Abstract
The biological safety cabinet is the one piece of laboratory and pharmacy equipment that provides protection for personnel, the product, and the environment. Through the history of laboratory-acquired infections from the earliest published case to the emergence of hepatitis B and AIDS, the need for health care worker protection is described. A brief description with design, construction, function, and production capabilities is provided for class I and class III safety cabinets. The development of the high-efficiency particulate air filter provided the impetus for clean room technology, from which evolved the class II laminar flow biological safety cabinet. The clean room concept was advanced when the horizontal airflow clean bench was manufactured; it became popular in pharmacies for preparing intravenous solutions because the product was protected. However, as with infectious microorganisms and laboratory workers, individual sensitization to antibiotics and the advent of hazardous antineoplastic agents changed the thinking of pharmacists and nurses, and they began to use the class II safety cabinet to prevent adverse personnel reactions to the drugs. How the class II safety cabinet became the mainstay in laboratories and pharmacies is described, and insight is provided into the formulation of National Sanitation Foundation standard number 49 and its revisions. The working operations of a class II cabinet are described, as are the variations of the four types with regard to design, function, air velocity profiles, and the use of toxins. The main certification procedures are explained, with examples of improper or incorrect certifications. The required levels of containment for microorganisms are given. Instructions for decontaminating the class II biological safety cabinet of infectious agents are provided; unfortunately, there is no method for decontaminating the cabinet of antineoplastic agents.
Full text
PDF









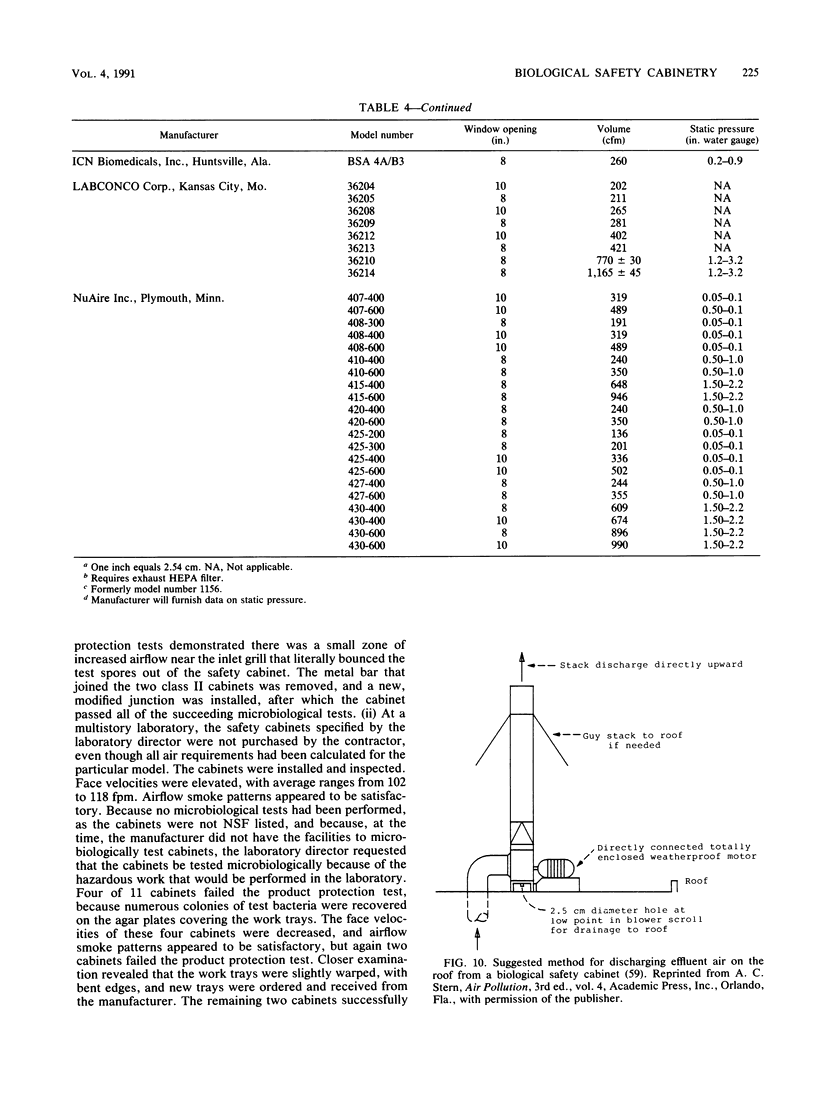





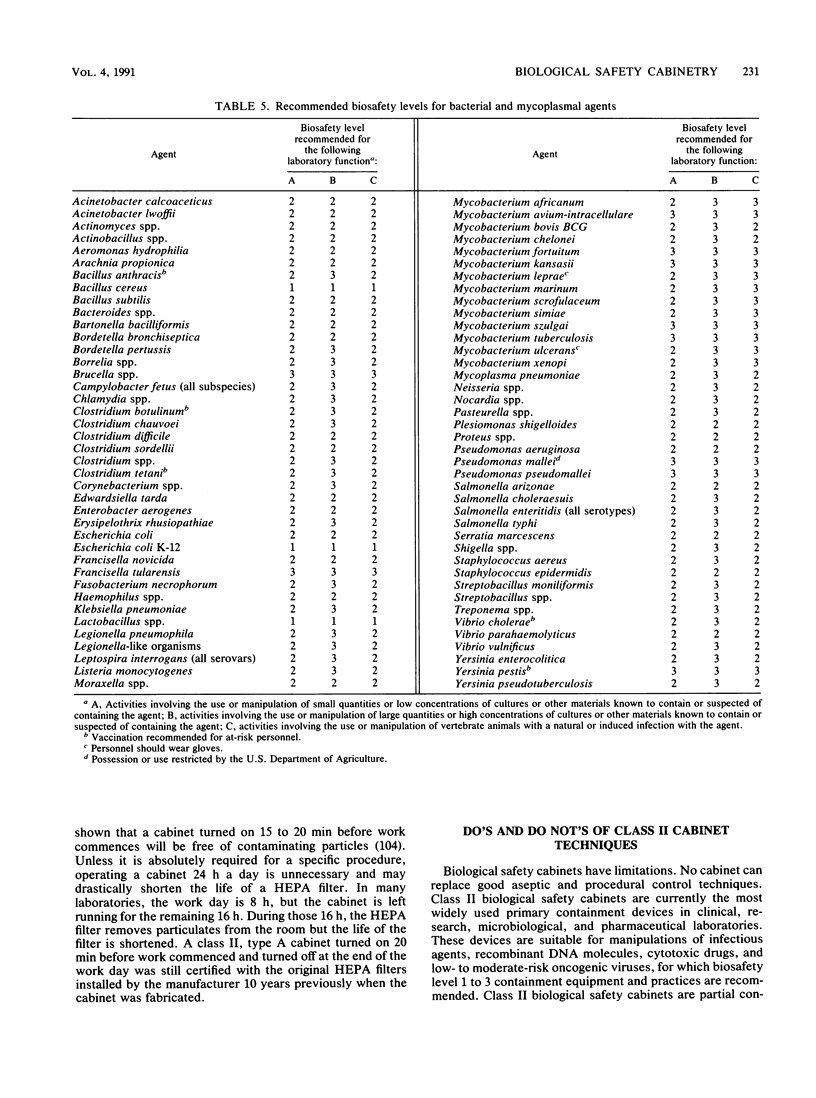
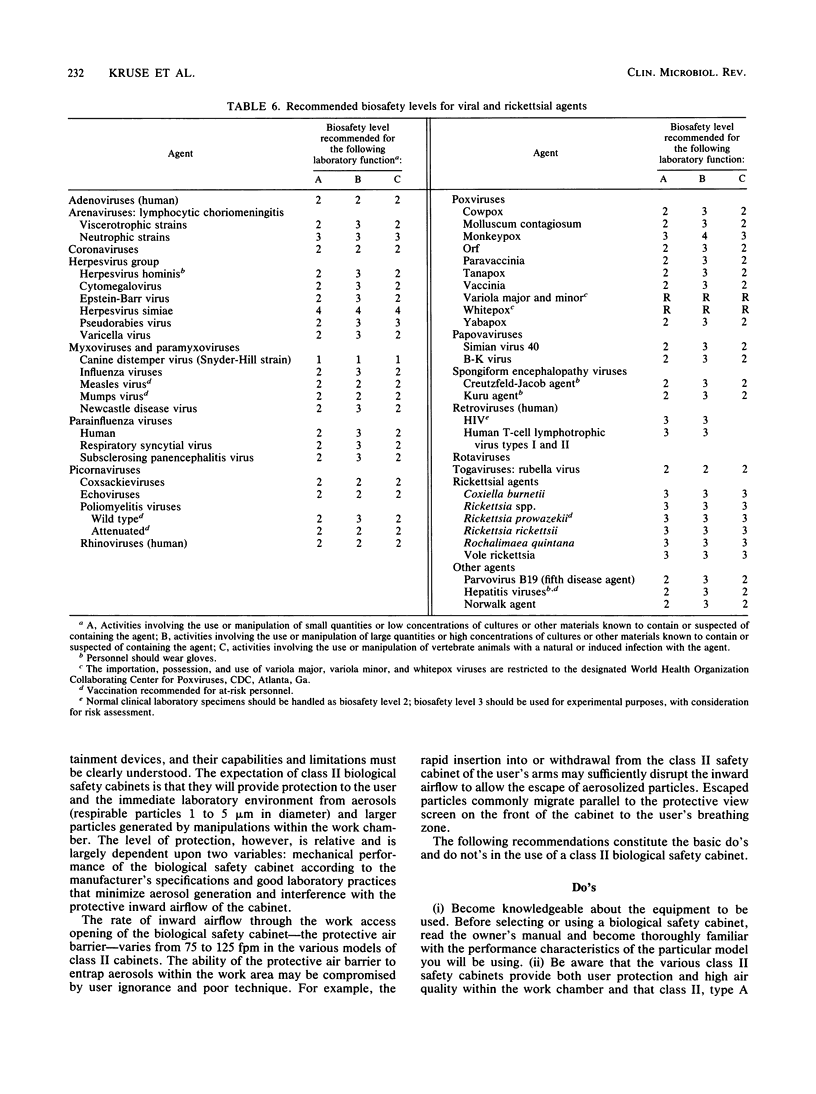
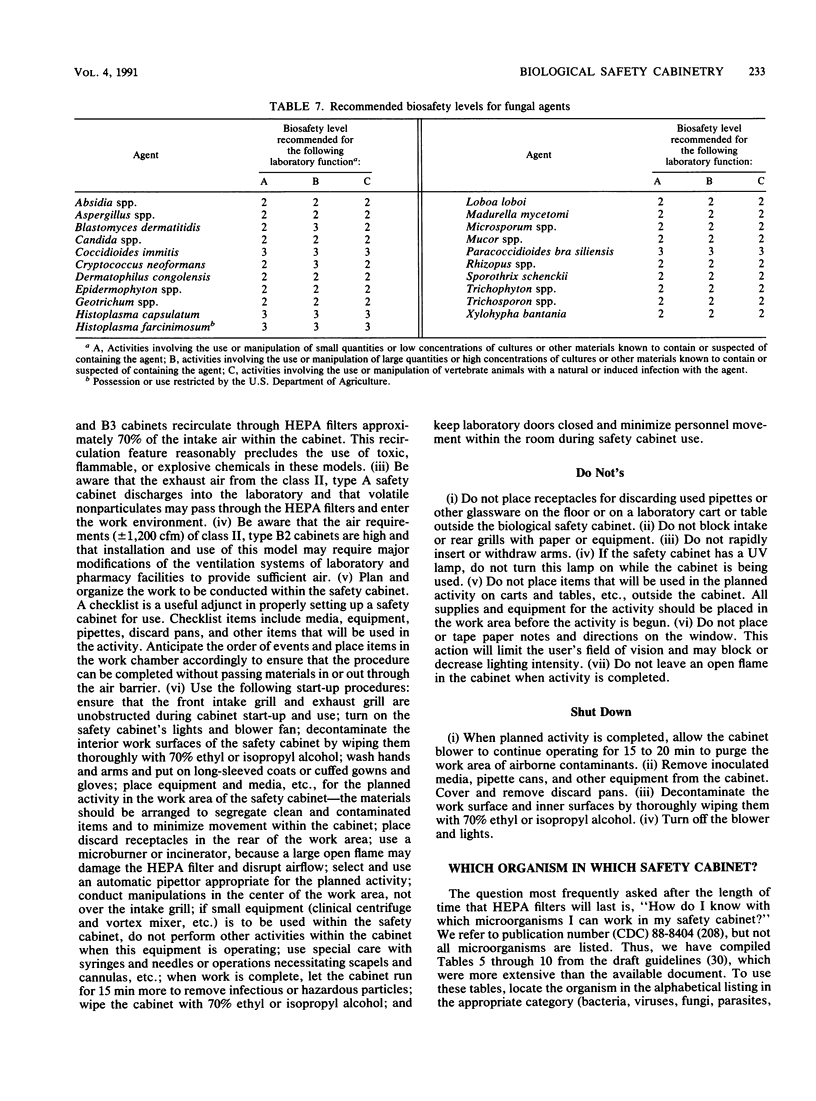






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. E., STEIN L., MOSS M. L., GROSS N. H. Potential infectious hazards of common bacteriological techniques. J Bacteriol. 1952 Oct;64(4):473–481. doi: 10.1128/jb.64.4.473-481.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers R. L., Walker R. J., Sabel F. L., McDade J. J. Development of a laminar air-flow biological cabinet. Am Ind Hyg Assoc J. 1969 Mar-Apr;30(2):177–185. doi: 10.1080/00028896909343106. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Anderson M., Brassington D., Bolger J. Development and operation of a pharmacy-based intravenous cytotoxic reconstitution service. Br Med J (Clin Res Ed) 1983 Jan 1;286(6358):32–36. doi: 10.1136/bmj.286.6358.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. W., Puckett W. H., Jr, Dana W. J., Nguyen T. V., Theiss J. C., Matney T. S. Risk of handling injectable antineoplastic agents. Am J Hosp Pharm. 1982 Nov;39(11):1881–1887. [PubMed] [Google Scholar]
