Abstract
Myelodysplastic syndromes (MDS) are a group of disorders characterized by variable cytopenias and ineffective hematopoiesis. Hematopoietic stem cells (HSCs) and myeloid progenitors in MDS have not been extensively characterized. We transplanted purified human HSCs from MDS samples into immunodeficient mice and show that HSCs are the disease-initiating cells in MDS. We identify a recurrent loss of granulocyte-macrophage progenitors (GMPs) in the bone marrow of low risk MDS patients that can distinguish low risk MDS from clinical mimics, thus providing a simple diagnostic tool. The loss of GMPs is likely due to increased apoptosis and increased phagocytosis, the latter due to the up-regulation of cell surface calreticulin, a prophagocytic marker. Blocking calreticulin on low risk MDS myeloid progenitors rescues them from phagocytosis in vitro. However, in the high-risk refractory anemia with excess blasts (RAEB) stages of MDS, the GMP population is increased in frequency compared with normal, and myeloid progenitors evade phagocytosis due to up-regulation of CD47, an antiphagocytic marker. Blocking CD47 leads to the selective phagocytosis of this population. We propose that MDS HSCs compete with normal HSCs in the patients by increasing their frequency at the expense of normal hematopoiesis, that the loss of MDS myeloid progenitors by programmed cell death and programmed cell removal are, in part, responsible for the cytopenias, and that up-regulation of the “don’t eat me” signal CD47 on MDS myeloid progenitors is an important transition step leading from low risk MDS to high risk MDS and, possibly, to acute myeloid leukemia.
Keywords: myelodysplasia, blood disorders, aging, monosomy 7, cancer stem cell
The World Health Organization (WHO) defines myelodysplastic syndromes (MDS) as a heterogeneous group of related clonal diseases characterized by variable cytopenias due to ineffective hematopoiesis and increased risk of progression to acute myeloid leukemia (AML) (1–4). To date, functional and diagnostic evaluations of immature hematopoietic cells in MDS have predominantly relied on unpurified or partially purified bone marrow cells (most frequently CD34+ cells; reviewed in ref. 5), which have limited power to identify cell lineage specific alterations. We have taken advantage of the purification of highly enriched HSCs and committed myeloid progenitors (6–9) via fluorescence activated cell sorting (FACS) to characterize hematopoietic subsets in primary MDS patient bone marrow samples with the goal of increasing the understanding of MDS pathogenesis.
Recent fluorescence in situ hybridization (FISH) and gene expression data from purified HSCs from 5q- MDS patients have suggested an HSC origin for MDS (10–13). HSCs from MDS patients (MDS HSCs) are relatively resistant to lenalidomide and decitabine treatment, because even patients with cytogenetic remission, as determined by FISH on peripheral blood cells, can maintain a significant MDS HSC burden (12, 13). Nevertheless, HSCs from MDS patients have not been shown to initiate the disease itself. Additionally, it is not clear whether isolated MDS HSCs exhibit increased apoptosis and aberrant proliferation similar to that observed in total bone marrow cells (14–17).
We and others have shown that the binding of cell surface calreticulin (CRT) on a target cell to the low-density lipoprotein receptor-related protein (LRP1) receptor on macrophages is an important mechanism for the phagocytosis of target cells, including apoptotic cells and cancer cells (18–20). We have also shown that the concomitant up-regulation of antiphagocytic CD47 on the surface of cancer cells is critical for their evasion of immunosurveillance (18). In addition, blocking CD47 ligation of its receptor SIRPα allows for the prophagocytic cell surface CRT to signal phagocytosis and clearance of tumor cells (18). Although cell surface CD47 expression is increased in numerous tumor types, including AML (21–24), these molecules have not been well characterized in MDS.
In this study, we demonstrate that low risk MDS HSCs (from bone marrow samples from patients with refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, and myelodysplastic syndrome not otherwise specified but with fewer than 5% blasts) can transplant the clonal disease into immunodeficient mice. We also show that fewer granulocyte-macrophage progenitors (GMPs) are present in low risk MDS bone marrow compared with normal and non-MDS bone marrow samples, a quantitative finding that can be used to aid diagnosis of low risk MDS in the clinical setting. This reduction in GMPs is likely secondary to increased programmed cell death and programmed cell removal by macrophages, the latter of which is due to up-regulation of CRT. In high risk MDS [refractory anemia with excess blasts (RAEB), including both WHO RAEB-1 and RAEB-2 subtypes], we show that the frequency of myeloid progenitors in the bone marrow is higher than in low risk MDS, likely due to their evasion of phagocytosis by up-regulation of CD47.
Results
MDS is thought to be a disease of HSCs, leading to variable cytopenias due to ineffective production of mature granulocyte, erythroid, and/or megakaryocyte (platelet) populations (1). However, there is little information regarding the status of the most primitive HSC and progenitor compartments in MDS. We used flow cytometry based on immunophenotypic markers to evaluate the distribution and frequency of HSC (Lin−CD34+CD38−CD90+CD45RA−) (6, 25–29) and committed myeloid progenitor populations (8) in low risk MDS bone marrow. The use of age-matched controls is crucial in studies such as this, because most patients with MDS are elderly, and normal bone marrow changes with age, with increased frequency of immunophenotypic HSCs in normal elderly bone marrow compared with young (30). We find that the frequencies of HSCs were not significantly higher in low risk MDS bone marrow than in similarly aged (elderly), nonanemic normal controls (Fig. 1A), suggesting that MDS HSCs expand at the expense of normal HSCs.
