Abstract
Recent work has suggested that beta-lactam antibiotics might directly affect eukaryotic cellular functions. Here, we studied the effects of commonly used beta-lactam antibiotics on rodent and human T cells in vitro and in vivo on T-cell–mediated experimental autoimmune diseases. We now report that experimental autoimmune encephalomyelitis and adjuvant arthritis were significantly more severe in rats treated with cefuroxime and other beta-lactams. T cells appeared to mediate the effect: an anti-myelin basic protein T-cell line treated with cefuroxime or penicillin was more encephalitogenic in adoptive transfer experiments. The beta-lactam ampicillin, in contrast to cefuroxime and penicillin, did not enhance encephalomyelitis, but did inhibit the autoimmune diabetes developing spontaneously in nonobese diabetic mice. Gene expression analysis of human peripheral blood T cells showed that numerous genes associated with T helper 2 (Th2) and T regulatory (Treg) differentiation were down-regulated in T cells stimulated in the presence of cefuroxime; these genes were up-regulated in the presence of ampicillin. The T-cell protein that covalently bound beta-lactam antibiotics was found to be albumin. Human and rodent T cells expressed albumin mRNA and protein, and penicillin-modified albumin was taken up by rat T cells, leading to enhanced encephalitogenicity. Thus, beta-lactam antibiotics in wide clinical use have marked effects on T-cell behavior; beta-lactam antibiotics can function as immunomodulators, apparently through covalent binding to albumin.
Keywords: autoimmunity, T-cell differentiation, immune regulation, multiple sclerosis
Beta-lactam antibiotics have been in use for decades for the treatment of bacterial infections. Investigation of their mechanism of action revealed that beta-lactam antibiotics bind covalently to bacterial proteins (penicillin-binding proteins), hindering cell-wall synthesis (1). It was initially thought that beta-lactam antibiotics would not be able to directly affect mammalian cells because mammalian cells do not make cell walls. On theoretical grounds, however, beta-lactam compounds might also bind eukaryotic cellular proteins and thereby affect their functions. Indeed, recent screening of various compounds in models of amyotrophic lateral sclerosis led to the discovery that beta-lactam antibiotics could increase the expression of neuronal glutamate transporter in cultured mammalian cells (2). Moreover, ceftriaxone was found to protect animals from several forms of glutamate-induced toxicity (2). Thus, a compound evolved by yeast apparently to inhibit competing bacterial cells (1) was demonstrated to induce gene expression in eukaryotic neuronal cells.
We initiated this study to examine the effects of beta-lactam antibiotics on T-cell functions in vitro and in vivo. Surprisingly, we found that cefuroxime enhanced adjuvant-induced arthritis (AA) and experimental autoimmune encephalomyelitis (EAE), both passive and active, in Lewis rats, and ampicillin inhibited type I diabetes in nonobese diabetic (NOD) mice. Penicillin was found to bind intracellular albumin; cefuroxime was found to suppress the expression of genes of the Th2 and Treg differentiation pathways; and ampicillin was found to induce increased expression of these genes.
Results
Enhancement of Actively Induced EAE and AA by Cefuroxime Treatment.
