Abstract
Deposition of amyloid-β (Aβ) in cerebral arteries, known as cerebral amyloid angiopathy (CAA), occurs both in the setting of Alzheimer’s disease and independent of it, and can cause cerebrovascular insufficiency and cognitive deficits. The mechanisms leading to CAA have not been established, and no therapeutic targets have been identified. We investigated the role of CD36, an innate immunity receptor involved in Aβ trafficking, in the neurovascular dysfunction, cognitive deficits, and amyloid accumulation that occurs in mice expressing the Swedish mutation of the amyloid precursor protein (Tg2576). We found that Tg2576 mice lacking CD36 have a selective reduction in Aβ1-40 and CAA. This reduced vascular amyloid deposition was associated with preservation of the Aβ vascular clearance receptor LRP-1, and protection from the deleterious effects of Aβ on cerebral arterioles. These beneficial vascular effects were reflected by marked improvements in neurovascular regulation and cognitive performance. Our data suggest that CD36 promotes vascular amyloid deposition and the resulting cerebrovascular damage, leading to neurovascular dysfunction and cognitive deficits. These findings identify a previously unrecognized role of CD36 in the mechanisms of vascular amyloid deposition, and suggest that this scavenger receptor is a putative therapeutic target for CAA and related conditions.
Keywords: cerebral blood flow, blood–brain barrier, pericytes, smooth muscle cells, tight junction
There is increasing evidence that alterations in the structure and function of cerebral blood vessels contribute to the brain dysfunction underlying Alzheimer’s disease (AD) (1, 2). Whereas resting cerebral blood flow (CBF) is reduced early in course of AD (3, 4), the increase in CBF induced by brain activity (functional hyperemia), a vital mechanism matching the metabolic demands of active neurons with the delivery of nutrients through blood flow, is suppressed (5, 6). With disease progression, deposition of amyloid-β (Aβ) in cerebral blood vessels, a condition known as cerebral amyloid angiopathy (CAA), damages cerebrovascular cells, weakens vessel walls, and disrupts vascular function further (7). CAA also occurs independent of AD and has emerged as a frequent cause of brain hemorrhage, silent infarct, and cognitive impairment (8, 9).
Studies in mice overexpressing mutated forms of the amyloid precursor protein (APP) have demonstrated that Aβ peptides, especially Aβ1-40, which accumulates preferentially in cerebral blood vessels, alter cerebrovascular function, resulting in vasoconstriction, impaired functional hyperemia, and inability of the endothelium to regulate vascular tone (10–12).These studies have raised the possibility that Aβ, in addition to damaging neurons and glia, also threatens the cerebral blood supply and increases the brain’s susceptibility to hypoxia-ischemia (1, 13). Furthermore, considering that vascular transport is a key pathway for clearance of Aβ from the brain (14), these vascular alterations also may enhance the accumulation of Aβ in brain and cerebral blood vessels.
Converging evidence indicates that Aβ exerts its deleterious vascular effects by producing reactive oxygen species (ROS) (11, 15, 16) through the enzyme NADPH oxidase (17–20). A key signaling pathway through which Aβ activates NADPH oxidase in cerebral blood vessels involves CD36 (21), a critical innate immunity receptor that binds Aβ and is present in endothelial cells and microglia/macrophages (22, 23). Genetic deletion of CD36 was shown to abrogate Aβ-induced vascular oxidative stress and cerebrovascular dysfunction in 3-mo-old mice overexpressing the Swedish mutation of APP (Tg2576) (21). However, those studies were performed in young mice before they developed Aβ deposition and cognitive impairment, and the impact of the cerebrovascular improvement afforded by CD36 deletion on amyloid load, CAA, and cognitive function could not be established.
To address this issue, we examined the role of CD36 in neurovascular regulation, Aβ deposition, and cognitive function in Tg2576 mice at an advanced age. We found that elderly Tg2576 mice lacking CD36 showed marked improvement in cerebrovascular function and cognitive performance in the Y maze test. Surprisingly, these beneficial vascular and cognitive effects were linked to a selective reduction in CAA, but not in amyloid plaques. Our findings implicate CD36 in the mechanisms of CAA and suggest that improved cerebrovascular health is able to preserve cognitive function irrespective of parenchymal amyloid burden.
Results
CD36 Deletion Improves Neurovascular Function in Aged Tg2576 Mice.