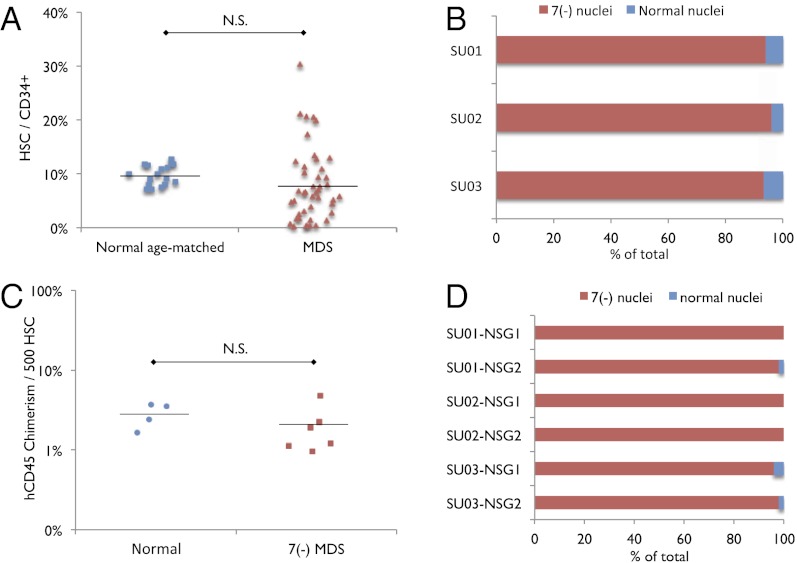
Fig. 1.
Immunophenotypic HSCs from low risk MDS bone marrow are not increased in frequency and monosomy 7 MDS HSCs reconstitute clonal disease in immunodeficient mice. (A) Frequency of immunophenotypic HSCs within the CD34+ population in normal age-matched (n = 18) and low risk MDS (n = 45) bone marrow samples. N.S., no significance. (B) Percentage of HSCs with nuclei exhibiting deletion of chromosome 7 and normal karyotype in monosomy 7 MDS patients (n = 3; SU001–SU003). One hundred fifty nuclei were analyzed for each sample. (C) Human CD45+ chimerism per 500 transplanted HSCs from normal bone marrow controls (n = 4) and monosomy 7 bone marrow samples (n = 3) into sublethally irradiated NSG newborn mice (one recipient per normal HSC sample; two recipients per monosomy 7 sample; 1,500–3,000 HSCs transplanted per recipient). N.S., no significance. (D) Percentage of successfully engrafted human CD45+ cells at 12–16 wk after transplant, with nuclei exhibiting deletion of chromosome 7 and normal karyotype. HSCs were sorted from three monosomy 7 bone marrow samples (SU001–SU003) and xenotransplanted into two recipients each (NSG1, NSG2). Fifty human CD45+ nuclei were analyzed from each xenotransplant recipient.
Previously, it has been shown that the HSC compartment in MDS is enriched for cytogenetically abnormal cells, including in two MDS samples with monosomy 7 (10, 11, 13). We evaluated three MDS samples harboring a clonal monosomy 7 cytogenetic abnormality. Consistent with previous reports, we detect this cytogenetic abnormality by FISH in more than 95% of the cells within the immunophenotypically defined HSC population (Fig. 1B). We purified these HSCs by FACS and xenotransplanted 1,500–3,000 of them into sublethally irradiated immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) pups (31). Human CD45+ cells were found in all recipient mice bone marrow (Fig. 1C), reflecting successful engraftment of these MDS HSCs. FACS-purified human CD45+ cells from engrafted mice harbored the monosomy 7 cytogenetic abnormality, as detected by FISH (Fig. 1D), definitively showing that HSCs from these MDS samples can transplant and initiate this clonal disease. Interestingly, the frequency of cells derived from the abnormal MDS clone was similar to that observed in the original patient bone marrow sample, indicating that the MDS clone did not exhibit reduced reconstitution ability in the context of transplant.
Because the frequency of HSCs in low risk MDS is not significantly different compared with normal age-matched controls and simple loss of HSCs cannot directly explain the several cytopenias that are found in this disease, we hypothesized that the loss of downstream myeloid progenitors plays a role in the ineffective hematopoiesis characteristic of MDS. We evaluated by flow cytometry the distribution and frequency of immunophenotypic common myeloid progenitors (CMPs; Lin−CD34+CD38+CD123+CD45RA−), granulocyte-macrophage progenitors (GMPs; Lin−CD34+CD38+CD123+CD45RA+), and megakaryocyte-erythroid progenitors (MEPs; Lin−CD34+CD38+CD123−CD45RA−) (8) in low risk MDS and normal bone marrow controls (Fig. 2A and Fig. S1). We have shown that the relative distribution of these myeloid progenitor populations does not change with age (30). A recent study has shown a relative increase in CMP frequency in MDS (13). We find that low risk MDS bone marrow exhibits alterations in myeloid progenitor distribution, with significant reduction of GMP frequency in low risk MDS compared with normal controls (P < 10−13) and non-MDS bone marrow disorders exhibiting at least one cytopenia (“Other GMP”; P < 10−10) (Fig. 2A). When the frequency of GMPs as a percentage of total myeloid progenitors [GMP/Total CD34+CD38+] was calculated, it was decreased by an average of 2.6-fold (Fig. 2A) and was accompanied by concomitant increases in relative CMP frequency compared with normal controls (Fig. S1B). Interestingly, the loss of GMPs in low risk MDS bone marrow was not regularly accompanied by absolute neutropenia in the peripheral blood. Notably, the most common cytopenia in MDS is anemia, but we found that low risk MDS MEPs are not significantly reduced in frequency relative to the total myeloid progenitor population (Fig. S1C). We do not yet have markers that define pure megakaryocytic or pure erythroid committed progenitors in humans, and although we suspect these populations will be altered in the bone marrow of MDS patients with respective thrombocytopenias and anemias, we cannot test that hypothesis directly at this time.