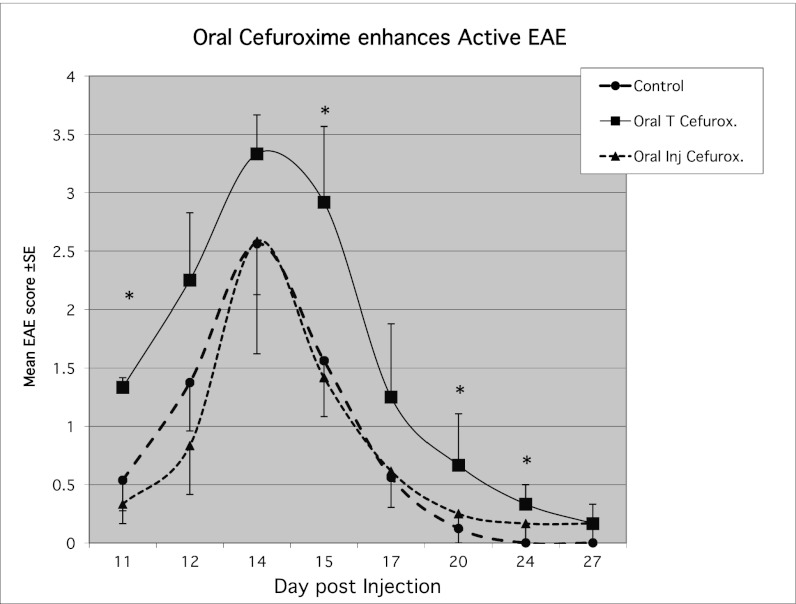
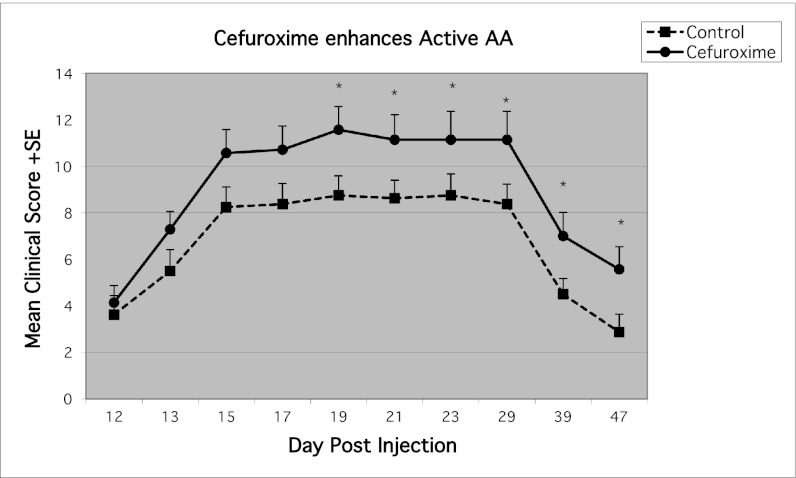
To test the effects of antibiotics in vivo, we induced active EAE and treated the injected rats with oral cefuroxime axetil in the drinking water from day 7 postinduction; the daily dose was 50 mg/kg, in the range of therapeutic pediatric human doses (3). For a control antibiotic, we administered orally the i.v. cefuroxime sodium preparation, which is not absorbed into the circulation. The rats that received oral cefuroxime developed significantly more severe EAE (Fig. 1). To extend our results to another experimental autoimmune disease, we induced AA in 16 rats (4). On day 12 postinjection, the rats were divided into two groups of eight rats each with similar disease scores; one group was injected i.p. with cefuroxime 5 mg (25 mg/kg) on day 12 and every other day to day 29. We chose a treatment regime (once every 2 d) that is different from the antibacterial dosing regime (three daily injections) to differentiate the immunomodulating effect from an antibacterial effect. The rats that had been injected with cefuroxime showed significantly more severe arthritis scores (Fig. 2). Thus, the enhancing effects of cefuroxime were manifested in two experimental autoimmune diseases.
Fig. 1.
Oral cefuroxime increases the severity of actively induced EAE. Rats, four in each group, were injected with GpMBP/CFA to induce active EAE. Test rats were given cefuroxime in the drinking water; we dissolved one 500-mg tablet in 500 mL of drinking water to administer 50 mg/kg. Control rats were given orally the i.v. preparation of cefuroxime sodium that is not absorbed from the gastrointestinal tract (45); a second control group was given water without antibiotics. Rats given the oral cefuroxime preparation (cefuroxime axetil) showed significantly more severe EAE than the two control groups. Asterisks indicate significant (P < 0.05) changes between cefuroxime and control groups.
Fig. 2.
