Abstract
Elastic fiber assembly requires deposition of elastin monomers onto microfibrils, the mechanism of which is incompletely understood. Here we show that latent TGF-β binding protein 4 (LTBP-4) potentiates formation of elastic fibers through interacting with fibulin-5, a tropoelastin-binding protein necessary for elastogenesis. Decreased expression of LTBP-4 in human dermal fibroblast cells by siRNA treatment abolished the linear deposition of fibulin-5 and tropoelastin on microfibrils. It is notable that the addition of recombinant LTBP-4 to cell culture medium promoted elastin deposition on microfibrils without changing the expression of elastic fiber components. This elastogenic property of LTBP-4 is independent of bound TGF-β because TGF-β–free recombinant LTBP-4 was as potent an elastogenic inducer as TGF-β–bound recombinant LTBP-4. Without LTBP-4, fibulin-5 and tropoelastin deposition was discontinuous and punctate in vitro and in vivo. These data suggest a unique function for LTBP-4 during elastic fibrogenesis, making it a potential therapeutic target for elastic fiber regeneration.
Keywords: connective tissue, development, extracellular matrix
Elastic fibers are ubiquitous extracellular matrix (ECM) components responsible for tissue elasticity. Elastic fibers are composed of an amorphous core of cross-linked elastin and a mantle of fibrillin-rich microfibrils (1, 2). Degradation of elastic fibers causes emphysema, arterial stiffness, and loose skin. The turnover rate of elastic fibers is very low, and there is no known method to effectively regenerate elastic fibers. The process of elastic fiber assembly involves synthesis and secretion of tropoelastin (elastin monomer), formation of tropoelastin microaggregates, initial cross-linking of elastin, deposition of microaggregated elastin on microfibrils, followed by late cross-linking of elastin by lysyl oxidase (2–4). Microfibrils are composed of polymers of fibrillin-1 and -2 as well as several associated molecules, such as microfibril-associating glycoproteins and latent TGF-β–binding proteins (LTBPs) (2, 5, 6), but the roles of these associated molecules in elastic fiber assembly remain obscure. Although microfibrils provide a scaffold for elastin deposition, microfibrils can also form macroaggregates devoid of elastin (5). Therefore, a key question for elastic fiber formation and regeneration is what promotes elastin deposition on microfibrils.
Several microfibril-associating molecules have been implicated in elastogenesis, including LTBP-4. Mice lacking LTBP-4S, the major form of LTBP-4 in lung and intestine (7), were reported to show severe defects in elastic fiber formation in the intestine, lung, and pulmonary artery (8, 9), indicating crucial function of LTBP-4 in elastic fiber assembly. LTBPs are large ECM proteins that share structural homology with fibrillins, and play major roles in regulating TGF-β bioavailability. Although the TGF-β precursor protein is cleaved into the N-terminal prodomain called latency-associated peptide (LAP) and the C-terminal mature TGF-β, TGF-β is kept inactive by continued noncovalent association with LAP (TGF-β–LAP). In addition, LAP is bound to LTBPs through disulfide bonds; thus TGF-β is secreted from cells in the form of a large latent complex consisting of TGF-β–LAP-LTBP (6, 10, 11). Among the four members of the LTBP family, LTBP-1, -3, and -4 bind LAP and target latent TGF-β to extracellular matrix. LTBP-1, -2, and -3 are not considered necessary for elastogenesis as judged by the phenotypes of mice or humans deficient in these genes (6). LTBP4 homozygous mutations in humans cause defective elastic fiber formation, resulting in cutis laxa and emphysema (12). However, how LTBP-4 is involved in elastic fiber assembly is largely unknown.
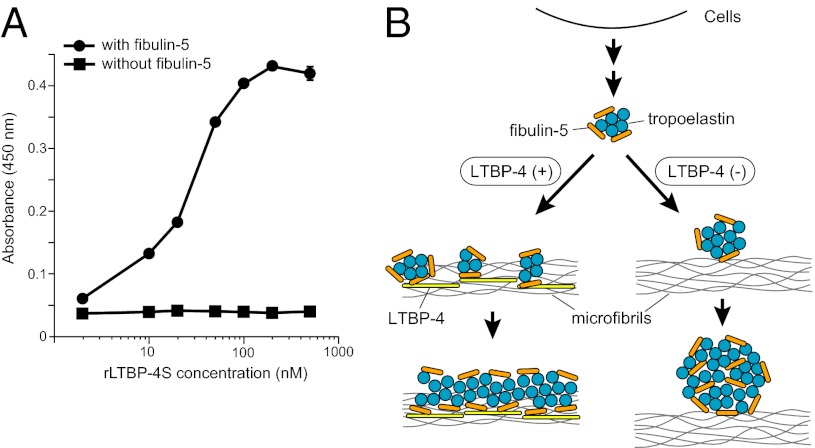
We and others previously reported that the secreted protein fibulin-5 is necessary for elastic fiber assembly (13, 14). Fbln5 (the fibulin-5 gene) knockout mice exhibit stiff and tortuous aortae, emphysematous lungs, and loose skin due to disorganized elastic fibers. Fibulin-5 interacts with both tropoelastin (14) and lysyl oxidase–like enzymes (15, 16), and facilitates elastin deposition onto microfibrils and subsequent cross-linking (16). To understand the molecular mechanism of fibulin-5–dependent elastin deposition on microfibrils, we examined the binding of fibulin-5 with microfibril-associated molecules. In addition to tropoelastin and lysyl oxidase–like enzymes, fibulin-5 interacts with several other molecules, such as integrins (13, 17, 18), elastin microfibril interface located protein (EMILIN) (19), apolipoprotein(a) (20), extracellular superoxide dismutase (21), LTBP-2 (22), and fibrillin-1 (23). However, these interactions do not account for the fiber organizing function of fibulin-5, because mice deficient in these molecules do not phenocopy Fbln5 knockout mice (24–27).