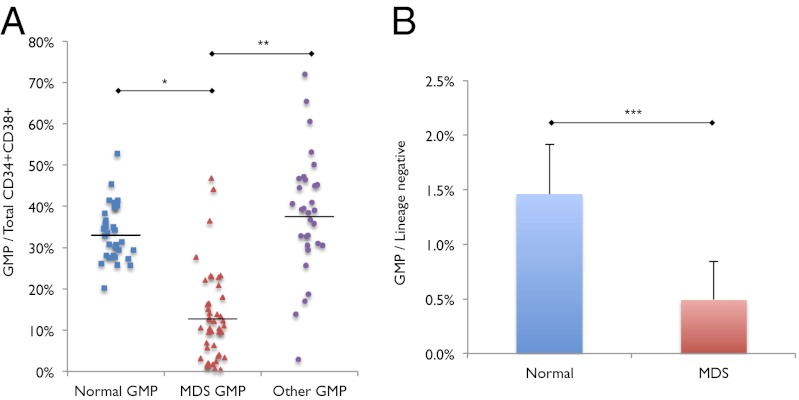
Fig. 2.
Decreased GMP frequency in low risk MDS. (A) Frequency of GMPs out of total myeloid progenitors (CMPs + GMPs + MEPs) in normal (n = 34), low risk MDS (n = 46; MDS), and non-MDS with at least one cytopenia bone marrow samples (n = 32; Other). *P < 10−13, **P < 10−10. (B) Frequency of GMPs out of total lineage negative bone marrow mononuclear cells in normal and low risk MDS bone marrow samples. ***P < 0.0006. Error bars represent one SD. “MDS” label represents low risk MDS samples.
Accurate absolute quantification of HSC and progenitor numbers from human bone marrow samples is challenging because of the inherent variability in bone marrow aspiration technique, which always collects a varying proportion of cells from the peripheral blood. Therefore, to better approximate absolute numbers of myeloid progenitor subsets in the bone marrow, we compared frequencies of CMPs, GMPs, and MEPs within the lineage-negative population, which are more likely to reflect hematopoietic cells from the bone marrow than total cells collected from bone marrow aspiration. We find that low risk MDS GMPs are decreased in frequency, on average by 3.0-fold, within the lineage-negative population compared with normal (P < 0.0006) (Fig. 2B), whereas low risk MDS CMPs and MEPs are not increased in frequency within the lineage-negative population (Fig. S1 D and E), suggesting that one of the defects in MDS hematopoiesis is not due to an absolute expansion of the CMP or MEP populations, but a significant absolute reduction in the GMP population.
Because we observed a consistent, statistically significant decrease in the frequency of GMPs among total myeloid progenitors, we tested whether GMP frequency could be used as a clinically useful tool to diagnose low risk MDS. Using the immunophenotypic markers described above for GMPs, CMPs, and MEPs (ref. 8 and Fig. S1A), we find that a maximum threshold of 24.0% GMPs among total myeloid progenitors yielded a sensitivity of 92% and a specificity of 89% for correctly identifying low risk MDS patients. Using this threshold of 24.0% GMPs for low risk MDS diagnosis, we evaluated the myeloid progenitor profile of 40 consecutive bone marrow samples submitted for evaluation of MDS and correctly categorized 100.0% of 28 non-MDS samples (false positive rate of 0.0) and 83.3% of 12 low risk MDS samples in a blinded fashion (false negative rate of 0.067).
To further determine whether the decreased frequency of GMPs is an inherent feature of low risk MDS, we transplanted FACS-purified HSCs from low risk MDS into sublethally irradiated NSG pups and analyzed the distribution of myeloid progenitors in the bone marrow of engrafted mice. As controls, we transplanted FACS-purified HSCs from normal human bone marrow samples. Human CD45+ chimerism was detected in mice transplanted with low risk MDS HSCs and normal HSCs (Fig. 3A). In mice transplanted with low risk MDS HSCs, engrafted cells were predominantly derived from the myeloid lineage, as evidenced by CD13+ and/or CD33+ staining, and minimally in the B-lymphoid lineage, as evidenced by CD19+ staining, whereas mice transplanted with normal HSCs exhibited comparatively more robust engraftment in the B-lymphoid lineage (Fig. 3B). Human CD34+ cells were also detected in the bone marrow of engrafted mice, and GMPs as a frequency of human CD34+ cells were decreased in mice transplanted with low risk MDS HSCs compared with mice transplanted with normal HSCs (Fig. 3C). These data indicate that the low risk MDS HSCs can transfer a human graft that recapitulates features present in low risk MDS patient bone marrow samples. Furthermore, it suggests that the relative loss of GMP is due to a cell-intrinsic defect in low risk MDS HSCs.
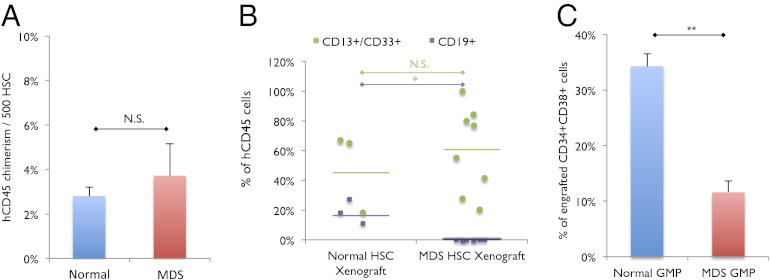
Fig. 3.
Low risk MDS HSCs transplanted into immunodeficient mice yield decreased GMPs. (A) Engraftment of 1,500–3,000 HSCS from normal (n = 4) and low risk MDS (n = 4) bone marrow samples transplanted into NSG newborn mice at 16 wk after transplant (one recipient per normal HSC sample; two recipients per low risk MDS sample), as represented by percentage of human CD45+ chimerism per 500 human HSCs transplanted. N.S., no significance. (B) Lymphoid (CD19+) and myeloid (CD13+/CD33+) engraftment as a percentage of hCD45+ cells in NSG newborn mice at 12–16 wk after transplant. *P < 0.03; N.S. no significance. (C) Frequency of GMPs out of total human CD34+CD38+ cells engrafted in NSG newborn mice at 16 wk after transplant. **P < 0.0007. Error bars represent one SD. “MDS” label represents low risk MDS samples.
