Abstract
Chloroplast heat shock protein 90 (Hsp90C) represents a highly conserved subfamily of the Hsp90 family of molecular chaperones whose function has not been defined. We identified Hsp90C as a component that interacts with import intermediates of nuclear-encoded preproteins during posttranslational import into isolated chloroplasts. Hsp90C was specifically coprecipitated with a complex of protein import components, including Tic110, Tic40, Toc75, Tic22, and the stromal chaperones, Hsp93 and Hsp70. Radicicol, an inhibitor of Hsp90 ATPase activity, reversibly inhibited the import of a variety of preproteins during translocation across the inner envelope membrane, indicating that Hsp90C functions in membrane translocation into the organelle. Hsp90C is encoded by a single gene in Arabidopsis thaliana, and insertion mutations in the Hsp90C gene are embryo lethal, indicating an essential function for the chaperone in plant viability. On the basis of these results, we propose that Hsp90C functions within a chaperone complex in the chloroplast stroma to facilitate membrane translocation during protein import into the organelle.
The function of chloroplasts in photosynthesis, and the metabolism of amino acids, lipids, and secondary metabolites, positions them at the central hub of plant metabolism and signaling (1). Chloroplast biogenesis and function rely on the coordinate expression of genes from both the plastid and nuclear genomes, and several thousand nuclear-encoded proteins are imported into the organelle following their synthesis on cytoplasmic ribosomes. The majority of these proteins are synthesized as preproteins with an amino-terminal cleavable targeting signal, the transit peptide, which directs their import into chloroplasts through multiprotein membrane complexes or translocons located in the outer (TOC) and inner (TIC) chloroplast envelope membranes (2, 3). During or shortly after transport through the TOC and TIC translocons, proteins fold and assemble or engage secondary targeting pathways within the organelle that mediate sorting and insertion to the inner envelope and thylakoid membrane systems (4).
The targeting and translocation of preproteins at the chloroplast envelope is facilitated by a variety of molecular chaperones (5). Cytosolic molecular chaperones, Hsc70 and Hsp90, have been proposed to assist in delivery of preproteins to the TOC receptors, Toc34 and Toc159, and the intrinsic GTPase activities of the receptors initiate outer membrane translocation via the β-barrel membrane channel, Toc75 (2, 3). Preprotein translocation occurs simultaneously through the TOC and TIC complexes with the assistance of Tic22, a chaperone in the intermembrane space (6, 7), and the putative channel components, Tic20 and Tic21 (2, 3). The TIC machinery interacts with three ATP-driven molecular chaperones in the stroma (3, 5). Members of the Hsp70 family, cpHsp70, and the Hsp100 AAA+ (ATPases associated with various cellular activities) family, Hsp93, associate with the TIC translocon in the stroma to facilitate import.
Two functionally overlapping genes encode both cpHsp70 (cpHsp70-1 and -2) and Hsp93 (Hsp93-III and -V) in Arabidopsis. Mutants with reduced or altered cpHsp70 or Hsp93 activity exhibit reduced levels of protein import and marked defects in chloroplast biogenesis (8–11), leading to proposals that both chaperones function as molecular motors for preprotein translocation across the envelope. The chloroplast also contains an Hsp60/GroEL chaperonin family member, cpn60, which is proposed to function in the folding of newly imported preproteins downstream of cpHsp70 and Hsp93 (12). All three chaperone systems have been shown to directly or indirectly immunoprecipitate with the major TIC component, Tic110 (10, 12–14). Tic110 also contains a docking site for preproteins at the stromal side of the inner membrane (15). At least one cochaperone, the integral inner membrane protein, Tic40, participates in import by facilitating the interaction of Hsp93, Tic110, and preproteins during the import reaction (9, 16, 17). Together, these observations have led to the proposal that the stromal domain of Tic110 functions as a scaffold to assemble the chloroplast chaperone network at the TIC translocon and mediate transfer of the preprotein from the translocon channel to the chaperones (2, 3, 5).
Despite strong evidence for their participation in protein import, the precise roles of individual chaperones in membrane translocation, protein folding, or suborganellar targeting has not been defined. In this study, we attempted to obtain a more complete accounting of the factors associated with protein import intermediates as a foundation for understanding the functions of the chloroplast import-associated chaperone network. We identified chloroplast heat shock protein 90, Hsp90C (18, 19), a member of the Hsp90 family of molecular chaperones, as a component of purified complexes containing protein import intermediates. We demonstrate that Hsp90C specifically associates with the TOC–TIC apparatus and several preproteins during protein import. Hsp90C is encoded by a single essential gene in Arabidopsis thaliana, indicating a requirement of Hsp90C for chloroplast function. Radicicol, an inhibitor of Hsp90 ATPase activity, reversibly blocked the import of a variety of nuclear-encoded preproteins at early protein import intermediates. On the basis of these results, we propose that Hsp90C cooperates with TIC components and other molecular chaperones to drive transport of preproteins into chloroplasts.
Results
Hsp90C Is Associated with Protein Import Intermediates.
The analysis of the function of the chloroplast chaperone network in protein translocation and protein folding are challenging because of the difficulties in isolating intermediates at the later stages of import. To address this issue, we took advantage of the unique import characteristics of the precursor to Arabidopsis Tic40 (preatTic40). PreatTic40 is nuclear encoded and our previous studies showed that it is imported into the stroma and accumulates as a soluble intermediate, int-atTic40, en route to insertion in the inner envelope membrane (20). We imported a FLAG epitope-tagged version of urea denatured recombinant preatTic40, preatTic40–3xFLAG (Fig. 1A), into isolated chloroplasts. After 30 min of import, high levels of the soluble intermediate, int-atTic40–3xFLAG, accumulate in the stroma (Fig. S1A, lane 1). PreatTic40–3xFLAG and mature atTic40–3xFLAG also are present in the chloroplast membrane fraction (Fig. S1A, lanes 2 and 3).
