Abstract
The strict tropism of many pathogens for man hampers the development of animal models that recapitulate important microbe–host interactions. We developed a rhesus macaque model for studying Neisseria–host interactions using Neisseria species indigenous to the animal. We report that Neisseria are common inhabitants of the rhesus macaque. Neisseria isolated from the rhesus macaque recolonize animals after laboratory passage, persist in the animals for at least 72 d, and are transmitted between animals. Neisseria are naturally competent and acquire genetic markers from each other in vivo, in the absence of selection, within 44 d after colonization. Neisseria macacae encodes orthologs of known or presumed virulence factors of human-adapted Neisseria, as well as current or candidate vaccine antigens. We conclude that the rhesus macaque model will allow studies of the molecular mechanisms of Neisseria colonization, transmission, persistence, and horizontal gene transfer. The model can potentially be developed further for preclinical testing of vaccine candidates.
Keywords: commensal Neisseria, nonhuman primate model, type IV pili
Microbe–host interactions are often refractory to investigation because of the exquisite tropism of many bacteria for the human host. Host-restriction has prevented the development of animal models that recapitulate colonization, transmission, and persistence; hampered studies of horizontal gene transfer (HGT); and prevented preclinical testing of vaccine candidates. One means to circumvent host restrictions is to develop an animal model of infection using a related species indigenous to that animal. For example, infections by human pathogens, such as Bordetella pertussis and Chlamydia trachomatis, have been effectively modeled in animals with Bordetella bronchiseptica and Chlamydia muridarum, respectively (1, 2).
The genus Neisseria and the rhesus macaque provide us with this opportunity. Neisseria are Gram-negative, diplococcal β-Proteobacteria. This genus includes >10 human-adapted species, two of which are pathogens, and an unknown number of species that naturally colonize animals. These species are highly related: >40% of the genes in pathogenic species are also present in commensals, and many of these encode virulence genes and antibiotic resistance alleles. The species are naturally competent and transfer genetic information to each other in vitro (3, 4). HGT is widely assumed to play an important role in the spread of antibiotic resistance among pathogenic Neisseria; however, there is little information on the extent to which HGT occurs in vivo.
Pathogens Neisseria meningitidis and Neisseria gonorrhoeae have an exquisite tropism for man. These pathogens do not naturally infect animals; inoculated into nonhuman hosts, the pathogens will colonize only for brief periods (5, 6). Neisseria species have been isolated from a number of animals (7–10), suggesting it may be possible to develop a model for studying Neisseria–host interactions in which there are no host-restriction barriers to overcome.
The rhesus macaque (RM) has ∼93% DNA sequence identity to man, is similar physiologically and anatomically to man, and expresses orthologs of many human receptors for the pathogenic Neisseria. Neisseria macacae, isolated from the RM oropharynx, is closely related to human commensals Neisseria sicca and Neisseria mucosa (11, 12). We explored the feasibility of developing a RM model for studying Neisseria–host interactions using species indigenous to this animal.
We report that N. macacae encode many orthologs of virulence factors and vaccine antigens of N. meningitidis and N. gonorrhoeae. At least two species of Neisseria inhabit the RM oropharynx. RM Neisseria persist for at least 6 y in their monkey hosts; they recolonize animals after laboratory passage and are transmitted between animals. Finally, RM Neisseria are naturally competent and transfer genetic markers to each other shortly after inoculation, in the absence of selection. We conclude that the RM model provides an excellent opportunity for studying commensal Neisseria colonization, transmission, long-term persistence, and HGT. This model has the potential for allowing a better understanding of asymptomatic carriage by pathogenic Neisseria (13, 14). We discuss the promise of this animal model for preclinical testing of vaccine antigens and persistence of antibiotic resistant determinants in a population.
Results
RM Neisseria Encodes Orthologs of Virulence Factors and Vaccine Antigens.
N. macacae was isolated from a RM in 1983 (12). A BLASTP search of its genome revealed genes encoding closely related orthologs of 61 of 70 factors hypothesized or demonstrated to play a role in virulence of human pathogenic Neisseria, including proteins involved in the biogenesis of the type IV pilus (Tfp), an appendage that functions in DNA transformation, colonization, motility, and host cell signaling (Tables S1 and S2). Also present are genes encoding current meningococcal vaccine components such as fHbp, GNA1030, GNA2091, NadA, and Porin P1 (Table S3), and promising vaccine antigens, such as LctP (15). These findings lend additional support to previous reports indicating the genetic relatedness of N. macacae to human-adapted Neisseria.
Survey Reveals Multiple Neisseria Species Colonize RMs.
We determined whether Neisseria are common inhabitants of RMs. In 2003, we surveyed 23 RMs at the Oregon National Primate Research Center. Seventeen animals were culture-positive for Neisseria (SI Materials and Methods). Five culture-negative RMs had been treated previously with enrofloxacin (Baytril) for a Gram-negative infection. Thus, Neisseria are a common inhabitant of the RM.