Numerous prior studies characterizing CD34+ cells, of which only a small fraction are putative HSCs, in the bone marrow of MDS patients revealed two hallmarks of MDS—increased apoptosis and a concomitant increase in the proliferative fraction; however, these data provide no specific information regarding whether HSCs or specific progenitor populations are pathologically affected in MDS. We evaluated apoptosis by measuring annexin V staining, and we find that normal and low risk MDS HSCs showed similar frequencies of annexin V-positive cells (Fig. 4A), but Lin−CD34+CD38+ cells, comprised of myeloid and lymphoid progenitors, showed a statistically significant 2.3-fold increase in the frequency of annexin V-positive cells (P < 0.03) (Fig. 4B). CMPs, GMPs, and MEPs from low risk MDS all exhibited statistically significant increases in the frequency of annexin V-positive cells (P < 0.02) (Fig. 4 C–E). These data indicate that although MDS is a disease originating in the HSC, the increased apoptosis observed in MDS does not manifest in HSCs but rather in downstream myeloid progenitors.
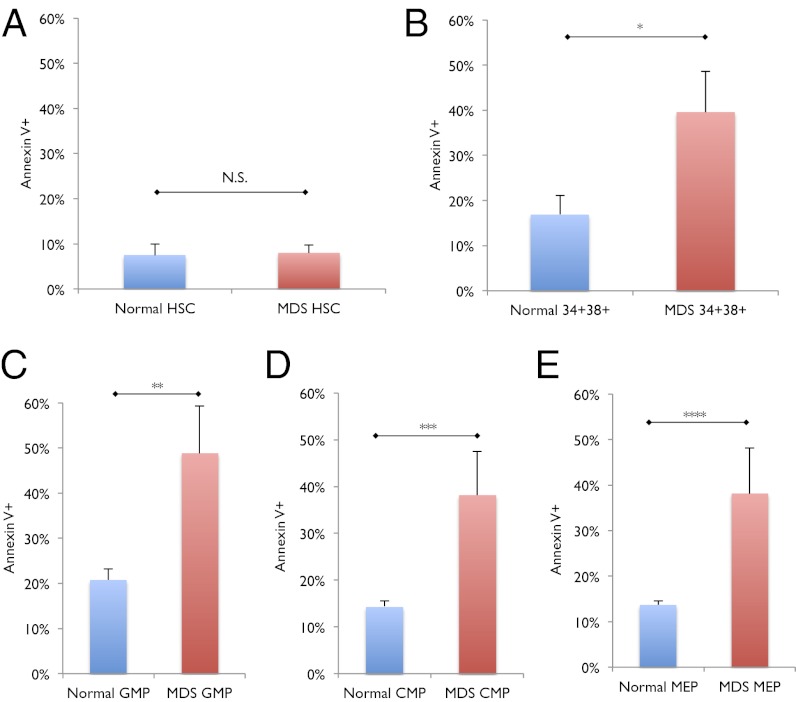
Fig. 4.
Increased apoptosis in low risk MDS GMPs but not in MDS HSCs. (A) Frequency of Annexin V-positive cells in normal (n = 11) and low risk MDS (n = 14) HSCs. N.S. no significance. (B) Frequency of Annexin V-positive cells in normal (n = 11) and low risk MDS (n = 14) CD34+CD38+ myeloid progenitor cells. *P < 0.03. (C) Frequency of Annexin V-positive cells in normal (n = 11) and low risk MDS (n = 14) GMPs. **P < 0.02. (D) Frequency of Annexin V-positive cells in normal (n = 11) and low risk MDS (n = 14) CMPs. ***P < 0.02. (E) Frequency of Annexin V-positive cells in normal (n = 11) and low risk MDS (n = 14) MEPs. ****P < 0.02. Error bars represent one SD. “MDS” label represents low risk MDS samples.
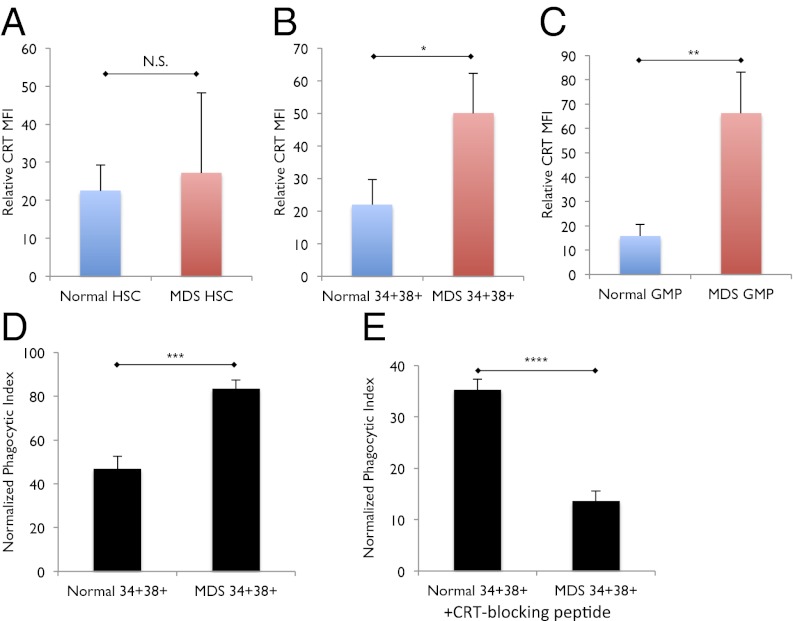
In addition to increased apoptosis, another mechanism that may contribute to the reduced frequency of GMPs observed in low risk MDS patients is increased phagocytosis. We find that cell surface expression of the prophagocytic marker CRT is similar between normal and low risk MDS HSCs (Fig. 5A). In contrast, we find that cell surface CRT expression is on average 2.3-fold higher on low risk MDS bone marrow CD34+CD38+ oligolineage progenitor cells compared with normal (P < 0.05) (Fig. 5B and Fig. S2A). Furthermore, low risk MDS GMPs exhibit significantly higher cell surface CRT expression compared with normal GMPs (P < 0.02) (Fig. 5C). These findings suggest that increased CRT expression on progenitor cells, and GMPs in particular, is a definitive feature of low risk MDS.