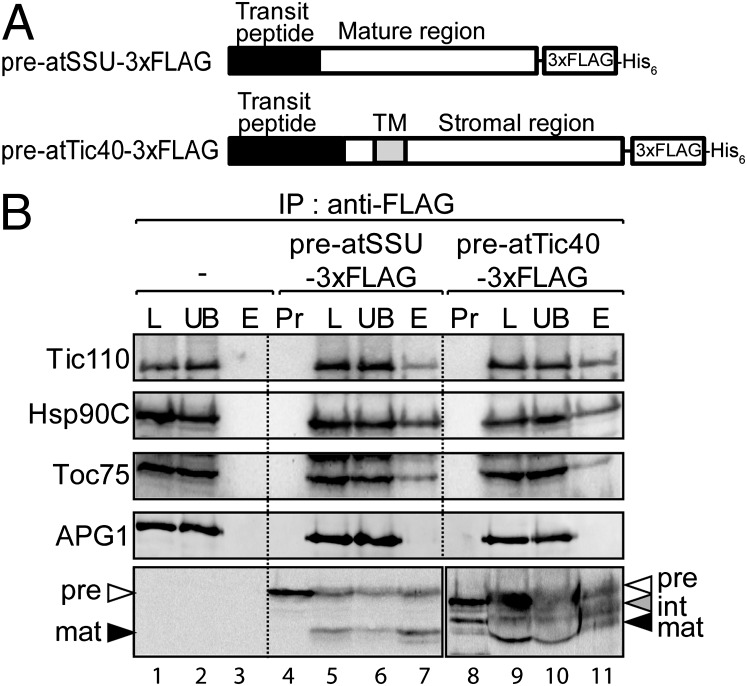
Fig. 1.
Hsp90C is associated with preprotein import intermediates. (A) Diagrams of the preproteins used in this study. (B) Urea-denatured preatSSU–3xFLAG and preatTic40–3xFLAG were incubated with isolated pea chloroplasts under import conditions. After the reaction, the chloroplasts were isolated and treated with 1 mM DSP in the dark for 15 min. Chloroplasts (1 mg of chlorophyll) were dissolved in buffer containing 1% Triton X-100 and immunoprecipitated with M2 Flag antibody. Equivalent fractions of total chloroplast detergent extract (L), unbound material (UB), and immunoaffinity eluates (E), were resolved by SDS/PAGE and the resolved polypeptides were visualized by immunoblotting with antisera to chloroplast proteins as indicated to the Left of each panel. Lanes 4 and 8 of the Lower panels contain 10% of the preprotein added to each import reaction (Pr). Gels in the Lower panels were loaded with 10% of the material in the Upper panels and were probed with M2 FLAG antibody to detect preprotein intermediates.
Chloroplasts from the 30-min import reaction were treated with dithiobis[succinimidyl propionate] (DSP), a thiol-cleavable, homobifunctional, and amine-reactive cross-linking agent to stabilize protein complexes (13). The chloroplasts were solubilized, and proteins subjected to immunoaffinity purification using immobilized FLAG epitope antibodies, and bound proteins were eluted by FLAG peptide. Immunoblots showed that Tic110, Hsp93, and cpHsp70 coprecipitated with the atTic40–3xFLAG intermediates, whereas control proteins of the inner envelope membrane (albino or pale green mutant 1, APG1) and thylakoid membrane (oxygen-evolving complex 23, OE23) were not detected in the precipitates (Fig. S1B, lane 2). These results establish the specificity of the immunoprecipitation reactions. A duplicate sample of the atTic40–3xFLAG immunoprecipitate was resolved by SDS/PAGE, silver-stained, and select protein bands were identified by MALDI TOF/TOF mass spectrometry. In addition to known TOC–TIC components (Fig. S1C, lane 5), we identified a major ∼83-kDa band as the chloroplast homolog of the Hsp90 (heat shock protein 90) family of molecular chaperones, designated Hsp90C (Fig. S2). Although Hsp90C is present in chloroplasts from all plant species examined (18, 19), its role in chloroplast function and biogenesis has not been examined in detail. Our data suggested that Hsp90C is involved in the import or suborganellar targeting of atTic40.
To confirm specific association of Hsp90C with preprotein import intermediates, we raised antibodies to recombinant Hsp90C (Fig. S3) and probed complexes immunoprecipitated with import intermediates of preatTic40–3xFLAG and a second preprotein, preatSSU–3xFLAG. PreatSSU–3xFLAG is a modified version of the precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) containing the FLAG epitope and His6 tag (Fig. 1A). Unlike atTic40, mature SSU is a soluble stromal protein, and previous studies have defined specific intermediates at several stages of import through the TOC–TIC complexes in in vitro import reactions (13, 21–25). Import intermediates for both preproteins coprecipitated Hsp90C as well as Tic110 and Toc75 (Fig. 1B, lanes 7 and 11). The interactions were specific because mock immunoprecipitates with anti-FLAG of chloroplasts lacking either preprotein intermediate failed to pull down Hsp90C or the TOC–TIC components (Fig. 1B, lane 3). In addition, APG1 was not coimmunoprecipitated with either protein import intermediate (Fig. 1B, lanes 7 and 11). Together, these data support the conclusion that Hsp90C specifically associates with nuclear-encoded proteins during import into chloroplasts.
Hsp90C Is Associated with TIC Components and Stromal Molecular Chaperones.
Hsp90C is a soluble protein that is primarily localized to the chloroplast stroma (Fig. S3C) (18). Therefore, we tested whether a fraction of Hsp90C associates with the TOC–TIC machinery at envelope membranes as part of the TIC-associated chaperone complex. To this end, total chloroplast extracts were immunoaffinity purified with anti-Hsp90C after treatment with or without DSP. Immunoblots showed that Hsp90C complexes contained Tic110 and Hsp93 in the absence or presence of cross-linker (Fig. 2A, lanes 3 and 7). Although the amount of Tic110 coeluted with Hsp90C did not increase detectably in the presence of cross-linker, coeluted Hsp93 increased markedly after cross-linking. CpHsp70 and very low amounts of endogenous Tic40 were only detected in complexes that were stabilized with DSP (Fig. 2A, lanes 3 and 7). The TOC channel component, Toc75, did not markedly coelute with Hsp90C under either condition (Fig. 2A, lanes 3 and 7). The APG1 control also was absent from both fractions. The immunoaffinity purification of chloroplast membrane extracts with anti-Hsp90C confirmed that a fraction of Hsp90C interacts with Tic110, Hsp93, cpHsp70, and Tic40 at the envelope membrane (Fig. 2B, lane 3). The ability of Hsp90C to interact stably with Tic110 in the absence or presence of cross-linker suggests that Hsp90C associates with the import-associated chaperone complex via an interaction with Tic110.
