Abstract
Assessing the extent to which population subdivision during cladogenesis is necessary for long-term phenotypic evolution is of fundamental importance in a broad range of biological disciplines. Differentiating cladogenesis from anagenesis, defined as evolution within a species, has generally been hampered by dating precision, insufficient fossil data, and difficulties in establishing a direct link between morphological changes detectable in the fossil record and biological species. Here we quantify the relative frequencies of cladogenesis and anagenesis for macroperforate planktic Foraminifera, which arguably have the most complete fossil record currently available, to address this question. Analyzing this record in light of molecular evidence, while taking into account the precision of fossil dating techniques, we estimate that the fraction of speciation events attributable to anagenesis is <19% during the Cenozoic era (last 65 Myr) and <10% during the Neogene period (last 23 Myr). Our central conclusion—that cladogenesis is the predominant mode by which new planktic Foraminifera taxa become established at macroevolutionary time scales—differs markedly from the conclusion reached in a recent study based solely on fossil data. These disparate findings demonstrate that interpretations of macroevolutionary dynamics in the fossil record can be fundamentally altered in light of genetic evidence.
Keywords: punctuated equilibrium, phyletic gradualism, lineage, morphospecies
Biological evolution proceeds through two distinct modes: anagenesis, which occurs within a species, and cladogenesis, which occurs during speciation and results in the subdivision of a species into two reproductively isolated, independently evolving taxa (1, 2). Distinguishing between these modes is essential to determining the role of population subdivision in evolutionary dynamics (3, 4). This topic has received considerable attention in paleontology (1, 2), perhaps because the fossil record provides the only direct record of long-term morphological change (5), but its importance extends to other biological disciplines. For example, in population genetics, models only predict that phenotypic change is contingent upon or associated with the evolution of reproductive isolation under specific circumstances (3). Thus, a primary role for cladogenesis may highlight the importance of particular speciation modes (2), metapopulations dynamics (6), or adaptive peaks in fitness landscapes (7, 8). In community ecology, both evolutionary modes may influence biodiversity, but the mechanisms differ: only cladogenesis results in an increase in diversity (9–11), but anagenesis may help maintain diversity if it results in niche differences that facilitate species coexistence (12, 13).
Remarkably few studies have attempted to quantify the relative frequencies of anagenesis and cladogenesis for entire species assemblages (e.g., refs. 5, 14, 15). Addressing this question is challenging due to insufficient fossil data for most taxa and the difficulties in relating morphological change to genetic divergence and the evolution of reproductive isolation (16). Fossil data can only be used to distinguish anagenesis from cladogenesis, at the assemblage level, to the extent that four general assumptions are upheld (5): (i) morphologically distinct taxa (hereafter morphospecies) that coexist temporally are reproductively isolated, and therefore independently evolving; (ii) ancestor–descendant relationships among morphospecies are inferred accurately; (iii) the vast majority of morphospecies have been identified; and (iv) the temporal range of each morphospecies, as defined by its first appearance datum (FAD) and last appearance datum (LAD), is estimated with known precision. Together these assumptions imply that the mode of evolution may be anagenetic if the FAD of the descendant coincides with the LAD of the ancestor within the bounds of the dating precision. Conversely, the mode is cladogenetic if, after accounting for dating precision, the FAD of the descendant still precedes the LAD of the ancestor, because this implies that the ancestor and descendant existed concurrently for some time period. Overlap in the temporal ranges of the ancestor and descendant has long been considered the “best de facto evidence of true cladogenesis” (17).
The fossil record for planktic Foraminifera—a group of single-celled eukaryotes that occupy marine pelagic zones throughout the world (18)—arguably adheres to assumptions i–iv better than that of any other group, providing an unprecedented opportunity to assess the relative frequencies of anagenesis and cladogenesis. Early fossil studies suggested that Foraminifera morphospecies generally arise through gradual, anagenetic change (19, 20). However, more recent studies of selected taxa indicate that cladogenesis may be far more prevalent than previously thought (21, 22). These recent studies highlight the need to integrate fossil data with other forms of evidence, particularly molecular data (23).
Here we assess the relative frequencies of anagenesis and cladogenesis for Cenozoic macroperforate planktic Foraminifera. We do so by analyzing the recently published phylogeny of Aze et al. (24), using a unique approach that incorporates assumptions i–iii above while taking into account uncertainties in the precision of the FAD and LAD estimates (assumption iv). This phylogeny summarizes current understanding of ancestor–descendant relationships among extinct and extant morphospecies based on the work of multiple researchers. It is anchored by a comprehensive compilation of FAD and LAD estimates (24), most of which were obtained using the most precise dating techniques currently available (25–27). Interpreting this phylogeny in light of molecular evidence for extant members of the Foraminifera (28–31), we calculate upper-bound estimates for the fractions of speciation events that may be the result of anagenesis for the Cenozoic era (last 65 Ma) and for the Neogene period (last 23 Ma), as described in Methods. Before presenting the results of our calculations, we consider evidence for and against assumptions i–iv for planktic Foraminifera. We conclude by considering the limitations of our approach and the potential implications of our findings.
Evaluating Assumptions
Adherence to assumptions i–iv above requires a largely complete fossil record. The Cenozoic fossil record for planktic Foraminifera is among the most well resolved for any group in terms of both taxonomic and temporal resolution owing to: high abundance and global ubiquity in marine pelagic zones (18), high preservation potential of their calcium carbonate shells in marine sediments, extensive spatial and temporal coverage of sampling, and a consistent and well-established system of taxonomic classification (24).