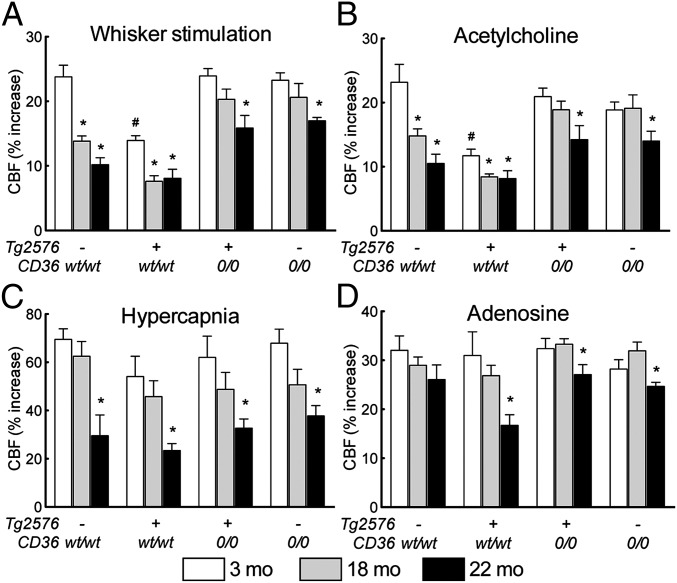
We first investigated whether lack of CD36 mitigates cerebrovascular dysfunction in older Tg2576 mice, at the age marked by extensive amyloid deposition in brain and cerebral blood vessels (1, 24). In nontransgenic littermates, the increase in somatosensory cortex CBF induced by neural activity (whisker stimulation) was greatest at age 3 mo and was progressively attenuated in 18- and 22 mo-old mice (P < 0.05, ANOVA; n = 5/group) (Fig. 1A), reflecting the cerebrovascular dysfunction associated with aging. Similarly, the increases in CBF induced by neocortical application of the endothelium-dependent vasodilators acetylcholine and bradykinin, and by hypercapnia, a response mediated by both neural and vascular factors (25), were reduced in older nontransgenic mice as well (P < 0.05) (Fig. 1 B and C and Fig. S1A). In Tg2576 mice expressing WT CD36 (Tg2576/CD36wt/wt), these responses were attenuated already at age 3 mo, with the reduction becoming more pronounced at 18 mo and 22 mo (Fig. 1 B and C and Fig. S1A). The increase in CBF evoked by the Ca2+ ionophore A23187 or the nitric oxide donor SNAP was attenuated starting at age 3 mo (Fig. S1 B and C), whereas the CBF response to adenosine was disrupted only at 22 mo (Fig. 1D), reflecting amyloid-induced cerebrovascular damage at this age. However, in Tg2576 mice lacking CD36 (Tg2576/CD360/0), these cerebrovascular alterations were markedly improved at all ages (Fig. 1 and Fig. S1). Interestingly, CD36 deletion rescued the cerebrovascular dysfunction in nontransgenic mice as well, in full at 18 mo and partially at 22 mo (Fig. 1 and Fig. S1), implicating CD36 in the cerebrovascular alterations induced by aging (26). These observations indicate that CD36 deletion counteracts the cerebrovascular dysfunction in Tg2576 mice even at an advanced age.
Fig. 1.
CD36 deletion in 3-, 18-, and 22-mo-old Tg2576 mice improves attenuation in the increased CBF produced by neural activity (whisker stimulation) (A), endothelium-dependent vasodilator acetylcholine (B), hypercapnia (C), and smooth muscle relaxant adenosine (D) (n = 5/group). The attenuation in the CBF responses induced by aging in CD36wt/wt littermates is also ameliorated by CD36 deletion. *P < 0.05 from 3 mo; #P < 0.05 from CD36wt/wt, ANOVA and Tukey test.
CD36 Deletion Reduces Amyloid Angiopathy, But Not Amyloid Plaques.
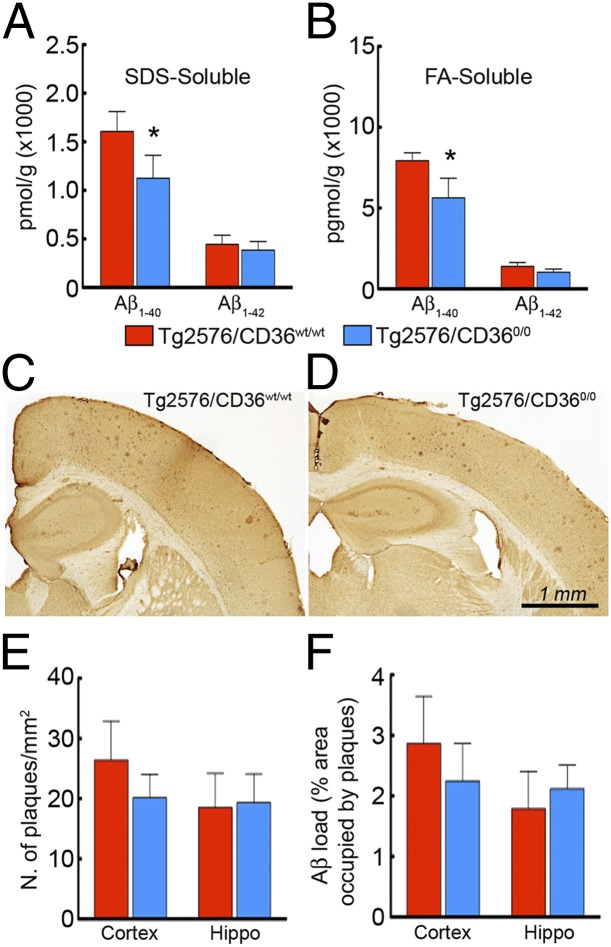
Accumulation of Aβ in cerebral blood vessels plays an important role in the neurovascular dysfunction of aged Tg2576 mice (20, 27). Consequently, we examined whether the rescue in neurovascular and vasomotor function observed in aged Tg2576 mice could be related to reduction in vascular amyloid deposition. We measured levels of SDS-soluble and formic acid (FA)-soluble (SDS-insoluble) Aβ in the brains of 18-mo-old Tg2576 mice with and without CD36 deletion. We found a reduction in Aβ1-40, but not in Aβ1-42, in Tg2576 mice lacking CD36 at 18 mo, but not at 22 mo (Fig. 2 A and B and Fig. S2 A and B).