- Avis K. E., Levchuk J. W. Special considerations in the use of vertical laminar-flow workbenches. Am J Hosp Pharm. 1984 Jan;41(1):81–87. [PubMed] [Google Scholar]
- BARBEITO M. S., ALG R. L., WEDUM A. G. Infectious bacterial aerosol from dropped Petri dish cultures. Am J Med Technol. 1961 Nov-Dec;27:318–322. [PubMed] [Google Scholar]
- Barbeito M. S., Taylor L. A. Containment of microbial aerosols in a microbiological safety cabinet. Appl Microbiol. 1968 Aug;16(8):1225–1229. doi: 10.1128/am.16.8.1225-1229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan D., Marback R. C. Laminar-airflow equipment certification: what the pharmacist needs to know. Am J Hosp Pharm. 1984 Jul;41(7):1343–1349. [PubMed] [Google Scholar]
- CHATIGNY M. A. Protection against infection in the microbiological laboratory: devices and procedures. Adv Appl Microbiol. 1961;3:131–192. doi: 10.1016/s0065-2164(08)70509-4. [DOI] [PubMed] [Google Scholar]
- Chabner B. A. Second neoplasm--a complication of cancer chemotherapy. N Engl J Med. 1977 Jul 28;297(4):213–215. doi: 10.1056/NEJM197707282970411. [DOI] [PubMed] [Google Scholar]
- Cloak M. M., Connor T. H., Stevens K. R., Theiss J. C., Alt J. M., Matney T. S., Anderson R. W. Occupational exposure of nursing personnel to antineoplastic agents. Oncol Nurs Forum. 1985 Sep-Oct;12(5):33–39. [PubMed] [Google Scholar]
- Connor T. H., Theiss J. C., Anderson R. W., Puckett W. H., Matney T. S. Re-evaluation of urine mutagenicity of pharmacy personnel exposed to antineoplastic agents. Am J Hosp Pharm. 1986 May;43(5):1236–1239. [PubMed] [Google Scholar]
- Coriell L. L., McGarrity G. J. Biohazard hood to prevent infection during microbiological procedures. Appl Microbiol. 1968 Dec;16(12):1895–1900. doi: 10.1128/am.16.12.1895-1900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudi C. B., Stephens B. L., Maier P. Possible occupational hazards associated with the preparation/administration of antineoplastic agents. NITA. 1982 Jul-Aug;5(4):264–265. [PubMed] [Google Scholar]
- D'Arcy P. F. Reactions and interactions in handling anticancer drugs. Drug Intell Clin Pharm. 1983 Jul-Aug;17(7-8):532–538. doi: 10.1177/106002808301700707. [DOI] [PubMed] [Google Scholar]