Assumptions i and ii are generally consistent with molecular studies, but exceptions have been observed. Analyses of small subunit ribosomal DNA (SSU rDNA) data for 19 extant morphospecies (of ∼50) indicate that morphospecies are generally monophyletic, and that ancestor–descendant relationships among morphospecies are accurately inferred from fossils at lower levels of the phylogeny (33, 34). One noted exception to monophyly among extant morphospecies is Globigerinoides ruber, which is now recognized as comprising two distinct clades on the basis of molecular evidence (35). Inconsistencies between molecular and fossil data are largely confined to the placement of higher-order clades, where SSU rDNA divergences are high and relationships are difficult to resolve (36). Although not all ancestor–descendant relationships are yet well established (24), the general concordance of molecular- and fossil-based methods at lower taxonomic levels suggests that the “stratophenetic” method of delineating ancestor–descendant relationship by tracing specimens of intermediate morphology back through time (37) is quite robust. More generally, this concordance is consistent with data indicating that shell traits often have a genetic basis that is sufficiently strong and conservative to allow discrimination among clades at macroevolutionary time scales (Table 1). Given that the shell characteristics used for planktic Foraminifera identification are the same for extinct and extant taxa, the findings of molecular studies are relevant for interpreting fossil assemblages (30). In particular, they suggest that assumptions i and ii are also upheld for extinct morphospecies.
Table 1.
Foraminiferal morphological characters that have been demonstrated to reflect genetically distinct populations
| Morphological character | Independent evidence |
| Test outline | Test outlines differ among genetically, ecologically, and biogeographically distinct populations within Globigerinella siphonifera (42) and Truncorotalia truncatulinoides (31, 54). |
| Chamber morphology | Compression of the final two chambers can be used to discriminate among genetically distinct populations within Globigerinoides ruber (49). |
| Coiling direction | Sinistral and dextral forms of the morphospecies Neogloboquadrina pachyderma correspond to ecologically and genetically distinct species with different temperature tolerances and biogeographic distributions (68, 69). |
| Relative frequencies of sinistral and dextral forms vary among genetically, ecologically, and biogeographically distinct populations of Truncorotalia truncatulinoides (31). | |
| Porosity | Populations of Orbulina universa that vary in porosity are genetically distinct and have different biogeographic distributions (28, 42, 43). |
Test outline and chamber morphology have been used historically to differentiate morphospecies. Coiling direction has only recently been confirmed as a character of taxonomic significance (68). Porosity is not currently recognized as a diagnostic character.
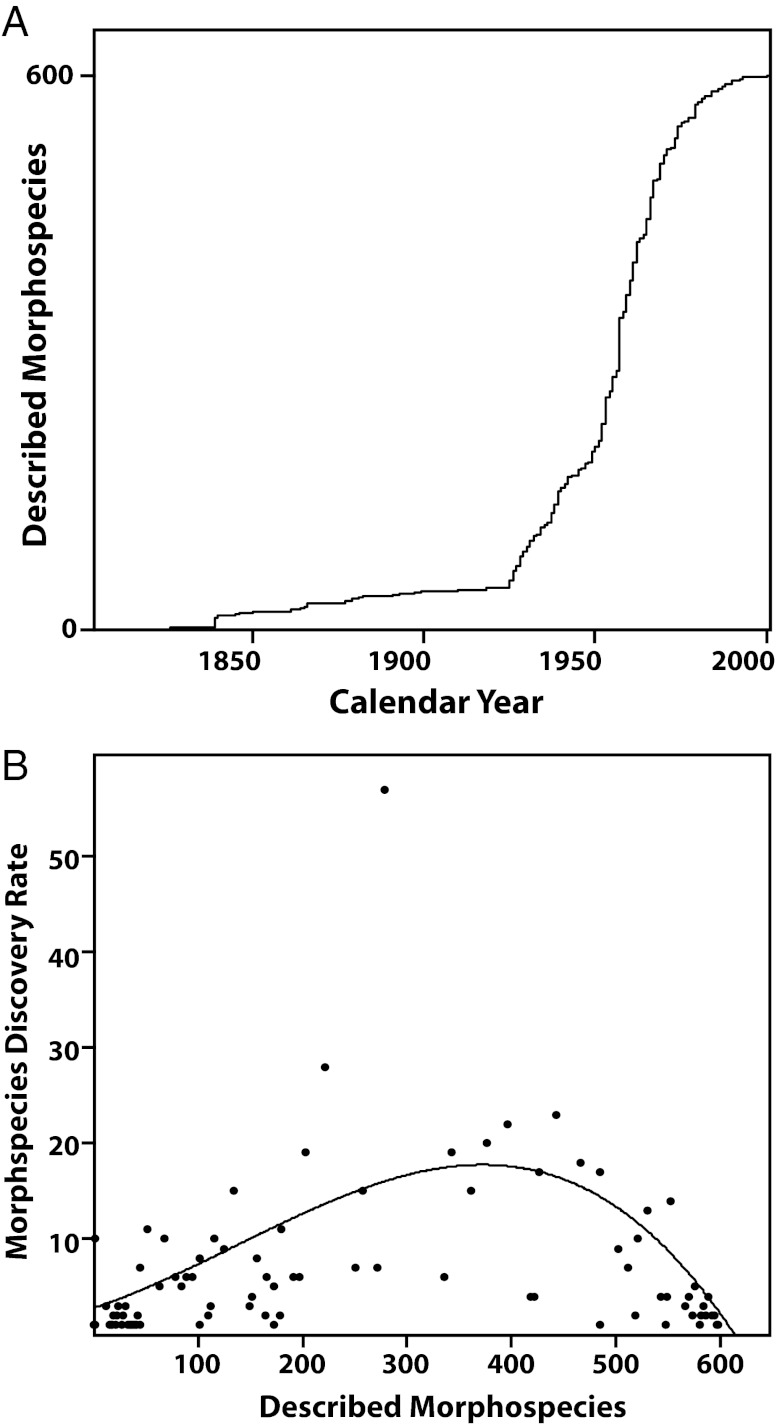
Assumption iii is supported by compiled data (38) presented in Fig. 1, which provides clear evidence of an asymptote in the rate of discovery of new morphospecies between 1826 and 2001 (Fig. 1A). Analyzing these data using the method proposed by Bebber et al. (39), we estimate that ∼96% [95% confidence interval (CI) of 96–100%] of all planktic Foraminifera morphospecies (both extinct and extant) have already been described (Fig. 1B). Consequently, it is unsurprising that recent taxonomic work has focused primarily on clarifying and refining phylogenetic relationships among already described morphospecies (40, 41) and subdividing morphospecies on the basis of finer-scale morphological differences (28, 31, 42, 43) (Table 1).
