Abstract
B-cell lymphoma–6 protein (Bcl-6) is a corepressor for inflammatory mediators such as vascular cell adhesion molecule–1 and monocyte chemotactic protein–1 and –3, which function to recruit monocytes to vascular endothelial cells upon inflammation. Poly [ADP ribose] polymerase 1 (PARP-1) is proinflammatory, in part through its binding at the Bcl-6 intron 1 to suppress Bcl-6 expression. We investigated the mechanisms by which PARP-1 dissociates from the Bcl-6 intron 1, ultimately leading to attenuation of endothelial inflammation. Analysis of the PARP-1 primary sequence suggested that phosphorylation of PARP-1 Serine 177 (Ser-177) by AMP-activated protein kinase (AMPK) is responsible for the induction of Bcl-6. Our results show that AMPK activation with treatment of 5-aminoimidazole-4-carboxamide ribonucleotide, metformin, or pulsatile shear stress induces PARP-1 dissociation from the Bcl-6 intron 1, increases Bcl-6 expression, and inhibits expression of inflammatory mediators. Conversely, AMPKα suppression or knockdown produces the opposite effects. The results demonstrate an anti-infamatory pathway linking AMPK, PARP-1, and Bcl-6 in endothelial cells.
Keywords: monocyte adhesion, THP-1 cells, inflammatory co-repressor, vascular dysfunction, endothelium
Toxins, disturbed blood flow, hyperlipidemia, and hyperglycemia often elicit vascular inflammation by triggering the expression and release of proinflammatory cytokines such as TNF-α and IL-1β to increase the expression of adhesion molecules and chemoattractants such as vascular cell adhesion molecule–1 (VCAM-1) and monocyte chemotactic proteins (MCPs) in endothelial cells (ECs) (1–3). Inhibition of these cytokines mitigates endothelial inflammation. For example, clinical trials using anti–TNF-α monoclonal antibodies (mAbs) to treat various inflammatory diseases not only alleviates the targeted diseases but also improves vascular function (4, 5). AMP-activated protein kinase (AMPK), which is activated under various environmental and metabolic stresses, exerts anti-inflammatory effects on vasculature, in part through inhibition of chemoattractants and adhesion molecules by regulating STAT3, NF-κB, and p300 pathways (6–9).
Poly [ADP ribose] polymerase 1 (PARP-1) is an abundant and ubiquitous nuclear protein that uses NAD+ to synthesize poly(ADP)-ribose (PAR) to “PARylate” itself and other nuclear proteins (10). PARP-1 also acts as a transcriptional regulator via direct interaction of its zinc finger domains with the sequence TTGATATAAAT within target genes (11). PARP-1 is involved in multiple cellular functions—e.g., repair of DNA single- and double-strand breaks, mitosis, cell fate after genotoxic insults, etc. (11–15). PARP-1 knockdown is anti-inflammatory since it acts as a coactivator of NF-κB–mediated transcription to activate proinflammatory pathways such as p38 MAPK and JNK and inhibit B-cell lymphoma–6 (Bcl-6)–exerted anti-inflammatory function (11, 15, 16). In vivo, PARP-1 knockout mice are protected from septic shock and hyperglycemia-induced endothelial dysfunction (17, 18). Furthermore, reduction of atherosclerosis has been found in PARP-1 knockout mice in Apolipoprotein E (ApoE)-null background (19).
The transcriptional repressor Bcl-6 contains a C-terminal zinc finger domain, a central domain that interacts with the histone deacetylases, and an N-terminal poxvirus and zinc finger domain that associates with various corepressors (20, 21). Dissociation of Bcl-6 from apo-peroxisome proliferator-activated receptor (PPAR)–δ promotes Bcl-6 promoter binding and inhibition of MCP-1, MCP-3, and macrophage inflammatory protein (MIP)–1β in macrophages (22, 23). PARP-1 represses Bcl-6 transcription by sequence-specific binding to the first intron of the Bcl-6 gene, and PARP-1 knockdown induces the expression of Bcl-6 (11).
Because the DNA binding domain of PARP-1 has a consensus sequence for AMPK phosphorylation and the activated AMPK is anti-inflammatory, we proposed that AMPK facilitates PARP-1 suppression of Bcl-6. Thus, this study investigates (1) the effects of AMPK phosphorylation of PARP-1 on its DNA binding (2), increase in Bcl-6 expression by the dissociation of phosphorylated PARP-1 from the Bcl-6 intron 1, and (3) suppression of inflammatory genes by the increase in Bcl-6.
