Significance
In mammalian cells DNA polymerase ζ (polζ) appears critical for bypass of DNA damage and was expected to be important for UV-induced skin carcinogenesis. To investigate the response to UV radiation, we engineered mice lacking polζ in the epidermis, circumventing a requirement for embryonic development. These mice were much more sensitive to UVB radiation than predicted, failed to mount skin-regenerative responses, and did not develop UV-induced skin tumors. Even unirradiated polζ-deficient keratinocytes had a marked proliferation defect and increased chromosomal breaks. Thus in rapidly proliferating cells, polζ maintains levels of DNA breaks below a lethal threshold.
Keywords: DNA replication, double-strand breaks, UV radiation, carcinogenesis
Abstract
DNA polymerase ζ (polζ) is critical for bypass of DNA damage and the associated mutagenesis, but also has unique functions in mammals. It is required for embryonic development and for viability of hematopoietic cells, but, paradoxically, skin epithelia appear to survive polζ deletion. We wished to determine whether polζ functions in a tissue-specific manner and how polζ status influences skin tumorigenesis. Mice were produced in which Rev3L (the catalytic subunit of polζ) was deleted in tissues expressing keratin 5. Efficient epidermal deletion of Rev3L was tolerated but led to skin and hair abnormalities, accompanied by evidence of DNA breaks. Unchallenged mice developed tumors in keratin 5-expressing tissues with age, consistent with the chromosomal instability accompanying a polζ defect. Unexpectedly, mice with the Rev3L deletion were much more sensitive to UVB radiation than mice defective in other DNA repair genes. Following irradiation, polζ-defective mice failed to mount skin-regenerative responses and responded to stress by mobilizing melanocytes to the epidermis. However, they did not develop skin tumors after chronic UVB irradiation. To determine the proliferative potential of polζ-deficient skin epithelia, keratinocytes were isolated and examined. These keratinocytes harbored chromosomal gaps and breaks and exhibited a striking proliferation defect. These results can be unified by a model in which slowly dividing cells accumulate replication-associated DNA breaks but otherwise survive Rev3L deletion, but functional polζ is essential for responses requiring rapid proliferation, both in cell culture and in vivo. The results reveal a biological role for mammalian polζ in tolerating DNA damage and enabling proliferative responses in vivo.
Fast, efficient genomic duplication requires that DNA be completely intact and in the B form, because replicative DNA polymerases cannot synthesize using a damaged DNA template (1, 2). When such a template is encountered, replication halts, and either a double-strand break (DSB) forms after replication fork collapse or the lesion is bypassed by translesion synthesis (TLS) polymerases or by template switching. After such damage tolerance, the DNA can be repaired. The relative importance of each pathway for maintaining genomic stability and preventing carcinogenesis is unknown.
TLS is mediated by specialized DNA polymerases (reviewed in ref. 3). DNA polymerase ζ (polζ, catalytic subunit REV3L) stands out as the most important DNA polymerase for bypass of lesions in template DNA. Polζ plays a major role in the bypass of many types of DNA damage, including pyrimidine(6-4)pyrimidone photoproducts induced by UV radiation (4, 5) as well as lesions formed by chemical damaging agents such as cisplatin and benzo[a]pyrene (4). Polζ also can be used for bypass of the frequent endogenously formed abasic sites in DNA (4). In the yeast Saccharomyces cerevisiae, spontaneous and DNA damage-induced mutagenesis is highly dependent on polζ function (6), and the enzyme is involved in the bypass of common impediments to DNA replication (7), including ribonucleotides accidentally incorporated during DNA synthesis (8).
S. cerevisiae can survive without polζ, but the enzyme clearly has unique functions in mammals because Rev3L-knockout mouse embryos die in midgestation (9–11). To circumvent this difficulty and to investigate the function of polζ in vivo, mice containing loxP recombination sites flanking essential Rev3L exons have been used for conditional disruption of polζ function. In a previous study we used Cre recombinase controlled by the mouse mammary tumor virus (MMTV) promoter, which expresses in a mosaic fashion in hematopoietic and epithelial cells. Normal hematopoietic cells did not survive MMTV-Cre–mediated Rev3L disruption, even in a p53-defective background (12). Similarly, inactivation of Rev3L by CD21-Cre or CD19-Cre caused a loss of mouse B-cell viability (13, 14). On the other hand, a mosaic of viable Rev3L-deleted and nondeleted cells was present in skin epithelia of MMTV-Cre mice. Cells that had deleted Rev3L were present in adult ear, skin, salivary gland, and mammary gland (12). Therefore, deletion of Rev3L in epidermally derived mammalian cells unexpectedly appeared compatible with viability.
These observations raise several important questions. First, are the requirements for polζ tissue specific, so that epidermal cells have a requirement for polζ that is fundamentally different from that of hematopoietic cells? Second, what is the importance of mammalian polζ in defense against UV radiation in vivo? Investigation of radiation sensitivity in mammalian cells has been limited previously to immortalized cells in culture. Third, if polζ is important for bypass of UV radiation damage, how does it modulate skin carcinogenesis in mammals?
Humans and mice defective in the DNA nucleotide excision repair (NER) pathway (NER) or in DNA polymerase η (polη) have a greatly elevated predisposition to UV radiation-induced skin cancer. A major contributor to this tumorigenesis is the accumulation of point mutations in key driver genes. Polζ-depleted cells in culture are reported to have reduced UV-induced point mutations (5, 6). It is possible that suppression of UV-induced mutagenesis in key genes for skin carcinomas would diminish tumorigenesis in polζ-deleted epidermis. On the other hand, the observed increase in the frequency of chromosome rearrangements in Rev3L-deleted cells (13, 15, 16) might accelerate cancer development. Mice efficiently deleting Rev3L in keratin 5-expressing epithelia were used in this study, allowing us to ask definitively whether normal epithelial tissues can develop and renew normally in vivo in the absence of polζ. We find a major biological role of polζ in the defense against UV radiation-induced DNA damage in vivo. The results show that mammalian polζ is important not only for tolerating DNA lesions but also for allowing cells to proliferate after damage.