Fig. 2.
CD36 deletion attenuates Aβ1-40, but not Aβ1-42 or plaque load, in 18-mo-old Tg2576 mice. (A and B) SDS-soluble Aβ1-40 (A) and FA-soluble (SDS-insoluble) Aβ1-40 (B) are reduced in Tg2576/CD360/0 mice (n = 5/group; P < 0.05). (C–F) The distribution of amyloid plaques, assessed using 4G8 immunocytochemistry (C and D), and the number per square millimeter (E) and percent occupied area (F) do not differ between Tg2576/CD36wt/wt and Tg2576/CD360/0 mice (n = 5/group; P > 0.05).
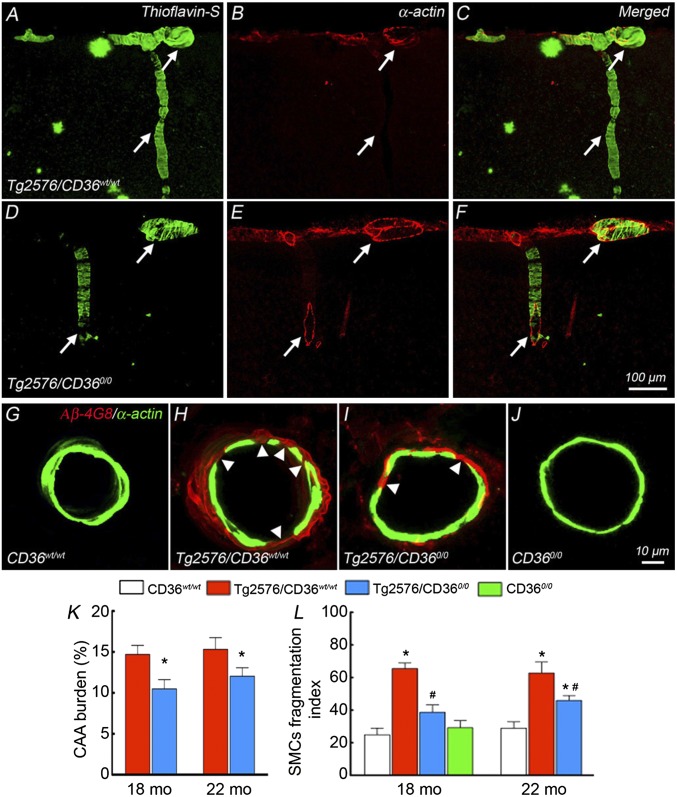
Considering that Aβ1-40 is associated predominately with blood vessels (7), we examined vascular amyloid deposition to determine whether CAA is selectively reduced in Tg2576 mice lacking CD36. In Tg2576/CD36wt/wt mice, we found thioflavin-S+ deposits and Aβ1-40 immunoreactivity in association with somatosensory cortex pial arterioles, identified by the smooth muscle marker α-actin (Fig. 3 A–C and Fig. S3 Aa–Ac). The amyloid deposits were associated with fragmentation of smooth muscle cells in cerebral resistance arterioles, reflecting Aβ-induced structural damage to the vasomotor apparatus (Fig. 3 G–J). In Tg2576/CD360/0 mice, vascular thioflavin-S+ deposits, vascular Aβ1-40, and smooth muscle cell fragmentation were reduced at both 18 mo and 22 mo (Fig. 3 D–F, H, I, K, and L and Fig. S3 Ad–Af and B). In contrast, neocortical and hippocampal amyloid burden, expressed as number of amyloid plaques per square millimeter and percent area occupied by the plaques, did not differ between Tg2576/CD36wt/wt mice and Tg2576/CD360/0 mice (Fig. 2 C–F and Fig. S2 C–F).
Fig. 3.
CD36 deletion selectively attenuates CAA and reduces smooth muscle cell fragmentation in 18- and 22-mo-old Tg2576 mice. Confocal images illustrate thioflavin-S+ vessels, identified by double-labeling with the smooth muscle marker α-actin in the somatosensory cortex. (A–C) Dense amyloid deposits are seen in pial and penetrating arterioles (arrows) in Tg2576 mice. (D–F) CAA load is attenuated in Tg2576 mice lacking CD36. (G–J) Smooth muscle cells are fragmented in Tg2576 mice compared with WT mice (G and H, arrowheads), which is attenuated by CD36 deletion (I and J). (K) CAA burden, expressed as percent of α-actin+ vessels that also display thioflavin-S, is attenuated in Tg2576 mice lacking CD36 (n = 5/group). *P < 0.05 from Tg2576/CD36wt/wt. (L) The number of smooth muscle cell fragments, reflecting smooth muscle damage and expressed as fragmentation index (SI Materials and Methods), is normalized in Tg2576 mice lacking CD36 at age 18 mo and reduced in these mice at age 22 mo (n = 40–60 arterioles/group). *P < 0.05 from CD36wt/wt; #P < 0.05 from Tg2576/CD36wt/wt.
Given that CD36 in microglia may play a role in microglial phagocytosis and amyloid clearance (28), we investigated the relationship between microglial cells and amyloid deposits in aged Tg2576 mice with and without CD36. We found that the number of microglial cells and their concentration around thioflavin-S+ blood vessels and amyloid plaques did not differ between Tg2576/CD36wt/wt mice and Tg2576/CD360/0 mice (Figs. S4 and S5). Therefore, the reduced vascular amyloid accumulation afforded by CD36 deletion cannot be attributed to selective recruitment of perivascular microglia resulting in increased amyloid sequestration or catabolism.