Results
AMPK Phosphorylation of PARP-1 Attenuates Its Binding to the Bcl-6 Intron 1.
Analysis of PARP-1 protein revealed an AMPK phosphorylation consensus sequence at Ser-177 in its DNA binding domain (Fig. 1A). Negatively charged PO4− incorporation onto this domain would create electrostatic repulsion and reduce the affinity of PARP-1 for DNA. Thus, we postulated that phosphorylation may suppress PARP-1 binding to the conserved first intron of Bcl-6 (11) Fig. 1B). To investigate whether AMPK can phosphorylate PARP-1, in vitro kinase assays were performed using active AMPKα2, PARP-1, and [γ-32P]ATP. Autoradiography showed [32P] incorporation onto PARP-1 (Fig. 1C). Further, in vitro kinase assays showed that a peptide with S177A substitution was not able to cause such phosphate incorporation, which occurred when using a peptide identical to the native PARP-1 sequence spanning Ser-177 and the SAMS peptide, a standard AMPK substrate (Fig. 1D).
Fig. 1.
AMPK phosphorylates PARP-1 to decrease its binding to the Bcl-6 intron 1. (A) PARP-1 domains with conserved Ser-177 of the AMPK phosphorylation sequence. (B) Bcl-6 intron 1 with PARP-1 DNA binding sequence. (C) Autoradiograph and Coomassie blue stain of PARP-1 in kinase assays using AMPKα2 and [γ-32P]ATP. (D) Kinase assay with PARP-1 peptides containing Ser-177, S177A, or SAMS as control. (E) ChIP assay of PARP-1 binding to Bcl-6 intron 1 in HUVECs, AMPK+/+, or −/− MEFs, treated with AICAR (1 mM) or metformin (1 mM). (F) Bcl-6 intron 1 binding site used to pull down PARP-1 from AICAR-treated AMPK+/+ or −/− MEFs. (G) ChIP using anti-FLAG to assess binding to Bcl-6 intron 1 in HUVECs transfected with FLAG-tagged native, S177A, or S177D PARP-1 plasmids with control or AMPKα siRNA, then treated with AICAR, metformin, or Compound C (20 nM). *P < 0.05.
We next examined whether AMPK-phosphorylated PARP-1 modulates PARP-1 binding to the Bcl-6 intron 1. Chromatin immunoprecipitation (ChIP) assay using primers to the PARP-1 binding site in the Bcl-6 intron 1 (Table S1) showed that 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) or metformin decreased PARP-1 binding to the Bcl-6 intron 1 in human umbilical vein endothelial cells (HUVECs) (Fig. 1E). Similar results were observed with wild-type (WT) but not AMPK-null, mouse embryonic fibroblast cells (MEFs) (Fig. 1E). To further validate regulation of PARP-1 binding by AMPK phosphorylation, a biotinylated nucleotide segment of Bcl-6 intron 1, including the PARP-1 binding sequence, was conjugated to streptavidin beads to pull out PARP-1 from cell lysates. Phosphorylation by AMPK should decrease PARP-1 affinity for the nucleotide sequence. As expected, AICAR decreased PARP-1 binding to the DNA sequence in WT, but not in AMPK-null, MEFs (Fig. 1F). To determine if phosphorylation of PARP-1 Ser-177 mediates interaction between PARP-1 and the Bcl-6 intron 1, we overexpressed the WT PARP-1, dephosphomimetic S177A, or phosphomimetic S177D PARP-1 mutants in HUVECs. ChIP assays (Fig. 1G) revealed that binding of PARP-1 S177D to Bcl-6 intron 1 was weaker than that of PARP-1 S177A with or without AICAR treatment.
AMPK Phosphorylation of PARP-1 Leads to Bcl-6 Transactivation.