- Dorr F. A., Coltman C. A., Jr Second cancers following antineoplastic therapy. Curr Probl Cancer. 1985 Feb;9(2):1–43. doi: 10.1016/s0147-0272(85)80033-7. [DOI] [PubMed] [Google Scholar]
- Falck K., Gröhn P., Sorsa M., Vainio H., Heinonen E., Holsti L. R. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet. 1979 Jun 9;1(8128):1250–1251. doi: 10.1016/s0140-6736(79)91939-1. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fineberg H. V. Education to prevent AIDS: prospects and obstacles. Science. 1988 Feb 5;239(4840):592–596. doi: 10.1126/science.3340845. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Gallelli J. F., Mahar H. Chemical destruction and disposal of antineoplastic drugs. Am J Hosp Pharm. 1986 Jul;43(7):1679–1679. [PubMed] [Google Scholar]
- Gresham G. A. Hepatitis in the post-mortem room. Proc R Soc Med. 1973 Aug;66(8):797–798. doi: 10.1177/003591577306600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist N. R., Emslie J. A. Infections in British clinical laboratories, 1984-5. J Clin Pathol. 1987 Aug;40(8):826–829. doi: 10.1136/jcp.40.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANEL E., Jr, ALG R. L. Biological hazards of common laboratory procedures. II. The hypodermic syringe and needle. Am J Med Technol. 1955 Nov-Dec;21(6):343–346. [PubMed] [Google Scholar]
- HILLEMAN M. R., TAYLOR R. O. A safe high-speed blendor for processing infectious materials. J Lab Clin Med. 1958 Jun;51(6):977–980. [PubMed] [Google Scholar]
- Hanson R. P., Sulkin S. E., Beuscher E. L., Hammon W. M., McKinney R. W., Work T. H. Arbovirus infections of laboratory workers. Extent of problem emphasizes the need for more effective measures to reduce hazards. Science. 1967 Dec 8;158(3806):1283–1286. doi: 10.1126/science.158.3806.1283. [DOI] [PubMed] [Google Scholar]
- Harper G. J. An assessment of environmental contamination arising from the use of equipment for carrying out the ELISA technique in microtitre plates. J Clin Pathol. 1983 Jan;36(1):110–113. doi: 10.1136/jcp.36.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington J. M., Shannon H. S. Incidence of tuberculosis, hepatitis, brucellosis, and shigellosis in British medical laboratory workers. Br Med J. 1976 Mar 27;1(6012):759–762. doi: 10.1136/bmj.1.6012.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C. A delayed complication of cancer therapy--cancer. J Natl Cancer Inst. 1979 Aug;63(2):275–277. [PubMed] [Google Scholar]
- Harrison B. R. Developing guidelines for working with antineoplastic drugs. Am J Hosp Pharm. 1981 Nov;38(11):1686–1693. [PubMed] [Google Scholar]
- Harstad J. B., Filler M. E. Evaluation of air filters with submicron viral aerosols and bacterial aerosols. Am Ind Hyg Assoc J. 1969 May-Jun;30(3):280–290. doi: 10.1080/00028896909343122. [DOI] [PubMed] [Google Scholar]