Fig. 5.
Cell-surface CRT is increased on and is necessary for the phagocytosis CD34+CD38+ myeloid progenitors in low risk MDS. (A) Cell-surface CRT expression, as expressed by mean fluorescence intensity (MFI) relative to fluorescence-minus-one (FMO) control, on normal (n = 11) and low risk MDS (n = 14) HSCs. N.S., no significance. (B) Cell-surface CRT expression, as expressed by MFI relative to FMO control, on normal (n = 11) and low risk MDS (n = 14) CD34+CD38+ myeloid progenitor cells. *P < 0.05. (C) Cell-surface CRT expression, as expressed by MFI relative to FMO control, on normal (n = 11) and low risk MDS (n = 14) GMPs. **P < 0.02. (D) Phagocytosis of normal and low risk MDS CD34+CD38+ myeloid progenitors (phagocytic index = no. of engulfed green cells / no. of macrophages × 100). ***P < 0.02. (E) Phagocytosis of normal and low risk MDS CD34+CD38+ myeloid progenitors treated with CRT-blocking peptide. ****P < 0.003. Error bars represent one SD. “MDS” label represents low risk MDS samples.
Interestingly, although low risk MDS CMPs also demonstrate an increased, albeit not statistically significant, cell surface CRT expression (Fig. S2B), their progeny, low risk MDS MEPs, do not show this increase in cell surface CRT expression (Fig. S2C). It is not clear whether the low risk MDS MEPs are CRT-low because CRT is not translocated to the surface of these cells, or if the CRT-high MEPs have already been removed by phagocytosis.
Because specific progenitor populations within low risk MDS bone marrow cells exhibit increased cell surface CRT expression, we explored the hypothesis that these cells would be more susceptible to CRT-mediated phagocytosis by macrophages. Upon incubation with normal human macrophages in vitro, CD34+CD38+ cells from low risk MDS were on average 1.8-fold more robustly phagocytosed than CD34+CD38+ cells from normal bone marrow (P < 0.02) (Fig. 5D and Fig. S3A). Interestingly, CD34− cells from low risk MDS were not as readily phagocytosed by human macrophages in vitro compared with both CD34+CD38+ cells from low risk MDS and CD34+CD38+ and CD34− cells from normal bone marrow (Fig. S4).
To further explore the role of CRT-mediated phagocytosis in low risk MDS, we determined whether inhibition of cell surface CRT prevents phagocytosis of low risk MDS CD34+CD38+ progenitor cells by human macrophages. Although phagocytosis of CD34+CD38+ cells from low risk MDS bone marrow samples was robust at baseline, incubation of low risk MDS and normal CD34+CD38+ cells with a CRT-blocking peptide, which acts by inhibiting the CRT–LRP interaction that signals phagocytosis, resulted in significant abrogation of phagocytosis by an average of 2.6-fold (P < 0.003) (Fig. 5E, Fig. S3B). These findings support the idea that increased cell surface CRT expression on low risk MDS CD34+CD38+ progenitor cells results in their increased CRT-mediated programmed cell removal.
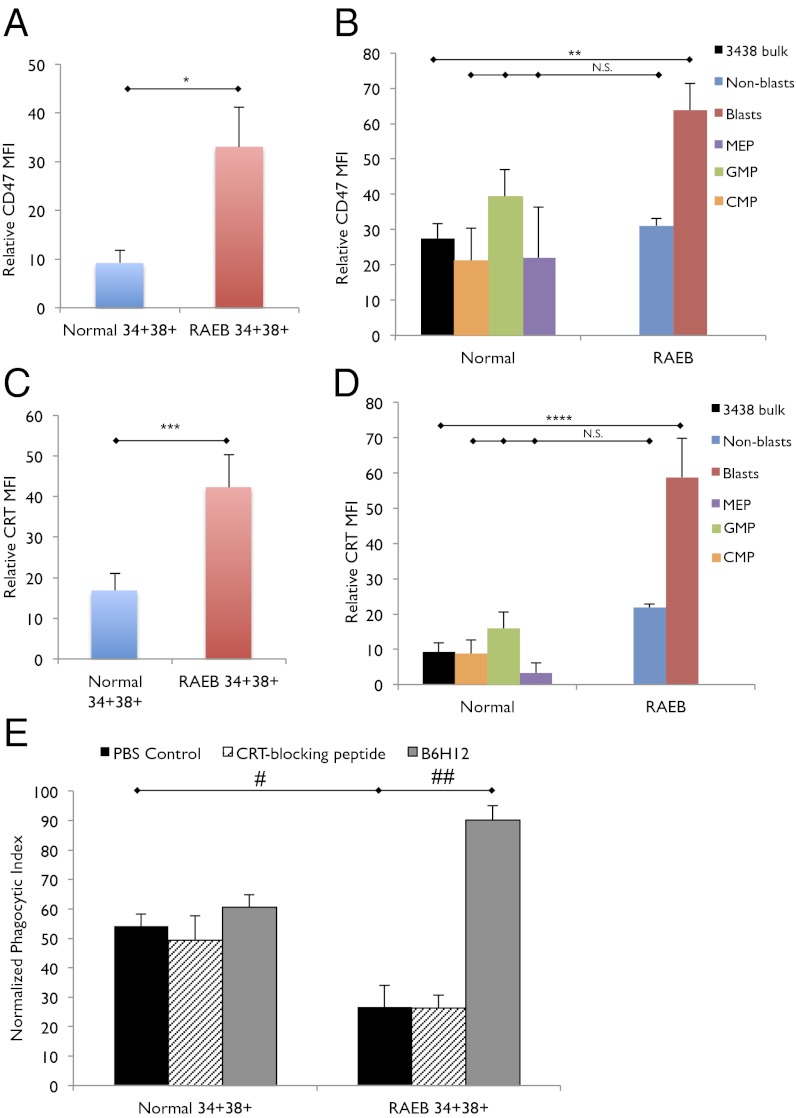
Given that CD47 is an antiphagocytic marker that counterbalances the prophagocytic signal from cell surface CRT, we investigated the expression of CD47 on CD34+CD38+ progenitors from low risk MDS as well as high risk MDS (RAEB). Of note, the immunophenotypic GMP population is not reduced in RAEB, and this population is predominantly composed of blasts (Fig. S5A). We find that CD47 expression is not significantly increased on low risk MDS CD34+CD38+ progenitors compared with normal CD34+CD38+ progenitors (Fig. S5B). CD47 expression on low risk MDS CMPs, GMPs, and MEPs is similar to their normal counterparts as well (Fig. S5C). However, when bone marrow samples from patients with high risk MDS (RAEB, including RAEB-1 and RAEB-2) were evaluated, CD34+CD38+ progenitors were found to express an average 3.6-fold increase in CD47 compared with this same immunophenotypic population in low risk MDS and normal bone marrow samples (P < 0.03) (Fig. 6A and Fig. S5D). Moreover, the blasts within the CD34+CD38+ population from RAEB exhibit significantly increased expression of CD47 (P < 0.03) (Fig. 6B). Of note, CD34+CD38+ cells from RAEB also express increased cell surface CRT compared with normal CD34+CD38+ cells (P < 0.04) (Fig. 6C), and it is the blast population that has significantly higher expression of cell surface CRT (P < 0.04) (Fig. 6D).