CD36 Deletion Rescues Alterations in Pericytes in Tg2576 Mice.
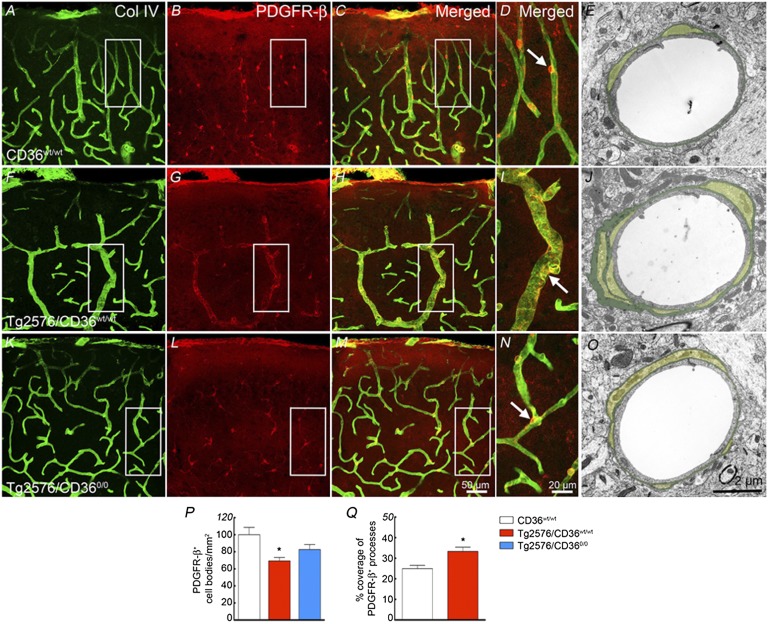
Aβ has toxic effects on pericytes (29, 30), and pericyte damage has been implicated in the neurovascular dysfunction associated with AD (31). Thus, we investigated whether pericytes are altered in Tg2576 mice and if so, whether the effect is dependent on CD36. In the somatosensory cortex of nontransgenic littermates, platelet-derived growth factor receptor-β (PDGFR-β) immunocytochemistry identified pericytes in association with cerebral microvessels (Fig. 4 A–D). In brain sections double-labeled with PDGFR-β and the basement membrane marker collagen IV (Col IV), pericyte cell bodies and processes were closely apposed to the vessel wall (Fig. 4 A–D). EM confirmed and expanded these findings using PDGFR-β as a pericyte marker. At the ultrastructural level, pericytes were embedded within the vascular wall, enveloped by the vascular basement membrane (Fig. 4E). In Tg2576/CD36wt/wt mice, the number of pericytes was reduced, but the remaining cells had elongated and hypertrophic processes surrounding the vessel wall and outlining its contours (Fig. 4 F–I and P). Ultrastructurally, these cells exhibited extensive processes encased by a thickened vascular basement membrane, increasing the vessel area covered by pericyte processes (Fig. 4 J and Q). These alterations were less marked in Tg2576/CD360/0 mice, in which pericyte number and morphology were partially normalized (Fig. 4 K–P). These findings indicate that CD36 deletion ameliorates the alterations in pericyte number and morphology occurring in Tg2576 mice.
Fig. 4.
CD36 deletion rescues the alterations in pericytes in Tg2576 mice. Pericytes, identified by PDGFR-β immunostaining, and microvessels, identified by the basement membrane marker Col IV, in 18-mo-old CD36wt/wt (A–E), Tg2576/CD36wt/wt (F–J), and Tg2576/CD360/0 (K–O) mice. (G and P) In Tg2576/CD36wt/wt mice (n = 5/group), pericyte cell bodies are reduced in number (P), but their processes are hypertrophic and envelop the capillaries outlining their contours (G). (J and Q) On EM, the vascular basal lamina is thickened and the pericyte processes are more extensive (J), such that the area of the vessel covered by processes is increased (n = 130–143 vessels from 3 mice/group) (Q). These alterations are ameliorated in Tg2576 mice lacking CD36 in K–Q (P < 0.05 from CD36wt/wt).
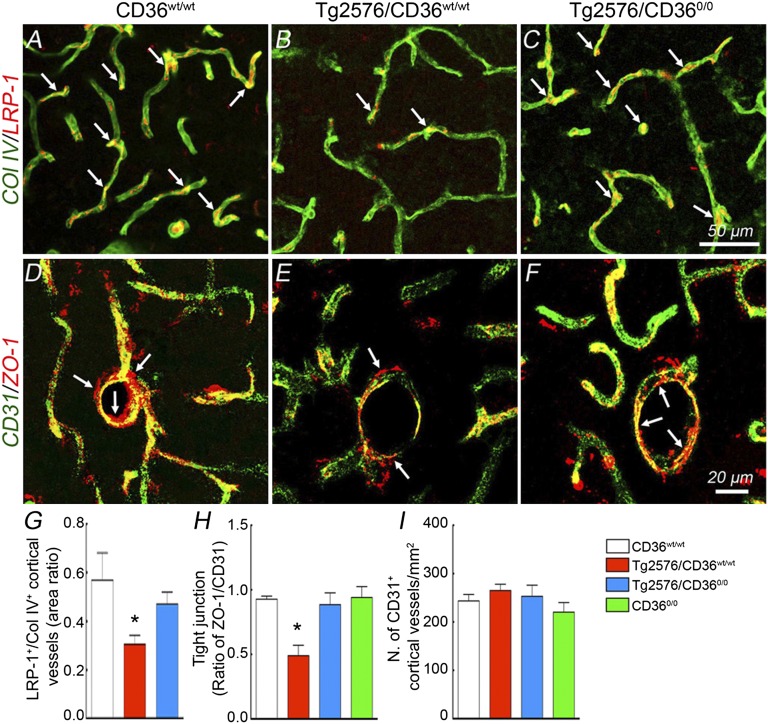
CD36 Deletion Counteracts Loss of Vascular LPR-1 and ZO-1 in Tg2576 Mice.