- Hirst M., Tse S., Mills D. G., Levine L., White D. F. Caution on handling antineoplastic drugs. N Engl J Med. 1983 Jul 21;309(3):188–189. doi: 10.1056/NEJM198307213090319. [DOI] [PubMed] [Google Scholar]
- Hoy R. H., Stump L. M. Effect of an air-venting filter device on aerosol production from vials. Am J Hosp Pharm. 1984 Feb;41(2):324–326. [PubMed] [Google Scholar]
- Huddleson I. F., Munger M. A Study of an Epidemic of Brucellosis Due to Brucella melitensis. Am J Public Health Nations Health. 1940 Aug;30(8):944–954. doi: 10.2105/ajph.30.8.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J. Report of an Outbreak of Q Fever at the National Institute of Health : II. Epidemiological Features. Am J Public Health Nations Health. 1947 Apr;37(4):431–440. [PMC free article] [PubMed] [Google Scholar]
- Hunt J. Can we risk finding out? Nurs Mirror. 1984 Feb 1;158(5):33–34. [PubMed] [Google Scholar]
- Jacobson J. T., Orlob R. B., Clayton J. L. Infections acquired in clinical laboratories in Utah. J Clin Microbiol. 1985 Apr;21(4):486–489. doi: 10.1128/jcm.21.4.486-489.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagun O., Ryan M., Waldron H. A. Urinary thioether excretion in nurses handling cytotoxic drugs. Lancet. 1982 Aug 21;2(8295):443–444. doi: 10.1016/s0140-6736(82)90474-3. [DOI] [PubMed] [Google Scholar]
- KRUSE R. H. Potential aerogenic laboratory hazards of Cocidioides immitis. Am J Clin Pathol. 1962 Feb;37:150–158. doi: 10.1093/ajcp/37.2.150. [DOI] [PubMed] [Google Scholar]
- Kenny C., Parkin J., Underhill G., Shah N., Burnell B., Osborne E., Jeffries D. J. HIV antigen testing. Lancet. 1987 Mar 7;1(8532):565–566. doi: 10.1016/s0140-6736(87)90210-8. [DOI] [PubMed] [Google Scholar]
- Kenny M. T., Sabel F. L. Particle size distribution of Serratia marcescens aerosols created during common laboratory procedures and simulated laboratory accidents. Appl Microbiol. 1968 Aug;16(8):1146–1150. doi: 10.1128/am.16.8.1146-1150.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg M. L., Quinn M. J. Airborne drug levels in a laminar-flow hood. Am J Hosp Pharm. 1981 Sep;38(9):1301–1303. [PubMed] [Google Scholar]
- Kruse R. H., Wedum A. G. Cross infection with eighteen pathogens among caged laboratory animals. Lab Anim Care. 1970 Jun;20(3):541–560. [PubMed] [Google Scholar]
- Kuehne R. W. Biological containment facility for studying infectious disease. Appl Microbiol. 1973 Sep;26(3):239–243. doi: 10.1128/am.26.3.239-243.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw J. L., Connor T. H., Theiss J. C., Anderson R. W., Matney T. S. Permeability of latex and polyvinyl chloride gloves to 20 antineoplastic drugs. Am J Hosp Pharm. 1984 Dec;41(12):2618–2623. [PubMed] [Google Scholar]