Fig. 1.
Number of planktic foraminiferal taxa described from 1826 to 2001 (data from ref. 38). (A) Cumulative number of described species is plotted as a function of time. The curve is asymptotic, indicating that there remain few undescribed species as of 2001. (B) Statistical approach of Bebber et al. (39) is used to estimate the asymptotic number of species by fitting a function that describes how the annualized rate of discovery (y axis) varies in relation to the number of species already described (x axis). The estimated total number of species corresponds to the number of species at which the rate of species discovery is equal to 0. The line is a cubic function that was fitted to the data using ordinary-least-squares regression. The model predicts a total of 622 planktic Foraminifera taxa (95% CI: 598–625) with 598 taxa discovered as of 2001.
Assigning precision estimates (assumption iv) to the FAD and LAD data, in part, entails consideration of three sources of dating error: disturbance of the sediments in which the taxon occurs after initial deposition or during collection, resulting in a mixing of sediments of varying age; regional diachroneity of taxon occurrence due to expansion and contraction of a taxon’s geographic range; and resolution of the chronostratigraphic techniques used to date the sediments (44). The first two sources affect dating at particular locations, but are largely controlled for when estimating FAD and LAD because consensus estimates are obtained by selecting the data deemed to be most reliable from among multiple locations (24). Chronostratigraphic resolution is therefore the primary source of error and, importantly, varies with sediment age. For the Neogene period (last 23 Ma), changes in sediment properties through time can be detected and correlated with Milankovitch glacial–interglacial cycles, resulting in a dating resolution of ±0.005 Ma to ±0.046 Ma (45). By contrast, for the Paleogene period (65–23 Ma ago), the most accurate dating method makes use of cycles in geomagnetic polarity, resulting in a dating resolution of ±0.380 Ma (46). As these dating methods have known levels of precision, they can be used to assign maximum precision estimates to the FAD and LAD data, following assumption iv.
Despite evidence in support of assumptions i–iv, there are two limitations in using morphospecies-level FAD and LAD data to distinguishing anagenesis from cladogenesis. First, Foraminifera specimens are generally assigned to morphospecies based on their similarity to designated holotypes (47). Whereas the holotype concept is inherently static and typological, a population is a dynamic entity composed of individuals of varying morphology (37). If individuals at the extremes of the morphotype distribution of an evolving population, rather than the mean morphotype, are used to estimate FAD and LAD, overlap in the temporal ranges of the “ancestor” and “descendant” of a single population undergoing anagenesis may occur (Fig. 2A). This phenomenon, which we refer to as “typological error,” can be avoided by defining FAD and LAD on the basis of frequency distributions of morphotypes, but detailed morphometric studies of this type are rare (e.g., ref. 48). In the absence of data to directly estimate the magnitude of typological error, we consider a range of values for the total error, defined as the sum of the dating error and the typological error, as well as other forms of evidence (Methods).
Fig. 2.
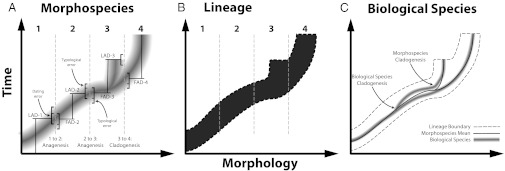
Graphical depiction of morphological evolution at the levels of (A) morphospecies, (B) lineage, and (C) biological species, for a hypothetical lineage. (A) Darker shading corresponds to morphotypes of higher abundance in morphospace for an evolving population. Dashed vertical lines represent typological divisions of the morphospace into discrete morphospecies. Based on assumptions i–iv, we identify the transitions between morphospecies 1 and 2, and between morphospecies 2 and 3, as potentially anagenetic, because the FAD and LAD values overlap within our error margins. Typological errors may broaden the estimated temporal overlap between ancestor and descendant, and must therefore also be accounted for. As the LAD of morphospecies 3 and FAD of morphospecies 4 do not overlap, and the two forms occupy distinguishable peaks in morphospace, they represent distinct morphospecies, implying that a cladogenetic event occurred. (B) All four morphospecies are assigned to the same lineage, despite morphospecies 3 and 4 having distinguishable frequency distributions in morphospace, because adjacent frequency distributions exhibit some overlap. Had there been unoccupied morphospace between morphospecies 3 and 4, this would have represented a cladogenetic event at the lineage level (24). (C) Selected “cryptic” biological species within morphospecies are represented as thin lines. Molecular evidence indicates that a morphospecies generally comprises multiple cryptic biological species (30). Consequently, the cladogenetic event at the morphospecies level may occur at the same time as the cladogenetic event at the biological species level or may occur later, as depicted in the figure.
The second challenge is that a number of studies have presented molecular evidence that morphospecies generally contain multiple “cryptic” genotypes that correspond to distinct biological species (30, 35, 49). Whereas these findings lend further support to the argument that Foraminifera morphospecies are reproductively isolated (assumption i), the existence of cryptic biological species limits what can be inferred from morphospecies-level data. In particular, it implies the following: (i) a single anagenetic event at the morphospecies-level may correspond to multiple cladogenetic events at the biological species level (13); (ii) a cladogenetic event at the biological species level may occur at the time of morphospecies-level divergence or may precede it (Fig. 2C), as has been proposed for the Fohsella lineage on the basis of isotopic evidence (22); and (iii) there are many instances of cladogenesis at the biological species level that leave no trace in the fossil record at the morphospecies level. For all three of these reasons, morphospecies-level data cannot be used to assess the relative frequencies of anagenesis and cladogenesis at the biological species level if such species are cryptic, as is the case for planktic Foraminifera.