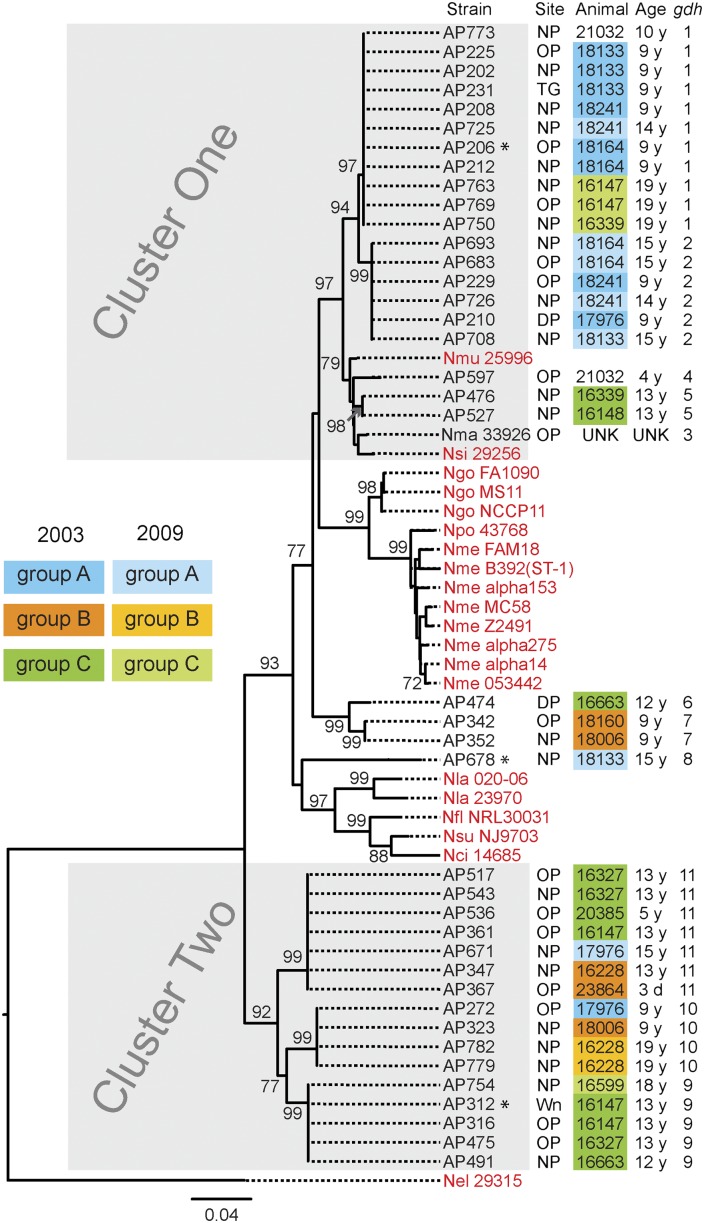
Forty RM-derived Neisseria were typed for their housekeeping gene gdh (16), an approach used successfully to group Neisseria species into monophyletic clusters (17) (Table S4). A comparison of their gdh sequences with those from human Neisseria species and N. macacae allowed us to construct a phylogenetic tree (Fig. 1). The gdh alleles of RM Neisseria clustered primarily at two locations in the tree, which we term cluster one and cluster two. Cluster one isolates were closely related to N. macacae and human commensals N. sicca and N. mucosa. Cluster two isolates fall between the cluster one strains and N. elongata, a human commensal distantly related to human pathogens N. meningitidis and N. gonorrhoeae (4, 11, 18). These results suggest that multiple species of Neisseria colonize RMs.
Fig. 1.
gdh sequences resolve RM Neisseria into two clusters. A rooted neighbor-joining tree constructed with gdh sequences from 40 RM Neisseria isolates (present study), N. macacae (Nma; ATCC 33926), and 20 human-adapted Neisseria (in red). Shown for each RM Neisseria strain are its strain number, its site of isolation, the animal from which it was cultured, the age of the animal, and its gdh allele number. Cluster one and cluster two RM Neisseria strains are in gray boxes. Housing groups of the animals are color coded: blue, group A; orange, group B; and green, group C. Neisseria strains cultured from animals in the same housing group is identified by the housing group color. Darker shade of a color indicates the 2003 survey; lighter shade indicates the 2009 survey. Three strains marked with an asterisk were inoculated into animals during this study. DP, dental plaque; Nci, N. cinerea (ATCC 14685); Nel, N. elongata (ATCC 29315); Nfl, N. flavescens (NRL30031); Ngo, N. gonorrohoeae (FA1090, MS11 and NCCP11); Nla, N. lactamica (20-060, ATCC 23970); Nme, N. meningitidis (FAM18, B392, α153, MC58, Z2491, α275, α14, and 053422), Nmu, N. mucosa (Nmu ATCC 25996); NP, nasopharynx; Npo, N. polysaccharea (Npo ATCC 43768); Nsi, N. sicca (ATCC 29256); Nsu, N. subflava (NJ9703); OP, oropharynx; TG, tongue; Wn, wound. Bootstrap values >70% are indicated (2,000 replications).
Different sites in the same animal were colonized with Neisseria bearing an identical gdh allele (Fig. 1 and Table S5). Six of 16 (37%) RMs were colonized with two Neisseria strains bearing distinct gdh alleles (Table S5). RMs housed together were frequently colonized with Neisseria bearing the same gdh alleles (Fig. 1). For example, four animals in housing group A were colonized with strains bearing gdh alleles 1 and 2 (cluster one) (Fig. 1). Two of six animals in housing group C were colonized with strains bearing gdh allele 5, and three had strains with gdh alleles 9 or 11 (cluster one and cluster two; groups A and C RMs) (Fig. 1). Thus, the same Neisseria strains can colonize multiple sites in an animal, and multiple strains can simultaneously colonize an animal.