Here we report that LTBP-4 orchestrates elastin deposition onto microfibrils by binding with fibulin-5, which is bound to tropoelastin. We show that LTBP-4 interacts with fibulin-5 specifically through the four-cysteine domains of LTBP-4 and the C-terminal domain of fibulin-5. Knockdown of LTBP4 by siRNA in human dermal fibroblasts abolished linear deposition of fibulin-5 and elastin on microfibrils, yielding a small number of punctate aggregates of these proteins. It is intriguing to note that the addition of recombinant LTBP-4 to LTBP4 knockdown cell cultures strongly induced elastic fiber assembly to a level surpassing that observed in control cell cultures. We also demonstrate that the elastogenic activity of LTBP-4 is not dependent on associated TGF-β, but is attributed to LTBP-4 itself.
Results
LTBP-4 Interacts with Fibulin-5.
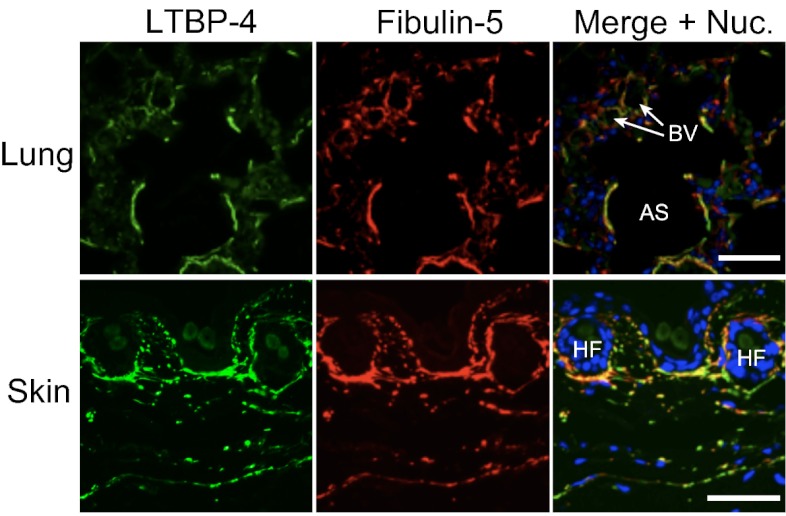
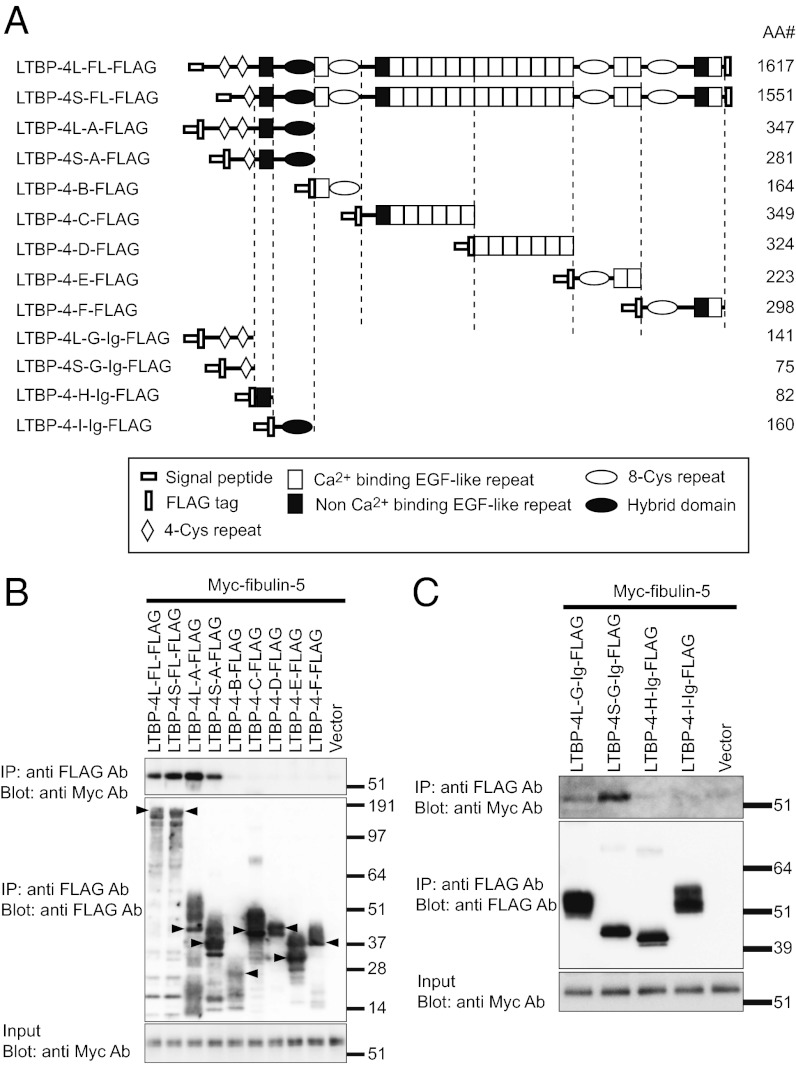
Elastic fibers have been reported to be defective in lungs, intestines, skin, and pulmonary arteries of Ltbp4S−/− mice (8, 9). We also found tortuous and stiff aortae with severely disorganized elastic lamellae in Ltbp4S−/− mice (Fig. S1 A–D). These phenotypes, together with the emphysematous lungs, were very similar to the phenotypes of Fbln5 knockout mice (13, 14). The phenotypic resemblance of Ltbp4 and Fbln5 knockout mice led us to study the interaction of fibulin-5 and LTBP-4. We found that LTBP-4 colocalizes with fibulin-5 in mouse lung and skin tissues (Fig. 1). In vitro binding assays using recombinant proteins of FLAG-tagged LTBP-4 and Myc-tagged fibulin-5 revealed interaction of these proteins (Fig. 2 A–C). When LTBP-4S and LTBP-4L, a transcript variant in the N-terminal region, were divided into five fragments, only the N-terminal fragments of LTBP-4S and -L interacted with fibulin-5 (Fig. 2B). We further subdivided the binding domain of LTBP-4 and fibulin-5 and found that the four-cysteine domains at the N terminus of LTBP-4S and L specifically interact with fibulin-5 (Fig. 2C). On the other hand, the C-terminal domain of fibulin-5 is involved in this interaction, because deletion of the C-terminal domain abolished the binding, and a C-terminal domain-Fc fusion protein was sufficient to bind with LTBP-4S (Fig. S2 A–C). These data demonstrate the specificity of binding between fibulin-5 and LTBP-4. The binding of fibulin-5 and LTBP-4 was confirmed with a solid-phase binding assay (Fig. S3A, and the purity of recombinant proteins was shown by SDS/PAGE in Fig. S4 A and B).