Results
Mice Survive Epithelial Deletion of Rev3L but Have Skin Tissue Abnormalities.
Mice containing a floxed allele of Rev3L (12, 15) coupled with either a wild-type allele or a knockout Rev3L allele (Fig. 1A) were bred with transgenic mice expressing Cre recombinase from a keratin 5 promoter (BK5.Cre mice). This promoter drives gene deletion at high efficiency (≥95%) in the basal epithelia of skin epidermis, hair follicles, sebaceous glands, thymus, and mammary gland (17, 18), but not in hematopoietic tissues. The efficiency of Cre activity was monitored using the membrane-Tomato/membrane-Green (mT/mG) Cre reporter gene (15, 19) (Fig. 1B) and by PCR of genomic DNA (Fig. S1). Control BK5.Cre;Rev3L+/lox and BK5.Cre;Rev3L+/+ mice retained one or two functional alleles of Rev3L, respectively, while still expressing keratin 5-driven Cre recombinase.
Fig. 1.
Abnormal skin epithelia and increased morbidity in aging Rev3L mice. (A) Schematic of (i) wild-type, (ii) knockout, (iii) floxed-intact, and (iv) floxed-deleted alleles of the murine Rev3L gene (12, 15). Vertical bars represent exons (red bars contain part of polymerase motif I and all of motif V); triangles are loxP sites. (B) mT/mG skin and salivary gland expressing RFP (no Cre activity) or GFP (Cre activity) in BK5.Cre transgenic mice. (C) Genotype percentages from a cross of male BK5.Cre;Rev3L+/lox and female Rev3L+/− mice. The BK5.Cre transgene and the floxed Rev3L allele are linked on chromosome 10. (D) Representative photograph of 12-d-old mouse pups with the genotypes BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/−. (E) H&E-stained skin sections from 7- to 10-wk-old mice. Arrows show the interfollicular epidermis, and arrowheads point to hair follicles. (Scale bars: 100 μm.) (F) Quantification of total interfollicular epidermal cells per millimeter of skin from BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, and BK5.Cre;Rev3L+/+ mice (n = 5). (G) Kaplan–Meier morbidity-free survival curve of BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice with increasing age (n = 19). *P < 0.05. Data points are mean ± SEM.
Mice deleting polζ in basal epithelium (BK5.Cre;Rev3L−/lox) were viable but were underrepresented at weaning (Fig. 1C). In addition, they displayed defects in skin and hair growth. The BK5.Cre;Rev3L−/lox pups were recognizable by a thinner hair coat (Fig. 1D), with hair becoming more normal in appearance by 8 wk of age. Rev3L-deleting mice did not maintain a synchronous hair cycle and showed degenerative changes in hair follicles as they aged (Figs. 1E and 2B). The interfollicular epidermis of the experimental mice displayed significantly fewer cells per millimeter of skin (Fig. 1F) compared with control mice. Thus, deletion of Rev3L leads to a less cellular epidermis and to hair follicle abnormalities.
Fig. 2.
Epithelial carcinomas in skin deleting Rev3L and suppressed response to TPA treatment. (A) Pathological findings in BK5.Cre;Rev3L mice. One mouse with plasmacytoma was not examined for the other features. In a few mice from each group, some of the small sebaceous glands could not be located in histological sections and were not assessed for pathology. (B) Representative histologic sections from normal skin or Zymbal’s gland and from tumors in BK5.Cre;Rev3L−/lox mice. (Scale bars: 100 μm.) (C–E) BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice were treated with 0.85, 1.7, or 3.4 nmol TPA twice weekly for 2 wk (n = 4). (C) Percent BrdU-positive cells, normalized to vehicle control. (D) Total interfollicular epidermal cell number per millimeter of skin, normalized to vehicle control. (E) Epidermal thickness after TPA treatment. *P ≤ 0.05; **P < 0.01. Data points are mean ± SEM.
Aged Rev3L-Deleting Mice Develop Squamous Cell Carcinomas.
To determine the long-term phenotypic effects of an epithelium-specific Rev3L knockout, experimental BK5.Cre;Rev3L−/lox and control BK5.Cre;Rev3L+/lox mice were monitored as they aged. The experimental mice displayed a shortened morbidity-free survival (Fig. 1G) compared with the controls. The reason for this shorter survival was primarily the development of squamous cell carcinomas (SCCs) in specialized sebaceous glands (clitoral and preputial glands, Meibomian glands of the eyelid, and Zymbal’s glands of the ear) and skin (Fig. 2 A and B and Fig. S2), which developed in ∼90% of the mice. All these target organs contain keratin 5-expressing tissues and thus were deleting Rev3L. In each type of sebaceous gland, there was a continuum of lesions ranging from hyperplasia to frank neoplasia. Even in Rev3L-deleting mice that did not have tumors in these sebaceous glands, these organs often had inflammatory and dysplastic changes not seen in control mice. The body weights of Rev3L-deleting and control mice at the time of death were not significantly different (31.0 ± 1.3 g for BK5.Cre;Rev3L−/lox mice and 36.2 ± 1.7 g for BK5.Cre;Rev3L+/lox mice); however, the spleens of the experimental mice were four times larger than the spleens of control mice (3.4 ± 0.52% of total body weight for BK5.Cre;Rev3L−/lox mice and 0.76 ± 0.08% of total body weight for BK5.Cre;Rev3L+/lox; P < 0.001). The larger spleens may be a response to increased inflammation in sebaceous glands and tumors. Therefore, the loss of epithelial Rev3L drives tumor development and promotes inflammation in specialized sebaceous glands. In addition, 3 of 22 experimental mice had fully developed lymphoma or plasmacytoma by age 390 d, whereas control mice >491 d of age had no fully developed instances (although 5 of 20 control mice had indications of possible early lymphoma by this time).