Methods
Given the central importance of temporal coexistence of the ancestor and descendant in establishing cladogenesis, following assumption i, we conservatively assume that an ancestor–descendant pair represents a potential anagenetic event unless both taxa are extant, which implies temporal coexistence and hence cladogenesis, or evidence exists to establish temporal coexistence at some time in the past. In our analysis, we consider three forms of evidence, in sequence, that can be used to establish an event as cladogenetic: stratigraphic evidence that the FAD of the descendant is older than the LAD of the ancestor; morphometric evidence that the ancestor and descendant exhibit distinguishable peaks in morphospace during the time period of range overlap; and/or phylogenetic evidence that an ancestor gave rise to several descendants over a short time period. In instances where detailed investigation of a particular speciation event fails to uncover evidence of cladogenesis, we identify that event as putative anagenesis (e.g., ref. 50).
In the first stage of analysis, we assess whether there is stratigraphic evidence of temporal range overlap for each ancestor–descendant pair after accounting for dating and typological errors. We calculate overlap using the FAD and LAD data compiled by Aze et al. (24). This compilation comprises predominantly recent studies, but also includes some older studies that use dating techniques that are less precise than those now available for Neogene events. For the Neogene, we therefore assume a dating error of ±0.1 Ma, which is similar in magnitude to estimates reported for Neogene diatoms (±0.08 Ma) (51). For the Paleogene, we assume a dating error of ±0.4 Ma based on the chronostratigraphic resolution (46). For typological error, direct estimates are unavailable, so we instead assume total errors (= dating errors + typological errors) of ±0.3 Ma for the Neogene and ±1.2 Ma for the Paleogene. We thus identify an event as cladogenetic at this stage of the analysis if temporal range overlap exceeds a total-error margin of 0.6 Ma for a Neogene event or 2.4 Ma for a Paleogene event. These margins were selected based on the maximum reported range-overlap values for putative anagenetic events during the Neogene (0.4 Ma for Globorotalia terminalis–Globorotalia sphericomiozea) (48) and Paleogene (1.5 Ma for Morozovella angulata–Morozovella conicotruncata) (52). Our chosen total-error margins exceed these values by 50% and are therefore expected to be conservative.
In the second stage, we consider morphometric evidence. We use this evidence in two ways. First, for events where range overlap is less than the total-error margin, but exceeds the dating-error margin (i.e., 0.2 Ma and 0.8 Ma for Neogene and Paleogene events, respectively), we can refute typological error and thus identify an event as cladogenetic, if there exists empty morphospace between the ancestor and descendant (24). This is because typological error can only occur if the frequency distributions of morphotypes for the ancestor and descendant exhibit overlap in morphospace (Fig. 2).
Second, we can identify an event as cladogenetic where, in the same sediment sample, ancestor and descendant have frequency distributions in morphospace that are statistically distinguishable, because this implies that the two taxa were contemporaneous (21, 48, 50). This morphometric approach is supported by theory (20) and by detailed ecological and genetic studies of extant planktic Foraminifera taxa (28, 31, 42, 43, 53, 54).
In the final stage, we consider phylogenetic evidence for cases where a single ancestral morphotype produces two or more descendants within the bounds of our total-error margins. Given our assumption that ancestor–descendant relationships are accurate (assumption ii), for an ancestral taxon to have given rise to two descendants sequentially, it must have remained extant after the emergence of the first descendant to give rise to the second descendant. Thus, the ancestor must have existed concurrently with its first descendant, implying that a cladogenetic event occurred.
Results and Discussion
Of 337 Cenozoic speciation events (24), 31.2% are identified as potentially anagenetic (95% CI: 26.4–36.2%; 105 events) based solely on stratigraphic evidence (Table S1). Restricting consideration to the 122 Neogene events, which are dated at higher precision, 18.9% are identified as potentially anagenetic based only on stratigraphic evidence (95% CI: 12.3–26.2%; 23 events). Incorporating morphological and phylogenetic evidence reduces the Cenozoic estimate from 31.2 to 19.0% (95% CI: 14.8–23.1%; 64 of 337 events), including seven putative anagenetic events, and the Neogene estimate from 18.9 to 9.8% (95% CI: 4.9–15.6%; 12 of 122 events), including one putative anagenetic event (Table S1).
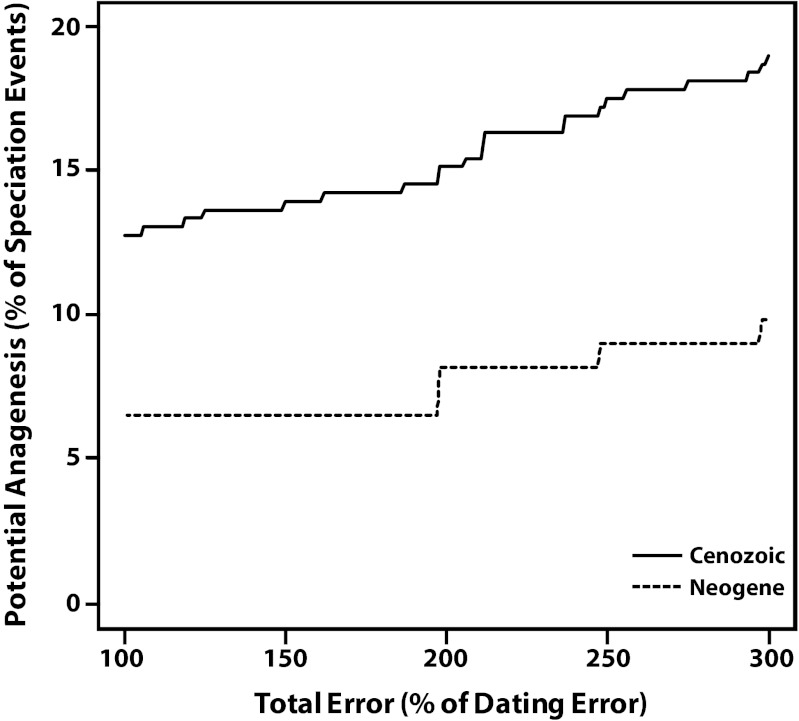
These estimates are sensitive to the assumed magnitudes of typological errors (Fig. 3). For example, if morphological and phylogenetic evidence are considered, but typological errors are assumed to be negligible, the estimated fraction of speciation events that are potentially anagenetic is reduced further to 12.8% for Cenozoic events (95% CI: 9.2–16.3%; 43 events), and to 6.6% for Neogene events (95% CI: 2.5–11.5%; 8 events).