Fig. 1.
LTBP-4 colocalizes with fibulin-5 in mouse tissues. Immunostaining of WT mouse lung at P0 (sagittal section) and mouse skin at 8 wk using anti–LTBP-4 antibody and anti–fibulin-5 antibody, indicating colocalization of these molecules. The lung figure presents LTBP-4 and fibulin-5 localization in terminal air sacs (AS). The circular structures present small blood vessels (BV). (Scale bars, 50 µm.) HF, hair follicle.
Fig. 2.
In vitro binding assay, showing interaction of LTBP-4 with fibulin-5. (A) Domain structure of the full-length LTBP-4 and the LTBP-4 truncation mutants. These mutants were expressed as N-terminal FLAG-tagged proteins flanked by the preprotrypsin signal sequence. LTBP-4L-G, LTBP-4S-G, LTBP-4-H, and LTBP-4-I were constructed as C-terminal fusion proteins with the Fc region of human IgG. The amino acid lengths (AA#) of the constructs except IgG are indicated. The expression vectors were independently transfected into 293T cells. Transfected cells were cultured in serum-free medium for 48 h, and the cell lysates and the conditioned media were harvested. Mixtures of the media and cell lysates (B), or the media alone (C) were incubated with each other, and these reactants were subjected to immunoprecipitation with anti-FLAG antibody. The immunoprecipitants were separated by SDS/PAGE, and analyzed by Western blotting. (B) Interaction of fibulin-5 with full-length LTBP-4L and -4S and with the N-terminal domains of LTBP-4L and -4S, but not with other parts of LTBP-4. Expected bands for the LTBP-4 constructs are indicated by arrowheads. (C) Interaction of fibulin-5 with LTBP-4L and -4S fragments containing four-cysteine domains, but not with other N-terminal fragments.
Recombinant LTBP-4S Protein Promotes Elastic Fiber Assembly.
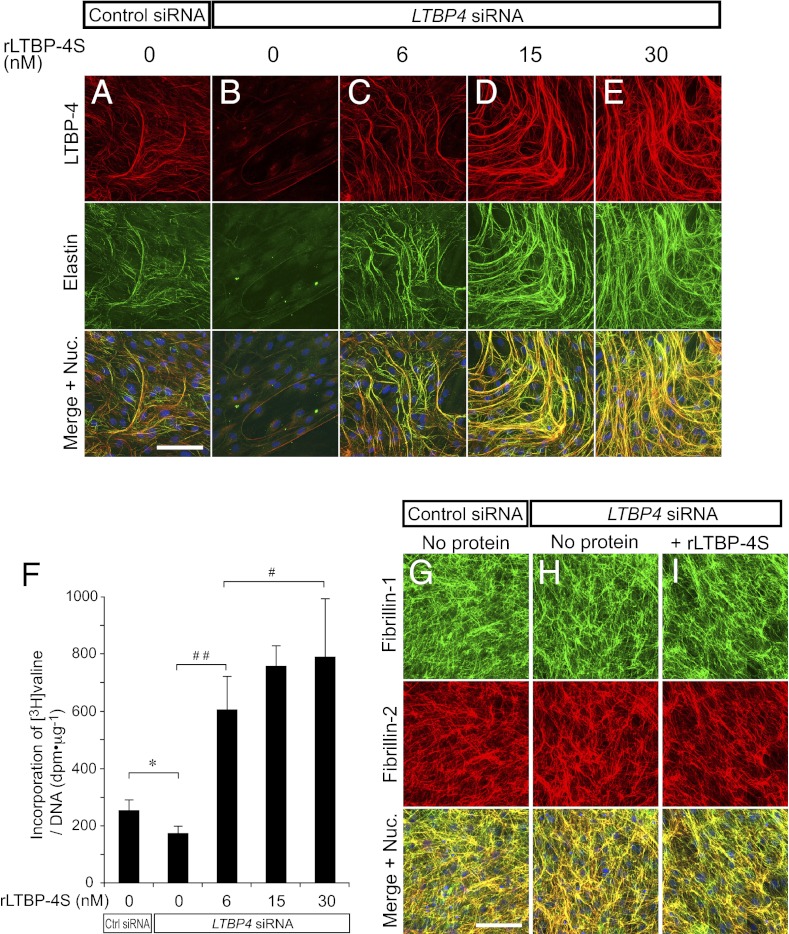
To study the function of LTBP-4 in elastic fiber assembly, we used neonatal human dermal fibroblasts (HDFs) that develop an elastic fiber meshwork when maintained at confluence (We use the term “elastic fibers” to refer to fibers containing linearly assembled and insoluble elastin as well as microfibrils that serve as scaffolds for linear deposition of elastin). We confirmed that gene knockdown with LTBP4 siRNA transfection achieved more than 90% reduction of LTBP4 mRNA expression at day 7 of culture, and remained at greater than 80% reduction at day 14 (Fig. S5A). Control siRNA transfection did not affect elastic fiber development (Fig. S6 A and B), as elastin was deposited linearly along LTBP-4–positive microfibrils in the ECM of control cells (the specificity of antibodies is shown in Fig. S4 C–E). However, LTBP4 knockdown abolished deposition of elastin (Fig. 3 A and B). The addition of rLTBP-4S at 6 nM restored linear deposition of elastin on microfibrils, excluding the possibility that the knockdown result was an off-target effect (Fig. 3C and Fig. S7C). To our surprise, the addition of rLTBP-4S at higher concentrations induced enhanced elastic fiber assembly in a dose-dependent manner to levels that exceeded assembly observed in control cells (Fig. 3 C–E). Elastin staining fully colocalized with LTBP-4 staining, and there was virtually no linear elastin deposition on LTBP-4–negative microfibrils (Fig. 3 A–E and Fig. S7 A–E). To quantify the amount of mature (i.e., cross-linked) elastic fibers induced by rLTBP-4S, we metabolically labeled newly synthesized elastin with [3H]-valine, and measured the incorporation of [3H]-valine into the culture NaOH-insoluble fraction, which corresponds to the amount of mature elastic fibers (28). As shown in Fig. 3F, we detected significant, dose-dependent incorporation of [3H]-valine into the insoluble fraction upon adding rLTBP-4 to the medium. This result demonstrated that the elastin-positive fibers induced by rLTBP-4 were mature and cross-linked. No overt differences in the configuration of microfibrils were observed after LTBP4 knockdown or addition of rLTBP-4S, as indicated by fibrillin-1 and -2 antibody staining (Fig. 3 G–I), suggesting that rLTBP-4S turned elastin-negative microfibrils to elastin-positive fibers. Moreover, LTBP4 knockdown or addition of rLTBP-4S did not affect the mRNA expression levels of elastic fiber components including FBN1 (fibrillin-1), FBLN4 and -5 (fibulin-4 and -5), LOX (lysyl oxidase), and LOXL1 (lysyl oxidase-like 1), whereas ELN (elastin) mRNA modestly increased 4 d after