for technical support. This research was supported by National Institutes of Health Grant R21 AI095780 (to L.P.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.DeBach P. The competitive displacement and coexistence principles. Annu Rev Entomol. 1966;11:183–212. [Google Scholar]
- 2.Mack RND, et al. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10(3):689–710. [Google Scholar]
- 3.Reitz SR, Trumble JT. Competitive displacement among insects and arachnids. Annu Rev Entomol. 2002;47:435–465. doi: 10.1146/annurev.ento.47.091201.145227. [DOI] [PubMed] [Google Scholar]
- 4.Lounibos LP. Competitive displacement and reduction. J Am Mosq Control Assoc. 2007;23(2, Suppl):276–282. doi: 10.2987/8756-971x(2007)23[276:cdar]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs JH, Hughes EA, Eichold BH., 2nd Replacement of Aedes aegypti by Aedes albopictus in Mobile, Alabama. J Am Mosq Control Assoc. 1991;7(3):488–489. [PubMed] [Google Scholar]
- 6.O’Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32(4):554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 7.Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science. 1987;236(4805):1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 8.Craig GB. The diaspora of the Asian Tiger Mosquito. In: Knight BN, editor. Biological Pollution: The Control and Impact of Invasive Exotic Species. Indianapolis, IN: Indiana Academy of Sciences; 1993. pp. 101–120. [Google Scholar]
- 9.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 10.Paupy C, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10(3):259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 11.Juliano SA. Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology. 1998;79(1):255–268. [Google Scholar]
- 12.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol Lett. 2005;8(5):558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasci RS, Hare SG, Willis FS. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and laboratory. J Am Mosq Control Assoc. 1989;5(3):416–421. [PubMed] [Google Scholar]
- 14.Tripet F, et al. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg. 2011;85(2):265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan L, et al. Aedes aegypti and Aedes albopictus in Bermuda: Extinction, invasion, invasion and extinction. Biol Invasions. 2010;12:3277–3288. [Google Scholar]
- 16.Klowden MJ, Chambers GM. Reproductive and metabolic differences between Aedes aegypti and Ae. albopictus (Diptera: Culicidae) J Med Entomol. 1992;29(3):467–471. doi: 10.1093/jmedent/29.3.467. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JJ, Fukuda T, Becnel JJ. Seasonality, prevalence and pathogenicity of the gregarine Ascogregarina taiwanensis (Apicomplexa: Lecudinidae) in mosquitoes from Florida. J Am Mosq Control Assoc. 1994;10(3):413–418. [PubMed] [Google Scholar]
- 18.Ribeiro JM, Spielman A. The satyr effect: A model predicting parapatry and species extinction. Am Nat. 1986;128(4):513–528. [Google Scholar]
- 19.Ribeiro JM. Can satyrs control pests and vectors? J Med Entomol. 1988;25(6):431–440. doi: 10.1093/jmedent/25.6.431. [DOI] [PubMed] [Google Scholar]
- 20.Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J Am Mosq Control Assoc. 1994;10(1):88–92. [PubMed] [Google Scholar]
- 21.Udovic D. Frequency dependent selection, disruptive selection and the evolution of reproductive isolation. Am Nat. 1980;116(5):621–641. [Google Scholar]
- 22.Zouros E, D'Entremont CJ. Sexual isolation among populations of Drosophila mojavensis: Response to pressure from a related species. Evolution. 1980;34(3):421–430. doi: 10.1111/j.1558-5646.1980.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 23.Markow T. Courtship behavior and control of reproductive isolation between Drosophila mojavensis and D. arizonensis. Evolution. 1981;35(5):1022–1026. doi: 10.1111/j.1558-5646.1981.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 24.Markow T, Fogleman JC, Heed WB. Reproductive isolation in Sonoran Desert Drosophila. Evolution. 1983;37(3):649–652. doi: 10.1111/j.1558-5646.1983.tb05585.x. [DOI] [PubMed] [Google Scholar]
- 25.McLain DK, Rai KS. Ethological divergence in allopatry and asymmetrical isolation in the South Pacific Aedes scutellaris subgroup. Evolution. 1985;39(5):998–1108. doi: 10.1111/j.1558-5646.1985.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 26.McLain DK, Rai KS. Reinforcement for ethological isolation in the Southeast Asian Aedes albopictus subgroup (Diptera: Culicidae) Evolution. 1986;40(6):1346–1350. doi: 10.1111/j.1558-5646.1986.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 27.Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: The Yellow Fever Mosquito. Am Entomol. 1991;37(1):14–26. [Google Scholar]
- 28.Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc. 1986;2(2):217–219. [PubMed] [Google Scholar]
- 29.Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(Suppl):1–40. [PubMed] [Google Scholar]