To test the response to moderate inflammation, the skin of Rev3L-deleting and control mice was treated with the tumor-promoting agent 12-O-tetradecanoylphorbol-13-acetate (TPA) (20). Although higher concentrations of TPA enhanced alopecia in the BK5.Cre;Rev3L−/lox mice, it did not induce tumor formation after 40 wk of treatment. Treatment with TPA two times per week for 2 wk stimulated epidermal DNA synthesis by fourfold in control mice but by less than 1.5-fold in Rev3L-deleting mice (Fig. 2C). There was a small but significant increase in epidermal cell number in both control and experimental mice after treatment with the highest concentration of TPA (Fig. 2D), but there was less epidermal thickening in the Rev3L-deleting skin (Fig. 2E). These data suggest a lack of sustained replicative potential in Rev3L-deleting skin in response to TPA. As in normal tissues, an inflammatory stimulus alone does not suffice to induce tumorigenesis in Rev3L-deleting skin.
Rev3L-Deleting Mice Are Extremely Sensitive to UV Radiation.
UV radiation of normal mouse skin causes a well-established cascade of responses (21), including protective thickening and replacement of damaged cells. We assessed the response of polζ-deleted epithelia to UVB radiation. Unexpectedly, Rev3L-deleting skin is extremely UV sensitive. Initial UV radiation-induced epidermal damage was followed by massive epidermal disruption because the skin and hair follicles were unable to regenerate normally.
BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, and BK5.Cre;Rev3L+/+ mice were irradiated with a single dose of 1,800 J/m2 UVB (approximately twice the minimal erythemal/edemal dose for the FVB/N strain), and skin sections were examined in cohorts of mice after 1–10 d. For the first few days, both control and experimental mice showed moderate erythema and edema, with skin in control mice resuming normal appearance and thickness by day 8. Erythema, edema, and inflammatory cell infiltration became progressively more severe in experimental mice. Starting at 48 h there was marked degeneration of the epidermis and hair follicles with widespread ulceration in irradiated areas by 8–10 d (Fig. 3 A and B). Five days after UV irradiation, numerous dramatic changes were evident in polζ-deleting skin. In some areas there was epidermal thickening without an increase in cell number (described below). In other areas the epidermis was completely lost. There also were small foci of epidermal proliferation. Higher radiation doses produced even more rapid and severe epidermal loss in polζ-deleting mice.
Fig. 3.

Acute UV radiation severely affects Rev3L-deleting skin. (A) Representative H&E-stained skin sections from 7- to 10-wk-old BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice at 24, 48, 72, 120, or 192 h after 1,800 J/m2 UVB radiation. (Scale bars: 100 μm.) Arrows point to areas demonstrating inflammatory cell influx. (B) Representative H&E-stained skin sections from 7- to 10-wk-old BK5.Cre;Rev3L−/lox mice showing additional observed phenotypes at 120 h after 1,800 J/m2 UVB radiation. (Scale bars: 100 μm.) (C and D) Quantification of total interfollicular epidermal cells per millimeter of skin (C) or epidermal thickness (D) from BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, or BK5.Cre;Rev3L+/+ mice that were untreated (No UV) or at various times after UVB radiation (n = 4). *P < 0.05; **P < 0.01. Data points are mean ± SEM.
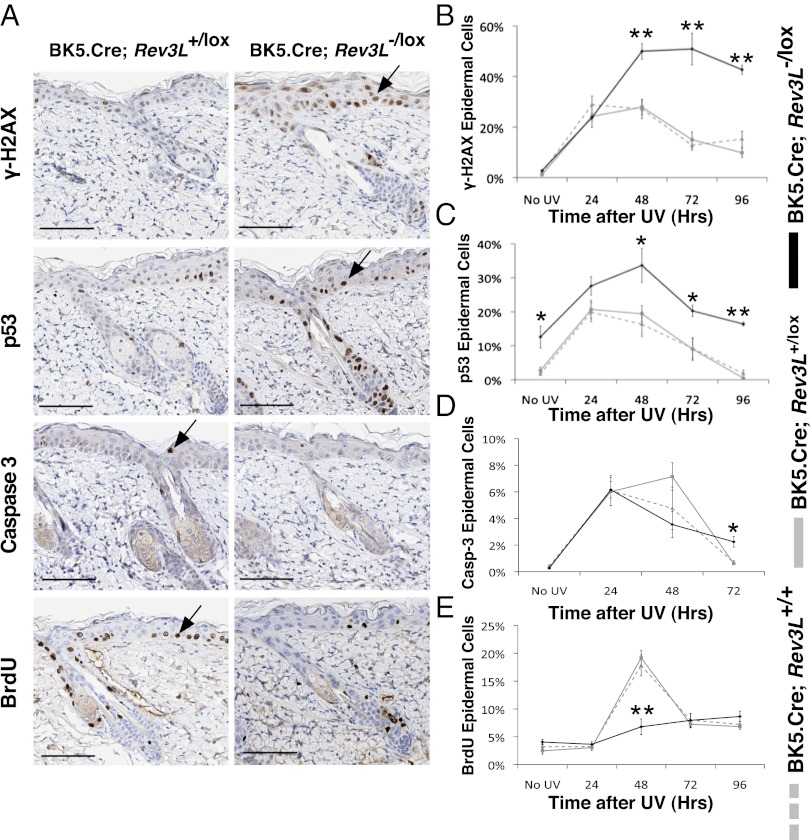
UV radiation-induced epidermal thickening in experimental mice during the first 24–120 h was caused by cell swelling and intercellular edema rather than by an increase in cell number (Fig. 3 C and D). The DNA damage and stress markers γ-H2AX and p53 (measured by immunohistochemistry) increased in all mice after UVB radiation (Fig. 4 A–C). Expression of these stress markers persisted longer at a higher level in polζ-deleting skin. Epidermal proliferation as measured by BrdU incorporation was enhanced 48 h after irradiation in control mice but not in experimental mice (Fig. 4E). Caspase-3 staining was seen in the epidermis of control and experimental mice (Fig. 4D), indicating apoptosis at frequencies comparable to those observed previously at similar doses of UVB radiation (21, 22). Much subsequent epidermal loss in Rev3L-deleting mice may be caused by nonapoptotic mechanisms.