- Larsh H. W., Schwarz J. Accidental inoculation blastomycosis. Cutis. 1977 Mar;19(3):334-5, 337. [PubMed] [Google Scholar]
- Lassila O., Toivanen A., Nordman E. Immune function in nurses handling cytostatic drugs. Lancet. 1980 Aug 30;2(8192):482–482. doi: 10.1016/s0140-6736(80)91920-0. [DOI] [PubMed] [Google Scholar]
- Lauer J. L., VanDrunen N. A., Washburn J. W., Balfour H. H., Jr Transmission of hepatitis B virus in clinical laboratory areas. J Infect Dis. 1979 Oct;140(4):513–516. doi: 10.1093/infdis/140.4.513. [DOI] [PubMed] [Google Scholar]
- Levy B. S., Harris J. C., Smith J. L., Washburn J. W., Mature J., Davis A., Crosson J. T., Polesky H., Hanson M. Hepatitis B in ward and clinical laboratory employees of a general hospital. Am J Epidemiol. 1977 Oct;106(4):330–335. doi: 10.1093/oxfordjournals.aje.a112469. [DOI] [PubMed] [Google Scholar]
- Macher J. M., First M. W. Effects of airflow rates and operator activity on containment of bacterial aerosols in a class II safety cabinet. Appl Environ Microbiol. 1984 Sep;48(3):481–485. doi: 10.1128/aem.48.3.481-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matney T. S., Nguyen T. V., Connor T. H., Dana W. J., Theiss J. C. Genotoxic classification of anticancer drugs. Teratog Carcinog Mutagen. 1985;5(5):319–328. doi: 10.1002/tcm.1770050502. [DOI] [PubMed] [Google Scholar]
- McDade J. J., Sabel F. L., Akers R. L., Walker R. J. Microbiological studies on the performance of a laminar airflow biological cabinet. Appl Microbiol. 1968 Jul;16(7):1086–1092. doi: 10.1128/am.16.7.1086-1092.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. D., Songer J. R., Sullivan J. F. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am Ind Hyg Assoc J. 1987 Mar;48(3):271–275. doi: 10.1080/15298668791384733. [DOI] [PubMed] [Google Scholar]
- Ng L. M., Jaffe N. Possible hazards of handling antineoplastic drugs. Pediatrics. 1970 Oct;46(4):648–649. [PubMed] [Google Scholar]
- Nikula E., Kiviniitty K., Leisti J., Taskinen P. J. Chromosome aberrations in lymphocytes of nurses handling cytostatic agents. Scand J Work Environ Health. 1984 Apr;10(2):71–74. doi: 10.5271/sjweh.2355. [DOI] [PubMed] [Google Scholar]
- Norppa H., Sorsa M., Vainio H., Gröhn P., Heinonen E., Holsti L., Nordman E. Increased sister chromatid exchange frequencies in lymphocytes of nurses handling cytostatic drugs. Scand J Work Environ Health. 1980 Dec;6(4):299–301. doi: 10.5271/sjweh.2605. [DOI] [PubMed] [Google Scholar]
- PHILLIPS G. B., REITMAN M. Biological hazards of common laboratory procedures. IV. The inoculating loop. Am J Med Technol. 1956 Jan-Feb;22(1):16–17. [PubMed] [Google Scholar]