Fig. 6.
CD47 is up-regulated on and necessary for the evasion of phagocytosis by CD34+CD38+ myeloid progenitors in high risk RAEB stage of MDS. (A) CD47 expression, as expressed by MFI relative to FMO control, on normal (n = 9) and RAEB (n = 7) CD34+CD38+ myeloid progenitor cells. *P < 0.03. (B) CD47 expression, as expressed by MFI relative to FMO control, on normal (n = 9) CD34+CD38+ myeloid progenitor cells (3438 bulk), normal MEP, normal GMP, normal CMP, and RAEB (n = 7) CD34+CD38+ blasts and RAEB CD34+CD38+ nonblasts. **P < 0.03; N.S. no significance. (C) Cell surface CRT expression, as expressed by MFI relative to FMO control, on normal (n = 9) and RAEB (n = 7) CD34+CD38+ myeloid progenitor cells. ***P < 0.04. (D) Cell surface CRT expression, as expressed by MFI relative to FMO control, on normal CD34+CD38+ myeloid progenitor cells (n = 9) and RAEB CD34+CD38+ blasts (n = 7) and RAEB CD34+CD38+ nonblasts (n = 7). ****P < 0.04; N.S., no significance. (E) Phagocytosis of normal and RAEB CD34+CD38+ myeloid progenitor cells treated with PBS control, CRT-blocking peptide, or anti-CD47 antibody (B6H12). #P < 0.03, ##P < 0.006. Error bars represent one SD.
These observations lead us to hypothesize that CD34+CD38+ cells from RAEB are able to evade phagocytosis by human macrophages because of up-regulation of CD47. Indeed, incubation with human macrophages showed that CD34+CD38+ cells from RAEB are not phagocytosed with the same efficiency as CD34+CD38+ cells from low risk MDS (P < 0.03) (Fig. 6E). Importantly, addition of a blocking anti-CD47 antibody (B6H12) resulted in significantly increased phagocytosis of CD34+CD38+ cells from RAEB (P < 0.006) (Fig. 6E and Fig. S6). In contrast, addition of CRT-blocking peptide did not alter the ability of macrophages to phagocytose CD34+CD38+ cells from RAEB (Fig. 6E). These findings suggest that transformation of low risk MDS to high risk MDS (RAEB) likely involves the up-regulation of antiphagocytic CD47 signal to counterbalance the expression of prophagocytic cell surface CRT. It is conceivable that CD47 up-regulation is the ultimate event in the progression of low risk MDS to high risk MDS and, possibly, AML, and that the increased expression of CD47 on MDS blasts heralds emergence of the fully malignant clone.
Discussion
We have examined the composition of HSC and myeloid progenitor populations from bone marrow samples of MDS patients and age-matched normal and non-MDS cytopenic controls. The HSC population in low risk MDS patient bone marrow is not increased in frequency, on average, compared with age-matched normal control HSC populations. However, the frequency of HSCs in low risk MDS bone marrow shows greater variance than HSCs from normal age-matched bone marrow, which may reflect how well the low risk MDS HSC population from a given patient is expanding into existing or possibly new HSC niches due to differences in the genetic subtypes of low risk MDS. The sample size was not sufficient to identify significant clinical correlations with low risk MDS HSC frequency. Additionally, MDS bone marrow samples harboring a clonal cytogenetic marker, such as monosomy 7, contain immunophenotypic HSCs that are composed almost entirely of the MDS clone, but a small fraction of the HSC population are not part of the MDS clone and, therefore, may represent normal or even pre-MDS clones. Together, these data suggest that MDS HSCs are expanding at the expense of normal HSC, much as we have shown for immunophenotypic HSCs in the chronic phase of CML (32).
We show that purified immunophenotypic low risk MDS HSCs can initiate clonal MDS in xenotransplanted immunodeficient mice. We have demonstrated that normal elderly (age 65 y and older) HSCs in the same xenotransplantation model are inherently biased toward myeloid differentiation and exhibit limited lymphoid differentiation potential compared with normal young (age 20–35 y) HSCs (30). Because MDS prevalence increases with age, it is possible that the bias of normal elderly HSCs away from lymphoid differentiation predisposes them to develop into MDS HSCs, which we have shown to exhibit even poorer generation of lymphoid progeny, a phenotype that has been described in human MDS patients (33). Low risk MDS HSC xenografts also generate reduced frequencies of GMPs, consistent with the hypothesis that MDS HSCs are not only the disease initiating cell in MDS but also suggests that lineage bias and the phenotype of ineffective hematopoiesis are largely cell intrinsic. We cannot completely exclude the possibility that the mouse recipient microenvironment may contribute to these phenotypes.