LTBP4 knockdown (Fig. S5C). Abrogated elastin deposition in the absence of LTBP-4 and enhanced elastic fiber assembly by rLTBP-4S was also observed in cultures of adult HDFs (Fig. S6 C–E) and of wild-type and Ltbp4S−/− mouse embryonic fibroblasts (MEFs) (Fig. S8 A–C). These data indicate that a substantial amount of microfibrils were formed and sufficient amounts of other elastic fiber components were expressed even in the absence of LTBP-4: however, microfibrils and elastic fiber components including elastin could not be assembled as elastic fibers without LTBP-4. Upon addition of rLTBP-4S, elastin and added rLTBP-4S colocalized with fibrillin-1 (Figs. S7 and S8I). This suggests that LTBP-4 promoted elastic fiber assembly by facilitating tropoelastin deposition on microfibrils (we term this property of LTBP-4 an “elastogenic” property).
Fig. 3.
Recombinant LTBP-4S protein (rLTBP-4S) promotes elastic fiber assembly without changing the configuration of microfibrils. (A–E) HDFs were transfected with control siRNA or LTBP4 siRNA and cultured in 10% serum-containing media supplemented with rLTBP-4S as indicated. The cells were fixed 13 d after transfection and stained with anti–LTBP-4 and anti-elastin antibodies. Bottom images were produced by superimposition of the Top and Middle images, together with Hoechst 33258 nuclear staining. Note even more elastic fiber assembly by rLTBP-4S-supplemented LTBP4 knockdown (KD) cells than control KD cells, which express a normal level of LTBP-4. (Scale bar, 100 µm.) Nuc., nuclei. (F) Quantitation of insoluble (i.e., cross-linked and mature) elastin produced by the knockdown cells cultured with the indicated amounts of rLTBP-4S added to the medium. Cells were metabolically labeled with [3H]valine during the culture period, and the radioactivity of NaOH-insoluble fractions was quantitated. The radioactivity count was corrected by the amount of total DNA of the cells of duplicate plates. Insoluble elastin was significantly decreased in LTBP4 KD culture (*P = 0.0006, Student's t test; n = 6, Ctrl KD; n = 8, LTBP4 KD). Addition of rLTBP-4S in LTBP4 KD culture increased insoluble elastin in a dose-dependent manner above the level of Ctrl KD (#P < 0.05, ##P < 0.01, analysis of variance, Tukey test, n = 8). Error bars represent SD; Ctrl, control. (G–I) HDFs were transfected with control siRNA or LTBP4 siRNA and cultured in 10% serum containing media with or without rLTBP-4S (30 nM). The cells were fixed 13 d after transfection and stained with anti–fibrillin-1 and -2 antibodies. (Scale bar, 100 µm.)
Recombinant LTBP-4S Protein Contains TGF-β–LAP.
We next investigated whether the elastogenic property of LTBP-4 is due to the function of LTBP-4 itself or to TGF-β associated with LTBP-4, as TGF-β is reported to increase expression of ECM components including elastic fibers (29), and LTBP-4 is reported to be a modulator of extracellular TGF-β levels (12, 30). Because we obtained rLTBP-4S from 293T cells stably expressing LTBP-4S, the rLTBP-4S might contain considerable amounts of TGF-β–LAP endogenously expressed by 293T cells, although LTBP-4 is reported to bind much less TGF-β–LAP than do LTBP-1 or -3 (31, 32). To assess the amount of TGF-β bound to rLTBP-4S, we heated rLTBP-4S to release active TGF-β and measured it using a cell-based TGF-β bioassay system (33). We determined that the TGF-β activity bound to 48 nM of rLTBP-4S corresponded to that of 52 pM of active TGF-β1 (Fig. S9A), a concentration sufficient to induce mRNA expression of ECM components (34). Although addition of recombinant LTBP-4S did not affect the expression of elastic fiber components at 4 d of culture (Fig. S5C), it is difficult to know if this is the case throughout the culture period. Instead of monitoring all of the components throughout the culture period, we decided to make recombinant LTBP-4 without TGF-β, thereby eliminating the possible contribution of TGF-β by LTBP-4. To remove TGF-β activity from rLTBP-4S, we coexpressed LTBP-4 and an engineered empty LAP (eLAP) that does not contain TGF-β by introducing a stop codon at the cleavage site between LAP and TGF-β1. We presumed that the overexpressed eLAP in 293T cells would bind rLTBP-4S, excluding the binding of endogenous TGF-β–LAP in a dominant negative manner. As expected, rLTBP-4S purified from eLAP-overexpressing cells (rLTBP-4S + eLAP) did not show TGF-β activity (Fig. S9A).