- PIKE R. M., SULKIN S. E., SCHULZE M. L. CONTINUING IMPORTANCE OF LABORATORY-ACQUIRED INFECTIONS. Am J Public Health Nations Health. 1965 Feb;55:190–199. doi: 10.2105/ajph.55.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. J., Bond W. W., Marshall J. H., Favero M. S., Raij L. An air sampling technique for hepatitis B surface antigen. Health Lab Sci. 1976 Oct;13(4):233–237. [PubMed] [Google Scholar]
- Pike R. M. Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annu Rev Microbiol. 1979;33:41–66. doi: 10.1146/annurev.mi.33.100179.000353. [DOI] [PubMed] [Google Scholar]
- Pike R. M. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab Sci. 1976 Apr;13(2):105–114. [PubMed] [Google Scholar]
- Pike R. M. Past and present hazards of working with infectious agents. Arch Pathol Lab Med. 1978 Jul;102(7):333–336. [PubMed] [Google Scholar]
- Power L. A., Anderson R. W., Cortopassi R., Gera J. R., Lewis R. M., Jr Update on safe handling of hazardous drugs: the advice of experts. Am J Hosp Pharm. 1990 May;47(5):1050–1060. [PubMed] [Google Scholar]
- Public Health Weekly Reports for APRIL 18, 1930. Public Health Rep. 1930 Apr 18;45(16):843–918. [PMC free article] [PubMed] [Google Scholar]
- REID D. D. Incidence of tuberculosis among workers in medical laboratories. Br Med J. 1957 Jul 6;2(5035):10–14. doi: 10.1136/bmj.2.5035.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN M., ALG R. L., MILLER W. S., GROSS N. H. Potential infectious hazards of laboratory techniques. III. Viral techniques. J Bacteriol. 1954 Nov;68(5):549–554. doi: 10.1128/jb.68.5.549-554.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN M., FRANK M. A., ALG R., WEDUM A. G. Infectious hazards of the high speed blendor and their elimination by a new design. Appl Microbiol. 1953 Jan;1(1):14–17. doi: 10.1128/am.1.1.14-17.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN M., MOSS M. L., HARSTAD J. B., ALG R. L., GROSS N. H. Potential infectious hazards of laboratory techniques. I. Lyophilization. J Bacteriol. 1954 Nov;68(5):541–544. doi: 10.1128/jb.68.5.541-544.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN M., MOSS M. L., HARSTAD J. B., ALG R. L., GROSS N. H. Potential infectious hazards of laboratory techniques. II. The handling of lyophilized cultures. J Bacteriol. 1954 Nov;68(5):545–548. doi: 10.1128/jb.68.5.545-548.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN M., PHILLIPS G. B. Biological hazards of common laboratory procedures. I. The pipette. Am J Med Technol. 1955 Nov-Dec;21(6):336–342. [PubMed] [Google Scholar]
- REITMAN M., PHILLIPS G. B. Biological hazards of common laboratory procedures. III. THe centrifuge. Am J Med Technol. 1956 Jan-Feb;22(1):14–16. [PubMed] [Google Scholar]