In 2009, the same animals (when possible) were surveyed again. In both surveys group A animals were colonized with Neisseria bearing gdh alleles 1 and 2. In 2003, group B animals carried Neisseria with alleles 7, 10, and 11; however, in 2009 they only carried Neisseria with allele 10. Only three group C animals were available for the 2009 survey. Two of three RMs had isolates with different gdh alleles in the two studies. Thus, Neisseria bearing the same gdh alleles can persist in a population of animals for as long as 6 y. Whether they were carried continuously by the same animals or lost and reacquired by the animals over time cannot be distinguished in this study.
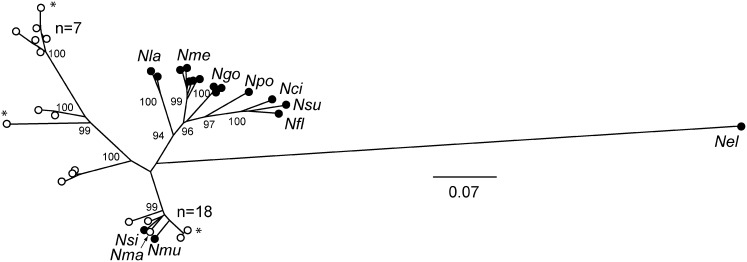
The gdh gene tree in Fig. 1 suggests the existence of multiple Neisseria species in RMs. We performed multilocus sequence typing (MLST) to obtain further evidence for this hypothesis. MLST analysis resolved Neisseria into at least two clusters similar to the gdh gene tree (Fig. 2). RM Neisseria species include those closely related to human commensal species N. mucosa and N. sicca and at least one other group more distantly related to human Neisseria species. This result suggests that at least two species of Neisseria colonize RMs. Like humans, RMs are often colonized simultaneously with multiple species of Neisseria, and these bacteria can be acquired and lost with time (19–22).
Fig. 2.
MLST resolves RM Neisseria strains into multiple clusters. An unrooted neighbor-joining tree constructed with concatenated MLST sequences (abcZ, adk, aroE, fumC, gdh, pdhC, pgm) from a subset (n = 31) of RM Neisseria analyzed in Fig. 1, N. macacae (Nma, ATCC 33926), and 17 human-adapted Neisseria. Three strains marked with an asterisk were inoculated into animals during this study. Nci, N. cinerea (ATCC 14685); Nel, N. elongata (ATCC 29315); Nfl, N. flavescens (NRL30031); Ngo, N. gonorrohoeae (FA1090, MS11, and NCCP11); Nla, N. lactamica (20-060, ATCC 23970); Nme, N. meningitidis (FAM18, MC58, Z2491, α14, and 053422); Nmu, N. mucosa (Nmu ATCC 25996); Nsi, N. sicca (ATCC 29256); Npo, N. polysaccharea (Npo ATCC 43768); Nsu, N. subflava (NJ9703). Bootstrap values are indicated for the major branches (2,000 replications). Human-adapted Neisseria are indicated by black circles; rhesus-derived Neisseria by open circles.
Characterization of RM Neisseria Species for Natural Competence.
Human-adapted Neisseria are competent for DNA transformation and engage in intra- and interspecies HGT in vitro (3, 4, 23). We determined whether RM Neisseria strains from different parts of the MLST tree are also naturally competent. Derivatives of AP206, AP312, and AP678 that spontaneously developed resistance in vitro to rifampicin (RifR) or streptomycin (SmR) were isolated. Their rpoB and rpsL alleles were sequenced to identify the mutations that conferred resistance to Rif or Sm, respectively. When antibiotic-sensitive RM Neisseria were incubated with DNA from antibiotic-resistant derivatives, they acquired resistance to the relevant antibiotic; they remained antibiotic-sensitive when incubated with DNA pretreated with DNaseI, or medium alone (Table 1). These data show that RM Neisseria species are competent for DNA transformation.
Table 1.
DNA transformation of rhesus macaque Neisseria strains
| Recipient strain | DNA | Transformation frequency* (× 10−4) |
| AP678-RifR | AP678-SmR | 3.15 ± 0.21 |
| AP678-RifR | AP678-SmR + DNase | 0.000098 ± 0.00003 |
| AP678-RifR | No DNA | 0.00001 ± 0.000005 |
| AP206-RifR | AP206-SmR | 3.12 ± 2.4 |
| AP206-RifR | AP206-SmR + DNase | < 0.0012 ± 0.0006 |
| AP206-RifR | No DNA | < 0.00002 ± 0.000004 |
| AP312-SmR | AP312-RifR | 0.33 ± 1.8 |
| AP312-SmR | AP312-RifR + DNase | 0.00015 ± 0.000081 |
| AP312-SmR | No DNA | 0.00025 ± 0.000038 |
*Transformation frequencies are expressed as the number of RifR or SmR cfu/total cfu. Values are averaged from three independent experiments ± SEM ‘‘<’’: transformation frequency below the limit of detection.