Because Bcl-6 transcription is inhibited by PARP-1 binding to its intron 1 (11), we reasoned that AMPK-induced phosphorylation of PARP-1 Ser-177 may affect Bcl-6 transactivation. Hence, we constructed a luciferase reporter Bcl-6-Luc driven by the Bcl-6 promoter conjugated to its native intron 1 and a mutant Bcl-6-Luc(Δ) reporter with the PARP-1 binding sequence deleted. As shown in Fig. 2A, AICAR and metformin increased the luciferase activity in HUVECs transfected with Bcl-6-Luc reporter. Adding the AMPK inhibitor Compound C with metformin blocked the luciferase activity. In contrast, HUVECs transfected with Bcl-6-Luc(Δ) maintained a high level of luminescence regardless of treatments. Parallel ChIP experiments (Fig. 2B) using primers specific to Bcl-6-Luc revealed that AICAR or metformin decreased the binding of PARP-1 to the Bcl-6 intron 1 compared with control and metformin + Compound C. The binding of PARP-1 to Bcl-6-Luc(Δ) was much less than Bcl-6-Luc under all conditions. To further confirm that AMPK phosphorylation of PARP-1 leads to Bcl-6 transcription, the Bcl-6-Luc–transfected HUVECs were infected with a virus encoding constitutively active AMPK (Ad-AMPK-CA) or a dominant negative form of AMPK (Ad-AMPK-DN). Bcl-6-Luc luciferase activity was greater in HUVECs infected with Ad-AMPK-CA than Ad-AMPK-DN; it was also higher in HUVECs transfected with phosphomimetic PARP-1 S177D than the PARP-1 S177A mutant (Fig. 2C).
Fig. 2.
Bcl-6 expression is enhanced by AMPK phosphorylation of PARP-1. (A and B) HUVECs were transfected with Bcl-6-Luc or Bcl-6-Luc(Δ) constructs, then treated with AICAR, metformin (met), or metformin + Compound C for luciferase assay or ChIP using anti-PARP-1 and primers for Bcl-6-Luc or Bcl-6-Luc(Δ). (C) HUVECs transfected with Bcl-6-Luc, infected with Ad-AMPK-CA or Ad-AMPK-DN, and cotransfected with S177A or S177D for luciferase activity. (D–F) Bcl-6 mRNA levels in HUVECs transfected with control, AMPKα, or PARP-1 siRNA, then treated with met or AICAR. (G) Bcl-6 expression in HUVECs treated with metformin or metformin + Compound C. (H) Bcl-6 expression in HUVECs transfected with control, AMPKα siRNA, or PARP-1 siRNA, then treated with AICAR. (I) Bcl-6 and Flag-PARP-1 expression in HUVECs transfected with PARP-1, S177A, or S177D, then treated with AICAR or AICAR + Compound C. *P < 0.05.
Since AMPK phosphorylation of PARP-1 increased Bcl-6 transcription, we measured endogenous Bcl-6 mRNA level upon AMPK activation. Consistent with the luciferase activity assay using the Bcl-6 reporter constructs, AMPK activation by metformin (Fig. 2D) or AICAR (Fig. 2E) increased Bcl-6 mRNA levels in HUVECs. AMPKα siRNA transfection abolished this induction (Fig. 2 D and E). Importantly, PARP-1 knockdown increased the level of Bcl-6 mRNA irrespective of treatment (Fig. 2F). Metformin and AICAR also increased Bcl-6 protein level (Fig. 2 G and H), and this was blocked upon AMPK inhibition with Compound C (Fig. 2G) or AMPK knockdown (Fig. 2H, Upper). Consistent with the results in Fig. 2F, PARP-1 knockdown produced a sustained increase in Bcl-6 (Fig. 2H, Lower). To demonstrate the effects of PARP-1 Ser-177 phosphorylation on Bcl-6 expression, we overexpressed the WT PARP-1, dephosphomimetic S177A, or phosphomimetic S177D in HUVECs. AICAR increased Bcl-6 expression in cells overexpressing WT PARP-1, but not in those expressing the S177A mutant (Fig. 2I, Upper). PARP-1 S177D still sustained high levels of Bcl-6 expression under AICAR treatment (Fig. 2I, Lower). Together, the data in Figs. 1 and 2 suggest that AMPK phosphorylation of PARP-1 at Ser-177 decreases the binding of PARP-1 to the Bcl-6 intron 1, thereby lifting the transcriptional repression of Bcl-6 mRNA.
AMPK-Dependent Induction of Bcl-6 Inhibits VCAM-1, MCP-1, and MCP-3 Transcription.
Bcl-6 is a transcriptional repressor of several adhesion molecules and inflammatory mediators, including VCAM-1, MCP-1, and MCP-3 (23, 24). Using ChIP, we observed increased Bcl-6 binding to the promoters of VCAM-1, MCP-1, and MCP-3 in HUVECs treated with AICAR or metformin, but not when AMPKα had been knocked down (Fig. 3A). Further, AICAR and metformin attenuated the mRNA expression of VCAM-1, MCP-1, and MCP-3, except when AMPKα was knocked down (Fig. 3B). To verify that AMPK modulates this Bcl-6–induced transcriptional repression via PARP-1, we knocked down PARP-1 to increase Bcl-6 expression, as illustrated in Fig. 2H. This led to inhibition of VCAM-1, MCP-1, and MCP-3 expression (Fig. 3C). In complementary experiments, knockdown of Bcl-6 enhanced the expression of VCAM-1, MCP-1, and MCP-3, with or without metformin (Fig. 3D).