Elastogenic Activity of LTBP-4 Is Independent from Bound TGF-β.
To assess whether TGF-β bound to rLTBP-4S accounts for the elastogenic activity of rLTBP-4S, we first tested the effect of active TGF-β1 on LTBP4 knockdown HDFs. Addition of active TGF-β1 to the culture medium at 60 pM, which is a higher concentration of TGF-β than in medium containing rLTBP-4S at 30 nM, did not restore elastic fiber assembly (Fig. S9F); whereas, the addition of 30 nM of rLTBP-4S induced more elastic fiber assembly than that observed in cultures of control cells (Fig. S9D). Next, we added 30 nM of rLTBP-4S + eLAP to the culture medium of LTBP4 knockdown HDFs, and found that HDFs supplemented with rLTBP-4S + eLAP induced as much elastic fiber assembly as HDFs supplemented with rLTBP-4S (Fig. S9 D and E). These data suggest that the elastogenic property of LTBP-4 is not dependent on associated TGF-β, but can be attributed to LTBP-4 itself.
LTBP-4 Is Required for Linear Deposition of Fibulin-5 and Elastin on Microfibrils.
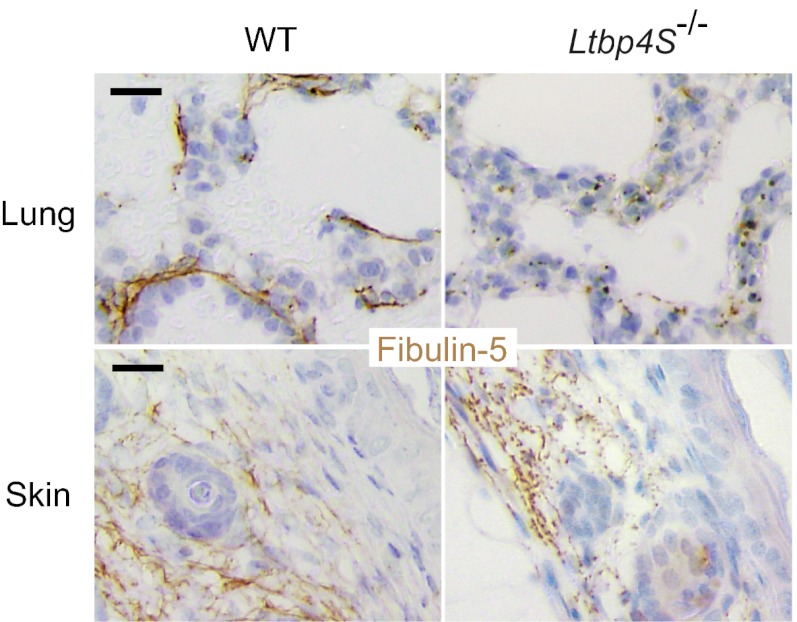
Immunostaining for fibulin-5 and LTBP-4 in the ECM of HDFs revealed that fibulin-5 specifically colocalized with LTBP-4 on microfibrils (Fig. 4A). When LTBP4 was knocked down by siRNA, deposition of fibulin-5 was abolished (Fig. 4B). The addition of rLTBP-4S not only rescued but augmented the linear deposition of fibulin-5 (Fig. 4C). This was not accompanied by an increase of FBLN5 mRNA expression (Fig. S5C), which suggests that LTBP-4 is necessary for fibulin-5 deposition on microfibrils. A requirement for LTBP-4 for fibulin-5 deposition was also observed in cultures of adult HDFs (Fig. S6 F–H) and of MEFs from Ltbp4S−/− and wild-type mouse embryos (Fig. S8 D–F). On the other hand, fibulin-5 is not necessary for LTBP-4 deposition, because knockdown of FBLN5 did not affect deposition of LTBP-4 (Fig. 4E). Although FBLN5 knockdown and LTBP4 knockdown both resulted in abrogated fibulin-5 deposition, there were clear differences when recombinant fibulin-5 (rFibulin-5) was added in the culture media. The added rFibulin-5 in FBLN5 knockdown cell cultures deposited linearly on microfibrils positive for LTBP-4 staining (Fig. 4F). However, when rFibulin-5 was added to LTBP4 knockdown cell cultures, the added protein did not deposit linearly and displayed primarily a punctate pattern (Fig. 4D). The addition of rFibulin-5 to LTBP4 knockdown cells also caused increased elastin deposition, but only in a punctate pattern (Fig. S10D). We observed this punctate deposition in the ECM of LTBP4 knockdown cells even without rFibulin-5 addition, although the amount of aggregates was very low (Fig. 3B and Fig. S10B). The punctate deposition of elastin in the absence of LTBP-4 was far less efficient than the linear deposition of elastin in the presence of LTBP-4 found in the ECM of control cells (Fig. S10 A and B). To substantiate and refine the light microscopy evidence of linear and punctuated protein deposition, we examined by electron microscopy the matrix made by LTBP4 knockdown cell culture, with or without recombinant LTBP-4 or fibulin-5. Tannic acid was used to stain elastin. Fig. S10 E–G shows that microfibrils of LTBP4 knockdown cultures were not stained by tannic acid and addition of recombinant LTBP-4 caused dark staining of microfibril bundles with fine elastin particles; whereas larger aggregates of elastin were observed in the cultures supplemented with recombinant fibulin-5. As shown in Fig. 5, fibulin-5 immunostaining in the skin and the lung of Ltbp4S−/− mice exhibits a punctate pattern, in contrast to the linear staining pattern along microfibrils in wild-type mice tissues. Therefore, loss of LTBP-4 abolishes linear deposition and causes punctate deposition of fibulin-5 both in vitro and in vivo.
Fig. 4.