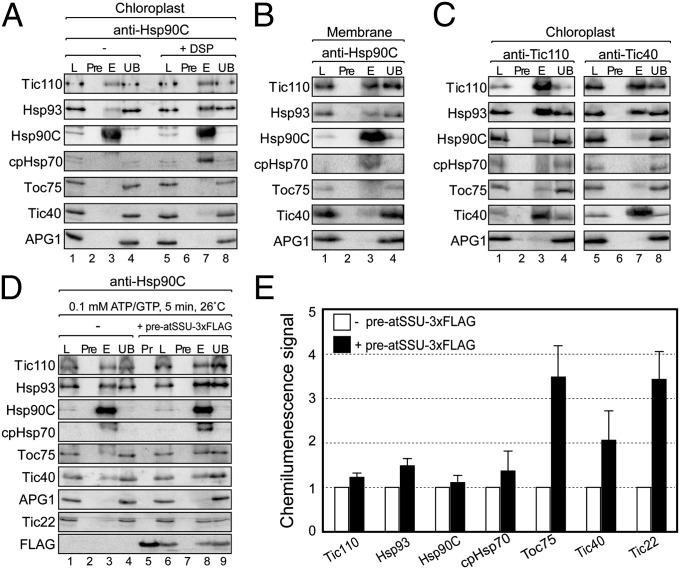
Fig. 2.
Hsp90C is associated with TOC–TIC supercomplexes. (A) Isolated pea chloroplasts (200 μg of chlorophyll) from untreated chloroplasts (−) or DSP-treated chloroplasts (+DSP) were dissolved in buffer containing 1% Triton X-100 and sequentially applied to preimmune IgG sepharose and anti-Hsp90C sepharose. The unbound fraction (UB) and eluates from the preimmune IgG (Pre) or anti-Hsp90C (E) sepharose were resolved by SDS/PAGE and immunoblotted with antibodies to the proteins indicated at the Left of the panels. (B) Total membrane fraction from DSP-treated chloroplasts was subjected to immunoaffinity chromatography and immunoblot analysis as in A. (C) DSP-treated chloroplasts were subjected to immunoaffinity chromatography using preimmune IgG sepharose, anti-Tic110 sepharose, or anti-Tic40 sepharose and immunoblotted as in A. (D) Isolated chloroplasts (200 μg of chlorophyll) were incubated with urea-denatured preatSSU–3xFLAG (lanes 6–9). Chloroplasts were treated with 1 mM DSP and subjected to sequential immunoaffinity chromatography as described in A. Lane 5 contains 10% of the preprotein added to each import reaction (Pr). (E) Quantification of proteins coeluting with Hsp90C in lanes 3 and 8 of D. Mean of the amounts measured in the absence of preatSSU–3xFLAG (D, lane 3) was set at 1 for each sample (white bars). Chemilumenescence signals from the immunoblots in the presence of preatSSU–3xFLAG (D, lane 8) were quantified and plotted as a ratio to the amount coeluting in the absence of preatSSU–3xFLAG (black bars). Error bars represent the SE (n = 3).
Tic110 and Tic40 are integral membrane components of the TIC complex, and they are both proposed to mediate interactions of molecular chaperones with the translocon (9–11, 14, 16, 17). The data in Fig. 2A suggest that Hsp90C binds to TIC complexes via an interaction with Tic110 independent of Tic40 or other known import-associated chaperones. To examine this in more detail, we assessed Tic110 and Tic40 coprecipitation with Hsp90C to investigate their roles in Hsp90C binding to TIC complexes. Hsp90C coeluted with Tic110 (Fig. 2C, lane 3), but very low levels of Hsp90C were detected in anti-Tic40 immunoaffinity eluates (Fig. 2C, lane 7). Interestingly, high levels of Tic40 also coeluted with anti-Tic110, and large amounts of Tic110 coeluted with anti-Tic40 (Fig. 2C, lanes 3 and 7). These data are consistent with an Hsp90C interaction with Tic110 and suggest that separate pools of Tic110 might interact with Hsp90C and Tic40. Collectively, these results demonstrate that Hsp90C is a component of the import-associated chaperone network, likely associating with TIC complexes independent of other known chaperones at the inner envelope membrane via a direct or indirect interaction with Tic110.
Preprotein import stimulates the formation of TOC–TIC supercomplexes to facilitate simultaneous translocation across the outer and inner envelope membranes (21–23, 25). To test whether Hsp90 participated in active supercomplexes, chloroplasts were incubated with saturating concentrations of purified recombinant preatSSU–3xFLAG and 0.1 mM ATP/GTP to form a translocation intermediate spanning both TOC and TIC complexes. The extracts were immunoaffinity purified with anti-Hsp90C and immunoblotted to detect TOC–TIC components (Fig. 2D). Quantification of the chemilumenescence signals indicated that the levels of Tic110, Hsp93, and cpHsp70 in Hsp90C in immunoaffinity eluates were not significantly changed by the import intermediate relative to samples purified in the absence of preatSSU–3xFLAG (Fig. 2E). However, the amounts of Tic22, Tic40, and Toc75 that coprecipitate with Hsp90C increased (Fig. 2 D, lane 3 versus 8, and E). These data support the hypothesis that Hsp90 participates in active TOC–TIC supercomplexes during preprotein translocation.
Hsp90C Is Essential for Viability.