Fig. 4.
Acute UV radiation causes activation of epidermal p53 and γ-H2AX but does not increase proliferation in Rev3L-deleting mice. (A) Representative immunohistochemical sections of γ-H2AX–, p53-, caspase 3-, or BrdU-stained skin sections from 7- to 10-wk-old BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice 48 h after 1,800 J/m2 UVB radiation. Arrows point to representative positively stained cells. (Scale bars: 100 μm.) (B–E) Quantification of percent of γ-H2AX– (B), p53- (C), caspase 3- (D), or BrdU- (E) positive interfollicular epidermal cells in skin sections from BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, or BK5.Cre;Rev3L+/+ mice that were untreated (No UV) or at various times after UVB radiation (n = 4). *P < 0.05; **P < 0.01. Data points are mean ± SEM.
The behavior of melanocytes after UV irradiation was examined in pigmented mice of mixed C57BL6/J and FVB/N background. BK5.Cre;Rev3L−/lox animals developed pronounced epidermal pigmentation. This tanning response, unusual for mice, was associated with migration of melanocytes into the epidermis of irradiated Rev3L-deleting skin (Fig. S3). Melanocytes do not delete Rev3L with K5.Cre expression, and so the observed melanocyte migration apparently occurs in response to signals initiated following damage to polζ-deleting keratinocytes.
Pronounced disintegration of hair follicles occurred after irradiation of polζ-deleting skin. Increased DNA synthesis in hair follicles was observed 24 h after UV radiation but declined rapidly thereafter (Fig. 5 A and B), whereas DNA-break signaling increased concomitantly (Fig. 5 C and D). Hair follicles then disappeared. In marked contrast, hair follicles in control mice showed no morphologic evidence of damage (Fig. 3A).
Fig. 5.
Acute UV radiation causes activation of follicular p53 and γ-H2AX, and chronic UV radiation causes skin loss. (A) Representative immunohistochemical sections of BrdU-stained skin from 7- to 10-wk-old BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice 24 h after a single dose of 1,800 J/m2 UVB radiation. Arrows point to representative positively stained cells. (Scale bars: 100 μm.) (B–D) Quantification of percent of BrdU- (B), γ-H2AX– (C), or p53- (D) positive hair follicle cells in skin sections from BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, or BK5.Cre;Rev3L+/+ mice that were untreated (No UV) or at various times after UVB radiation (n = 4). (E) Kaplan–Meier morbidity-free survival curve of BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, and BK5.Cre;Rev3L+/+ mice with increasing UV radiation dose (n = 17). (F) p53 mutant patches in BK5.Cre;Rev3L mice. Mutation frequency is calculated as the length of p53 mutant focus per total skin length. *P < 0.05; **P < 0.01. Data points indicate mean ± SEM.
Chronic UV Radiation Is Poorly Tolerated in Rev3L-Deleting Skin and Does Not Induce Tumors.
Other mouse models that show an acute sensitivity to UV radiation include those with deficiencies in genes responsible for NER or the TLS DNA polη. When chronically UV irradiated, such mice develop SCCs much more rapidly than their wild-type littermates (Table S1). To determine if a lack of Rev3L leads to an increased incidence of UV radiation-induced tumor formation, chronic UV irradiation experiments were initiated following standard protocols. We found that a low-dose exposure of Rev3L-deleting mice (50 J/m2 UVB radiation three times weekly) was barely tolerated. Even at this very low dose, Rev3L-deleting mice sometimes showed excessive reddening of the dorsal skin; these mice were removed from the study and returned when they had recovered.
No experimental or control mice developed tumors after 52 wk of this low-dose regimen. Control mice were not notably affected by the low dose, but BK5.Cre;Rev3L−/lox mice were progressively lost from the study because of the formation of nonhealing cutaneous ulcers (Fig. 5E). Irradiation of Rev3L-deleting mice with higher doses (e.g., 100 J/m2 three times weekly) caused extensive skin ulceration and was not tolerated for longer than 5 wk. As a control for UV radiation-induced skin tumor formation, BK5.Cre;Rev3L+/+ mice were irradiated with a higher-dose protocol of UVB for up to 50 wk, which induced SCCs in 7 of 16 mice.
Patches of epidermal cells with stabilized, mutant p53 expression commonly occur in response to UV radiation and are believed to represent cell populations from which skin tumors emerge (23). The p53 mutations seen in these patches are point mutations detected with antibody PAb240, which recognizes a commonly exposed epitope in a mutant conformation created by a variety of amino acid substitutions. Complete inactivation of p53 is not detected by this approach. In the present studies, no p53 mutant patches as detected by PAb240 staining were observed in the skin of control mice at low cumulative doses of UVB (4,000 J/m2), but many such patches were observed after high-dose UV radiation (>150 kJ/m2). In Rev3L-deleting mice some p53 mutant patches were observed after low doses of UV radiation (Fig. 5F). Even though there is a decreased rate of point mutagenesis in the absence of Rev3L, such p53-defective clones should have a significant selective advantage for proliferation of Rev3L-deleted cells (15). However, UVB irradiation dramatically compromises epidermal integrity in Rev3L-deleting mice, suggesting progression of these patches to form a tumor is prevented by the high radiation sensitivity and severely impaired proliferative potential of the polζ-deleting cells and tissues.
Intrinsic Renewal Defect and Accumulation of Endogenous DNA Damage in Rev3L-Deleting Keratinocytes.