LTBP-4 is necessary for linear deposition of fibulin-5 to microfibrils. HDFs were transfected with control siRNA (A), LTBP4 siRNA (B–D), or FBLN5 siRNA (E, F), and cultured for 13 d in 10% serum containing media with or without recombinant proteins as indicated [rLTBP-4S 30 nM (C) or rFibulin-5 60 nM (D, F)], followed by immunostaining with anti–LTBP-4 and anti–fibulin-5 antibodies. Bottom images were produced by superimposition of the Top and Middle images, together with Hoechst 33258 nuclear staining. Note the punctate pattern of rFibulin-5 deposition on microfibrils in LTBP4 KD culture, in contrast to the linear deposition of rFibulin-5 in the presence of LTBP-4.
Fig. 5.
Punctate pattern of fibulin-5 deposition in the absence of LTBP-4 in vivo. Immunohistochemistry of the lung and the skin tissues from 7-d-old wild-type and Ltbp4S−/− mice was performed using anti–fibulin-5 antibody. (Scale bars, 20 µm.)
LTBP-4 Interacts with Tropoelastin Indirectly Through Binding of LTBP-4 with Fibulin-5 and Fibulin-5 with Tropoelastin.
Finally, we examined whether the ability of LTBP-4 to facilitate tropoelastin deposition to microfibrils is mediated by direct interaction of LTBP-4 and tropoelastin. We performed solid-phase binding assays using recombinant tropoelastin as an immobilized protein and rLTBP-4S and rFibulin-5 as soluble ligands. As shown in Fig. S3B, rLTBP-4S did not interact with tropoelastin, whereas rFibulin-5 strongly interacted with tropoelastin. However, rLTBP-4S efficiently bound to the tropoelastin-coated plate in the presence of rFibulin-5 (Fig. 6A), indicating that LTBP-4 binding to tropoelastin is dependent on fibulin-5. Together with the result that LTBP-4 interacts with fibulin-5 (Fig. 2, Figs. S2 and S3A), this result suggests that LTBP-4 does not tether tropoelastin directly, but through interaction of LTBP-4 with fibulin-5 and fibulin-5 with tropoelastin.
Fig. 6.
LTBP-4 does not directly interact with elastin, but interacts with fibulin-5-bound elastin. (A) Solid-phase binding assays using recombinant tropoelastin as an immobilized protein and rLTBP-4S as a soluble ligand, in the presence (500 nM) or absence of rFibulin-5 (means ± SD, n = 3). (B) A proposed model for the role of LTBP-4–fibulin-5 interaction in elastic fiber assembly. In the presence of LTBP-4, microaggregation of tropoelastin, which is tethered by fibulin-5, deposits linearly on microfibrils. Subsequent coalescence of tropoelastin takes place on microfibrils, resulting in thickening of elastic fibers. In the absence of LTBP-4, tropoelastin/fibulin-5 complex cannot linearly deposit on microfibrils, and misplaced aggregates of tropoelastin/fibulin-5 grow to form globular structures.
Discussion
In this study, we investigated the essential role of LTBP-4 in elastic fiber assembly. Our proposed model for the molecular mechanism of elastic fiber organization by LTBP-4 is illustrated in Fig. 6B. In the presence of LTBP-4, elastin, which is tethered by fibulin-5, deposits linearly on microfibrils through direct interaction of LTBP-4 with fibulin-5. In the absence of LTBP-4, the elastin/fibulin-5 complex cannot linearly deposit on microfibrils, and misplaced aggregates of elastin/fibulin-5 grow to form globular structures. It was reported that subcritical concentrations (120 µg/mL) of tropoelastin spontaneously aggregate to form ∼200–300-nm particles at 37 °C (35). Indeed, by electron microscopy we observed 200–300-nm aggregates of elastin in LTBP4 knockdown HDF cultures supplemented with rFibulin-5 (Fig. S10G). LTBP-4 may prevent this globular aggregation by trapping fibulin-5/elastin complex on microfibrils before the aggregates grow, thereby permitting subsequent coalescence of elastin to occur on microfibrils. This results in thickening of elastic fibers. It is also possible that LTBP-4 controls self-association of elastin by an unknown mechanism. In either case, LTBP-4 is the molecule necessary for linear assembly of elastin on microfibrils through interaction with fibulin-5. It has been reported that elastin appears to be virtually excluded from microfibril bundles in both Ltbp4S−/− mice (9, 36) and Fbln5−/− mice (37) as revealed by transmission electron microscopy. Similar observations were made in the tissues of human patients homozygous for LTBP4 and FBLN5 mutations, both of which yield cutis laxa, a loose skin syndrome (12, 38). Our model explains why LTBP-4 deficiency and fibulin-5 deficiency cause very similar phenotypes even at the electron microscopic level.
It was reported that fibulin-5 directly interacts with the N-terminal fragments of fibrillin-1 with high affinity (23, 39), which seems to be inconsistent with our result that fibulin-5 deposits poorly in a linear configuration on microfibrils in the absence of LTBP-4. However, the binding affinity between fibulin-5 and fibrillin-1 fragments may not be proportional to the interaction efficiency between fibulin-5 and fibrillin-1 microfibrils in cell culture, because the N terminus of fibrillin-1 is considered to be folded and bound with the C terminus of a second fibrillin-1 (40), and the binding sites of fibrillin-1 for LTBP-4 and for fibulin-5 are not the same (41). Therefore, the fibrillin-1–binding site for fibulin-5 might be hidden, whereas the binding site for LTBP-4 might be available when assembled as microfibrils. It is also possible that LTBP-4 might interact with microfibrils through binding with an intervening molecule, such as fibronectin, that can interact with fibrillin-1 at a site different from the N terminus (42). Nevertheless, granular aggregates of elastin and fibulin-5 observed in the absence of LTBP-4 seemed to associate with microfibrils, perhaps indicating residual binding of fibrillins and fibulin-5 or of fibrillins and tropoelastin (43).