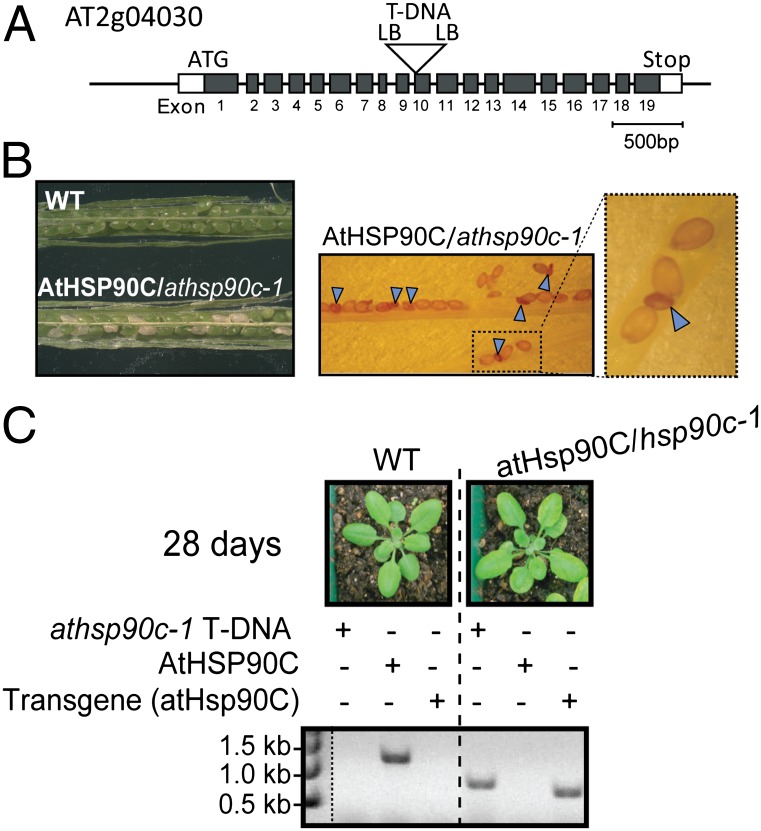
To investigate the in vivo function of Hsp90C, we examined Arabidopsis mutants lacking a functional HSP90C gene. A single-copy gene located on chromosome II (AT2g04030) encodes Arabidopsis Hsp90C (Fig. 3A). We obtained two independent T-DNA insertion lines, SALK_120525 (hsp90c-1) and SALK_012235 (hsp90c-2), but we could not obtain homozygous hsp90c-1 hsp90c-2 plants, consistent with annotation of these as lethal insertion mutations in public databases (Chloroplast Function Database; http://rarge.psc.riken.jp/chloroplast/) (26). The SeedGenes database (http://www.seedgenes.org/) reported that two additional insertion lines in HSP90C, emb 1956-2 and emb 1956-1, are embryo defective at the heart stage of development (27). Consistent with these observations, the siliques from the F1 generation of the hsp90c-1 plants contained ∼25% albino and aborted seeds that fail to develop properly (Fig. 3B, arrowsheads). These data confirm that Hsp90C is essential for viability. To provide additional evidence that the defects in the insertion lines were directly linked to HSP90C, we transformed heterozygous hsp90C-1 plants with the HSP90C cDNA under control of a 1185-bp genomic fragment corresponding to its putative native promoter. Segregation and phenotypic analysis of these plants demonstrated that the transgene fully complemented the lethal phenotype of hsp90C-1 (Fig. 3C). These data confirm that the HSP90C gene is essential in Arabidopsis, consistent with a critical role for the chaperone in chloroplast biogenesis and function.
Fig. 3.
atHsp90C is essential for viability. (A) Schematic representation of the AtHSP90C gene (AT2g04030). The position of the T-DNA insertion in athsp90c-1 (SALK_120525) is indicated by an open triangle. Protein-coding exons are depicted by black boxes, and untranslated regions are depicted by white boxes. LB, the T-DNA left border. (B) Micrographs of dissected siliques from AtHSP90C/athsp90c-1 heterozygous plants. Aborted embryos/seeds are indicated by arrowheads. (C) Complementation of homozygous of hsp90c-1 with the atHsp90C cDNA under control of a 1185-bp genomic fragment from upstream of the AT2g04030 coding region (atHsp90C). Visual phenotype and genotypes of wild type and hsp90c-1 plants transformed with the atHsp90C cDNA were confirmed by PCR analysis of genomic DNA from plants with primer sets specific for the cDNA, T-DNA insertion, and endogenous gene (SI Materials and Methods and Table S1).
Radicicol, an Inhibitor of Hsp90C ATPase Activity, Blocks Preprotein Import.
The lethality of the HSP90C insertional mutants limited our ability to directly test Hsp90C’s function in vivo and in isolated chloroplasts. Consequently, we tested the effects of radicicol, a specific inhibitor of the Hsp90 N-terminal ATP binding site (28), in in vitro import reactions with isolated chloroplasts. A previous study demonstrated that radicicol inhibited the ATPase activity of Hsp90C isolated from the green algae, Chlamydomonas reinhardtii (29). Radicicol also inhibited purified recombinant Arabidopsis Hsp90C ATPase activity, with 95% inhibition observed at 100 μM radicicol (Fig. S4). Radicicol did not inhibit the ATPase activities of purified recombinant Hsp93 and AtcpHsp70-2 (Fig. S4), demonstrating its specificity for Hsp90C.
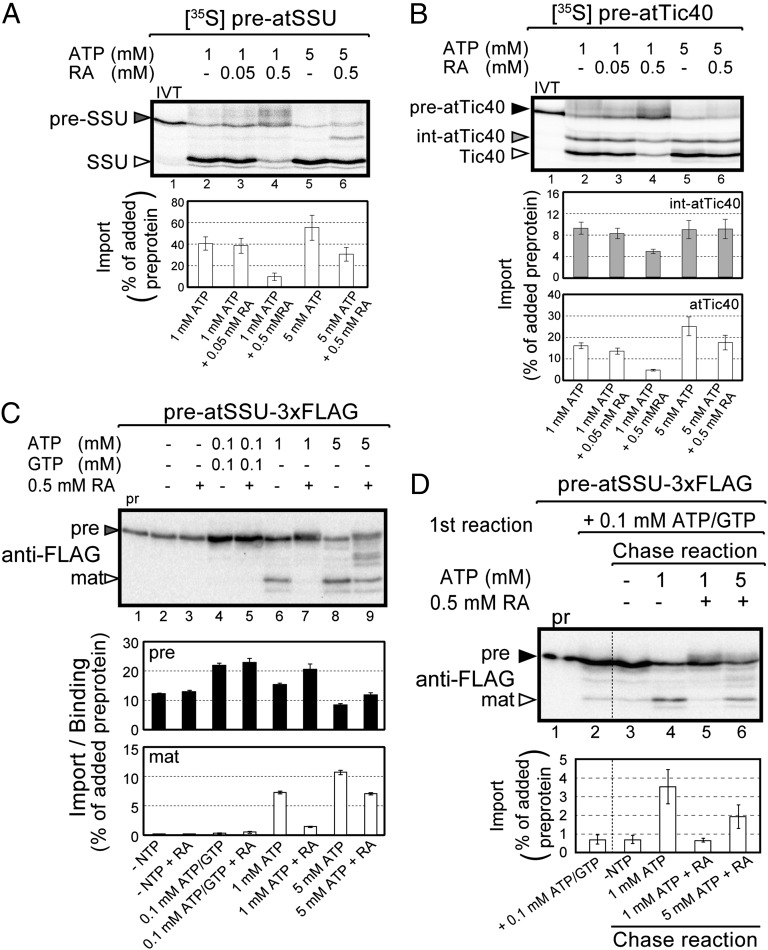
To investigate whether radicicol inhibited protein import, isolated chloroplasts were preincubated with the inhibitor before initiating the import reaction. Radicicol decreased the levels of import of in vitro translated preatSSU and preatTic40, with maximum inhibition of 76% and 69% observed at 500 μM radicicol, respectively (Fig. 4 A and B, lane 2 versus 4). As a competitive inhibitor of ATPase activity (28), the effects of radicicol are predicted to decrease in the presence of increasing concentrations of ATP. Indeed, increasing the concentration of ATP from 1 mM to 5 mM in the presence of 500 μM radicicol fully reversed inhibition of preatTic40 import (Fig. 4B, lane 4 versus 6) and partially reversed the inhibition of pre-SSU import to 75% of uninhibited levels (Fig. 4A, lane 4 versus 6). The relatively higher levels of radicicol required to inhibit import compared with those required to inhibit isolated Hsp90C likely reflects the presence of 1 mM ATP necessary to drive import in the assay. We also cannot discount the possibility that the chloroplast envelope represents a significant permeability barrier to the inhibitor. Taken together, these data are consistent with a direct role for Hsp90C in protein import.