To clarify the mechanisms underlying the extreme sensitivity of Rev3L-deleting skin epithelium to UV radiation, we isolated keratinocytes from the skin of BK5.Cre;Rev3L mice. We hypothesized that they would not be able to thrive following a stimulus that normally would induce rapid proliferation. Within 5 d of isolation from skin, there were far fewer BK5.Cre;Rev3L−/lox keratinocytes than control cells, and senescence was apparent (Fig. 6 A–C), with more cells harboring multiple nuclei (Fig. S4) and fewer undergoing DNA synthesis (Fig. 6D). Potent proliferation signals therefore invoke a senescence barrier in polζ-defective keratinocytes, as they do in mouse embryonic fibroblasts (15). To determine whether epithelial cells from BK5.Cre;Rev3L−/lox mice harbor chromosomal aberrations, metaphase spreads were analyzed from keratinocytes of 3-d-old mice. There was an eightfold increase in the number of gaps and breaks (chromatid-type aberrations) per metaphase, compared with controls (Fig. 6 E and F). There was no significant difference in chromosomal-type aberrations (dicentrics and double minutes; Table S2) or in aneuploidy and polyploidy (Fig. S5). Consistent with the observation of gaps and breaks in the chromosomes, the BK5.Cre;Rev3L−/lox keratinocytes displayed twice as many DNA DSBs [cells containing foci of both p53-binding protein 1 (53BP1) and phosphorylated histone H2A.x (γ-H2AX)] as controls (Fig. 6 G and H).
Fig. 6.
Growth defects and DNA breaks in Rev3L-deleting keratinocytes. (A) Representative phase-contrast photographs of BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes 5 d after isolation. (B) Quantification of cells with senescent morphology in BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes 5 d after isolation (n = 7). (C) Quantification of cells per square millimeter of BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes 5 d after isolation (n = 7). (D) BrdU incorporation, normalized to total cells and compared with incorporation from a single population doubling of a reference cell population, of BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes at 1, 3, and 5 d after isolation (n = 4). (E) Chromosome spreads from BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes 3 d after isolation. Arrows indicate chromosome breaks or gaps (n = 6). (F) Quantification of gaps and breaks per metaphase in BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes (n = 6). (G) 53BP1 and γ-H2AX immunofluorescence of keratinocytes derived from BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice 3 d after isolation (n = 7). (H) Quantification of BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox keratinocytes containing both 53BP1 and γ-H2AX foci 3 d after isolation (n = 7). *P < 0.05; **P < 0.01. Data points are mean ± SEM.
Evidence of breaks and genomic stress also was apparent in the skin of unchallenged experimental and control mice. There was elevated p53 staining in the interfollicular epidermis of BK5.Cre;Rev3L−/lox mice compared with controls and possibly elevated γ-H2AX staining (P = 0.053) (Fig. 7 A and B, and Fig. S6). Therefore, similar to Rev3L-deleting cells in culture (15, 16, 24–27), DSBs and chromosomal gaps and breaks are present in Rev3L-deleting epidermal cells in vivo. The evidence suggests that proliferation leads to an increase in DNA breaks in Rev3L-deleting cells. These breaks then activate cell-stress responses (such as p53 stabilization) that eventually lead to cell death.
Fig. 7.
Increased DNA breaks in Rev3L-deleting mouse skin. (A and B) Percentage of (A) p53-positive cells and (B) γ-H2AX–positive cells in the interfollicular epidermis of 7- to 10-wk-old BK5.Cre;Rev3L−/lox and BK5.Cre;Rev3L+/lox mice (n = 5). (C) Quantification of BrdU label-retaining follicular cells at 30, 60, or 90 d after BrdU injection in BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, or BK5.Cre;Rev3L+/+ mice (n = 6). (D) Quantification of the percent of follicles that stain for K15 at 30, 60, or 90 d after BrdU injection in BK5.Cre;Rev3L−/lox, BK5.Cre;Rev3L+/lox, or BK5.Cre;Rev3L+/+ mice (n = 6). *P < 0.05, **P < 0.01. Data points are mean ± SEM. (E) Model for thresholds of tolerance in Rev3L-deficient cells and mice. Two factors contribute to cytotoxicity in Rev3L-deleting cells: increasing DNA damage (x-axis) and increasing proliferation (y-axis). Together, these factors cause DNA breaks (dotted blue diagonal line) in cells lacking Rev3L. Any DNA damage or proliferation above a threshold level (dotted black horizontal and vertical lines) initiates cell-death programs (likely signaled by p53). When DNA damage and proliferation are below these threshold levels (i.e., in the lower left-hand quadrant of graph), the cells can survive. However, they still experience DNA breaks which cause genomic instability and can lead to cancer formation. The levels of proliferation and DNA damage caused by different stimuli are noted by arrows on the x- and y-axes.
To determine whether epidermal stem cells are rapidly exhausted in unchallenged BK5.Cre;Rev3L−/lox mice, 10-d-old mouse pups were injected with BrdU, and label-retaining cells in the stem cell niche (bulge region) of the hair follicle were quantified after 30, 60, and 90 d. No significant change in the number of BrdU label-retaining cells of any of the genotypes was observed (Fig. 7C and Fig. S7). At 30 and 60 d there was a small but significant decrease in hair follicles that stained with keratin 15 (K15, marking the stem cell niche) in the experimental mice compared with controls (Fig. 7D and Fig. S7). However, the epidermal and follicular defects in BK5.Cre;Rev3L−/lox mice do not measurably exhaust the skin stem cell compartment after 3 mo. This observation may indicate that Rev3L-defective stem cells do not respond well to stimuli for renewal. A similar defect in epidermal stem cell functionality is seen with third-generation telomerase-deficient (Terc−/−) mice (28, 29). Such mice have epidermal defects but no increased turnover of label-retaining stem cells in unchallenged skin. When Terc−/− mice are treated with TPA, telomerase-deficient skin has a higher number of label-retaining cells compared with controls because these cells cannot proliferate in response to the stimulus.