As Tfp play an essential role in neisserial DNA transformation, and pilus biogenesis genes are present in the N. macacae genome (Tables S1 and S2), we determined whether RM Neisseria produce Tfp. RM strains AP206, AP312, and AP678 have genes that encode Tfp PilE orthologs (accession numbers KC422436, KC422438, and KC422437), and they produce pili-like fibers, as judged by scanning electron microscopy (Fig. S1). Subunits of the fibers from these strains migrate at 13–15 kDa in SDS/PAGE, consistent with predicted molecular weight of their PilE orthologs (Fig. S1D). AP678 Tfp reacted with monoclonal antibody SM1 (Fig. S1E). mAb SM1 recognizes the epitope EYYLN, which is highly conserved among Tfp of the human pathogenic Neisseria (24). Tryptic digestion and MALDI/TOF MS of the AP678 “pilin” agreed with the theoretical masses predicted for AP678 PilE (Fig. S1 F and G). Equally important, nonpiliated variants of AP678 cannot be transformed (wild-type AP678, 4.22 × 10−4 SEM ± 4.31 × 10−5; nonpiliated AP678, 6.48 × 10−9 SEM ± 1.87 × 10−9; n = 3). Taken together, these observations indicate that RM Neisseria produce Tfp.
Experimental Challenge Demonstrates RM Neisseria Recolonize Animals After Laboratory Passage and Are Transmitted Between Animals.
The antibiotic resistant derivatives of AP206, AP312, and AP678 were used to establish colonization of RM (Fig. S2). Two animals were assigned to the experimental cohort, and two to the negative control cohort. At the beginning of the experiment (phase I) (Fig. 3), all animals were colonized with Neisseria, and these isolates were sensitive to Rif and Sm. The animals were treated with Baytril, which successfully reduced the preexisting Neisseria (day 0).
Fig. 3.
Animal studies of RM Neisseria reveal colonization, transmission, persistence, and HGT. Timeline of the experiment is given in days. Asterisk above the day indicates the time animals were cultured for Neisseria. (A) Listed are the inoculated animals, the RM Neisseria strain inoculated into each animal on days 0 and 7, the antibiotic resistance phenotype of the inoculated strain (see abbreviations below), and the gdh allele of the inoculated strain in parenthesis. Orange bar over timeline shows duration of enrofloxacin (Baytril) treatment. The boxed text over the timeline indicate the days when the two monkeys in each cohort were housed together (paired housing) or individually (separate housing). (B) The three phases of the experiment are blocked in different colors. Phase I: reduction of preexisting Neisseria from animals with Baytril. Phase II: inoculation and monitoring of colonization of inoculated strains. Phase III: monitoring persistence, transmission, and HGT. Listed below each sampling day and next to each animal are the antibiotic resistance phenotypes and gdh alleles of the Neisseria strains recovered. N, sensitive to both antibiotics; nc, not cultured; nd, not determined; R, rifampicin resistant; RS, rifampicin and streptomycin resistant; S, streptomycin resistant. “-” Indicates that no Neisseria were recovered from swabs collected on that day. Swabs taken on days 0 and 7 were taken before inoculation procedure.
In phase II, day 0, the animals were housed separately to prevent physical contact. Each experimental animal was inoculated in the nostrils and at multiple sites in the oral cavity with one of the strains discussed above (a total of 2.5 × 108 to 3 × 1010 cfu per animal). Lower dose titrations were not performed because of constraint of time and resources. It should be noted that colonization occurred with the lowest dose. Animal RM 29410 was inoculated with AP678-RifR, and animal RM 29411 with AP312-SmR. Tongue and throat swabs were then taken from the animals at various times to detect colonization (Fig. 3). Control animals were not inoculated but sampled throughout the study.
On day 3, many colonies of SmR Neisseria were cultured from RM 29411; however, on day 7, only one SmR colony was cultured. RifR Neisseria was not recovered from RM 29410 at any time. Fearing that AP312-SmR colonization of RM 29411 was waning, and that AP678-RifR failed to colonize RM 29410, both animals were reinoculated. On day 7, RM 29411 received a second dose of AP312-SmR, and RM 29410 with a different strain, AP206-RifR. At day 14, both animals were robustly colonized with SmR or RifR Neisseria whose gdh alleles matched those of the inoculum (Fig. 3 and Table S6). These results indicate that RM Neisseria can recolonize animals after laboratory passage. Whether the “eclipse” period is typical of Neisseria colonization or a quirk of the experiment is unclear.
Control (uninoculated) animals were culture-negative for Neisseria until day 7. The gdh alleles of Neisseria recovered on day 14 matched or were closely related to those of their preexisting Neisseria, indicating Baytril lowered the preexisting flora to an undetectable level but did not eradicate it.
In phase III, the animals were monitored for transmission, long-term carriage, and HGT. On day 17, animals in each cohort were again housed together. Until day 72, when the experiment terminated, they were cultured periodically for SmR, RifR, or SmR/RifR Neisseria, and the gdh alleles of the isolates were sequenced (Fig. 3). On day 28, both experimental animals were colonized with AP312-SmR and at least one of the RifR strains. From day 42 onward, both animals harbored all three inoculating strains. Thus, RM Neisseria can be transmitted between cage-mates within 14 d, and they can persist for over 2 mo in their natural primate host.
Neither SmR nor RifR Neisseria was cultured from the negative control cohort. The Neisseria isolated from these animals had gdh alleles matching those in the preexisting flora. It should be noted that Neisseria bearing two new gdh alleles were cultured from the negative control cohort during this time (Fig. 3 and Table S6).
HGT in RM Neisseria in Vivo.