Low density lipoprotein receptor-related protein-1 (LRP-1) is involved in the brain-to-blood vascular clearance of Aβ and is reduced in cerebral vessels of mice overexpressing APP (14, 32). Because vascular amyloid and the vasculotropic Aβ1-40 peptide were reduced in Tg2576/CD360/0 mice, we examined whether CD36 deletion may rescue the cerebrovascular expression of LRP-1. In Tg2576/CD36wt/wt mice LPR-1 expression was reduced compared with WT littermates (Fig. 5 A, B, and G). Similarly, the tight junction protein zonula occludens-1 (ZO-1) was reduced (Fig. 5 D, E, and H). LPR-1 and ZO-1 expression were improved in Tg2576/CD360/0 mice (Fig. 5 C, F–H). The rescue of LRP-1 and ZO-1 expression was not related to any changes in the number of vascular profiles in the groups of mice studied (Fig. 5I).
Fig. 5.
CD36 deletion rescues the reduced LRP-1 and ZO-1 in the somatosensory cortex of Tg2576 mice. LRP-1 (A–C) and ZO-1 (D–F) immunofluorescence in WT (CD36wt/wt), Tg2576 (Tg2576/CD36wt/wt), and Tg2576 mice lacking CD36 (Tg2576/CD360/0). (G–I) Quantification of LRP-1, ZO-1, and number of microvessels, identified by the endothelial marker CD31. Microvascular expression of LRP-1 (A, B, and G, n = 5/group; P < 0.05) and ZO-1 (D, E, and H, n = 5/group; P < 0.05) is suppressed in Tg2576 mice, an effect rescued by CD36 deletion (C, F, G, and H; P > 0.05 from CD36wt/wt). (I) The number of cortical microvessels does not differ between groups (n = 5/group; P > 0.05).
CD36 Deletion Improves Y Maze Performance in Tg2576 Mice.
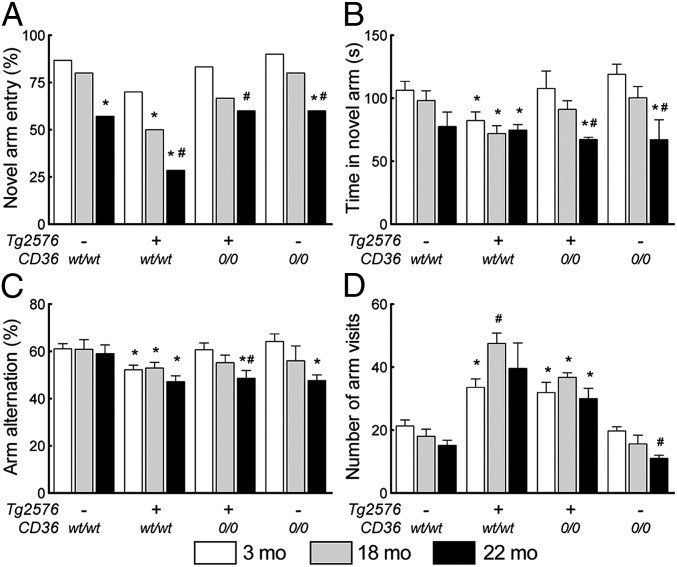
Finally, we used a two-trial spatial-memory task in a Y maze (20, 33, 34) to determine whether the reduced neurovascular dysfunction and CAA in Tg2576/CD360/0 mice are associated with improved cognitive performance. We chose the Y maze test for its high sensitivity to the behavioral dysfunction of Tg2576 mice (35). As described elsewhere (20), in the first trial (acquisition), mice were placed in the Y maze with the third arm blocked. During the second trial (retrieval), performed 30 min later, the closed arm was open, allowing the mice to explore all arms of the maze. Novel arm entries, time spent in the novel arm, arm alternation, and total arm visits were recorded. Nontransgenic littermates (n = 10/group) tended to ignore the novel arm (P < 0.05, χ2 test) and spend less time in it (P < 0.05, ANOVA) only at 22 mo of age (Fig. 6A). In contrast, Tg2576/CD36wt/wt mice (n = 10–15 group) spent less time in the novel arm and demonstrated reduced arm alternation already at 3 mo (P < 0.05, ANOVA) (Fig. 6 B and C) and reduced entry in the novel arm at both 18 mo and 22 mo (P < 0.05, χ2 test) (Fig. 6A). The total number of arm visits was increased in Tg2576 mice (P < 0.05) (Fig. 6D), reflecting increased locomotor activity. These alterations are consistent with the reported behavioral deficits in Tg2576 mice (20, 35). However, novel arm entries, time spent in the novel arm, and arm alteration were comparable between in Tg2576/CD360/0 mice and nontransgenic littermates or CD360/0 mice (P > 0.05), with reductions seen only at age 22 mo (Fig. 6 A–C). The number of total arm visits was reduced in Tg2576/CD360/0 mice, but only partially (P > 0.05 from nontransgenic and CD360/0 littermates) (Fig. 6D). These results indicate that Tg2576 mice lacking CD36 are protected from the cognitive dysfunction induced by APP overexpression.