Fig. 3.
AMPK induction of Bcl-6 attenuates the expression of VCAM-1, MCP-1, and MCP-3. (A and B) HUVECs were transiently transfected with control or AMPKα siRNA, then treated with AICAR or metformin for 1 h. ChIP assays were performed to determine the binding of Bcl-6 to VCAM-1, MCP-1, and MCP-3 promoters (A), and the abundance of VCAM-1, MCP-1, and MCP-3 mRNA was analyzed using qPCR (B). (C) HUVECs were transfected with control or PARP-1 siRNA, and levels of VCAM-1, MCP-1, or MCP-3 mRNA were determined. (D) HUVECs were transfected with control or Bcl-6 siRNA and then treated with or without metformin. Levels of VCAM-1, MCP-1, or MCP-3 mRNA were determined. (E) HUVECs were transfected with the MCP-1-Luc reporter (50 nM). After transfection, cells were infected with Ad-AMPK-CA or Ad-AMPK-DN [5 multiplicity of infection (MOI)] and lysed; luciferase activity was then measured. (F) Effects of AMPKα, PARP-1, and Bcl-6 knockdown and AICAR or metformin treatment on monocyte attachment to HUVECs. (G) Quantification of the results in F. *P < 0.05.
To confirm that AMPK phosphorylation of PARP-1 at Ser-177 suppresses these inflammatory genes, PARP-1 was knocked down in MEFs, which were then transfected with an MCP-1 promoter luciferase reporter together with the WT, S177A, or S177D PARP-1 constructs. After also infecting the cells with Ad-AMPK-CA or Ad-AMPK-DN, the MCP-1 promoter activity decreased in cells expressing activated AMPK and WT PARP-1 (Fig. 3E). MCP-1 promoter activity was higher in MEFs expressing S177A than those expressing S177D (Fig. 3E), regardless of coexpression of AMPK-CA or AMPK-DN. These results suggest that PARP-1 phosphorylation at Ser-177 plays a crucial role in suppressing MCP-1 transcription. Because VCAM-1, MCP-1, and MCP-3 are involved in recruiting monocytes to ECs, we investigated the AMPK–PARP-1–Bcl-6 cascade effect on monocyte recruitment to ECs. As illustrated in Fig. 3 F and G, monocyte adherence was prevented by AICAR and metformin, but increased by Compound C. Consistent with these findings, AMPKα and Bcl-6 knockdowns were associated with excessive monocyte–EC adhesion, while PARP-1 knockdown attenuated monocyte adhesion. The data in Fig. 3 suggest that the AMPK-induced phosphorylation of PARP-1 at Ser-177 reduces VCAM-1, MCP-1, and MCP-3 expression, thus attenuating monocyte recruitment to ECs.
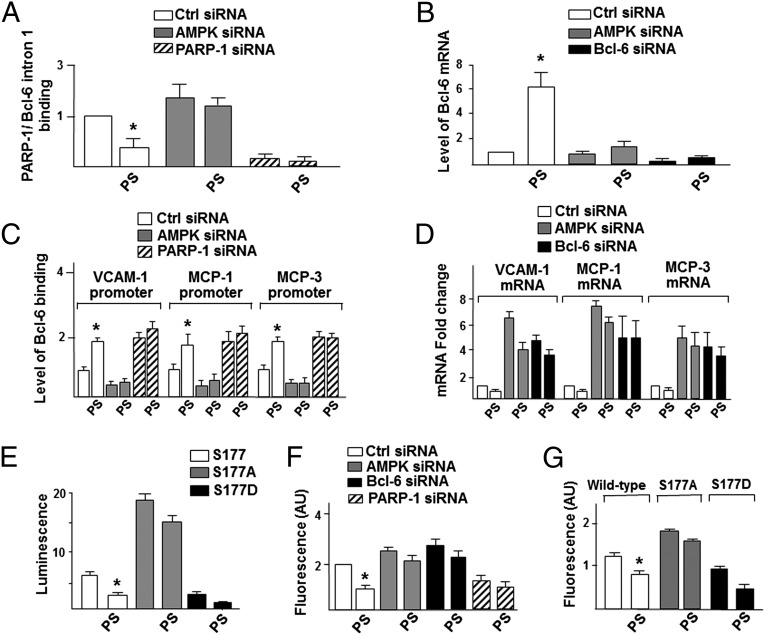
Pulsatile Shear Stress Induces the AMPK–PARP-1–Bcl-6 Anti-Inflammatory Cascade.