Our evaluation of low risk MDS HSCs and progenitors reveals that a significant decrease in the frequency of GMPs is a common characteristic of low risk MDS, both in our initial analysis and in our prospective, blind evaluation of 40 patient samples submitted for evaluation of MDS to a clinical flow cytometry service. Notably, in immunodeficient mice transplanted with low risk MDS HSCs, we observe a similar reduction in GMPs in the human myeloid progenitor profile reflected in the bone marrow of engrafted mice. Because the low risk MDS samples tested were randomly selected, these findings are likely true in multiple genetic subtypes of low risk MDS and that different subtypes of low risk MDS share certain common pathophysiologic characteristics; however, additional prospective studies using genetically characterized MDS samples will be required to verify this hypothesis.
We show that low risk MDS myeloid progenitors, and not low risk MDS HSCs, exhibit increased apoptosis, as illustrated by increased annexin V staining. In addition, CD34+CD38+ hematopoietic progenitor cells, including GMPs, from low risk MDS patients express increased cell-surface CRT, a prophagocytic receptor, and inhibition of cell-surface CRT reduces their phagocytosis by macrophages. Although CRT has been implicated as a prophagocytic signal in numerous tumor types (18), its expression had not been previously well characterized in MDS. Because CRT is not up-regulated at the most primitive level of the HSC but only downstream beginning at the more committed myeloid progenitor stages, the loss of progeny from maturing progenitors such as GMPs is likely the major source of cell loss in low risk MDS. By blocking the CRT–LRP interaction using a CRT-blocking peptide, we show that phagocytosis of low risk MDS progenitor cells can be abrogated. The increased phagocytosis and apoptosis of GMPs may explain the reduced frequency of GMPs in low risk MDS bone marrow and, possibly, also the neutropenia observed in the peripheral blood of 15–20% of MDS patients. The other cytopenias in MDS, anemias and thrombocytopenias, are not explained by a significant loss of MEPs. However, compared with normal MEPs, low risk MDS MEPs exhibit increased apoptosis, as evidenced by an increased frequency of annexin V-positive cells, which may account in part for the anemias and thrombocytopenias commonly observed in MDS patients. We predict that downstream pure erythroid and/or megakaryocytic progenitors exhibit increased programmed cell death and/or programmed cell removal in MDS. Because low risk MDS HSCs do not up-regulate CRT, they are not likely to be eliminated by phagocytosis and, therefore, persist and expand in the bone marrow, perpetuating disease.
The importance of macrophage-mediated phagocytosis in MDS pathogenesis is further supported by our finding that that CD34+CD38+ progenitor cells from high risk MDS (RAEB) bone marrow samples express increased CD47, a critical antiphagocytic marker that counteracts the prophagocytic cell surface CRT signal (18–24). We show that low risk MDS progenitor cells express increased levels of cell surface CRT but not CD47, compared with normal controls, and are therefore susceptible to phagocytosis and clearance by macrophages. In contrast, high risk MDS progenitor cells express higher levels of CD47, and can avoid phagocytosis, likely promoting their ability to propagate, and gradually take over the bone marrow. In hematopoiesis, only HSCs self-renew; therefore, we proposed (34–37), and in several cases have shown, that the accumulation of genetic and epigenetic changes characteristic of leukemia progression occur in clones of self-renewing HSCs, and not in the downstream progeny that cannot normally self-renew (32, 38–41). It is likely that at least some events in preleukemic progression trigger both programmed cell death and programmed cell removal (37). Here, we show that low risk MDS likely follow this progression toward high risk MDS and reveal a stage wherein the preleukemic HSC clones outcompete normal HSC clones, but, at their oligolineage progenitor stage, lose blood-forming capacity as a result of programmed cell death and programmed cell removal (37, 42), resulting in peripheral cytopenias. We propose that as the MDS clones lose programmed cell death, the cytopenias still result from programmed cell removal. Finally, in the progression of low risk MDS into high risk MDS and possibly AML, there is the loss of programmed cell removal by expression of the don’t eat me signal, CD47.
Materials and Methods
Please refer to SI Materials and Methods for further details.
Human Samples.
Human bone marrow samples represented the full spectrum of WHO subtypes of low risk MDS without excess blasts (n = 46; includes refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, and myelodysplastic syndrome not otherwise specified but with fewer than 5% blasts) and high risk MDS (n = 7; refractory anemia with excess blasts, includes both WHO RAEB-1 and RAEB-2 subtypes). Controls included a group of non-MDS–related cytopenias (n = 32) and young and elderly normal controls (n = 34).
Flow Cytometry and Cell Sorting.
A panel of antibodies was used for analysis and sorting of HSC and progenitor populations and analysis of human chimerism/engraftment. Cells were analyzed and sorted by using a FACSAriaII cytometer (BD Biosciences). FlowJo Software (Treestar) was used to analyze flow cytometry data.
Mouse Transplantation.
NSG prenatal day (P)0–P3 newborn pups were preconditioned with 100 rads of gamma irradiation 4–24 h before transplantation (9, 31, 43). Cells resuspended in PBS containing 2% (vol/vol) FCS were transplanted i.v. via the anterior facial vein by using a 30 or 31 gauge needle.
FISH.
Cells were sorted by FACS directly onto a slide for cytogenetic analysis by interphase FISH using Vysis LSI EGFR SpectrumOrange/CEP 7 SpectrumGreen Probe (Abbott Molecular), according to the manufacturer’s instructions.
In Vitro Phagocytosis Assay.
FACS-sorted CFSE-labeled normal bone marrow and MDS bone marrow CD34+CD38+ and CD34− cells were labeled with CFSE, incubated with PBS, CRT-blocking peptide (MBL International), or anti-CD47 antibody (clone B6H12, eBiosciences), and then coincubated with human peripheral blood-derived macrophages. Wells were examined under a Leica microscope by using an enhanced green fluorescent protein (GFP) filter set. Phagocytic index was calculated as number of ingested cells/number of macrophages × 100. At least 300 macrophages were counted per well.