Fig. 3.
Estimated fractions of speciation events that are potentially anagenetic for Cenozoic (solid lines) and Neogene (dashed lines) planktic Foraminifera, assuming varying levels of total error. Total errors are expressed as percentages of the dating errors (100% = ±0.1 Ma for the Neogene, ±0.4 Ma for Paleogene). Estimates take into account morphological and phylogenetic evidence (Table S1).
Before discussing the potential implications of our findings, it is important to first consider the effects of typological error. In the absence of data to directly estimate typological error, we estimate the total errors based on the maximum observed range overlap for ancestor–descendant pairs shown to be the result of anagenesis. Our estimates are necessarily crude given the paucity of detailed investigations on particular speciation events (Table S1). However, even if we allow the total errors to exceed our crude estimates by 50% (0.6 Ma range overlap for the Neogene, 2.4 Ma overlap for the Paleogene), our central conclusion that cladogenesis is the predominant mode is qualitatively unaffected (Fig. 3). Thus, our study highlights that range overlap may aid in distinguishing cladogenesis from anagenesis, particularly when combined with other forms of evidence. It also highlights the need for further inquiry into the issue of typological error, which will require detailed morphometric investigations (e.g., refs. 50, 55, 56).
Our estimates for the fractions of speciation events that are potentially anagenetic is quantitatively consistent with that of Wagner and Erwin (14), who estimated that anagenesis comprised at most 12.5% of Foraminifera speciation events using a phylogeny that was much smaller (40 morphospecies of globigerinids) and less precisely dated than the one used here. It is also consistent with more recent, detailed analyses of putative anagenetic events, which have subsequently been shown to result from cladogenesis. Hull and Norris (21), for example, demonstrated that the sequence between G. plesiotumida and G. tumida—which was once upheld as an example of phyletic gradualism (19)—included a third previously unrecognized morphotype, which arose through cladogenesis and gave rise to G. tumida over an exceptionally short time period (∼45,000 y). Our analysis also identifies the emergence of G. tumida as a cladogenetic event, because G. plesiotumida persists at sites other than the one where G. tumida first appeared for a period of 1.3 Ma (24). Another example is the evolution of late Neogene lineage Sphaeroidinella, which was once considered a prime example of anagenesis (57). However, subsequent work has shown that the emergence of the descendant taxon was restricted to a portion of the ancestral taxon’s range (58), implying that the event was cladogenetic.
We do not mean to imply that phenotypic evolution occurs only during cladogenesis; temporal changes in phenotype are well documented for morphospecies. Lazarus et al. (59), for example, showed that Truncorotalia crassaformis, Truncorotalia tosaensis, and Truncorotalia truncatulinoides all exhibited significant variation through time in test size and shape. However, these changes entailed random shifts in phenotype space about a mean, implying quasistasis, rather than directional changes in morphology. Using a random-walk model of phenotypic evolution, Hunt (60) was able to define and test for stasis, and found good support for it before and after the transition from G. plesiotumida to G. tumida. In light of such findings, our results could be viewed as supportive of Futuyma’s (3) qualified version of punctuated equilibrium, which predicts that phenotypic changes through time are generally ephemeral, owing to habitat changes and extinction and interbreeding among local populations, unless accompanied by the evolution of reproductive isolation, which allows phenotypic differences to persist over time scales long enough to be observed in the fossil record.
Our estimate for the relative frequency of anagenesis is far lower than the estimate of 40% reported by Aze et al. (24) using the same macroperforate planktic Foraminifera data. This pronounced difference is directly attributed to the different set of assumptions used in their study. In particular, Aze et al. (24) only tallied an event as cladogenetic if the descendant species was sufficiently differentiated that it exhibited no zone of overlap with its ancestor in morphospace. As depicted in the hypothetical example in Fig. 2C, morphospecies 3 and 4 fail this criterion, and would therefore be classified as part of the same lineage by Pearson and colleagues (24, 37, 61). There are important advantages to this lineage-based approach. In particular, it has allowed an objective and internally consistent phylogeny to be constructed and has yielded important insights (24, 32). However, because lineages are defined without considering other data, they can encompass genetically and ecologically distinct taxa (Fig. 4). Aze and colleagues (24) were aware of this issue, but explicitly chose not to incorporate genetic evidence because such data are currently only available for a subset of taxa. Thus, the fact that our study has reached an entirely different conclusion than that of Aze et al. (24) demonstrates that our interpretations of the same fossil data can be fundamentally altered in light of genetic evidence.