Fig. 6.
CD36 deletion ameliorates cognitive function in Tg2576 mice assessed using the Y maze (n = 10/group). (A–C) The reductions in novel arm entry (A), time spent in the novel arm (B), and arm alternation (C) observed in Tg2576/CD36wt/wt mice are partially rescued by CD36 deletion. (D) The number of arm visits is increased in Tg2576/CD36wt/wt mice, reflecting hyperactivity, an effect also attenuated by CD36 deletion. *P < 0.05 from CD36wt/wt; #P < 0.05 from 3 mo, χ2 test in A, ANOVA and Tukey test in B–D.
Discussion
We have demonstrated that CD36 deletion ameliorates cerebrovascular function in Tg2576 mice at an age marked by extensive amyloid deposition in brain and cerebral blood vessels. The improvement is observed in vascular responses initiated by neural activity (functional hyperemia), endothelium, and smooth muscle cells, reflecting a broad beneficial impact on vasomotor regulation and function. Underlying these vascular effects is a selective reduction of CAA, but not of amyloid plaques, as well as preservation of the phenotype of smooth muscle cells, endothelia, and pericytes. Importantly, CD36 deletion in Tg2576 mice is associated with improved cognitive function assessed using the Y maze test. Collectively, these findings implicate CD36 in the mechanisms of vascular amyloid deposition and provide insight into the pathobiology of CAA.
We previously reported that CD36 is required for the cerebrovascular dysfunction observed in 3-mo-old Tg2576 mice, an effect mediated by suppression of NADPH oxidase-dependent production of vascular ROS (19, 20). Those observations provide evidence that CD36 is the link between Aβ1-40 and NADPH oxidase-derived ROS resulting in neurovascular dysfunction. However, 3-mo-old Tg2576 mice exhibit no alterations in cerebrovascular structure, and no amyloid deposition in brain and blood vessels (11). Therefore, previous studies could not determine whether CD36 is involved in the full expression of the brain pathology observed in aged Tg2576 mice, including amyloid plaques, CAA, vascular damage, and cognitive dysfunction (20). In the present study, we have demonstrated that CD36 deletion has a profound effect in aged Tg2576 mice, ameliorating both neurovascular and cognitive function. Although we studied the cerebrovascular effects of CD36 deletion in Tg2576 mice only in the somatosensory cortex, similar neurovascular improvements likely occur in other brain regions in which Aβ levels are increased, such as the hippocampus.
Remarkably, the behavioral improvements were linked not to suppression of amyloid plaques accumulating in the brain parenchyma, but rather to a selective reduction in CAA and its detrimental vascular effects. Thus, in aged mice, the damage to smooth muscle cells and pericytes, key cells in vasomotor function, resulted in a global vascular impairment involving not only neurovascular coupling and endothelium-dependent responses, as in young mice, but also responses to agents acting specifically on vascular smooth muscles. CD36 deletion in Tg2576 mice attenuated the damage to smooth muscle cells and pericytes, resulting in functional improvement in the vasomotor apparatus and restoration of all cerebrovascular responses. CD36 deletion also increased pericyte numbers and improved pericyte morphology in Tg2576 mice. Considering the link between pericytes and the blood-brain barrier (BBB) (36), this finding may indicate an improvement in BBB function, as reflected by preservation of the BBB protein ZO-1 in Tg2576 mice lacking CD36. Preservation of the BBB is vital for the bidirectional transfer of molecules between blood and brain, as well as for homeostasis of the cerebral microenvironment (14). Thus, CD36 is involved in key upstream events leading to CAA and to the associated vascular damage and neurovascular dysfunction.
CD36 deletion reduces Aβ1-40 and CAA in Tg2576 mice, but has no affect on Aβ1-42 and amyloid plaques. This observation reveals a unique role for CD36 in vascular Aβ1-40 accumulation and amyloid deposition. Given that the effect of CD36 is restricted to Aβ1-40, it is unlikely that the attenuation is related to reduced Aβ cleavage from APP by secretases, because in that case reductions in both Aβ1-40 and Aβ1-42 would be anticipated (37). Rather, the selectivity of the reduction in Aβ1-40 points to an enhanced clearance or catabolism of the peptide. Although increased Aβ metabolism by the peptidase neprilysin could play a role (38), Aβ1-40 is rapidly removed from the brain by vascular transport and is less susceptible than Aβ1-42 to cleavage by neprilysin (39). Moreover, considering that Aβ1-40 is associated preferentially with blood vessels, it is conceivable that enhanced vascular clearance of Aβ1-40 contributes to the mechanisms by which CD36 deletion reduces CAA. Interestingly, the reduction in CAA is seen at both 18 and 22 mo of age, but the reduction in Aβ1-40 is seen only at 18 mo. This is most likely related to the massive increase in Aβ1-40 occurring in 22 mo-old Tg2576 mice (24), which overloads the amyloid clearance pathways. However, despite the increased amyloid production, CD36 is still able to protect the blood vessels and reduce the vascular accumulation of Aβ even at this advanced age.