Fig. 4.
Radicicol inhibits preprotein import into chloroplasts. (A and B) Isolated pea chloroplasts were incubated with in vitro translated [35S] preatSSU (A) or [35S] preatTic40 (B) and 1 or 5 mM ATP at 26 °C for 20 min (lanes 2–6) in the presence of different concentrations of radicicol as indicated. Lane 1 contains 10% of the [35S] preprotein added to each reaction (IVT). (C) Chloroplasts were incubated with urea-denatured preatSSU–3xFLAG in the presence or absence of radicicol and the indicated concentrations of nucleoside triphosphates (NTP). The chloroplasts were isolated, resolved by SDS/PAGE, and the precursor (pre) and mature (mat) forms of atSSU–3xFLAG were detected by immunoblotting with anti-FLAG. (D) Chloroplasts were incubated with urea-denature preatSS–3xFLAG in the presence of 0.1 mM ATP and GTP to promote formation of the early import intermediate (lane 2). In lanes 3–6, equivalent samples of chloroplasts to lane 2 were incubated with the indicated concentration of ATP and 500 μM radicicol to promote protein import. (C and D) Lane 1 contains 10% of the preprotein (pr) added to each reaction. (A–D) Each data bar represents the mean ± SEM (n = 3).
Interestingly, the levels of pre-SSU and preatTic40 bound to chloroplasts increased in the presence of radicicol (Fig. 4 A and B, compare lanes 2 and 4). The bound forms were protease sensitive (Fig. S5A, lane 4 versus 5 and lane 9 versus 10), suggesting that the inhibitor arrested import at an early intermediate stage in TOC–TIC translocation. To determine if the intermediate was productively engaged in the TOC–TIC translocons, chloroplasts from the radicicol-inhibited reactions were reisolated, washed, and incubated with ATP to test the import competence of the bound preproteins. A total of 80% of bound pre-SSU and 53% of bound preatTIC40 were imported (Fig. S5B, lane 3 versus 4 and lane 7 versus 8), suggesting that radicicol resulted in the accumulation of productive early intermediates in preprotein import.
We tested the import of urea denatured and purified recombinant preatSSU–3xFLAG to more precisely control the energetics of the reaction (24) and thereby define the stage of import affected by radicicol. Furthermore, previous studies have implicated cytosolic Hsp90 in the targeting of preproteins to the TOC translocon (30), and the use of urea-denatured recombinant pre-SSU–3xFLAG eliminated the possibility that the effect on import was due to inhibition of cytosolic Hsp90 present in the reticulocyte lysate of in vitro translated preproteins (31). Three general stages of import have previously been defined (2, 3, 21–25): energy-independent binding to the TOC receptors, early import intermediates spanning both TOC and TIC translocons formed in the presence of 0.1 mM ATP/GTP, and late import intermediates captured in the presence of high concentrations of ATP (>1 mM) that are fully translocated across the TOC translocon and remain engaged with the TIC complex. Radicicol did not inhibit energy-independent binding (Fig. 4C, lane 2 versus 3) or formation of the preatSSU–3xFLAG early import intermediate (Fig. 4C, lane 4 versus 5), but did inhibit the import of preatSSU–3xFLAG in the presence of 1 mM ATP (Fig. 4C, lane 6 versus 7). The inhibition of import was reversed by increasing the ATP concentration to 5 mM (Fig. 4C, lane 7 versus 9). In the absence of radicicol, the early import intermediate was imported and processed to its mature form when the ATP concentration was increased to 1 mM (Fig. 4D, lane 2 versus 4). By contrast, radicicol inhibited the import of the preatSSU–3xFLAG early import intermediate when the ATP concentration was increased to 1 mM (Fig. 4D, lane 2 versus 5). This inhibition was reversed if the ATP concentration in the import reaction was increased to 5 mM (Fig. 4D, lane 2 versus 6). Our results demonstrate that radicicol specifically inhibits a step subsequent to the formation of the early import intermediate. Taken together, these studies further support a direct role for Hsp90C in protein import and demonstrate that Hsp90 directly participates in the ATP-driven translocation across the outer and inner envelope membranes.