- REITMAN M., WEDUM A. G. Microbiological safety. Public Health Rep. 1956 Jul;71(7):659–665. [PMC free article] [PubMed] [Google Scholar]
- Rake B. W. Influence of crossdrafts on the performance of a biological safety cabinet. Appl Environ Microbiol. 1978 Aug;36(2):278–283. doi: 10.1128/aem.36.2.278-283.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. D., Ignoffo R., Lawrence J., Torti F. M., Koretz M., Anson N., Meier A. Adverse reactions to AMSA in medical personnel. Cancer Treat Rep. 1982 Oct;66(10):1885–1885. [PubMed] [Google Scholar]
- Rieche K. Carcinogenicity of antineoplastic agents in man. Cancer Treat Rev. 1984 Mar;11(1):39–67. doi: 10.1016/0305-7372(84)90016-1. [DOI] [PubMed] [Google Scholar]
- SILANT'EV E. I., ANKUDINOV V. A., KOLESOV S. G. [on anthrax immunity following the effect on the body of ionizing radiations]. Zh Mikrobiol Epidemiol Immunobiol. 1962 Nov;33:121–123. [PubMed] [Google Scholar]
- SLEPUSHKIN A. N. Epidemiologicheskoe izuchenie sluchaev laboratornogo zarazheniia virusom venesuel'skogo entsefalomielita loshadei. Vopr Virusol. 1959 May-Jun;4(3):311–314. [PubMed] [Google Scholar]
- SMITH G. S. Tuberculosis as a necropsy room hazard. J Clin Pathol. 1953 May;6(2):132–134. doi: 10.1136/jcp.6.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULKIN S. E., PIKE R. M. Survey of laboratory-acquired infections. Am J Public Health Nations Health. 1951 Jul;41(7):769–781. doi: 10.2105/ajph.41.7.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan S. G., Lindbohm M. L., Hornung R. W., Hemminki K. A study of occupational exposure to antineoplastic drugs and fetal loss in nurses. N Engl J Med. 1985 Nov 7;313(19):1173–1178. doi: 10.1056/NEJM198511073131901. [DOI] [PubMed] [Google Scholar]
- Severin M. J. Acquired immunodeficiency syndrome: more than a health-related dilemma. Clin Microbiol Rev. 1989 Oct;2(4):425–436. doi: 10.1128/cmr.2.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber S. M., Adamson R. H. Toxicity of antineoplastic agents in man, chromosomal aberrations antifertility effects, congenital malformations, and carcinogenic potential. Adv Cancer Res. 1975;22:57–155. doi: 10.1016/s0065-230x(08)60176-1. [DOI] [PubMed] [Google Scholar]
- Skinhöj P. Occupational risks in Danish clinical chemical laboratories. II. Infections. Scand J Clin Lab Invest. 1974 Feb;33(1):27–29. doi: 10.3109/00365517409114194. [DOI] [PubMed] [Google Scholar]
- Skinhøj P., Søeby M. Viral hepatitis in Danish health care personnel, 1974-78. J Clin Pathol. 1981 Apr;34(4):408–411. doi: 10.1136/jcp.34.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Hutton M. D. Tuberculosis in correctional institutions. JAMA. 1989 Jan 20;261(3):436–437. [PubMed] [Google Scholar]