Aβ can be removed from the brain through perivascular lymphatic-like drainage, a pathway impaired by vascular damage and CAA (40, 41). In addition, Aβ can be transported from brain to blood by LRP-1, a protein that acts in concert with P-glycoprotein, ApoE, ApoJ, and α2-macroglobulin to regulate brain Aβ homeostasis (14). LRP-1 down-regulation reduces brain-to-blood Aβ transport and promotes Aβ accumulation (42), whereas its up-regulation facilitates brain-to-blood Aβ clearance (43). Our finding that CD36 deletion rescues vascular LRP-1 expression in Tg2576 mice implicates this pathway in the mechanisms by which CD36 leads to Aβ1-40 accumulation. Indeed, LPR-1 has greater affinity for Aβ1-40 than for Aβ1-42 (44), possibly contributing to the selective reduction in Aβ1-40 afforded by the rescue of LRP-1 expression associated with CD36 deletion in Tg2576 mice. Thus, our data suggest that CD36 may promote CAA by reducing Aβ1-40 clearance, an effect resulting from vasomotor dysfunction, vascular damage, and down-regulation of vascular LPR-1. The cell type(s) responsible for the cerebrovascular effects of CD36 have not yet been identified, however. The study of mice with cell-specific deletion of CD36 will go a long way toward addressing this important question.
CAA is a major cause of cognitive decline, lobar cerebral hemorrhage, and cerebrovascular insufficiency in elderly persons (7, 13); however, the factors contributing to the amyloid accumulation specifically in blood vessels remain to be defined. Our present findings implicate CD36 in vascular amyloid deposition and provide insight into the mechanisms of CAA, a highly prevalent condition with no current treatment. Our data raise the possibility that CD36 is a putative therapeutic target for CAA. Approaches to down-regulation of CD36 signaling using antibodies blocking the Aβ binding site (21, 45) would offer the prospect of reducing vascular Aβ accumulation and ameliorating the devastating effects of CAA on cerebrovascular structure and function. Identification of the cell type(s) expressing CD36 and responsible for the Aβ accumulation could provide a cellular target for potential interventions to dampen CD36 signaling and forestall vascular Aβ accumulation.
In conclusion, we have demonstrated that CD36 deletion ameliorates neurovascular dysfunction in aged Tg2576 mice. The effect is related to a selective reduction of CAA, but not of amyloid plaques. The reduced vascular amyloid deposition results in a reduction of the attendant damage to smooth muscle cells and pericytes. Importantly, the improved neurovascular function is associated with cognitive improvement, as assessed by the Y maze test. Our findings reveal a previously unrecognized involvement of CD36 in CAA and provide insight into the pathobiology of this debilitating condition. They also provide evidence that ameliorating CAA can improve cognitive function independent of amyloid plaques, and point to CD36 as a presumptive therapeutic target for the vascular damage induced by amyloid accumulation in AD and CAA.
Materials and Methods
Mice.
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Transgenic mice expressing the Swedish mutation of APP (Tg2576) were crossed with CD360/0 mice as described previously (21). To minimize confounding effects of background heterogeneity and genetic modifiers, experiments were performed in age-matched littermates, male mice aged 3, 18, or 22 mo.
General Surgical Procedures.
As detailed elsewhere (11, 20, 21) and in SI Materials and Methods, mice were anesthetized (urethane-chloralose) and ventilated (SAR-830; CWE) (11, 20, 21). Arterial pressure and blood gases were controlled (Table S1). Rectal temperature was maintained at 37 °C.
Recording of CBF.
The somatosensory cortex was exposed (2 × 2 mm) and superfused with modified Ringer’s solution (pH 7.3–7.4) at 37 °C (20, 21, 46). CBF was recorded continuously at the site of superfusion with a laser Doppler probe (Vasamedic).
Experimental Protocol for CBF Experiments.
CBF recordings were started once physiological parameters were at steady state (Table S1). To study the increase in CBF produced by somatosensory activation, the whiskers were activated by side-to-side deflection for 60 s. Acetylcholine (10 µM), bradykinin (50 µM), A23187 (3 µM), SNAP (50 µM), or adenosine (400 µM) (all Sigma-Aldrich) was topically superfused for 3–5 min, and the resulting changes in CBF were monitored (20, 21). Response of CBF to hypercapnia was examined by elevating arterial pCO2 to 50–60 mmHg (20, 47).
Measurement of Aβ and Determination of Plaque or CAA Load.
Aβ1-40 and Aβ1-42 levels (SDS-soluble and FA-soluble) were determined using ELISA-based assays, as described previously (20, 21). The number of plaques per square millimeter and the percent area occupied by the plaques were determined stereologically in the somatosensory cortex and hippocampus in sections immunostained with the 4G8 antibody (20). CAA burden, expressed as percent vessels with CAA, was determined in sections stained with thioflavin-S and counterstained with the smooth muscle marker α-actin (20, 48, 49).
Immunofluorescence and EM.