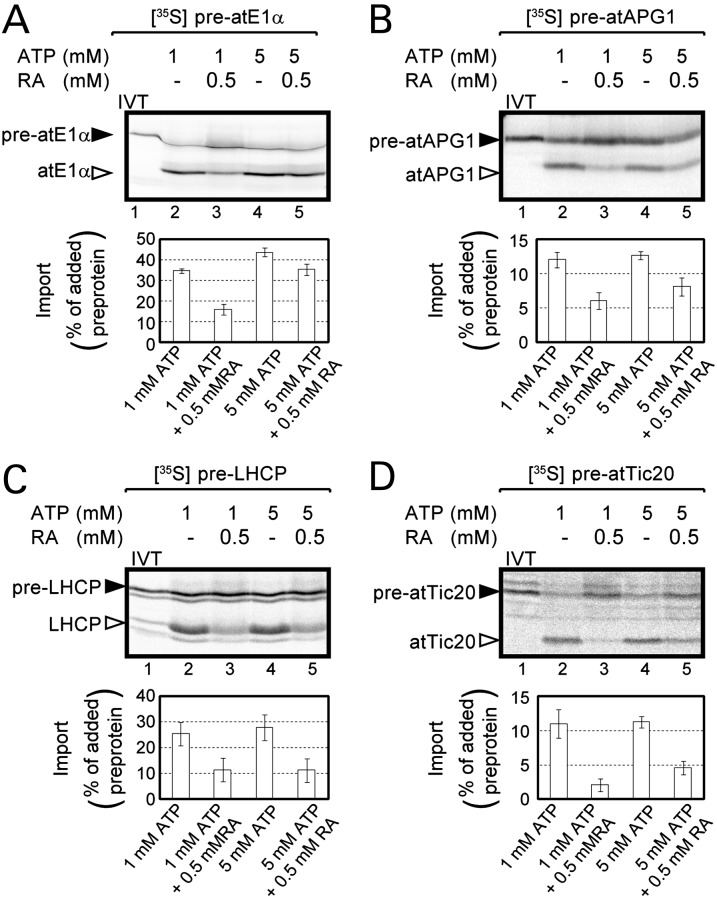
The TOC–TIC translocons mediate the import of proteins destined for multiple suborganellar compartments (4). Therefore, we tested whether or not Hsp90C participated as a general component of the import system by examining the effects of radicicol on the import of additional preproteins that are targeted to the chloroplast stroma (preatE1α), the inner membrane (preatAPG1 and preatTic20), and the thylakoid membrane [pre-light-harvesting chlorophyll a/b-binding protein (LHCP)]. Radicicol inhibited the import of all preproteins tested (Fig. 5 A–D, compare lanes 2 and 3), consistent with a general role for Hsp90C in TOC–TIC translocation. Interestingly, the inhibition of preatAPG1, pre-LHCP, and pre-Tic20 was less susceptible to reversal by 5 mM ATP (Fig. 5 B–D, lane 3 versus 5), suggesting that the import of these more hydrophobic membrane proteins are more sensitive to the function of Hsp90C.
Fig. 5.
Radicicol inhibits the import of a diverse set of nucleus-encoded chloroplast preproteins. (A–D) In vitro translated [35S] preatE1α (A), [35S] preatAPG1 (B), [35S] pre-LHCP (C), or [35S] preatTic20 (D) were incubated with pea chloroplasts and 1 mM ATP or 5 mM ATP in the presence or absence of radicicol at 26 °C for 20 min as described in the legend of Fig. 4A. Lane 1 contains 10% of the [35S] preprotein added to each reaction (IVT). Each data bar represents the mean ± SEM (n = 3).
Discussion
The Hsp90 family is a highly conserved group of molecular chaperones that perform diverse functions (32). Specific subfamilies of the Hsp90s exist in prokaryotes and multiple organelles in eukaryotes, including the cytosol, the ER (glucose-regulated protein 94, Grp94) (33), mitochondria (TNF receptor-associated protein 1, TRAP1) (34), and chloroplasts (Hsp90C) (18). Cytosolic Hsp90 has been studied extensively, and its participation, with a number of cochaperones, in the targeting, assembly, and stability of multiprotein complexes has revealed critical roles in protein folding, cellular signaling, and protein trafficking (35). TRAP1 is implicated in mitochondrial homeostasis of tumor cells (36), and Grp94 function is essential in mouse embryo development (37). In addition, Arabidopsis Grp94 is required for meristem function and supports the synthesis of a secretory protein in vivo (38, 39). Despite the fact that insertional mutants lacking Hsp90C expression are lethal in plants (Fig. 3) (26, 27), the specific function of Hsp90C activity in chloroplasts is much less understood. Our data demonstrate that chloroplast Hsp90C functions as a general component of the TOC/TIC pathway for protein import into chloroplasts. Coimmunoaffinity purification studies showed that Hsp90C associated with protein import intermediates en route to the stroma and the inner membrane (Figs. 1 and 2). The observation that radicicol inhibited the import of stromal, inner membrane, and thylakoid proteins strongly supports this conclusion (Figs. 4 and 5). The radicicol data also suggest that Hsp90C is required for ATP-dependent translocation of preproteins through TOC–TIC translocons, consistent with a role as a component of the translocation motor. Our studies provide evidence directly linking Hsp90C to an essential activity required for chloroplast biogenesis (Fig. 3).
There are precedents for the participation of members of the cytosolic Hsp90 in protein targeting and membrane translocation. Cytosolic Hsp90 has been shown to participate in the posttranslational targeting of preproteins to the TOC–TIC and translocase of the outer membrane-translocase of the inner membrane (TOM–TIM) import machinery of chloroplasts and mitochondria, respectively (30, 40). It was recently reported that cytosolic Hsp90 participates in translocation of antigen across the endosomal membrane into the cytosol in mammalian cells and also in dislocation of the cholera toxin A1 subunit from the ER into the cytosol (41, 42). We provide unique evidence for the involvement of an organellar Hsp90 in protein translocation (Figs. 1, 2, 4, and 5). Our work extends the diverse roles of Hsp90 chaperone function and suggests that Hsp90s play more general roles in protein translocation in a variety of systems.
Although studies on the function of Hsp90C are limited, the accumulated evidence suggests that the chaperone plays diverse functions within chloroplasts beyond its role in protein import. Overexpression of Hsp90C in Arabidopsis reduces tolerance to salt, drought, and oxidative stress (43, 44). Additionally, the cr88 mutant, a Gly-to-Arg substitution at position 646 of Hsp90C in Arabidopsis, alters responses to red light and constitutively delays chloroplast development (18). These findings led to proposals that Hsp90C also is involved in stress responses and functions in the transduction of plastid signals that regulate photosynthesis-related gene expression in a manner reminiscent of the role of cytosolic and other organelle Hsp90 family members in signal transduction pathways (33–35). Chlamydomonas Hsp90C associates with vesicle-inducing protein in plastid 1 (VIPP1), a protein implicated in thylakoid biogenesis or maintenance (45). Based on these reports, we hypothesize that Hsp90C plays roles in diverse organellar processes and functions in specific processes (e.g., protein import, assembly of macromolecular complexes, and organellar signaling) that are controlled by interactions with specific cochaperones, similar to the cytosolic Hsp90 system. To date, the cochaperones for the Hsp90C have not been identified.