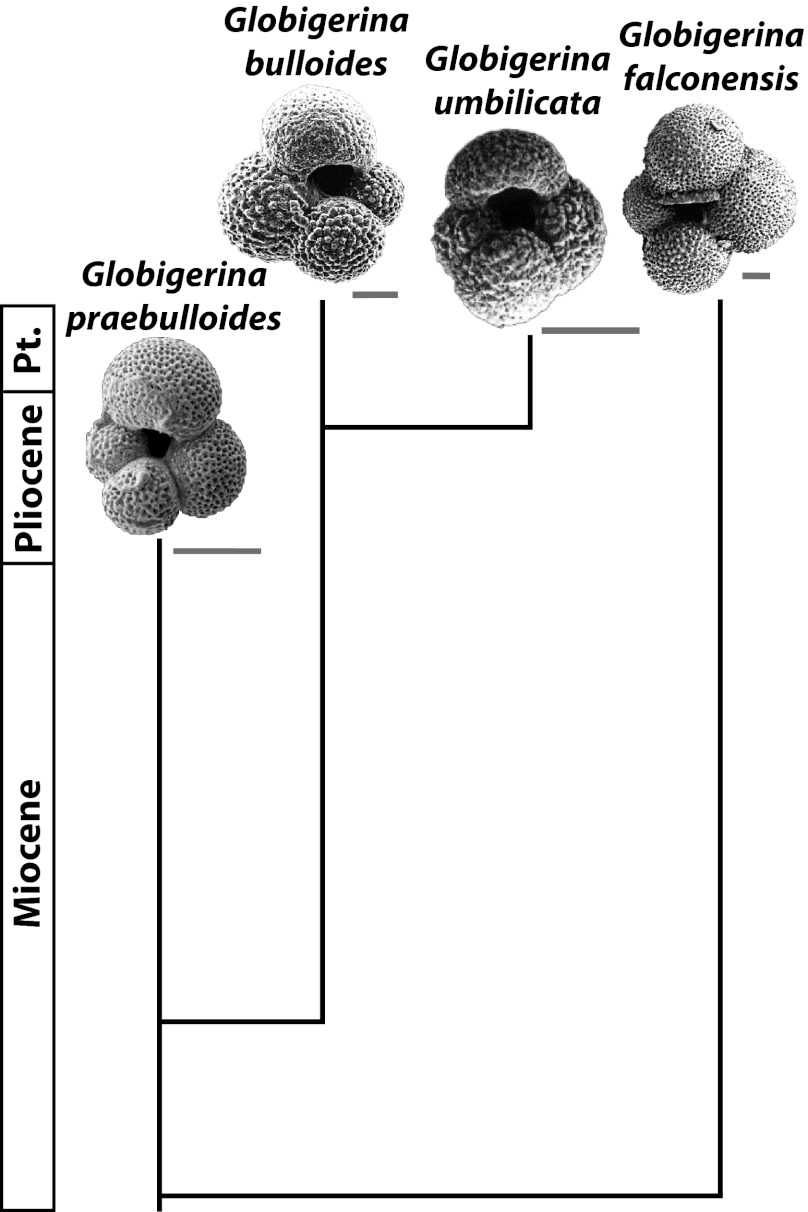
Fig. 4.
Phylogeny for the Globigerina lineage as per Aze et al. (24). Taxa are grouped in the same lineage based on overlap in the frequency distributions of morphotypes (24), but three lines of evidence indicate that Globigerina bulloides and Globigerina falconensis are reproductively isolated, and therefore independently evolving taxa: SSU rDNA genetic divergence is 22.9% (34), habitat preferences differ (62), only G. falconensis has symbionts (63). (Scale bars in gray, 100 µm for Globigerina praebulloides and Globigerina umbilicata and 50 µm for G. bulloides and G. falconensis.) Pt, Pleistocene. [G. praebulloides image reprinted from ref. 64, Copyright (2004), with permission from Elsevier. G bulloides image reprinted with permission from ref. 65. G. umbilicata image reprinted from ref. 66, Copyright (2003), with permission from Elsevier. G. falconensis image reprinted with permission from ref. 67.]
Supplementary Material
Acknowledgments
We thank John Alroy, Gene Hunt, and one anonymous reviewer for providing constructive feedback and commentary that improved the quality of the manuscript. L.C.S. and A.P.A. were supported under the Australian Research Council's Discovery Projects funding scheme (DP-110105280).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208302110/-/DCSupplemental.
References
- 1.Simpson GG. The Major Features of Evolution. Columbia Univ Press, New York; 1953. [Google Scholar]
- 2.Eldredge N, Gould SJ. In: Models in Paleobiology. Schopf TJM, editor. San Francisco: Freman, Cooper & Co; 1972. pp. 82–115. [Google Scholar]
- 3.Futuyma DJ. On the role of species in anagenesis. Am Nat. 1987;130(3):465–473. [Google Scholar]
- 4.Venditti C, Pagel M. Speciation as an active force in promoting genetic evolution. Trends Ecol Evol. 2010;25(1):14–20. doi: 10.1016/j.tree.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JBC, Cheetham AH. Tempo and mode of speciation in the sea. Trends Ecol Evol. 1999;14(2):72–77. doi: 10.1016/s0169-5347(98)01504-3. [DOI] [PubMed] [Google Scholar]
- 6.Eldredge N, et al. The dynamics of evolutionary stasis. Paleobiology. 2005;31(Suppl 2):133–145. [Google Scholar]
- 7.Estes S, Arnold SJ. Resolving the paradox of stasis: Models with stabilizing selection explain evolutionary divergence on all timescales. Am Nat. 2007;169(2):227–244. doi: 10.1086/510633. [DOI] [PubMed] [Google Scholar]
- 8.Lande R. The dynamics of peak shifts and the pattern of morphological evolution. Paleobiology. 1986;12(4):343–354. [Google Scholar]
- 9.Hubbell SP. A Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ: Princeton Univ Press; 2001. [Google Scholar]
- 10.Allen AP, Gillooly JF. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol Lett. 2006;9(8):947–954. doi: 10.1111/j.1461-0248.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 11.Allen AP, Gillooly JF, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci USA. 2006;103(24):9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacArthur RH. Geographical Ecology: Patterns in the Distribution of Species. New York: Harper & Row; 1972. [Google Scholar]
- 13.Alizon S, Kucera M, Jansen VAA. Competition between cryptic species explains variations in rates of lineage evolution. Proc Natl Acad Sci USA. 2008;105(34):12382–12386. doi: 10.1073/pnas.0805039105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner PJ, Erwin DH. In: New Approaches for Studying Speciation in the Fossil Record. Erwin DH, Anstey RL, editors. New York: Columbia Univ Press; 1995. pp. 87–122. [Google Scholar]
- 15.Stuessy TF, et al. Anagenetic evolution in island plants. J Biogeogr. 2006;33(7):1259–1265. [Google Scholar]
- 16.Hallam A. Speciation patterns and trends in the fossil record. Geobios. 1997;30(7):921–930. [Google Scholar]
- 17.Eldredge N. In: New Approaches for Studying Speciation in the Fossil Record. Erwin DH, Anstey RL, editors. New York: Columbia Univ Press; 1995. pp. 39–63. [Google Scholar]