Neisseria resistant to both Sm and Rif were cultured from several anatomical sites of both experimental animals 44 d after colonization by the inoculating strains. All SmR/RifR isolates had the rpsL allele from AP312-SmR and the rpoB allele from either AP206-RifR or AP678-RifR (Table 2). Numerous strain-specific polymorphisms in sequenced segments of rpoB, rpsL, and gdh of 758, 369, and 501 base pairs, respectively, allowed us to conclude that double-resistant isolates arose via HGT and not spontaneous mutation (Fig. S2). RifR, SmR, or SmR/RifR Neisseria were never isolated from the control animals.
Table 2.
rpoB, rpsL, and gdh alleles of RifR/SmR Neisseria strains isolated from experimental animals 72 d postinoculation
| Strain | rpoB-RifR | rpsL-SmR | gdh* | Animal | Site |
| AP1498 | 206 | 312 | 206 | 29410 | Tongue |
| AP1500 | 206 | 312 | 206 | 29410 | Hard palate |
| AP1496 | 678 | 312 | 678 | 29411 | Oropharynx |
| AP1497 | 678 | 312 | 678 | 29411 | Esophagus |
| AP1502 | 678 | 312 | 678 | 29411 | Cheek |
| AP1503 | 678 | 312 | 678 | 29411 | Tongue |
| AP1504 | 678 | 312 | 678 | 29411 | Nose |
rpoB, rpsL, and gdh alleles of double-resistant strains matched the indicated alleles from inoculation strains AP206, AP312, and AP678.
*The recipient strain was identified by sequencing the gdh allele of each RifR/SmR strain.
Colonization of the RM Nasal Cavity.
On the last day of the experiment, all animals were cultured for Neisseria at the inoculation sites (nose, tongue, and throat) as well as sites that had not received an inoculum. The latter sites include the epithelia covering the cribriform plate, ethmoturbinates, maxilloturbinates, and nasopharynx above the soft palate. AP312, AP206, and AP678 were cultured from both experimental animals at many of these sites (Table 3 and Table S6). We cannot rule out that the inoculation procedure may have introduced the bacteria to these locations. However, strains naturally transmitted between cage-mates had migrated to uninoculated sites high in the nasal cavity, areas thick with mucous and difficult to access. Neisseria was also cultured from all sites in the uninoculated controls; their gdh alleles matched those of the preexisting strains (Table S6).
Table 3.
Sites of inoculation of rhesus macaque Neisseria strains at day 0 or 7, and sites from which the strains were cultured 72 d postinoculation
| Strain | Animal | Resistance (gdh)* | Site |
| Rhesus Neisseria strains inoculated on day 0 or 7† | |||
| AP678 | 29410 | R(8) | Nose, oral cavity |
| AP206 | 29410 | R(1) | Nose, oral cavity |
| AP312 | 29411 | S(9) | Nose, oral cavity |
| Nasal cavity isolates isolated on day 72‡ | |||
| AP1430 | 29410 | R(8) | Ethmoturbinate |
| AP1525 | 29410 | S(9) | Ethmoturbinate |
| AP1506 | 29410 | R(8) | Maxilloturbinate |
| AP1523 | 29410 | S(9) | Maxilloturbinate |
| AP1519 | 29410 | S(9) | Nasal meatus |
| AP1528 | 29410 | R(8) | Nasal meatus |
| AP1529 | 29410 | R(1) | Nasal meatus |
| AP1426 | 29410 | R(1) | Cribriform plate |
| AP1505 | 29410 | R(8) | Cribriform plate |
| AP1520 | 29410 | S(9) | Cribriform plate |
| AP1479 | 29411 | R(8) | Ethmoturbinate |
| AP1515 | 29411 | S(9) | Ethmoturbinate |
| AP1475 | 29411 | R(1) | Maxilloturbinate |
| AP1512 | 29411 | S(9) | Maxilloturbinate |
| AP1513 | 29411 | S(9) | Nasal meatus |
| AP1526 | 29411 | R(8) | Nasal meatus |
| AP1527 | 29411 | R(1) | Nasal meatus |
| AP1473 | 29411 | R(8) | Cribriform plate |
| AP1511 | 29411 | S(9) | Cribriform plate |
*R and S: Resistance to rifampicin and streptomycin, respectively. gdh allele number is in parenthesis.
†Sites of inoculation at day 0 or 7 are listed.
‡Sites of culture 72 d postinoculation are listed.
Discussion
We have described an animal model that recapitulates several aspects of Neisseria–host interactions that are currently refractory to in vivo experimentation. Our system involves the infection of the rhesus macaque with Neisseria species indigenous to this animal. This approach circumvents host-restriction barriers, and will allow the dissection of molecular mechanisms underlying pharyngeal colonization, persistence, transmission, and HGT.
We presented evidence that Neisseria are a common inhabitant of the RM nasopharynx. At least two species and multiple strains of Neisseria naturally colonize RMs (Figs. 1 and 2). Strains with identical gdh alleles can persist in the same animals for extended periods (as long as 6 y) (Fig. 1), and strains can be introduced to and lost from an animal over time. Four different strains can colonize an animal simultaneously (Fig. 3). These observations closely parallel the epidemiological findings for Neisseria colonization in humans (19, 20). Simultaneous carriage of multiple species/strains is a prerequisite for the dissemination of antibiotic-resistant loci by HGT (see below).