Exacerbation of adjuvant-induced arthritis by cefuroxime treatment. Rats were injected with CFA at the base of tail to induce AA. On day 12, the rats were divided into two similar groups based on their clinical scores. The experimental group was injected i.p. with 5 mg of cefuroxime on day 12 and then every other day till day 29. Recipients of cefuroxime demonstrated more severe arthritis scores than the control group. Asterisks indicate significant (P < 0.05) changes between cefuroxime and control group.
Cefuroxime Treatment of Encephalitogenic T Cells Enhances Adoptive EAE.
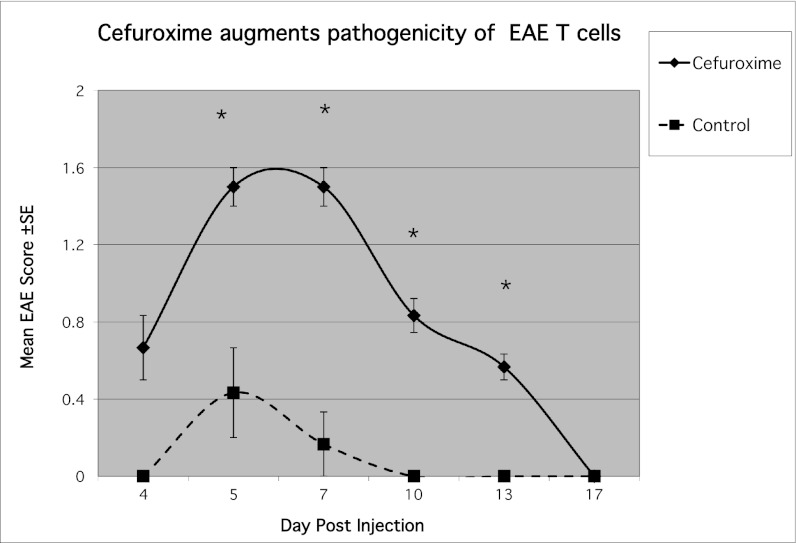
Treatment with cefuroxime in vivo could affect many different host agents involved in EAE or AA as well as influencing the rats’ bacterial flora (5). To test whether the antibiotic might directly modify the behavior of effector T cells, we stimulated an encephalitogenic T-cell line in the presence or absence of cefuroxime in vitro. The activated T cells were then washed to remove the antibiotic, and the T cells were injected into naïve recipient rats. We used the BP10 line attenuated by repeated stimulations to reduce its pathogenic potential, and to allow us to detect either suppression or enhancement of encephalitogenicity. We found that the presence of cefuroxime during T-cell activation markedly enhanced the manifestations of EAE in the recipient rats (Fig. 3). A similar enhancing effect was seen upon incubation of the BP10 line with 50 μg/mL of another beta-lactam antibiotic, penicillin. To rule out antigen-presenting cells (APC) as the target of the beta-lactam antibiotic (6), we stimulated the encephalitogenic line without APC using phorbol myristate acetate (PMA; 50 ng/mL) and ionomycin (500 ng/mL) in the presence or absence of cefuroxime. The EAE mediated by the T cells stimulated in the presence of cefuroxime was significantly more severe (Fig. S1), indicating that the antibiotic directly enhanced the encephalitogenicity of the T cells.
Fig. 3.
Cefuroxime enhances the pathogenicity of the BP10 line. The weakly encephalitogenic BP10 line was stimulated for 3 d with MBP in the presence or absence of cefuroxime (50 μg/mL). The stimulated T cells were then inoculated (107 per rat) i.p. to naïve rats, and EAE was scored. The recipients of the cefuroxime-treated cells showed significantly more severe disease scores; asterisks indicate significant (P < 0.05) changes between cefuroxime and control group.
Different Beta-Lactam Antibiotics Enhance EAE.
We tested several beta-lactam antibiotics (see Table S1 for a description of beta lactams used) for their effect on the adoptive transfer of EAE. We incubated the BP10 line with ceftriaxone, penicillin, or ampicillin (50 μg/mL). Ceftriaxone enhanced EAE severity, as did penicillin, but ampicillin treatment did not increase the severity of EAE (Fig. S2).
Ampicillin Protects NOD Mice from Diabetes.