Statistical Analysis.
Statistical analysis using Student’s t test and calculations of specificity and sensitivity were done by using Microsoft Excel and/or GraphPad Prism software.
Supplementary Material
Acknowledgments
We thank Ravindra Majeti, Mark Chao, Charles Chan, and Beverly Mitchell for helpful advice and discussions; Theresa Storm and Libuse Jerabek for excellent laboratory management; Adrianne Mosley, Aaron McCarty, and Charlene Wang for animal husbandry; Seth Karten for editing; and Ken Cheung for statistical advice. Support for this work was provided by the Stanford Medical Scientist Training Program (W.W.P.); Goldwater Scholarship (to J.V.P.); National Institutes of Health Grants R01AG029124 (to S.L.S. and I.L.W.), P01CA139490 (to I.L.W.), R01CA86065 (to I.L.W. and W.W.P), and K08 CA1295470 (to C.Y.P.); Department of Defense Grant W81XWH-11-1-0288 (to C.Y.P.); the Leukemia and Lymphoma Society (I.L.W., P.L.G., W.W.P., J.V.P., and K.S.); the William E. Walsh Leukemia Research Fund (P.L.G. and K.S.); and the Virginia and D. K. Ludwig Fund for Cancer Research (I.L.W., W.W.P., and J.V.P.).
Footnotes
Conflict of interest statement: W.W.P., C.Y.P., and I.L.W. filed US Patent Application Serial No. 13/508,319 entitled “Cell Surface Marker Expression in Hematopoietic Stem Cells and Progenitors for the Diagnosis, Prognosis, and Treatment of Myelodysplastic Syndromes;” I.L.W. filed US Patent Application Serial No. 12/321,215 entitled “Methods for Manipulating Phagocytosis Mediated by CD47;” and I.L.W. filed a patent application regarding therapeutic and diagnostic methods for manipulating phagocytosis through calreticulin and low-density lipoprotein-related receptors.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222861110/-/DCSupplemental.
References
- 1.Brunning R, et al. Myelodysplastic syndromes/neoplasms. In: Swerdlow S, et al., editors. WHO Classification of Tumours and Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: Intl Agency Res Cancer; 2008. [Google Scholar]
- 2.Hofmann WK, Koeffler HP. Myelodysplastic syndrome. Annu Rev Med. 2005;56:1–16. doi: 10.1146/annurev.med.56.082103.104704. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 4.Greenberg PL, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Loosdrecht AA, et al. Standardization of flow cytometry in myelodysplastic syndromes: Report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica. 2009;94(8):1124–1134. doi: 10.3324/haematol.2009.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89(7):2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida N, et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95(20):11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson L, et al. Involvement and functional impairment of the CD34(+)CD38(-)Thy-1(+) hematopoietic stem cell pool in myelodysplastic syndromes with trisomy 8. Blood. 2002;100(1):259–267. doi: 10.1182/blood-2001-12-0188. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson L, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110(8):3005–3014. doi: 10.1182/blood-2007-03-079368. [DOI] [PubMed] [Google Scholar]
- 12.Tehranchi R, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 2010;363(11):1025–1037. doi: 10.1056/NEJMoa0912228. [DOI] [PubMed] [Google Scholar]
- 13.Will B, et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood. 2012;120(10):2076–2086. doi: 10.1182/blood-2011-12-399683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza A, et al. Cell cycle kinetic studies in 68 patients with myelodysplastic syndromes following intravenous iodo- and/or bromodeoxyuridine. Exp Hematol. 1997;25(6):530–535. [PubMed] [Google Scholar]
- 15.Mundle SD, et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes. Blood. 1996;88(7):2640–2647. [PubMed] [Google Scholar]
- 16.Shetty V, et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk Res. 1996;20(11-12):891–900. doi: 10.1016/s0145-2126(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 17.Rajapaksa R, Ginzton N, Rott LS, Greenberg PL. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996;88(11):4275–4287. [PubMed] [Google Scholar]
- 18.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2(63):63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Orr AW, et al. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161(6):1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KS, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106(33):14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao MP, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71(4):1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94(10):5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Péault B, Weissman IL, Buckle AM, Tsukamoto A, Baum C. Thy-1-expressing CD34+ human cells express multiple hematopoietic potentialities in vitro and in SCID-hu mice. Nouv Rev Fr Hematol. 1993;35(1):91–93. [PubMed] [Google Scholar]
- 27.McCune JM, et al. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 28.Negrin RS, et al. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6(3):262–271. doi: 10.1016/s1083-8791(00)70008-5. [DOI] [PubMed] [Google Scholar]
- 29.Murray L, et al. Analysis of human hematopoietic stem cell populations. Blood Cells. 1994;20(2-3):364–369, discussion 369–370. [PubMed] [Google Scholar]
- 30.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108(50):20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CY, Majeti R, Weissman IL. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat Protoc. 2008;3(12):1932–1940. doi: 10.1038/nprot.2008.194. [DOI] [PubMed] [Google Scholar]
- 32.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 33.Sternberg A, et al. Evidence for reduced B-cell progenitors in early (low-risk) myelodysplastic syndrome. Blood. 2005;106(9):2982–2991. doi: 10.1182/blood-2005-04-1543. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA. 2000;97(13):7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 36.Weissman I. Stem cell research: Paths to cancer therapies and regenerative medicine. JAMA. 2005;294(11):1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 37.Chao MP, Majeti R, Weissman IL. Programmed cell removal: A new obstacle in the road to developing cancer. Nat Rev Cancer. 2012;12(1):58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson CH, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci USA. 2006;103(16):6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamsson AE, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA. 2009;106(10):3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jan M, et al. 2012. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4(149):149ra118.
- 42.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa F, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106(5):1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.