Survey of RMs for Neisseria species found that animals housed together often shared strains bearing the same gdh alleles. RMs enrolled in the inoculation studies were grooming pairs. Transmission of Neisseria strains between animals housed together is therefore likely the result of direct contact. Transmission is unlikely to be through aerosols, as the inoculated Neisseria strains were never detected in the pair of negative control RMs that were housed in the same room as the inoculated animals.
RM Neisseria is able to recolonize animals after laboratory passage. Our study suggests there may be an “eclipse” period 7–14 d after inoculation, during which the infecting strain could not be cultured from the animal. Whether this is a bona fide characteristic of RM Neisseria infection is unclear, although it recalls a similar behavior of N. gonorrhoeae upon human inoculation (25). RM Neisseria naturally transmitted from one animal to another were able to colonize areas high in the nasal cavity, such as ethmoid turbinates, maxilloturbinates, the nasal meatus, and the epithelium covering the cribriform plate. The ability of RM Neisseria to colonize the latter site is of particular interest. It has been proposed that pathogen N. meningitidis can gain access to the meninges and the central nervous system via the olfactory nerves that connect the nasal cavity to the brain through perforations in the cribriform plate (26).
Despite its importance to microbial pathogenesis and evolution, there is surprisingly little information about HGT in a natural setting. Our study shows that HGT occurred in two of three inoculating strains, in the absence of selection, and within 44 d after the strains established residency in the animal (Fig. 3 and Table 2). The rapid occurrence of HGT in RM Neisseria highlights an important public health problem. Commensals are reservoirs of antibiotic resistance determinants, and HGT likely plays an important role in their spread among the pathogens. Supporting this notion are the observations that a N. meningitidis strain responsible for an outbreak in the Midwest appears to have acquired its ciprofloxacin-resistant gyrA allele from commensal N. lactamica carried in the community (27); and that the cephalosporin resistance of some N. gonorrhoeae strains is because of mosaic penA alleles of commensal origin (28–30).
RM commensal Neisseria persist in their monkey host long-term. This finding is reminiscent of the findings from human inoculation studies with Neisseria lactamica (31). That study, moreover, suggests N. lactamica may impede N. meningitidis carriage by mechanisms distinct from humoral immunity. N. lactamica was hypothesized to block N. meningitidis carriage through competition or cell-mediated immunity. That study did not detect dual carriage of N. lactamica and N. meningitidis.
Although the RM model has limitations for Neisseria research (e.g., cost and small number of animals), it has several distinct advantages over the human colonization model. The RM model allows simultaneous carriage by multiple Neisseria species; it can therefore be used for in vivo HGT studies, particularly of antibiotic resistance determinants. Being a closed system, the RM model provides a means for studying not only colonization but also strain transmission dynamics between animals. Finally, it provides a means for performing competition experiments to identify important in vivo niches and genetic determinants important for long-term persistence.
The RM model may be adapted to study the molecular mechanisms of Neisseria pharyngeal colonization. Human-specific commensal and pathogenic Neisseria have 896 genes in common, many of which encode virulence factors (32). N. macacae harbor orthologs of Neisseria virulence factors, putative and established (Tables S1 and S2); their contribution to colonization, long-term carriage, and transmission can be examined via in vitro experimentation followed by animal testing of defined mutants or recombinant mutant libraries. Moreover, human pathogenic Neisseria virulence factors expressed in RM Neisseria carriage strains may be examined for virulence traits in the monkey host.
The RM model also holds promise for vaccine development. Rodent models have been used to identify antigens that stimulate a protective immune response against meningococcemia. The RM model may be useful for testing the efficacy of vaccine candidates against pharyngeal carriage. Serogroup C vaccines have successfully reduced carriage of N. meningitidis; this in turn has resulted in herd immunity, reducing the incidence of serogroup C meningococcal disease in unvaccinated individuals (33, 34).
Another advantage of the RM model is the close evolutionary and physiological relatedness of this animal to man. Vaccination of infant RMs with meningococcal outer membrane vesicle results in the development of broadly protective serum antibodies (35). Testing candidates in the RM should allow a better prediction of human antibody responses than in rodents.
Finally, it may be desirable to develop a vaccine with specificity for N. meningitidis. As mentioned, there is evidence that a healthy commensal Neisseria microflora could protect humans from colonization by pathogenic species (31). Children colonized by N. lactamica early in life are less likely to carry N. meningitidis at a later time (36). Our study shows that at least two species of Neisseria colonize RMs simultaneously for extended periods. This finding provides a means to test vaccine candidates for their ability to prevent carriage and for their specificity.
Materials and Methods
See SI Materials and Methods for additional details describing Neisseria culture and identification, PCR, oligonucleotides (Table S7), sequencing, phylogenetic and polymorphism analysis, bacterial strains, transformation assays, electron microscopy, Tfp purification, and proteomics.
Sample Collection and Culturing During Neisseria Surveys.