In the vasculature, ECs are subjected to various types of blood flow patterns. In particular, pulsatile flow (PS) activates AMPK (25) and is associated with anti-inflammatory effects (6–9). To explore the beneficial effect of the AMPK–PARP-1–Bcl-6 pathway under flow conditions, we examined the effects of PS on transactivation of Bcl-6 and inhibition of VCAM-1, MCP-1, and MCP-3. ChIP experiments confirmed that PS reduced PARP-1 binding to the Bcl-6 intron 1 (Fig. 4A) to induce Bcl-6 in ECs, but not when AMPKα was knocked down (Fig. 4B). As expected, PS also increased Bcl-6 binding to VCAM-1, MCP-1, and MCP-3 promoters and repressed their expression (Fig. 4 C and D). These effects were reverted if AMPKα or Bcl-6 had been knocked down (Fig. 4 C and D). Consistent with these findings, PS reduced the MCP-1 promoter activity in ECs cotransfected with WT (Fig. 4E) or S177D PARP-1, but not S177A PARP-1. In line with the results in Fig. 3G, PS also attenuated monocyte binding, but not when AMPKα and Bcl-6 had been knocked down. PARP-1 knockdown led to inhibition of monocyte adhesion (Fig. 4F). Furthermore, overexpression of PARP-1 S177D in ECs increased, while PARP-1 S177A decreased, monocyte adhesion under both static and PS conditions (Fig. 4G). Together, Fig. 4 confirms the role of PS-activated AMPK in its phosphorylation of PARP-1 in ECs to prevent monocyte recruitment.
Fig. 4.
PS induces the AMPK–PARP-1–Bcl-6 pathway. (A and B) HUVECs were transfected with control, AMPKα, PARP-1, or Bcl-6 siRNA and then subjected to PS for 2 h. A shows ChIP assays for the binding of PARP-1 to the Bcl-6 intron 1, and B is the level of Bcl-6 mRNA. (C) Cells were transfected with control, AMPKα, or PARP-1 siRNA and subjected to PS for 2 h. ChIP was performed to determine the binding of Bcl-6 to VCAM-1, MCP-1, and MCP-3 promoters. (D) Cells were transfected with control, AMPKα, or Bcl-6 siRNA and then subjected to PS for 2 h. The VCAM-1, MCP-1, and MCP-3 mRNA levels were quantified. (E) HUVECs were transfected with the MCP-1-Luc and FLAG–PARP-1, S177A, or S177D, and then subjected to PS, lysis, and luciferase activity measurement. (F) Cells were transiently transfected with control, AMPKα, PARP-1, or Bcl-6 siRNA, and then subjected to PS or static conditions and quantification of monocyte attachment. (G) Cells were transfected with FLAG–PARP-1, S177A, or S177D. After PS treatment, the monocyte attachment was quantified. *P < 0.05.
AMPK–PARP-1–Bcl-6 Pathway in the Vessel Wall in Vivo.
We next investigated whether the AMPK–PARP-1–Bcl-6 anti-inflammatory cascade is functional in the vasculature in vivo. Thoracic aortas were isolated from WT and AMPKα2−/− mice with or without metformin administration. ChIP assays indicated less PARP-1 bound to the Bcl-6 intron 1 in the aortas of metformin-treated WT than in the untreated control WT mice. This difference in PARP-1/Bcl-6 binding was not seen in the AMPKα2−/− mice (Fig. 5A). Consistent with this finding, Bcl-6 mRNA and protein levels increased with metformin treatment in the aortas of WT, but not AMPKα2−/−, mice (Fig. 5B). Because TNF-α is an inflammatory cytokine that mediates obesity-induced vascular inflammation, we tested whether metformin blocks TNF-α–induced VCAM-1, MCP-1, and MCP-3 expression. Compared with TNF-α alone, TNF-α administration with metformin increased Bcl-6 binding to the VCAM-1, MCP-1, and MCP-3 promoters in the aortas from WT, but not AMPKα2−/−, mice (Fig. 5C) to decrease expression of VCAM-1, MCP-1, and MCP-3 (Fig. 5D). Together, these results demonstrate that the AMPK–PARP-1–Bcl-6 anti-inflammatory cascade is functional in vivo.