- 18.Langer MR. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J Eukaryot Microbiol. 2008;55(3):163–169. doi: 10.1111/j.1550-7408.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 19.Malmgren BA, Berggren WA, Lohmann GP. Evidence for punctuated gradualism in the late neogene Globorotalia tumida lineage of planktonic foraminifera. Paleobiology. 1983;9(4):377–389. [Google Scholar]
- 20.Tabachnick RE, Bookstein FL. The structure of individual variation in Miocene globorotalia. Evolution. 1990;44(2):416–434. doi: 10.1111/j.1558-5646.1990.tb05209.x. [DOI] [PubMed] [Google Scholar]
- 21.Hull PM, Norris RD. Evidence for abrupt speciation in a classic case of gradual evolution. Proc Natl Acad Sci USA. 2009;106(50):21224–21229. doi: 10.1073/pnas.0902887106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris RD, Corfield RM, Cartlidge J. What is gradualism? Cryptic speciation in globorotaliid foraminifera. Paleobiology. 1996;22(3):386–405. [Google Scholar]
- 23.Norris RD. Pelagic species diversity, biogeography, and evolution. Paleobiology. 2000;26(4):236–258. [Google Scholar]
- 24.Aze T, et al. A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data. Biol Rev Camb Philos Soc. 2011;86(4):900–927. doi: 10.1111/j.1469-185X.2011.00178.x. [DOI] [PubMed] [Google Scholar]
- 25.Berggren WA, Kent DV, Swisher CC, III, Aubry MP. A revised Cenozoic geochronology and chronostratigraphy. Society for Sedimentary Geology Special Publication. 1995;54:129–212. [Google Scholar]
- 26.Berggren WA, Pearson PN. A revised tropical to subtropical Paleogene planktonic foraminiferal zonation. J Foraminiferal Res. 2005;35(4):279–298. [Google Scholar]
- 27.Wade BS, Pearson PN, Berggren WA, Pälike H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth Sci Rev. 2011;104(1–3):111–142. [Google Scholar]
- 28.de Vargas C, Norris R, Zaninetti L, Gibb SW, Pawlowski J. Molecular evidence of cryptic speciation in planktonic foraminifers and their relation to oceanic provinces. Proc Natl Acad Sci USA. 1999;96(6):2864–2868. doi: 10.1073/pnas.96.6.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darling KF, et al. Molecular evidence for genetic mixing of Arctic and Antarctic subpolar populations of planktonic foraminifers. Nature. 2000;405(6782):43–47. doi: 10.1038/35011002. [DOI] [PubMed] [Google Scholar]
- 30.Darling KF, Wade CM. The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotypes. Mar Micropaleontol. 2008;67(3–4):216–238. [Google Scholar]
- 31. Quillévéré F, et al. (2012) Global scale same-specimen morpho-genetic analysis of Truncorotalia truncatulinoides: A perspective on the morphological species concept in planktonic foraminifera. Palaeogeogr Palaeoclimatol Palaeoecol, 10.1016/j.palaeo.2011.03.013.
- 32.Ezard THG, Pearson PN, Aze T, Purvis A. The meaning of birth and death (in macroevolutionary birth-death models) Biol Lett. 2012;8(1):139–142. doi: 10.1098/rsbl.2011.0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vargas C, Zaninetti L, Hilbrecht H, Pawlowski J. Phylogeny and rates of molecular evolution of planktonic foraminifera: SSU rDNA sequences compared to the fossil record. J Mol Evol. 1997;45(3):285–294. doi: 10.1007/pl00006232. [DOI] [PubMed] [Google Scholar]
- 34.Stewart IA, Darling KF, Kroon D, Wade CM, Troelstra SR. Genotypic variability in subarctic Atlantic planktic foraminifera. Mar Micropaleontol. 2001;43(1–2):143–153. [Google Scholar]
- 35.Aurahs R, Grimm GW, Hemleben V, Hemleben C, Kucera M. Geographical distribution of cryptic genetic types in the planktonic foraminifer Globigerinoides ruber. Mol Ecol. 2009;18(8):1692–1706. doi: 10.1111/j.1365-294X.2009.04136.x. [DOI] [PubMed] [Google Scholar]
- 36.Aurahs R, et al. Using the multiple analysis approach to reconstruct phylogenetic relationships among planktonic foraminifera from highly divergent and length-polymorphic SSU rDNA sequences. Bioinform Biol Insights. 2009;3:155–177. doi: 10.4137/bbi.s3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson PN. A lineage phylogeny for the Paleogene Planktonic Foraminifera. Micropaleontology. 1993;39(3):193–232. [Google Scholar]
- 38.Stewart DRM, Pearson PN. 2000. PLANKRANGE: a database of planktonic foraminiferal ranges. Available at http://palaeoglybrisacuk/Data/plankrangehtml. (Last update: 12/1/2002). Accessed May 10, 2012.