We previously reported that LTBP-2 interacts with fibulin-5 and regulates elastic fiber assembly (22). In that report, we showed that LTBP-2 prevents fibulin-5 deposition on fibrillin-1–free microfibrils, and promotes fibulin-5 deposition on fibrillin-2–free microfibrils. Unlike LTBP-4, LTBP-2 knockdown in human dermal fibroblasts did not reduce fibulin-5 and elastin deposition on microfibrils, and recombinant LTBP-2 did not promote fibulin-5 and elastin deposition on microfibrils unless fibrillin-2 was knocked down. Therefore, in human dermal fibroblast cultures, LTBP-2 does not affect fibulin-5 and elastin deposition unless either fibrillin-1 or -2 is knocked down. As we did not knock down fibrillins in our current study, microfibrils made by the cells contain both fibrillin-1 and -2. Therefore, the presence of LTBP-2 did not affect fibulin-5 and elastin deposition.
Among the four LTBP family members, LTBP-1 and -3 strongly interact with LAP, through their third 8-cysteine domain (6). The same domain of LTBP-4 interacts only weakly with LAP (32), and LTBP-2 does not bind with LAP (31). We have shown that a small amount of TGF-β is associated with rLTBP-4S purified from 293T cells overexpressing LTBP4S cDNA, consistent with previous reports. Although LTBP-4 does not bind LAP as effectively as LTBP-1 or -3, lung fibroblasts from Ltbp4S−/− mice and skin fibroblasts from LTBP4 mutant humans were reported to show either decreased or increased activation of TGF-β, respectively, indicating that LTBP-4 is a modulator of extracellular TGF-β activity (12, 30). Therefore, it is important to distinguish between TGF-β–dependent and independent functions of LTBP-4. Our data showing hyperassembly of elastic fibers after the addition of rLTBP-4S and rLTBP-4S + eLAP, but not TGF-β, to LTBP4 knockdown cells demonstrate that the elastogenic activity of LTBP-4 is independent from bound TGF-β. These data substantiate the data of Dabovic and coworkers (9) demonstrating that the defects in TGF-β and in elastin observed in Ltbp4S−/− mice are separable.
In this study, we did not address potential cellular involvement in elastic fiber assembly. It is reported that globular elastin aggregates are actively remodeled by cells to form linear fiber structures (44). In addition, fibrillins are ligands of integrins and cell surface heparan sulfate proteoglycans (45, 46). Further study is needed to reconcile possible cellular involvement and our model for elastic fiber assembly.
Degradation of elastic fibers causes not only loose skin, but also aging-related diseases, such as emphysema, stiff arteries, and pelvic prolapse (47, 48). However, there is no known therapeutic intervention to regenerate elastic fibers. Although TGF-βs were reported to increase the amount of ECM proteins including elastic fibers (29), TGF-βs are multipotent cytokines that also are important modulators of cell growth, inflammation, and apoptosis (49, 50). Moreover, excessive TGF-β activity is reported to be deleterious in many disease states (49–51). LTBP-4S + eLAP provides a way to specifically increase elastic fibers without activating the TGF-β pathway. Our finding that LTBP-4 increases elastic fibers by promoting elastin deposition on microfibrils could be an approach toward regenerative treatment for diseases caused by elastic fiber degradation.
Materials and Methods
Mice.
All mice used in this study were maintained on normal laboratory diet. Ltbp4S−/− mice were previously described (8). All procedures were conducted according to the Guideline for Animal Experimentation at Kansai Medical University and the regulations of New York University Langone Medical Center Institutional Animal Care and Use Committee.
Antibodies and Immunodetection.
Primary antibodies used were: mouse anti-elastin monoclonal (1:100; Millipore), rabbit anti–fibrillin-1 polyclonal (1:100; Elastin Products Company), mouse anti-fibrillin-2 monoclonal (1:100, provided by L. Sakai), rabbit anti–fibulin-5 polyclonal (1:2,000, provided by T. Sasaki (Max Planck Institute for Biochemistry, Martinsried, Germany), Fig. 1; 1:100, previously described, ref. 16, Fig. 5), mouse anti–fibulin-5 monoclonal (1:200, previously described, ref. 22, Fig. 4, Figs. S4, S6, and S8), goat anti–LTBP-4 polyclonal (1:40 or 1:200; R&D Systems, Fig. 1, Fig. S8), rabbit anti–LTBP-4 polyclonal (1:200, made by immunizing rabbits with recombinant human LTBP-4, Figs. 3 and 4, Figs. S4, S6, S9, and S10) antibodies. See SI Materials and Methods for details.
Cell Culture.
The 293T cells, HDFs, and MEFs were maintained in DMEM (Invitrogen) supplemented with 2 mM glutamine, 100 units/100 mg⋅mL−1 penicillin/streptomycin, and 10% FBS at 37 °C in 5% CO2. HDFs from facial tissue excised for cleft lip repair of a 3-mo-old patient were provided by M.N. Adult HDFs were from a 56-y-old Caucasian (Cell Applications). Studies of human skin samples were approved by the ethical commission of Kansai Medical University and Kyoto University, and patients' informed consent was obtained. See SI Materials and Methods for details.
In Vitro Binding Assay.
The 293T cells were transfected using LipofectAMINE PLUS (Invitrogen) or FuGENE HD (Promega). After transfection, they were cultured in serum-free DMEM/F12 (Invitrogen). The mixture of conditioned media and cell lysates (Fig. 2B, Fig. S2 B and C) or conditioned media (Fig. 2C) were subjected to immunoprecipitation with anti-FLAG M2 affinity gel followed by Western blotting as described previously (22).
RNAi.
Duplex RNA oligonucleotides (Stealth Select RNAi) were purchased from Invitrogen. Control oligonucleotides were designed as a scrambled sequence of protein phosphatase PP2C gamma, a gene irrelevant to the extracellular matrix. Mixed siRNA duplexes (at final concentration 167 pM) were reverse transfected into HDFs using Lipofectamine RNAiMAX (Invitrogen). See SI Materials and Methods for details.
Solid-Phase Binding Assays.