Mice were anesthetized and perfused transcardially with a fixative (20, 21, 50). Brains were sectioned (40 μm), and free-floating sections were processed for immunofluorescence or EM. For confocal microscopy, fluorescently labeled images (α-actin, CD31, Col IV, ZO-1, LRP-1, and PDRFR-β) were acquired randomly in the somatosensory cortex underlying the cranial window (0.38 to −1.94 mm from the bregma) (20, 21). For EM, random portions of the somatosensory cortex were excised, and thin sections (65 nm) were examined on a Tecnai electron microscope (FEI) (50). Brain sections from Tg2576/CD36wt/wt and Tg2576/CD360/0 mice were processed under identical conditions and imaged and analyzed using identical settings (SI Materials and Methods).
Behavioral Assessment by the Y Maze.
Behavioral assessment was performed using the Y maze as described previously (20). After acclimatization to the apparatus, a mouse was placed into an arm of the maze (start arm) and allowed to explore only two of the arms for 5 min (training trial). The third arm, which remained closed, was randomly chosen in each trial. The closed arm was opened in the test trial, serving as the novel arm. After a 30-min intertrial interval, the mouse was returned to the same start arm and allowed to explore all three arms for 5 min (test trial). Sessions were videorecorded and replayed for determination of the parameters of interest by an observer blinded to mouse genotype.
Data Analysis.
Data are expressed as mean ± SEM. Two-group comparisons were analyzed using the two-tailed t test. Multiple comparisons were evaluated by ANOVA and the Tukey test. Differences in novel arm entries were analyzed using the χ2 test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (Grant NS37853, to C.I.), the American Heart Association (Grant 09SDG2060701, to L.P.), and the Alzheimer’s Association (Zenith Award, to C.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300021110/-/DCSupplemental.
References
- 1.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci Transl Med. 2011;3(77):sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codispoti KE, et al. Longitudinal brain activity changes in asymptomatic Alzheimer disease. Brain Behav. 2012;2(3):221–230. doi: 10.1002/brb3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luckhaus C, et al. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. Neuroimage. 2008;40(2):495–503. doi: 10.1016/j.neuroimage.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Rosengarten B, Paulsen S, Burr O, Kaps M. Neurovascular coupling in Alzheimer patients: Effect of acetylcholine-esterase inhibitors. Neurobiol Aging. 2009;30(12):1918–1923. doi: 10.1016/j.neurobiolaging.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, et al. Altered PET functional brain responses in cognitively intact elderly persons at risk for Alzheimer disease (carriers of the epsilon4 allele) Am J Geriatr Psychiatry. 2004;12(6):596–605. doi: 10.1176/appi.ajgp.12.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14(4):343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick PB, et al. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimberly WT, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72(14):1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 11.Iadecola C, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2(2):157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 12.Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20(12):1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380(6570):168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 16.Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci. 2005;25(48):11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrano A, et al. Amyloid beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15(5):1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 18.d’Uscio LV, et al. Activation of PPARδ prevents endothelial dysfunction induced by overexpression of amyloid-β precursor protein. Cardiovasc Res. 2012;96(3):504–512. doi: 10.1093/cvr/cvs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park L, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25(7):1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park L, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105(4):1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park L, et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci USA. 2011;108(12):5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281(30):20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 24.Kawarabayashi T, et al. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21(2):372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faraci FM. Reactive oxygen species: Influence on cerebral vascular tone. J Appl Physiol. 2006;100(2):739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 26.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27(12):1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 27.Robbins EM, et al. Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J Neurosci. 2006;26(2):365–371. doi: 10.1523/JNEUROSCI.3854-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Khoury JB, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao J, et al. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol. 2005;167(2):505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem. 1997;68(3):1135–1141. doi: 10.1046/j.1471-4159.1997.68031135.x. [DOI] [PubMed] [Google Scholar]
- 31.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem. 2000;73(1):31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- 34.Sarnyai Z, et al. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci USA. 2000;97(26):14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002;75(5):627–642. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 36.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masters CL, Selkoe DJ. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miners JS, Kehoe P, Love S. Neprilysin protects against cerebral amyloid angiopathy and Aβ-induced degeneration of cerebrovascular smooth muscle cells. Brain Pathol. 2011;21(5):594–605. doi: 10.1111/j.1750-3639.2011.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis. 2001;3(1):23–30. doi: 10.3233/jad-2001-3105. [DOI] [PubMed] [Google Scholar]
- 40.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18(2):253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell RD, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11(2):143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellano JM, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci USA. 2012;109(38):15502–15507. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43(3):333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iadecola C. Nitric oxide participates in the cerebrovasodilation elicited from cerebellar fastigial nucleus. Am J Physiol. 1992;263(5 Pt 2):R1156–R1161. doi: 10.1152/ajpregu.1992.263.5.R1156. [DOI] [PubMed] [Google Scholar]
- 47.Niwa K, et al. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283(1):H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 48.Van Dooren T, et al. Neuronal or glial expression of human apolipoprotein e4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice. Am J Pathol. 2006;168(1):245–260. doi: 10.2353/ajpath.2006.050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler DT, et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21(5):1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce JP, et al. Sex differences in the subcellular distribution of angiotensin type 1 receptors and NADPH oxidase subunits in the dendrites of C1 neurons in the rat rostral ventrolateral medulla. Neuroscience. 2009;163(1):329–338. doi: 10.1016/j.neuroscience.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.