- 39.Bebber DP, Marriott FHC, Gaston KJ, Harris SA, Scotland RW. Predicting unknown species numbers using discovery curves. Proc Biol Sci. 2007;274(1618):1651–1658. doi: 10.1098/rspb.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsson RK, Hemleben C, Berggren WA, Huber BT. 1999. Atlas of Paleocene planktonic Foraminifera. Smithsonian Contributions to Paleobiology (Smithsonian Institution Press, Washington)
- 41.Pearson PN, Olsson RK, Huber BT, Hemleben C, Berggren WA. 2006. Atlas of Eocene planktonic foraminifera. Cushman Foundation for Foraminiferal Research Special Publication (Cushman Foundation for Foraminiferal Research, Washington, DC)
- 42.Huber BT, Bijma J, Darling K. Cryptic speciation in the living planktonic foraminifer Globigerinella siphonifera (d'Orbigny) Paleobiology. 1997;23(1):33–62. [Google Scholar]
- 43.Morard R, et al. Morphological recognition of cryptic species in the planktonic foraminifer Orbulina universa. Mar Micropaleontol. 2009;71(3–4):148–165. [Google Scholar]
- 44.Pol D, Norell MA. Uncertainty in the age of fossils and the stratigraphic fit to phylogenies. Syst Biol. 2006;55(3):512–521. doi: 10.1080/10635150600755446. [DOI] [PubMed] [Google Scholar]
- 45.Hilgen FJ, et al. Extending the astronomical (polarity) time scale into the Miocene. Earth Planet Sci Lett. 1995;136(3–4):495–510. [Google Scholar]
- 46.Raffi I, et al. A review of calcareous nannofossil astrobiochronology encompassing the past 25 million years. Quat Sci Rev. 2006;25(23–24):3113–3137. [Google Scholar]
- 47.Scott GH. Holotypes in the taxonomy of planktonic foraminiferal morphospecies. Mar Micropaleontol. 2011;78(3–4):96–100. [Google Scholar]
- 48.Wei K-Y. Multivariate morphometric differentiation of chronospecies in the late Neogene planktonic foraminiferal lineage Globoconella. Mar Micropaleontol. 1987;12:183–202. [Google Scholar]
- 49.Aurahs R, Treis Y, Darling K, Kucera M. A revised taxonomic and phylogenetic concept for the planktonic foraminifer species Globigerinoides ruber based on molecular and morphometric evidence. Mar Micropaleontol. 2011;79(1–2):1–14. [Google Scholar]
- 50.Wei K-Y, Kennett JP. Phyletic gradualism and punctuated equilibrium in the Late Neogene planktonic foraminiferal clade Globoconella. Paleobiology. 1988;14(4):345–363. [Google Scholar]
- 51.Cody RD, Levy RH, Harwood DM, Sadler PM. Thinking outside the zone: High-resolution quantitative diatom biochronology for the Antarctic Neogene. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;260:92–121. [Google Scholar]
- 52.Corfield RM, Granlund AH. Speciation and structural evolution in the Palaeocene Morozovella lineage (planktonic Foraminiferida) J Micropalaeontology. 1988;7(1):59–72. [Google Scholar]
- 53.Healy-Williams N, Ehrlich R, Williams DF. Morphometric and stable isotopic evidence for subpopulations of Globorotalia truncatulinoides. J Foraminiferal Res. 1985;15(4):242–253. [Google Scholar]
- 54.de Vargas C, Renaud S, Hilbrecht H, Pawlowski J. Pleistocene adaptive radiation in Globorotalia truncatulinoides: Genetic, morphologic, and environmental evidence. Paleobiology. 2001;27(1):104–125. [Google Scholar]
- 55.Quillévéré F, Norris RD, Berggren WA, Aubry M-P. 59.2 Ma and 56.5 Ma: Two significant moments in the evolution of acarininids (planktonic foraminifera) GFF. 2000;122(1):131–132. [Google Scholar]
- 56.Soldan DM, Petrizzo MR, Silva IP, Cau A. Phylogenetic relationships and evolutionary history of the Paleogene genus Igorina through parsimony analysis. J Foraminiferal Res. 2011;41(3):260–284. [Google Scholar]
- 57.Malmgren BA, Kučera M, Ekman G. Evolutionary changes in supplementary apertural characteristics of the late Neogene Sphaeroidinella dehiscens lineage (planktonic foraminifera) Palaios. 1996;11(2):192–206. [Google Scholar]
- 58.Kucera M. Biochronology of the mid-Pliocene Sphaeroidinella event. Mar Micropaleontol. 1998;35(1):1–16. [Google Scholar]
- 59.Lazarus D, Hilbrecht H, Spencer-Cervato C, Thierstein H. Sympatric speciation and phyletic change in Globorotalia truncatulinoides. Paleobiology. 1995;21(1):28–51. [Google Scholar]
- 60.Hunt G. Gradual or pulsed evolution: When should punctuational explanations be preferred? Paleobiology. 2008;34(3):360–377. [Google Scholar]
- 61.Pearson PN. Cladogenetic, extinction and survivorship patterns from a lineage phylogeny: The Paleogene planktonic foraminifera. Micropaleontology. 1996;42(2):179–188. [Google Scholar]
- 62.Malmgren BA, Kennett JP. Biometric differentiation between recent Globigerina bulloides and Globigerina falconensis in the southern Indian Ocean. J Foraminiferal Res. 1977;7(2):130–148. [Google Scholar]
- 63.Hemleben C, Spindler M, Andersen OR. Modern Planktonic Foraminifera. New York: Springer; 1989. [Google Scholar]
- 64.Pearson PN, et al. Paleogene and Cretaceous sediment cores from the Kilwa and Lindi areas of coastal Tanzania: Tanzania Drilling Project Sites 1–-5. J Afr Earth Sci. 2004;39:25–62. [Google Scholar]
- 65.Kemle-von Mücke S, Hemleben C. 1999. South Atlantic Zooplankton, ed Boltovskoy D (Backhuys Publishers, Leiden, The Netherlands), pp 43–73.
- 66.Eguchi NO, Ujiie H, Kawahata H, Taira A. Seasonal variations in planktonic foraminifera at three sediment traps in the subarctic, transition and subtropical zones of the central North Pacific Ocean. Mar Micropaleontol. 2003;48(1–2):149–163. [Google Scholar]
- 67.Boltovskoy E, Watanabe S. Quaternary stratigraphy of two cores off the coast of Peru. Quaternary of South America and Antarctic peninsula. 1986;4:27–44. [Google Scholar]
- 68.Darling KF, Kucera M, Kroon D, Wade CM. A resolution for the coiling direction paradox in Neogloboquadrina pachyderma. Paleoceanography. 2006;21(2):PA2011. [Google Scholar]
- 69.Darling KF, Kucera M, Pudsey CJ, Wade CM. Molecular evidence links cryptic diversification in polar planktonic protists to Quaternary climate dynamics. Proc Natl Acad Sci USA. 2004;101(20):7657–7662. doi: 10.1073/pnas.0402401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.