Our coimmunoaffinity purification analysis showed that Hsp90C associated with Tic110 even in the absence of protein import (Fig. 2), demonstrating a role for Hsp90C as a component of the import-associated chaperone network, and suggesting that Tic110 serves as the docking site for Hsp90C at the translocon. It remains to be seen if the Hsp90C–Tic110 interaction is direct or indirect. Preprotein intermediates en route to the chloroplast stroma stimulated the formation TOC–TIC supercomplexes and complexes containing Hsp90C, Tic110, Hsp93, and cpHsp70, suggesting that the association of the chaperone network with Tic110 is dynamic and responsive to protein import. Tic40, the integral membrane cochaperone, was not a stable component of Hsp90C–TIC complexes (Fig. 2). Interestingly, the stromal region of Tic40 contains tetratricopeptide repeat (TPR), stress-inducible protein 1/Hsp70–Hsp90-organizing protein (Sti1p/Hop), and Hip (Hsp70-interacting protein) domains (16, 17), all of which mediate interactions between Hsp90s and their cochaperones in cytosolic systems (35). In chloroplasts, it has been shown that Tic40 regulates transit peptide–Tic110 interactions and stimulates Hsp93 ATP hydrolysis (17). Tic40 might also transiently participate in the formation of Hsp90C–TIC interactions (Fig. 2). Therefore, it is possible that Tic40 plays multiple roles in coordinating the assembly and function of the import-associated chaperone complexes.
We observed that cpHsp70 and Hsp93 coprecipitated with Hsp90C, expanding the components of the chaperone network coordinated by Tic110 in higher plant chloroplasts. Interestingly, Hsp90C functions in a complex with cpHsp70 in the stroma of Chlamydomonas chloroplasts (29, 45, 46), raising the possibility that these two chaperones also function together at TIC complexes during protein import in vascular plants. It was previously proposed that the cpHsp70 system and the Hsp93–Tic40 systems function in parallel during protein import based on epistasis analysis of combinations of cpHsp70, Hsp93, and Tic40 mutants (9, 10, 47). Although our data suggest that Hsp90C functions as a component of the translocation motor of a wide variety of preproteins, the different chaperones may act sequentially or exhibit some selectivity toward distinct preprotein substrates during import, depending upon the physical characteristics of the preprotein. The remarkable complexity of the import-associated chaperone network in chloroplasts compared with other organelle translocation systems suggests that the stromal chaperones do not simply function as components of the translocon motor, but participate in a series of events required for efficient import. Further investigation of the role of Hsp90C in protein import provides an excellent system for understanding the roles of the components of the import-associated chaperone network in protein targeting and chloroplast biogenesis.
Materials and Methods
Protein import experiments were performed with chloroplasts from 10–12-d-old pea seedlings (Pisum sativum var. Green Arrow) as previously described (13). Chloroplast import reactions were performed using [35S]methione-labeled preproteins and chloroplasts corresponding to 20 μg of chlorophyll. For generating early import intermediate with E. coli expressed preproteins, chloroplasts were depleted of internal ATP by incubation for 30 min in the dark in the presence of 400 nM nigericin (17). Energy-depleted chloroplasts corresponding to 40 μg of chlorophyll and purified recombinant pre-SSU-3xFLAG-His were incubated in the presence of 0.1 mM GTP and ATP for 5 min at 26 °C as described previously (18). SI Materials and Methods provides additional information related to the main text on the following topics: plasmid and DNA constructs, protein expression and purification, antibody production, chloroplast isolation, cross-linking, immunoprecipitation or immunoaffinity purification, in vitro translation and in vitro import assays, ATPase assays, and Arabidopsis transformation.
Supplementary Material
Acknowledgments
We thank Dr. Alice Cheung, Dr. Daniel Kita, Dr. Peter Chien, and Robert Vass for technical support. We thank Dr. Kenneth C. Cline, Dr. Daniel N. Hebert, and Dr. Hsou-min Li for providing plasmids. This work was supported by National Institutes of Health Grant 2RO1-GM061893 (to D.J.S.). H.I. is a recipient of a Japan Society for the Promotion of Science postdoctoral fellowship for research abroad.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. atHsp90C (AEC05778), preatSSU (AED94315), preatTic40 (AAP31939), pre-E1α (AEE27233), Hsp93 (AAA33680), and atcpHsp70-2 (AED95870)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219229110/-/DCSupplemental.
References
- 1.Lopez-Juez E, Pyke KA. Plastids unleashed: Their development and their integration in plant development. Int J Dev Biol. 2005;49(5-6):557–577. doi: 10.1387/ijdb.051997el. [DOI] [PubMed] [Google Scholar]
- 2.Inaba T, Schnell DJ. Protein trafficking to plastids: One theme, many variations. Biochem J. 2008;413(1):15–28. doi: 10.1042/BJ20080490. [DOI] [PubMed] [Google Scholar]
- 3.Li HM, Chiu CC. Protein transport into chloroplasts. Annu Rev Plant Biol. 2010;61:157–180. doi: 10.1146/annurev-arplant-042809-112222. [DOI] [PubMed] [Google Scholar]
- 4.Celedon JM, Cline K. Intra-plastid protein trafficking: How plant cells adapted prokaryotic mechanisms to the eukaryotic condition. Biochim Biophys Acta. 2013;1833(2):341–351. doi: 10.1016/j.bbamcr.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Perez U, Jarvis P. Molecular chaperone involvement in chloroplast protein import. Biochim Biophys Acta. 2013;1833(2):332–340. doi: 10.1016/j.bbamcr.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Tripp J, et al. Structure and conservation of the periplasmic targeting factor Tic22 protein from plants and cyanobacteria. J Biol Chem. 2012;287(29):24164–24173. doi: 10.1074/jbc.M112.341644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser S, et al. Tic22 is an essential chaperone required for protein import into the apicoplast. J Biol Chem. 2012;287(47):39505–39512. doi: 10.1074/jbc.M112.405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constan D, Froehlich JE, Rangarajan S, Keegstra K. A stromal Hsp100 protein is required for normal chloroplast development and function in Arabidopsis. Plant Physiol. 2004;136(3):3605–3615. doi: 10.1104/pp.104.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacheva S, et al. In vivo studies on the roles of Tic110, Tic40 and Hsp93 during chloroplast protein import. Plant J. 2005;41(3):412–428. doi: 10.1111/j.1365-313X.2004.02307.x. [DOI] [PubMed] [Google Scholar]