Various concentrations of recombinant FLAG-tagged LTBP-4S with or without 500 nM (30 μg mL−1) of recombinant Myc-tagged fibulin-5 were used as soluble ligands. Solid-phase binding assays using purified tropoelastin were performed as previously described (22). The primary antibody was anti-FLAG M2 antibody (Sigma-Aldrich), and the secondary antibody was HRP-conjugated anti-mouse IgG antibody (Pierce). Signals were detected with Substrate Reagent Pack (R&D Systems).
Statistics.
Results are expressed as the mean ±SD and statistical analysis was performed by Student's t test or Tukey test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Ms. A. Kimura for technical assistance. This work was supported by the Funding Program for Next Generation World-Leading Researchers in Japan, Japan Society for the Promotion of Science, Japan Science and Technology Agency, and Takeda Science Foundation (to T.N.), by Global Centers of Excellence Program “Center for Frontier Medicine,” Ministry of Education, Sport, Culture, Science, and Technology, Japan (to K.N.), and by the National Institutes of Health and National Marfan Foundation (to D.B.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215779110/-/DCSupplemental.
References
- 1.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: The elastic fiber. FASEB J. 1993;7(13):1208–1218. [PubMed] [Google Scholar]
- 2.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115(Pt 14):2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 3.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81(4):229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 4.Sato F, et al. Distinct steps of cross-linking, self-association, and maturation of tropoelastin are necessary for elastic fiber formation. J Mol Biol. 2007;369(3):841–851. doi: 10.1016/j.jmb.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: Multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19(2):151–154. doi: 10.1152/physiolgenomics.00092.2004. [DOI] [PubMed] [Google Scholar]
- 6.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113(2):410–418. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantola AK, Ryynänen MJ, Lhota F, Keski-Oja J, Koli K. Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol. 2010;223(3):727–736. doi: 10.1002/jcp.22082. [DOI] [PubMed] [Google Scholar]
- 8.Sterner-Kock A, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16(17):2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabovic B, et al. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219(1):14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J Biol Chem. 2005;280(9):7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 11.Koli K, Saharinen J, Hyytiäinen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52(4):354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Urban Z, et al. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85(5):593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa H, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 16.Hirai M, et al. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176(7):1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999;274(32):22476–22483. doi: 10.1074/jbc.274.32.22476. [DOI] [PubMed] [Google Scholar]
- 18.Lomas AC, et al. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem J. 2007;405(3):417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanetti M, et al. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol. 2004;24(2):638–650. doi: 10.1128/MCB.24.2.638-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapetanopoulos A, et al. Direct interaction of the extracellular matrix protein DANCE with apolipoprotein(a) mediated by the kringle IV-type 2 domain. Mol Genet Genomics. 2002;267(4):440–446. doi: 10.1007/s00438-002-0673-6. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen AD, et al. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95(11):1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 22.Hirai M, et al. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007;26(14):3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman LJ, et al. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388(Pt 1):1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouvard D, et al. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89(3):211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Sheppard D. Identification and molecular characterization of multiple phenotypes in integrin knockout mice. Methods Enzymol. 2007;426:291–305. doi: 10.1016/S0076-6879(07)26013-6. [DOI] [PubMed] [Google Scholar]
- 26.Shipley JM, et al. Developmental expression of latent transforming growth factor beta binding protein 2 and its requirement early in mouse development. Mol Cell Biol. 2000;20(13):4879–4887. doi: 10.1128/mcb.20.13.4879-4887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carta L, et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281(12):8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinek A, Zhang S, Smith AC, Callahan JW. Impaired elastic-fiber assembly by fibroblasts from patients with either Morquio B disease or infantile GM1-gangliosidosis is linked to deficiency in the 67-kD spliced variant of beta-galactosidase. Am J Hum Genet. 2000;67(1):23–36. doi: 10.1086/302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann N Y Acad Sci. 1990;580:225–232. doi: 10.1111/j.1749-6632.1990.tb17931.x. [DOI] [PubMed] [Google Scholar]
- 30.Koli K, et al. Disruption of LTBP-4 function reduces TGF-beta activation and enhances BMP-4 signaling in the lung. J Cell Biol. 2004;167(1):123–133. doi: 10.1083/jcb.200403067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11(8):2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J Mol Biol. 2005;345(1):175–186. doi: 10.1016/j.jmb.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Abe M, et al. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216(2):276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 34.Ignotz RA, Endo T, Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262(14):6443–6446. [PubMed] [Google Scholar]
- 35.Tu Y, Weiss AS. Transient tropoelastin nanoparticles are early-stage intermediates in the coacervation of human tropoelastin whose aggregation is facilitated by heparan sulfate and heparin decasaccharides. Matrix Biol. 2010;29(2):152–159. doi: 10.1016/j.matbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Dabovic B, et al. Control of lung development by latent TGF-β binding proteins. J Cell Physiol. 2011;226(6):1499–1509. doi: 10.1002/jcp.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, et al. Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix Biol. 2009;28(4):211–220. doi: 10.1016/j.matbio.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Q, et al. Fibulin-5 mutations: Mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15(23):3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 39.El-Hallous E, et al. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J Biol Chem. 2007;282(12):8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- 40.Marson A, et al. Homotypic fibrillin-1 interactions in microfibril assembly. J Biol Chem. 2005;280(6):5013–5021. doi: 10.1074/jbc.M409029200. [DOI] [PubMed] [Google Scholar]
- 41.Ono RN, et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem. 2009;284(25):16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314(13):2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Trask TM, et al. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem. 2000;275(32):24400–24406. doi: 10.1074/jbc.M003665200. [DOI] [PubMed] [Google Scholar]
- 44.Kozel BA, et al. Elastic fiber formation: A dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207(1):87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- 45.Sabatier L, et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20(3):846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez F, Dietz HC. Fibrillin-rich microfibrils: Structural determinants of morphogenetic and homeostatic events. J Cell Physiol. 2007;213(2):326–330. doi: 10.1002/jcp.21189. [DOI] [PubMed] [Google Scholar]
- 47.Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordrecht, Neth.) 2009;31(4):305–325. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168(2):519–528. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 50.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: The dark side of tissue repair. J Clin Invest. 1992;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holm TM, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332(6027):358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.