NOD mice spontaneously develop diabetes mellitus similar to type I diabetes in humans (7). Because ceftriaxone enhanced EAE, but ampicillin did not, we tested the effects of both beta-lactam antibiotics on the development of autoimmune diabetes in NOD mice. Groups of 10 mice were untreated or injected s.c. at weekly intervals with ceftriaxone (675 μg per mouse) or ampicillin (1,300 μg per mouse). The mice treated with ampicillin developed an incidence of diabetes of 30% at 5.7 mo, whereas the untreated control and ceftriaxone-injected mice manifested a 60% incidence of disease (Fig. S3; P = 0.05 control vs. ampicillin and P = 0.017 ceftriaxone vs. ampicillin). Thus, some beta-lactam antibiotics can have opposing effects on different T-cell–mediated autoimmune diseases in rodents: ampicillin down-regulates NOD mouse diabetes, but not rat EAE, and ceftriaxone up-regulates rat EAE, but not mouse diabetes. In experiments using beta lactams in vivo, it is possible that the effects were due to changes in the bacterial microbiome that is known to affect T-cell regulation (8).
Cefuroxime and Ampicillin Manifest Opposing Effects on Immune-Related Gene Expression in Human T Cells.
In view of the wide use of beta-lactam antibiotics in clinical medicine, we directed the mechanistic studies to a set of important human T-cell genes; we used the Human Autoimmune and Inflammatory Response Gene Array (SuperArray Bioscience Corp.). This array contains 367 genes, including cytokines; chemokines and their receptors; transcription factors; and signaling proteins. We purified CD4+ T cells from healthy human donors, stimulated them for 120 min with mitogenic plate-bound anti-CD3 antibody in the presence or absence of cefuroxime 50 μg/mL or ampicillin 50 μg/mL, and analyzed the effect on gene expression. Analysis of the results was performed using the GEArray analysis program. The results are shown in Table S2. Fifty-eight genes were found to be down-regulated significantly by cefuroxime, but most of these genes (56 of 58) were up-regulated by ampicillin. Interestingly, eight of these genes were reported to be down-regulated in the peripheral blood lymphocytes of multiple sclerosis patients in Israel (9), and 15 genes were down-regulated in the T cells of multiple sclerosis patients in Japan (10). The products of these genes included cytokines; chemokines and their receptors; signaling molecules; and transcription factors (Table S2). Many of the genes down-regulated by cefuroxime and up-regulated by ampicillin were reported to participate in Th2 and Treg pathways, and only a minority has been implicated in the Th1 pathway. Note that the cytokine gene TNF-α, considered to be proinflammatory, was found to have anti-inflammatory effects in knockout mice (11). The down-regulation of molecules in the Th2/Treg pathways by cefuroxime is consistent with its augmentation of EAE (12) and AA; in contrast, the up-regulation of these genes by ampicillin is consistent with its down-regulation of NOD diabetes (13).
Human T-Cell Protein of 67 kDa Specifically Binds Penicillin Covalently.