- 30.Moore CG. Aedes albopictus in the United States: Current status and prospects for further spread. J Am Mosq Control Assoc. 1999;15(2):221–227. [PubMed] [Google Scholar]
- 31.Hornby JA, Moore DE, Miller TW., Jr Aedes albopictus distribution, abundance, and colonization in Lee County, Florida, and its effect on Aedes aegypti. J Am Mosq Control Assoc. 1994;10(3):397–402. [PubMed] [Google Scholar]
- 32.Mekuria Y, Hyatt M. Aedes albopictus in South Carolina. J Am Mosq Control Assoc. 1995;11(4):468–470. [PubMed] [Google Scholar]
- 33.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 34.Britch SC, Linthicum KJ, Anyamba A, Tucker CJ, Pak EW. Mosquito Surveillance Team Long-term surveillance data and patterns of invasion by Aedes albopictus in Florida. J Am Mosq Control Assoc. 2008;24(1):115–120. doi: 10.2987/5594.1. [DOI] [PubMed] [Google Scholar]
- 35.Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull World Health Organ. 1971;45(6):847–850. [PMC free article] [PubMed] [Google Scholar]
- 36.Gubler DJ, Bhattachaya NC. Swarming and mating of Aedes (S.) albopictus in nature. Mosq News. 1972;32(2):219–223. [Google Scholar]
- 37.Roth LM. A study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti Linnaeus. Am Midl Nat. 1948;40(2):265–352. [Google Scholar]
- 38.Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009;323(5917):1077–1079. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leahy MG, Craig GB. Barriers to hybridization between Aedes aegypti and Aedes albopictus. Evolution. 1967;21(1):41–58. doi: 10.1111/j.1558-5646.1967.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 40.Gwadz RW, Craig GB., Jr Sexual receptivity in female Aedes aegypti. Mosq News. 1968;28(4):586–593. [Google Scholar]
- 41.Higgie M, Blows MW. The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution. 2008;62(5):1192–1203. doi: 10.1111/j.1558-5646.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 42.Dobzhansky T, Koller PC. An experimental study of sexual isolation in Drosophila. Biol Zent Bl. 1938;58:589–607. [Google Scholar]
- 43.Cator LJ, Harrington LC. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim Behav. 2011;82(4):627–633. doi: 10.1016/j.anbehav.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Endler JA. Natural Selection in the Wild. Princeton: Princeton Univ Press; 1986. [Google Scholar]
- 45.Thompson JN. Rapid evolution as an ecological process. Trends Ecol Evol. 1998;13(8):329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- 46.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leisnham PT, Lounibos LP, O’Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90(9):2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliano SA. Coexistence, exclusion or neutrality? A meta-analysis of competition between Aedes albopictus and resident mosquitoes. Israel J Ecol Evol. 2010;56(3–4):325–351. doi: 10.1560/IJEE.55.3-4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosq Control Assoc. 1989;5(2):219–225. [PubMed] [Google Scholar]
- 50.Rey JR, et al. Habitat segregation of mosquito arbovirus vectors in south Florida. J Med Entomol. 2006;43(6):1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4(5):e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18(3):215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 53.Wu F, et al. Distribution of Aedes albopictus (Diptera: Culicidae) in northwestern China. Vector Borne Zoonotic Dis. 2011;11(8):1181–1186. doi: 10.1089/vbz.2010.0032. [DOI] [PubMed] [Google Scholar]
- 54.Almeida APG, et al. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol. 2005;42(3):419–428. doi: 10.1093/jmedent/42.3.419. [DOI] [PubMed] [Google Scholar]
- 55.Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: Role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69(6):634–640. [PubMed] [Google Scholar]
- 56.Das BP, et al. Detection of dengue virus in wild caught Aedes albopictus (Skuse) around Kozhikode Airport, Malappuram District, Kerala, India. Dengue Bull. 2004;28:210–212. [Google Scholar]
- 57.Thenmozhi V, et al. Natural vertical transmission of dengue virus in Aedes albopictus (Diptera: Culicidae) in Kerala, a southern Indian state. Jpn J Infect Dis. 2007;60(5):245–249. [PubMed] [Google Scholar]
- 58.Mousseau TA. Intra- and interpopulational genetic variation. In: Mousseau TA, Sinervo B, Endler J, editors. Adaptive Genetic Variation in the Wild. New York: Oxford Univ Press; 2000. pp. 219–250. [Google Scholar]
- 59.Vlach JJ, Fussel EM. Keeping Aedes albopictus out of the Florida Keys. Wing Beats. 2003;14(4):36–38. [Google Scholar]
- 60.O'Meara GF, Gettman AD, Evans LF, Curtis GA. The spread of Aedes albopictus in Florida. Am Entomol. 1993;39(3):163–173. [Google Scholar]
- 61.Darsie RF, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: Univ of Florida Press; 2005. [Google Scholar]
- 62.Lounibos LP, et al. Differential survivorship of invasive mosquito species in south Florida cemeteries: Do site-specific microclimates explain patterns of coexistence and exclusion? Ann Entomol Soc Am. 2010;103(5):757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]