Fig. 5.
AMPK regulates Bcl-6 transactivation and the subsequent repression of VCAM-1, MCP-1, MCP-3 in vivo. (A and B) AMPK+/+ and AMPKα2−/− mice were injected with saline (Ctrl) or metformin (200 mg/kg) for 5 h. (A) The level of PARP-1 binding to the Bcl-6 intron 1 in extracted aortic tissues was analyzed using ChIP followed by qPCR. (B) The levels of Bcl-6 mRNA and protein were assessed using qPCR and immunoblotting, respectively. (C and D) AMPK+/+ and AMPKα2−/− mice were injected with TNF-α (25 µg/kg) alone for 5 h or with TNF-α (25 µg/kg) for 1 h followed by metformin (200 mg/kg) for 4 h. (C) The level of Bcl-6 binding to the promoters of VCAM-1, MCP-1, and MCP-3 in extracted aortic tissues was assessed using ChIP analysis followed by qPCR. (D) mRNA levels for VCAM-1, MCP-1, and MCP-3 were then assessed in the aorta using qPCR. (E) A graphic illustration of the anti-inflammatory effect of the AMPK–PARP-1–Bcl-6 pathway.
Discussion
Aberrant activity and/or expression of PARP-1 are present in ECs under abnormally elevated levels of radical oxygen species (ROS), TNF-α, or angiotensin II to cause vascular inflammation (26–28). Such proinflammatory conditions are linked to decreases in AMPK activity and Bcl-6 expression. It has been shown that the binding of PARP-1 to the Bcl-6 intron 1 decreases Bcl-6 expression (11) and that PARP-1 knockdown or AMPK activation becomes anti-inflammatory in many cell types, including ECs. Here, we report an anti-inflammatory pathway that links AMPK, PARP-1, and Bcl-6 together. Our results indicate that AMPK phosphorylates PARP-1 at Ser-177 to decrease its binding to Bcl-6 intron 1. This decrease in binding increases Bcl-6 expression, thus promoting its function as a corepressor to attenuate the transcriptional induction of proinflammatory molecules (e.g., VCAM-1, MCP-1, and MCP-3) in ECs. As summarized in Fig. 5E, AMPK activation and/or PARP-1 deficiency mediates an anti-inflammatory phenotype. The AMPK–PARP-1–Bcl-6 pathway deduced in this study plays a key role in the functional consequences of AMPK activation, PARP-1 phosphorylation, and Bcl-6 induction to reduce inflammation in ECs.
In lipid-laden macrophages, proinflammatory PPARδ sequesters Bcl-6 and prevents it from corepressing MCP-1 and MCP-3 transcription (22, 23). Deletion of PPARδ in transplanted bone marrow–derived macrophages increases the availability of Bcl-6 to lead to reduction of atherosclerotic lesion area in recipient mice (22). Treatment with the PPARδ agonist GW0742 increases the free Bcl-6 level to inhibit angiotensin II–induced vascular inflammation (29). Our current study demonstrates an alternative pathway in which AMPK phosphorylation of PARP-1 also leads to an increase in the level of Bcl-6. This notion is supported by the reduced atherosclerosis in both AMPKα1−/−/ApoE−/− and PARP-1−/−/ApoE−/− mice (19, 30). Mechanistically, this pathway is supported by the current findings that AMPK phosphorylation of PARP-1 and knockdown of PARP-1 have similar effects on induction of Bcl-6 (Fig. 2H) and on inhibition of expression of inflammatory markers (Fig. 4D). AMPK α2 has been reported to inhibit NF-κB to attenuate inflammatory cascades (31). On the other hand, AMPKα1 has been suggested to mediate the expression of proinflammatory cytokines or anti-apoptotic proteins via NF-κB (32, 33). Thus, it is likely that AMPKα2 phosphorylates PARP-1 with a higher affinity.
NF-κB is a transcription factor that activates inflammatory genes including VCAM-1, MCP-1, and MCP-3. Although AMPKα2 and Bcl-6 attenuate NF-κB activity (8, 34), it is unlikely that NF-κB plays a functional role in mediating Bcl-6 transactivation, given that the Bcl-6 promoter does not contain a “GGGACTTTCC” consensus NF-κB enhancer element. Instead, PARP-1 may act as a transcriptional corepressor by binding to the Bcl-6 intron 1 (11), and dissociation of PARP-1 from Bcl-6 DNA would restore Bcl-6 transcription to suppress NF-κB activity (34). It is noted that ERK1/2 phosphorylation of PARP-1 at Ser-372 and Threonine 373 is important for maximal PARP-1 enzymatic activity, and that JNK phosphorylation of PARP-1 mediates H2O2-induced cell death (35). However, since AMPK activation attenuates the ERK1/2 and JNK pathways (36, 37), it is unlikely that ERK1/2 and JNK are involved in mediating PARP-1 binding to the Bcl-6 intron 1 and transactivation of Bcl-6.