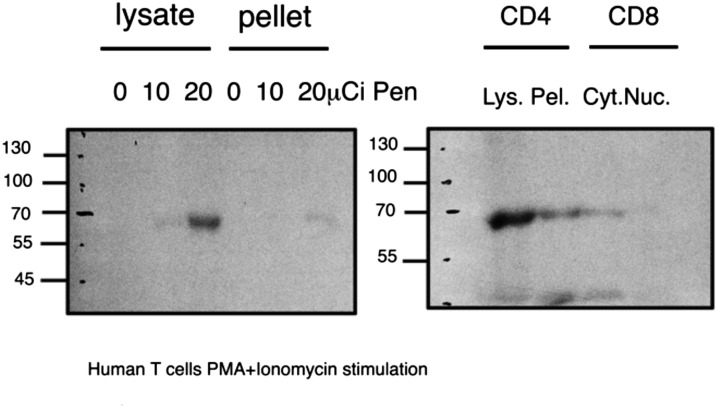
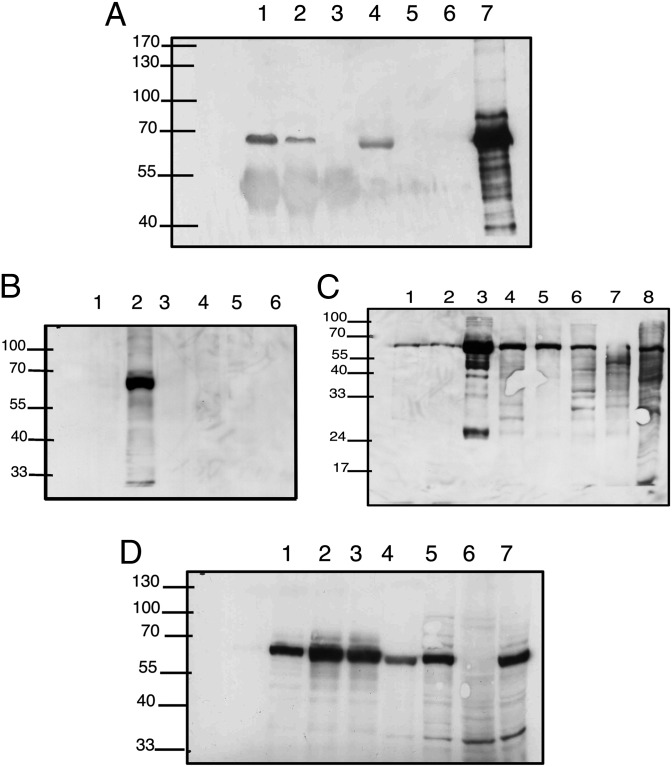
Penicillin and other beta-lactam antibiotics have been shown to inhibit bacterial cell-wall synthesis by binding covalently to specific penicillin-binding proteins and thus interfering with their enzymatic activity (1). We reasoned that beta-lactam antibiotics might affect T-cell behavior likewise by covalently binding a key T-cell protein. To test this hypothesis, we incubated purified CD4 or CD8 human T cells with 10 or 20 μCi of tritium-labeled beta-lactam benzylpenicillin (Amersham) for 3 d during stimulation with PMA and ionomycin. The stimulated T cells were collected and washed, and their proteins were subjected to SDS/PAGE separation. As seen in Fig. 4, a single major penicillin–protein radioactive band was detected at 67 kDa in lysates of both CD4 and CD8 T cells. The intensity of the band was stronger in CD4 T cells in the presence of 20-μCi concentration of penicillin.
Fig. 4.
Radioactively labeled penicillin binds a 67-kDa band in human T-cell lysates. CD4 and CD8 cells were separated and purified from healthy human donors, and incubated in stimulation medium with PMA and ionomycin and 3[H]benzylpenicillin for 72 h. Cell lysates were separated on SDS gels, and the dried gels were exposed in intensifying screens to X-ray film for 2 wk at –80 °C. (Left) CD4 T cells; (Right) CD4 and CD8 T cells. Cyt, cytoplasmic; Lys, total lysate; Nuc, nuclear fraction; Pel, pellet of total lysate.
Albumin Is the 67-kDa Penicillin-Binding Band.
We isolated the 67-kDa lactam-binding band by activating human T cells in the presence of biotinylated ampicillin or biotinylated ceftriaxone, lysed the cells, and purified the lysates by binding to a CaptAvidin column (Invitrogen). The fractions binding the beta-lactam antibiotics were eluted by applying carbonate/bicarbonate buffer or by free biotin. The isolated protein band was subjected to enzymatic digestion, and the resulting peptides were identified by mass spectrometry. The 67-kD protein from human CD4 T cells was identified as human serum albumin. Thus, albumin seems to be a lactam-binding protein in human T cells.