- 10.Su PH, Li HM. Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts. Plant Cell. 2010;22(5):1516–1531. doi: 10.1105/tpc.109.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi LX, Theg SM. A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell. 2010;22(1):205–220. doi: 10.1105/tpc.109.071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler F, Blobel G. Interaction of the protein import and folding machineries of the chloroplast. Proc Natl Acad Sci USA. 1996;93(15):7684–7689. doi: 10.1073/pnas.93.15.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akita M, Nielsen E, Keegstra K. Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking. J Cell Biol. 1997;136(5):983–994. doi: 10.1083/jcb.136.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba T, et al. Arabidopsis tic110 is essential for the assembly and function of the protein import machinery of plastids. Plant Cell. 2005;17(5):1482–1496. doi: 10.1105/tpc.105.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba T, Li M, Alvarez-Huerta M, Kessler F, Schnell DJ. atTic110 functions as a scaffold for coordinating the stromal events of protein import into chloroplasts. J Biol Chem. 2003;278(40):38617–38627. doi: 10.1074/jbc.M306367200. [DOI] [PubMed] [Google Scholar]
- 16.Chou ML, et al. Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J. 2003;22(12):2970–2980. doi: 10.1093/emboj/cdg281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou ML, Chu CC, Chen LJ, Akita M, Li HM. Stimulation of transit-peptide release and ATP hydrolysis by a cochaperone during protein import into chloroplasts. J Cell Biol. 2006;175(6):893–900. doi: 10.1083/jcb.200609172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao D, Froehlich JE, Zhang H, Cheng CL. The chlorate-resistant and photomorphogenesis-defective mutant cr88 encodes a chloroplast-targeted HSP90. Plant J. 2003;33(1):107–118. doi: 10.1046/j.1365-313x.2003.016011.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Schnell DJ. Reconstitution of protein targeting to the inner envelope membrane of chloroplasts. J Cell Biol. 2006;175(2):249–259. doi: 10.1083/jcb.200605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnell DJ, Kessler F, Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266(5187):1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- 22.Kouranov A, Schnell DJ. Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J Cell Biol. 1997;139(7):1677–1685. doi: 10.1083/jcb.139.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen KY, Li HM. Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts. Plant J. 2007;49(1):149–158. doi: 10.1111/j.1365-313X.2006.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue H, Akita M. Three sets of translocation intermediates are formed during the early stage of protein import into chloroplasts. J Biol Chem. 2008;283(12):7491–7502. doi: 10.1074/jbc.M709571200. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi S, et al. A 1-megadalton translocation complex containing Tic20 and Tic21 mediates chloroplast protein import at the inner envelope membrane. Plant Cell. 2009;21(6):1781–1797. doi: 10.1105/tpc.108.063552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myouga F, et al. The Chloroplast Function Database: A large-scale collection of Arabidopsis Ds/Spm- or T-DNA-tagged homozygous lines for nuclear-encoded chloroplast proteins, and their systematic phenotype analysis. Plant J. 2010;61(3):529–542. doi: 10.1111/j.1365-313X.2009.04074.x. [DOI] [PubMed] [Google Scholar]
- 27.Meinke D, Muralla R, Sweeney C, Dickerman A. Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci. 2008;13(9):483–491. doi: 10.1016/j.tplants.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Roe SM, et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 29.Willmund F, Schroda M. HEAT SHOCK PROTEIN 90C is a bona fide Hsp90 that interacts with plastidic HSP70B in Chlamydomonas reinhardtii. Plant Physiol. 2005;138(4):2310–2322. doi: 10.1104/pp.105.063578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qbadou S, et al. The molecular chaperone Hsp90 delivers precursor proteins to the chloroplast import receptor Toc64. EMBO J. 2006;25(9):1836–1847. doi: 10.1038/sj.emboj.7601091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB. Reconstitution of the steroid receptor.hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271(22):12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823(3):607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Marzec M, Eletto D, Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta. 2012;1823(3):774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altieri DC, Stein GS, Lian JB, Languino LR. TRAP-1, the mitochondrial Hsp90. Biochim Biophys Acta. 2012;1823(3):767–773. doi: 10.1016/j.bbamcr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 36.Kang BH, et al. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131(2):257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Wanderling S, et al. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18(10):3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishiguro S, et al. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J. 2002;21(5):898–908. doi: 10.1093/emboj/21.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein EM, et al. Plant endoplasmin supports the protein secretory pathway and has a role in proliferating tissues. Plant J. 2006;48(5):657–673. doi: 10.1111/j.1365-313X.2006.02904.x. [DOI] [PubMed] [Google Scholar]
- 40.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112(1):41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 41.Imai T, et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci USA. 2011;108(39):16363–16368. doi: 10.1073/pnas.1108372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor M, et al. Hsp90 is required for transfer of the cholera toxin A1 subunit from the endoplasmic reticulum to the cytosol. J Biol Chem. 2010;285(41):31261–31267. doi: 10.1074/jbc.M110.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song H, et al. Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses. Planta. 2009;229(4):955–964. doi: 10.1007/s00425-008-0886-y. [DOI] [PubMed] [Google Scholar]
- 44.Song H, et al. Overexpression of organellar and cytosolic AtHSP90 in Arabidopsis thaliana impairs plant tolerance to oxidative stress. Plant Mol Biol Rep. 2009;27:342–349. [Google Scholar]
- 45.Heide H, et al. Application of quantitative immunoprecipitation combined with knockdown and cross-linking to Chlamydomonas reveals the presence of vesicle-inducing protein in plastids 1 in a common complex with chloroplast HSP90C. Proteomics. 2009;9(11):3079–3089. doi: 10.1002/pmic.200800872. [DOI] [PubMed] [Google Scholar]
- 46.Willmund F, Dorn KV, Schulz-Raffelt M, Schroda M. The chloroplast DnaJ homolog CDJ1 of Chlamydomonas reinhardtii is part of a multichaperone complex containing HSP70B, CGE1, and HSP90C. Plant Physiol. 2008;148(4):2070–2082. doi: 10.1104/pp.108.127944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi LX, Theg SM. The motors of protein import into chloroplasts. Plant Signal Behav. 2011;6(9):1397–1401. doi: 10.4161/psb.6.9.16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.