Swabs (throat, tongue, nose, wound, and so forth) were cultured the same day or transported at ambient temperature in Amies gel with charcoal for culture the next day. Dental plaque scrapings were harvested with a sterile scalpel blade into GC Medium Base (GCB) and disrupted with a microtube pellet pestle (Kontes) before culture. Neisseria were grown at 37 °C, 5% CO2 with a humidified atmosphere. In general, swabs were used to directly inoculate agar plates. In some cases swabs were vortexed in GCB 20% (vol/vol) glycerol and before cryopreservation 25 μL was used to inoculate plates for culture. Neisseria species obtained during culture surveys (Table S4) were isolated on the following media: LBVT.SNR (19), chocolate agar (Difco) and 1% hemoglobin (wt/vol) (Oxoid), Trypticase soy agar (Difco) with 5% sheep blood (vol/vol), LB.SNR (LBVT.SNR without vancomycin and trimethoprim). Neisseria species isolated during the colonization study, including necropsy (Table S6), were isolated on GCB agar with Kellog’s supplements (37), chocolate agar, or LB agar (Lennox, Research Products International) with or without the following antibiotics: V, vancomycin, 3 μg/mL; T, trimethoprim, 3 μg/mL; Rif50, rifampicin, 50 μg/mL; Sm100, streptomycin, 100 μg/mL.
Animals, Housing, and Experimental Inoculations.
Age-matched female RM (Macaca mulatta) were of Indian or Chinese origin. RM ages, pairing, and summary of Neisseria flora are listed in Table S8. Nonhuman primate studies were performed in biosafety level 2 biocontainment at the Oregon National Primate Research Center (ONPRC) and the protocol was approved by the Oregon Health Sciences’ ONPRC Animal Care and use Committee and carried out in strict accordance with the recommendations of the National Institutes of Health’s (NIH) Guide for the Care and Use of Laboratory Animals and the US Animal Welfare Act. The ONPRC is an American Association for Accreditation of Laboratory Animal Care-accredited, NIH-supported nonhuman primate research facility, and has an approved Assurance (#3304–01) for the care and use of animals on file with the Office for Protection from Research Risks at the NIH. We specifically selected grooming pairs for this study. The experimental and negative control cohorts were housed indoors. Animals were full-paired (cohoused within the same cage) in cages facing each other 8-feet apart to prevent physical contact. To prevent inadvertent spread of Neisseria between animals, each cohort was swabbed by a separate animal technician. RMs were sedated with ketamine hydrochloride (5–10 mg/kg body weight) to collect swab samples. Gloves were changed before sampling each animal.
To reduce the level of preexisting Neisseria in RM, animals were given intramuscular injections of enrofloxacin (Baytril), 10 mg/kg, once daily for 2 wk. Before inoculation on day 0, animals were rested for 5 d to allow Baytril to clear from the blood stream and mucosal surfaces.
Regarding inoculations, on day 0 animal 29410 received 2.55 × 108 cfus of strain AP678-RifR and animal 29411 received 2.35 × 109 cfus of strain AP312-SmR. On day 7 animal 29410 received 1.35 × 1010 cfus of strain AP206-RifR and animal 29411 received 3.12 × 1010 cfus of strain AP312-SmR. A total of 2 mL of neisserial suspension in supplemented GCB was introduced into the nose (0.25 mL per nostril) and oral cavity (1.5 mL) using a plastic syringe while animals were in a supine position.
Supplementary Material
Acknowledgments
We thank A. Agellon for electron microscopy; E.T. Mullaney and H. Li for technical help, K. Andrews for recommendations on baytril treatment, C.J. Doane for animal care, and M. Fisher for sample collection (Oregon National Primate Research Center); R. Colligan for technical assistance with mass spectrometry; J. Frelinger for advice on study design; D. Yoder (BIO5 Institute Media Facility) for excellent materials; and H. Seifert and A. Koshy for suggestions on the manuscript. This work was sponsored by the Oregon National Primate Research Center, Supplemental Nonhuman Primate Pilot Program (S.W.W., N.J.W, A.M.W., and M.S.), and funds from the BIO5 Institute, the College of Medicine, and the Undergraduate Biology Research Program, University of Arizona.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the National Center of Biotechnology Information GenBank database, www.ncbi.nlm.nih.gov/RefSeq [accession nos. JX549156–JX549186 (abcZ); JX549187–JX549217 (adk); JX549311–JX549341 (aroE); KC422439–KC422440 (fHbp); JX549218–JX549248 (fumC); JX502556–JX502601 (gdh); JX549249–JX549279 (pdhC); JX549280–JX549310 (pgm); KC422436–KC422438 (pilE); JX549153–JX549155 (rpoB); and JX549150–JX549152 (rpsL)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217420110/-/DCSupplemental.