Metformin has been shown to improve an array of inflammatory events, including diabetes mellitus and postsurgical endotoxin–induced inflammation (38, 39). Further, AICAR administration provides anti-inflammatory effects in vascular cells in vitro and in vivo (6, 9, 40). The nonuniform distribution of atheroma in the arterial tree underscores the importance of the local flow patterns in inflammation. Athero-protective flow patterns often elicit anti-inflammatory phenotypes of endothelium. Clinically, the pleiotropic effects of statin on the vessel are attributed to the anti-inflammatory effect and enhanced bioavailability of nitric oxide (NO). A common effect of metformin, AICAR, athero-protective flow, and statin on ECs is AMPK activation (25, 41–43). Thus, AMPK not only serves as a master regulator in energy homeostasis but also a key player in anti-inflammation.
Since AMPK activation and/or PARP-1 deficiency mediates an anti-inflammatory phenotype, the AMPK–PARP-1–Bcl-6 pathway can be implicated in the intervention of other inflammation-related diseases such as metabolic syndrome and insulin insensitivity. For example, adipose tissue plays an integral role in endothelial inflammation through the release of TNF-α and subsequent expression of inflammatory cytokines and adhesion molecules exacerbating obesity-related vascular disease (44). Consistent with our hypothesis, metformin exhibited the effects of the AMPK–PARP-1–Bcl-6 anti-inflammatory cascade in adipocytes to mitigate TNF-α–induced inflammation (Fig. S1).
As summarized in Fig. 5E, PARP-1 binding to Bcl-6 intron 1 suppresses the transcription of Bcl-6. Upon activation of AMPKα2, PARP-1 becomes phosphorylated, leading to its dissociation from the Bcl-6 intron 1. This dissociation leads to transcriptional up-regulation of Bcl-6 and subsequent corepression of VCAM-1, MCP-1, and MCP-3 to result in an anti-inflammatory phenotype.
Materials and Methods
Experimental methods are described briefly here and given in greater detail in SI Materials and Methods. All cells were cultured according to standard protocols. Immunoblotting and ChIP assays were conducted according to recommended protocols published by ABcam. Luciferase experiments were conducted in accordance with standard protocols from Promega Corporation (catalog no. E1910). Monocyte binding was assessed using a Cytoselect adhesion assay kit according to the manufacturer’s instructions. A Victor2 plate reader was used for all experiments requiring quantification of luminescence. qPCR experiments were conducting using a Bio-Rad CFX96 real-time PCR detection system according to the manufacturer’s instructions. All primers used in ChIP and standard qPCR experiments are displayed in Tables S1 and S2.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Zhen Chen and Shankar Subramaniam for thoughtful discussion. Brendan Gongol expresses his gratitude for years of wisdom and support from his father Steve Gongol. This study was supported in part by Grant HL89940 and HL93731 (to J.S.) and HL080518 and HL108735 (to S.C.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222051110/-/DCSupplemental.