- Sotaniemi E. A., Sutinen S., Arranto A. J., Sutinen S., Sotaniemi K. A., Lehtola J., Pelkonen R. O. Liver damage in nurses handling cytostatic agents. Acta Med Scand. 1983;214(3):181–189. doi: 10.1111/j.0954-6820.1983.tb08593.x. [DOI] [PubMed] [Google Scholar]
- Stern E. L., Johnson J. W., Vesley D., Halbert M. M., Lawrence B. S., Williams L. E., Blume P. Aerosol production associated with clinical laboratory procedures. Am J Clin Pathol. 1974 Nov;62(5):591–600. doi: 10.1093/ajcp/62.5.591. [DOI] [PubMed] [Google Scholar]
- Sullivan J. F., Songer J. R., Estrem I. E. Laboratory-acquired infections at the National Animal Disease Center 1960--1976. Health Lab Sci. 1978 Jan;15(1):58–64. [PubMed] [Google Scholar]
- Taylor A. T., Wade A. E. Chemical carcinogenicity and the antineoplastic agents. Am J Hosp Pharm. 1984 Sep;41(9):1844–1848. [PubMed] [Google Scholar]
- Taylor L. A., Barbeito M. S., Gremillion G. G. Paraformaldehyde for surface sterilization and detoxification. Appl Microbiol. 1969 Apr;17(4):614–618. doi: 10.1128/am.17.4.614-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari P. L., Tonat K., DeChristoforo R., Gallelli J. F., Zimmerman P. F. Disposal of antineoplastic wastes at the National Institutes of Health. Am J Hosp Pharm. 1984 Jan;41(1):87–93. [PubMed] [Google Scholar]
- Vesley D., Hartmann H. M. Laboratory-acquired infections and injuries in clinical laboratories: a 1986 survey. Am J Public Health. 1988 Sep;78(9):1213–1215. doi: 10.2105/ajph.78.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDUM A. G. Bacteriological safety. Am J Public Health Nations Health. 1953 Nov;43(11):1428–1437. doi: 10.2105/ajph.43.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDUM A. G. Control of laboratory airborne infection. Bacteriol Rev. 1961 Sep;25:210–216. doi: 10.1128/br.25.3.210-216.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDUM A. G. LABORATORY SAFETY IN RESEARCH WITH INFECTIOUS AEROSOLS. Public Health Rep. 1964 Jul;79:619–633. [PMC free article] [PubMed] [Google Scholar]
- Waksvik H., Klepp O., Brøgger A. Chromosome analyses of nurses handling cytostatic agents. Cancer Treat Rep. 1981 Jul-Aug;65(7-8):607–610. [PubMed] [Google Scholar]
- Wedum A. G., Barkley W. E., Hellman A. Handling of infectious agents. J Am Vet Med Assoc. 1972 Dec 1;161(11):1557–1567. [PubMed] [Google Scholar]
- Wilson J. P., Solimando D. A., Jr Aseptic technique as a safety precaution in the preparation of antineoplastic agents. Hosp Pharm. 1981 Nov;16(11):575-6, 579-81. [PubMed] [Google Scholar]
- deWerk Neal A., Wadden R. A., Chiou W. L. Exposure of hospital workers to airborne antineoplastic agents. Am J Hosp Pharm. 1983 Apr;40(4):597–601. [PubMed] [Google Scholar]