These results provide an explanation for the extreme UV radiation sensitivity. In the absence of polζ, not only are mechanisms absent for bypass of UV-induced DNA lesions, but also the proliferative response of Rev3L-defective cells is impaired.
Discussion
Polζ Deletion Leads to Epithelial Abnormalities and Spontaneous Tumor Formation in Aging Rev3L-Deleted Tissues.
This study answers several long-standing questions about the function of polζ in mammalian cells. First, it has seemed puzzling that hematopoietic cells are largely inviable when polζ function is ablated, but epithelial cells can survive in tissues deleting Rev3L in a mosaic fashion (12). We show here that although epithelial tissues are viable when Rev3L is deleted efficiently, the epidermis is atypical, with reduced interfollicular cellularity and abnormalities in hair follicles. Unchallenged Rev3L-defective epidermis accumulates genomic damage leading to chromosome abnormalities. As a consequence, mice deleting Rev3L in keratin 5-expressing tissues develop spontaneous SCCs. These tumors arise predominantly in specialized sebaceous glands, all of which harbor keratin 5-expressing epithelia. The incremental accumulation of chromosomal alterations over time eventually leads to changes necessary for tumor formation. As a tumor suppressor (12), Polζ falls into the category of gene products that limit spontaneous tumor incidence by controlling the level of DNA breaks and chromosome rearrangements (30).
Frequent tumors in specialized sebaceous glands also are seen in mice predisposed for simultaneous loss of Rad51C and Tp53. These mice acquire Zymbal’s and preputial gland tumors after 400–440 d (31). In contrast, 35% of NER-defective xeroderma pigmentosum group A (Xpa−/−) mice (compared with 19% of Xpa+/+ control mice) form spontaneous tumors after 2 y, but none of these neoplasias arise in specialized sebaceous glands (32). Mice lacking the exonuclease activity of either DNA polymerase δ or ε have distinct mutator and cancer phenotypes, but neither group has an increased incidence of sebaceous gland tumors (33). The Rad51C-p53 model (31) has an increased load of DNA breaks because of a defect in DSB repair by homologous recombination. The Rev3L-deficient mice may phenocopy this defect, increasing the load of DSBs arising because of failure to resolve blocked forks and gaps in the absence of polζ. REV3L also might have a more direct role in repair of some DSBs by homologous recombination (34, 35).
Polζ Defect Confers Extreme UV Sensitivity.
Inactivation of polζ causes the epidermis to be exquisitely sensitive to UV radiation. As discussed further below, this sensitivity is a consequence of the dual role of polζ in bypassing lesions caused by UV radiation and in sustaining normal cell proliferation in UV radiation-damaged epidermis. It is striking that polζ-deleting skin appears to be the most UV radiation sensitive of any known mouse mutant, including prototypical models for UV radiation sensitivity. Table S1 summarizes results of studies for mice defective in NER genes and DNA polη. Haired NER-defective mice (shaved before irradiation) tolerate 6,000 J⋅m−2⋅wk−1, whereas albino hairless NER-defective mice tolerate 400 J⋅m−2⋅wk−1or more. In the present study, haired Rev3L-defective mice could tolerate only 150 J⋅m−2⋅wk−1. The epithelial dysfunction and radiation sensitivity documented here depend strictly on Rev3L disruption, because all experiments included isogenic mice retaining one allele of Rev3L and expressing BK5.Cre. It is unlikely that Cre recombinase contributes significantly to the increased sensitivity, because hairless mice constitutively deleting Ercc1 with the same BK5.Cre (18) show UV radiation sensitivity similar to that of other NER-knockout mice (Table S1). Mice expressing K5.Cre for epithelial deletion of the Stat3 gene tolerate 3,600–14,000 J⋅m−2⋅wk−1 UV radiation and respond with relatively normal epidermal hyperproliferation (36).
After irradiation for 7–30 wk, all mice deficient in XPA, XPC, or the cyclobutane pyrimidine dimer (CPD) bypass polymerase polη develop SCCs (Table S1). Irradiation of hairless mice deleting Ercc1 with BK5.Cre (375 J⋅m−2⋅wk−1) led to tumors in all animals by 20 wk (18). Similarly, mice defective for the genes responsible for Cockayne syndrome group A or B (Ercc8 and Ercc6, respectively) develop skin carcinomas by 40 wk. In marked contrast to NER-defective models, deletion of Rev3L in the skin did not increase the incidence of UV radiation-induced carcinomas.
Several factors likely contribute to the absence of UV radiation-induced tumors in Rev3L-deleting skin. Irradiated Rev3L-defective skin has increased cell death caused by chromosomal damage and a reduced ability to regenerate normally. UV radiation-induced DNA lesions caused by repeated irradiation present a continual challenge during DNA replication for Rev3L-deleted cells, even with mutant p53. Further, a Rev3L defect is expected to reduce the frequency of UV radiation-induced point mutations (4, 6, 37). Base change mutagenesis of genes such as p53 drives tumor formation in mouse models of UV carcinogenesis (23). Rapid formation of UV radiation-induced p53 mutant patches is correlated with tumor incidence in Xpa-, Xpc-, and Ercc6 (Csb)-deficient mice (23). In Rev3L-deleting skin, some p53 point mutant clones indeed are detected after low-dose chronic irradiation, and these may have a proliferative advantage. Formation of skin carcinomas did not take place, however. Instead, chronic irradiation of polζ-defective mice with even a relatively low dose of UV radiation leads to persistent skin ulceration. As a consequence, tumor clones may be unable to proliferate in polζ-defective skin because of heavily damaged stromal elements and inadequate recruitment of stem cells to repopulate the epidermis after radiation. We regularly observed slow or absent healing of adventitious skin wounds (nicks from shaving or wounds inflicted by cage mates), consistent with a deficiency in tissue regeneration after wounding.