References
- 1.Cotter PA, Miller JF. Genetic analysis of the Bordetella-host interaction. Ann N Y Acad Sci. 1996;797:65–76. doi: 10.1111/j.1749-6632.1996.tb52950.x. [DOI] [PubMed] [Google Scholar]
- 2.Miyairi I, Ramsey KH, Patton DL. Duration of untreated chlamydial genital infection and factors associated with clearance: Review of animal studies. J Infect Dis. 2010;201(Suppl 2):S96–S103. doi: 10.1086/652393. [DOI] [PubMed] [Google Scholar]
- 3.Catlin BW. [Interspecific transformation of Neisseria by culture slime containing deoxyribonucleate] Science. 1960;131(3400):608–610. doi: 10.1126/science.131.3400.608-a. [DOI] [PubMed] [Google Scholar]
- 4.Higashi DL, et al. N. elongata produces type IV pili that mediate interspecies gene transfer with N. gonorrhoeae. PLoS ONE. 2011;6(6):e21373. doi: 10.1371/journal.pone.0021373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect Immun. 2010;78(1):433–440. doi: 10.1128/IAI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor-Robinson D, Furr PM, Hetherington CM. Neisseria gonorrhoeae colonises the genital tract of oestradiol-treated germ-free female mice. Microb Pathog. 1990;9(5):369–373. doi: 10.1016/0882-4010(90)90071-w. [DOI] [PubMed] [Google Scholar]
- 7.Barrett SJ, Sneath PH. A numerical phenotypic taxonomic study of the genus Neisseria. Microbiology. 1994;140(Pt 10):2867–2891. doi: 10.1099/00221287-140-10-2867. [DOI] [PubMed] [Google Scholar]
- 8.Dent VE. Identification of oral Neisseria species of animals. J Appl Bacteriol. 1982;52(1):21–30. doi: 10.1111/j.1365-2672.1982.tb04368.x. [DOI] [PubMed] [Google Scholar]
- 9.Holmes B, et al. Neisseria weaveri sp. nov. (formerly CDC group M-5), from dog bite wounds of humans. Int J Syst Bacteriol. 1993;43(4):687–693. doi: 10.1099/00207713-43-4-687. [DOI] [PubMed] [Google Scholar]
- 10.Sneath PH, Barrett SJ. A new species of Neisseria from the dental plaque of the domestic cow, Neisseria dentiae sp. nov. Lett Appl Microbiol. 1996;23(5):355–358. doi: 10.1111/j.1472-765x.1996.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Bennett JS, et al. A genomic approach to bacterial taxonomy: An examination and proposed reclassification of species within the genus Neisseria. Microbiology. 2012;158(Pt 6):1570–1580. doi: 10.1099/mic.0.056077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedros NA, Hoke C, Chun P. Neisseria macacae sp. nov., a new Neisseria species isolated from the oropharynges of rhesus monkeys (Macaca mulatta) Int J Syst Bacteriol. 1983;33(3):515–520. [Google Scholar]
- 13.Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 2007;44(Suppl 3):S84–S101. doi: 10.1086/511422. [DOI] [PubMed] [Google Scholar]
- 14.Yazdankhah SP, Caugant DA. Neisseria meningitidis: An overview of the carriage state. J Med Microbiol. 2004;53(Pt 9):821–832. doi: 10.1099/jmm.0.45529-0. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, et al. Identification of novel antigens that protect against systemic meningococcal infection. Vaccine. 2005;23(32):4136–4141. [Google Scholar]
- 16.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JS, et al. Species status of Neisseria gonorrhoeae: Evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 2007;5:35. doi: 10.1186/1741-7007-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonjum T. Genus I. Neisseria Trevisan 1885, 105AL. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2005. 2nd Ed Vol 2C, pp 777–798. [Google Scholar]
- 19.Knapp JS, Hook EW., 3rd Prevalence and persistence of Neisseria cinerea and other Neisseria spp. in adults. J Clin Microbiol. 1988;26(5):896–900. doi: 10.1128/jcm.26.5.896-900.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sáez Nieto JA, Marcos C, Vindel A. Multicolonization of human nasopharynx due to Neisseria spp. Int Microbiol. 1998;1(1):59–63. [PubMed] [Google Scholar]
- 21.Bennett JS, et al. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect Immun. 2005;73(4):2424–2432. doi: 10.1128/IAI.73.4.2424-2432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckee CO, et al. Role of selection in the emergence of lineages and the evolution of virulence in Neisseria meningitidis. Proc Natl Acad Sci USA. 2008;105(39):15082–15087. doi: 10.1073/pnas.0712019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bovre K, Holten E. Neisseria elongata sp. nov., a rod-shaped member of the genus Neisseria. Re-evaluation of cell shape as a criterion in classification. J Gen Microbiol. 1970;60(1):67–75. doi: 10.1099/00221287-60-1-67. [DOI] [PubMed] [Google Scholar]
- 24.Virji M, Heckels JE, Potts WJ, Hart CA, Saunders JR. Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. J Gen Microbiol. 1989;135(12):3239–3251. doi: 10.1099/00221287-135-12-3239. [DOI] [PubMed] [Google Scholar]
- 25.Hobbs MM, et al. Experimental gonococcal infection in male volunteers: Cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front Microbiol. 2011;2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjölinder H, Jonsson AB. Olfactory nerve—A novel invasion route of Neisseria meningitidis to reach the meninges. PLoS ONE. 2010;5(11):e14034. doi: 10.1371/journal.pone.0014034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu HM, et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360(9):886–892. doi: 10.1056/NEJMoa0806414. [DOI] [PubMed] [Google Scholar]
- 28.Ito M, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother. 2005;49(1):137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osaka K, et al. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J Infect Chemother. 2008;14(3):195–203. doi: 10.1007/s10156-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 30.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34(2):115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 31.Evans CM, et al. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin Infect Dis. 2011;52(1):70–77. doi: 10.1093/cid/ciq065. [DOI] [PubMed] [Google Scholar]
- 32.Marri PR, et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS ONE. 2010;5(7):e11835. doi: 10.1371/journal.pone.0011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiden MC, Stuart JM. UK Meningococcal Carriage Group Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359(9320):1829–1831. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 34.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ. 2003;326(7385):365–366. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeberling O, et al. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine. 2011;29(29-30):4728–4734. doi: 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold R, Goldschneider I, Lepow ML, Draper TF, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137(2):112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 37.Kellogg DS, Jr, Cohen IR, Norins LC, Schroeter AL, Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.