References
- 1.Marui N, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murao K, et al. TNF-α stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276(2):791–796. doi: 10.1006/bbrc.2000.3497. [DOI] [PubMed] [Google Scholar]
- 3.Kirii H, et al. Lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 4.Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91(4):635–646. doi: 10.1038/clpt.2011.328. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: What have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 6.Ewart MA, Kohlhass CF, Salt IP. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to HAEC by AMPK. Arterioscler Thromb Vasc Biol. 2008;28:2255–2257. doi: 10.1161/ATVBAHA.108.175919. [DOI] [PubMed] [Google Scholar]
- 7.Nerstedt A, et al. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3) Diabetologia. 2010;53(11):2406–2416. doi: 10.1007/s00125-010-1856-z. [DOI] [PubMed] [Google Scholar]
- 8.Salminen A, Hyttinen JMT, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J Mol Med (Berl) 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Qiu J, Wang X, Zhang Y, Xia M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol. 2011;31(12):2897–2908. doi: 10.1161/ATVBAHA.111.237453. [DOI] [PubMed] [Google Scholar]
- 10.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP-1 and beyond. Nature. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrose HE, Papadopoulou V, Beswick RW, Wagner SD. Poly-(ADP-ribose) polymerase-1 (Parp-1) binds in a sequence-specific manner at the Bcl-6 locus and contributes to the regulation of Bcl-6 transcription. Oncogene. 2007;26(42):6244–6252. doi: 10.1038/sj.onc.1210434. [DOI] [PubMed] [Google Scholar]
- 12.Kong X, et al. Condensin I recruitment to base damage-enriched DNA lesions is modulated by PARP1. PLoS ONE. 2011;6(8):e23548. doi: 10.1371/journal.pone.0023548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashima L, et al. CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation. J Biol Chem. 2012;287(16):12975–12984. doi: 10.1074/jbc.M111.321828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muñoz-Gámez JA, et al. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5(1):61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- 15.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59(9):1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racz B, et al. Regulation of MKP-1 expression and MAPK activation by PARP-1 in oxidative stress: A new mechanism for the cytoplasmic effect of PARP-1 activation. Free Radic Biol Med. 2010;49(12):1978–1988. doi: 10.1016/j.freeradbiomed.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Oliver FJ, et al. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose)polymerase-1 deficient mice. EMBO J. 1999;18:444–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Soriano F, et al. Diabetic endothelial dysfunction: The role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7(1):108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 19.von Lukowicz T, et al. PARP1 is required for adhesion molecule expression in atherogenesis. Cardiovasc Res. 2008;78(1):158–166. doi: 10.1093/cvr/cvm110. [DOI] [PubMed] [Google Scholar]
- 20.Huynh KD, Bardwell VJ. The BCL-6 POZ domain and other POZ domains interact with the co-repressors N-CoR and SMRT. Oncogene. 1998;17(19):2473–2484. doi: 10.1038/sj.onc.1202197. [DOI] [PubMed] [Google Scholar]
- 21.Huynh KD, Fischle W, Verdin E, Bardwell VJ. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14(14):1810–1823. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CH, et al. Transcriptional repression of atherogenic inflammation: Modulation by PPARdelta. Science. 2003;302(5644):453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 23.Fan Y, et al. Suppression of pro-inflammatory adhesion molecules by PPAR-δ in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(2):315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 24.Toney LM, et al. BCL-6 regulates chemokine gene transcription in macrophages. Nat Immunol. 2000;1(3):214–220. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26(6):1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 26.Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol. 2008;28(4):711–717. doi: 10.1161/ATVBAHA.107.156406. [DOI] [PubMed] [Google Scholar]
- 27.Los M, et al. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13(3):978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Jiang Z, Lei P, Huang W, Yu X. Poly(ADP-ribose) polymerase-1 mediates angiotensin II-induced expression of plasminogen activator inhibitor-1 and fibronectin in rat mesangial cells. Kidney Blood Press Res. 2011;34(5):320–327. doi: 10.1159/000327344. [DOI] [PubMed] [Google Scholar]
- 29.Takata Y, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA. 2008;105(11):4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, et al. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: The role of uncoupling protein 2. PLoS ONE. 2011;6(9):e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ Res. 2010;106(6):1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-κ B pathway. J Immunol. 2007;179(8):5483–5492. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem. 2010;285(20):15346–15355. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Perez-Rosado A, et al. Bcl-6 represses NF-κB activity in diffuse large B-cell lymphomas. J Pathol. 2008;214:489–507. doi: 10.1002/path.2279. [DOI] [PubMed] [Google Scholar]
- 35.Luo X, Kraus WL. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26(5):417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata D, et al. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110(4):444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 37.Schulz E, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118(13):1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballero AE, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: A placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2004;89(8):3943–3948. doi: 10.1210/jc.2004-0019. [DOI] [PubMed] [Google Scholar]
- 39.Bergheim I, et al. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther. 2006;316(3):1053–1061. doi: 10.1124/jpet.105.092122. [DOI] [PubMed] [Google Scholar]
- 40.Li D, et al. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010;285(43):33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51(8):2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 42.Li X, et al. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(10):1789–1795. doi: 10.1161/ATVBAHA.108.172452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun W, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114(24):2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 44.Donato AJ, et al. TNF-α impairs endothelial function in adipose tissue resistance arteries of mice with diet-induced obesity. Am J Physiol Heart Circ Physiol. 2012;303(6):H672–H679. doi: 10.1152/ajpheart.00271.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.