Melanocyte migration to the epidermis and donation of melanin to keratinocytes is typical of human skin but is not a normal response in UV-irradiated mice. However, it has been observed as a stress response in Atm−/− mice exposed to ionizing radiation (38) and in Terc−/− mice with transgenic expression of TERF2, which have aberrant telomeres (39). In these instances damaged melanocyte stem cells are believed to differentiate terminally to melanocytes and undergo transit to the epidermis. Notably, in the keratin 5-Cre conditional Rev3L model studied here, polζ deletion takes place in the keratinocytes, not in the melanocytes. The melanocyte migration in irradiated polζ-deleting mouse epidermis therefore is triggered indirectly as a consequence of keratinocyte damage.
Thresholds of Tolerance to DNA Damage and Proliferation in Rev3L-Deficient Cells.
We propose that the phenotypes of Rev3L-deficient mouse cells in vivo and in vitro can be unified by considering two roles for polζ: a function in maintaining genomic integrity in normally proliferating cells and a function in DNA damage tolerance following UV irradiation. These ideas are illustrated schematically in Fig. 7E.
In all cells, endogenous DNA lesions and replication barriers arise continuously which require polζ function. For example, these barriers may include difficult-to-replicate DNA sequences (7) and ribonucleotides occasionally incorporated during DNA synthesis (8). In the absence of polζ, double-strand breaks and chromosome abnormalities accumulate. Rapidly proliferating cells such as those in the hematopoietic system cannot survive this genomic stress. In cell culture, there is a persistent stimulus to replicate DNA and divide, and so polζ is required for keratinocyte proliferation (this study) and proliferation of primary cultures of embryonic fibroblasts (15). Attenuation of damage-sensing checkpoints, for example by immortalization with T antigen, allows proliferation of Rev3L-defective cells in culture at the expense of an increased load of chromosomal aberrations (15).
In unchallenged epithelia, longer cell-cycling times allow more opportunities for alternative pathways of DNA damage tolerance or repair, and the tissues can survive, although genomic damage accumulates slowly with time and predisposes Rev3L-deleting tissues to tumor formation. However, if proliferation levels are too high (as with stimulation by placing cells into culture or when rapid regeneration is signaled following UV radiation), an excessive number of DNA breaks occur, and cell-death pathways are engaged. Acute UV radiation increases both the DNA damage load and proliferative pressure, resulting in rapid loss of cell viability with catastrophic tissue damage that does not support formation of UV radiation-induced tumors.
These observations may have implications for any therapies designed to inactivate polζ to sensitize tumors to DNA-damaging agents (40, 41). Strategies that target polζ for short periods of time, in conjunction with treatments that induce DNA damage and stimulate proliferation, likely would be highly effective. However, although temporary inhibition of polζ may be tolerated, caution must be used with longer-term suppression, because normal tissues would be expected to experience significant cellular toxicity, genome instability by chromosome breakage, and additional tumor formation.
Materials and Methods
Most experiments were completed with mice on a pure FVB/N strain background. Mice used for the aging and keratinocyte experiments were genetically ∼75% FVB/N mixed with C57BL6/J and 129/OLA strains. Detailed experimental procedures are described in SI Materials and Methods, encompassing mouse breeding, animal monitoring, necropsy and histology, tissue fluorescence, treatment of mice with TPA, keratinocyte culture, chromosome analysis, immunofluorescence, BrdU incorporation assay, label retaining cell assay, treatment of mice with UV radiation, p53 mutant clone analysis, and statistical analysis.
Supplementary Material
Acknowledgments
We thank David Trono, Maria Sandoval, and Rebecca Gray for expert assistance, Lidza Kalifa for initiating the TPA experiments, and Fernando Benavides and the Genetics Services core for congenics analysis and advice. We thank the Research Animal Support Facility, the Molecular Biology Core Facility, and the Integrated Imaging, Histology and Pathology Facility at Smithville for expert support. We appreciate discussion with our laboratory colleagues and Patrick Moore for comments on the manuscript. This research was supported by National Institutes of Health (NIH) Grant CA132840 from the National Cancer Institute, by Grant P30ES007784 from the National Institute of Environmental Health Sciences, by NIH Cancer Center Support Grant P30-CA016672 (University of Texas M. D. Anderson Cancer Center), and by the Grady F. Saunders, PhD Distinguished Professorship (to R.D.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217425110/-/DCSupplemental.
References
- 1.McCulloch SD, Kunkel TA. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008;18(1):148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt MW, Matsumoto Y, Loeb LA. High fidelity and lesion bypass capability of human DNA polymerase delta. Biochimie. 2009;91(9):1163–1172. doi: 10.1016/j.biochi.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange SS, Takata K, Wood RD. DNA polymerases and cancer. Nat Rev Cancer. 2011;11(2):96–110. doi: 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shachar S, et al. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. EMBO J. 2009;28(4):383–393. doi: 10.1038/emboj.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon JH, Prakash L, Prakash S. Error-free replicative bypass of (6-4) photoproducts by DNA polymerase zeta in mouse and human cells. Genes Dev. 2010;24(2):123–128. doi: 10.1101/gad.1872810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan GN, Wittschieben JP, Wittschieben BØ, Wood RD. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res. 2008;18(1):174–183. doi: 10.1038/cr.2007.117. [DOI] [PubMed] [Google Scholar]
- 7.Northam MR, Robinson HA, Kochenova OV, Shcherbakova PV. Participation of DNA polymerase zeta in replication of undamaged DNA in Saccharomyces cerevisiae. Genetics. 2010;184(1):27–42. doi: 10.1534/genetics.109.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzaro F, et al. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol Cell. 2012;45(1):99–110. doi: 10.1016/j.molcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito G, et al. Disruption of the Rev3l-encoded catalytic subunit of polymerase zeta in mice results in early embryonic lethality. Curr Biol. 2000;10(19):1221–1224. doi: 10.1016/s0960-9822(00)00726-0. [DOI] [PubMed] [Google Scholar]
- 10.Bemark M, Khamlichi AA, Davies SL, Neuberger MS. Disruption of mouse polymerase zeta (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr Biol. 2000;10(19):1213–1216. doi: 10.1016/s0960-9822(00)00724-7. [DOI] [PubMed] [Google Scholar]
- 11.Wittschieben J, et al. Disruption of the developmentally regulated Rev3l gene causes embryonic lethality. Curr Biol. 2000;10(19):1217–1220. doi: 10.1016/s0960-9822(00)00725-9. [DOI] [PubMed] [Google Scholar]
- 12.Wittschieben JP, et al. Loss of DNA polymerase zeta enhances spontaneous tumorigenesis. Cancer Res. 2010;70(7):2770–2778. doi: 10.1158/0008-5472.CAN-09-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenten D, et al. Pol zeta ablation in B cells impairs the germinal center reaction, class switch recombination, DNA break repair, and genome stability. J Exp Med. 2009;206(2):477–490. doi: 10.1084/jem.20080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly J, et al. Altered Ig hypermutation pattern and frequency in complementary mouse models of DNA polymerase ζ activity. J Immunol. 2012;188(11):5528–5537. doi: 10.4049/jimmunol.1102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange SS, Wittschieben JP, Wood RD. DNA polymerase zeta is required for proliferation of normal mammalian cells. Nucleic Acids Res. 2012;40(10):4473–4482. doi: 10.1093/nar/gks054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittschieben JP, Reshmi SC, Gollin SM, Wood RD. Loss of DNA polymerase zeta causes chromosomal instability in mammalian cells. Cancer Res. 2006;66(1):134–142. doi: 10.1158/0008-5472.CAN-05-2982. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39(1):52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 18.Doig J, et al. Mice with skin-specific DNA repair gene (Ercc1) inactivation are hypersensitive to ultraviolet irradiation-induced skin cancer and show more rapid actinic progression. Oncogene. 2006;25(47):6229–6238. doi: 10.1038/sj.onc.1209642. [DOI] [PubMed] [Google Scholar]
- 19.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 20.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat Protoc. 2009;4(9):1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YP, et al. Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer Res. 1999;59(18):4591–4602. [PubMed] [Google Scholar]
- 22.van Oosten M, et al. Differential role of transcription-coupled repair in UVB-induced G2 arrest and apoptosis in mouse epidermis. Proc Natl Acad Sci USA. 2000;97(21):11268–11273. doi: 10.1073/pnas.200226697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebel H, et al. Relationship between UV-induced mutant p53 patches and skin tumours, analysed by mutation spectra and by induction kinetics in various DNA-repair-deficient mice. Carcinogenesis. 2005;26(12):2123–2130. doi: 10.1093/carcin/bgi198. [DOI] [PubMed] [Google Scholar]
- 24.Van Sloun PP, et al. Involvement of mouse Rev3 in tolerance of endogenous and exogenous DNA damage. Mol Cell Biol. 2002;22(7):2159–2169. doi: 10.1128/MCB.22.7.2159-2169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks JK, et al. Differential roles for DNA polymerases eta, zeta, and REV1 in lesion bypass of intrastrand versus interstrand DNA cross-links. Mol Cell Biol. 2010;30(5):1217–1230. doi: 10.1128/MCB.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zander L, Bemark M. Immortalized mouse cell lines that lack a functional Rev3 gene are hypersensitive to UV irradiation and cisplatin treatment. DNA Repair (Amst) 2004;3(7):743–752. doi: 10.1016/j.dnarep.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda E, et al. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22(12):3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores I, Blasco MA. A p53-dependent response limits epidermal stem cell functionality and organismal size in mice with short telomeres. PLoS ONE. 2009;4(3):e4934. doi: 10.1371/journal.pone.0004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309(5738):1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 30.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov SG, Haines DC, Martin BK, Sharan SK. Loss of Rad51c leads to embryonic lethality and modulation of Trp53-dependent tumorigenesis in mice. Cancer Res. 2009;69(3):863–872. doi: 10.1158/0008-5472.CAN-08-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakane H, et al. Impaired spermatogenesis and elevated spontaneous tumorigenesis in xeroderma pigmentosum group A gene (Xpa)-deficient mice. DNA Repair (Amst) 2008;7(12):1938–1950. doi: 10.1016/j.dnarep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albertson TM, et al. DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice. Proc Natl Acad Sci USA. 2009;106(40):17101–17104. doi: 10.1073/pnas.0907147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, et al. REV1 and polymerase ζ facilitate homologous recombination repair. Nucleic Acids Res. 2012;40(2):682–691. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane DP, Shusterman M, Rong Y, McVey M. Competition between replicative and translesion polymerases during homologous recombination repair in Drosophila. PLoS Genet. 2012;8(4):e1002659. doi: 10.1371/journal.pgen.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DJ, Angel JM, Sano S, DiGiovanni J. Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene. 2009;28(7):950–960. doi: 10.1038/onc.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, et al. hREV3 is essential for error-prone translesion synthesis past UV or benzo[a]pyrene diol epoxide-induced DNA lesions in human fibroblasts. Mutat Res. 2002;510(1-2):71–80. doi: 10.1016/s0027-5107(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 38.Inomata K, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137(6):1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96(5):701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 40.Xie K, Doles J, Hemann MT, Walker GC. Error-prone translesion synthesis mediates acquired chemoresistance. Proc Natl Acad Sci USA. 2010;107(48):20792–20797. doi: 10.1073/pnas.1011412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doles J, et al. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc Natl Acad Sci USA. 2010;107(48):20786–20791. doi: 10.1073/pnas.1011409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.