Because albumin is a known contaminant in sequencing studies, we repeated the isolation of the 67-kD band from penicillin-treated cells using immunoprecipitation (IP) with anti-human serum albumin (anti-HSA) and Western blotting with an antibody that binds specifically to penicillin bound to proteins, Pen 9 (14). Fig. 5A shows the results of this experiment. The 67-kDa band appears in the IP of cytoplasmic and nuclear fractions; the band was absent from the lysate of the cytoplasmic fraction following immune precipitation (lane 5). In other words, IP with anti-HSA antibody resulted in the disappearance of the penicillin-protein band from the cytoplasmic lysate, suggesting that, except for albumin, there are no other proteins of a similar molecular weight that are modified by penicillin.
Fig. 5.
(A) Immunoprecipitation of 67-kDa band with anti-human serum albumin antibody. Nuclear (lane 1) or cytoplasmic (lane 2) lysates of human CD4 T cells treated with penicillin (control: no lysate, lane 3) were incubated with rabbit polyclonal anti-human albumin (Sigma), precipitated with protein A Sepharose, and run on an SDS gel. Lysates without the protein A bound complex (lane 4, nuclear; lane 5, cytoplasmic; lane 6, no lysate control; lane 7, positive control) were run. Western blot analysis was performed with monoclonal anti-penicillin (Pen 9). IP of the cytoplasmic fraction with anti-human albumin resulted in the disappearance of the penicilloyl band, indicating that the only labeled protein was albumin. (B) T cells express albumin modifiable by penicillin. Human CD3 cells were stimulated in vitro with PMA and ionomycin in the presence of the indicated antibiotics for 48 h. The cells were collected, washed, and lysed in lysis buffer, and the lysates were separated in SDS/PAGE. Proteins were transferred to nitrocellulose and probed with Pen 9 in standard Western blots. The Pen 9-labeled 67-kDa band is present only in penicillin-treated T cells. Lane 1 cells, untreated with antibiotics; lane 2, penicillin; lane 3, ampicillin; lane 4, cefuroxime; lane 5, chloramphenicol; and lane 6, vancomycin. (C) Detection of in vivo penicillin-labeled proteins. Lewis rats were injected with penicillin G; 120 min after injection, the rats were killed and various tissues were subjected to SDS/PAGE and Western blotting with monoclonal Pen 9 antibody. The 67-kDa band is present in all tissues and is most abundant in the serum sample. Lane 1, thymus; lane 2, spleen; lane 3, serum; lane 4, kidney; lane 5, intestine; lane 6, liver; lane 7, lung; lane 8, positive control human PBL. (D) Western blot analysis of various cell lines treated with penicillin shows the dominant 67-kDa band in most samples. Lane 1, mouse mesenchymal stem cells; lane 2, mouse immature dendritic cells; lane 3, mouse mature dendritic cells; lane 4, Jurkat; lane 5, human acute lymphoblastic leukemia (ALL) line MOLT-4; lane 6, FAO rat hepatoma cell line; lane 7, CEM human ALL line. The band is absent from a hepatoma cell line that was documented to have undergone dedifferentiation and lost its albumin expression (16).
Analysis of T-Cell Beta-Lactam Binding by Anti-Penicilloyl–Albumin Antibody.
We used a monoclonal anti-penicilloyl–albumin antibody to further confirm that the beta-lactam binding molecule produced by human T cells is albumin. When penicillin binds covalently to a protein, the beta-lactam ring binds to a lysine residue (15). A monoclonal antibody called Pen 9 is specific to the thiazolidine ring of penicillin bound to albumin (14). To test the reactivity of Pen 9 in our system, we activated purified human T cells by mitogenic treatment in the presence of the beta-lactam antibiotics penicillin and ampicillin or with other families of antibiotics in culture and tested the lysates by Western blot with Pen 9. Fig. 5B shows that the Pen 9 reacted specifically to a major protein of the penicillin-treated T cells and not with the other antibiotics present during T-cell activation. Note that Pen 9 did not bind to the ampicillin-treated T cells; apparently the albumin molecule modified by ampicillin does not present the specific epitope presented by the penicillin–albumin molecule.
Pen 9 Monoclonal Antibody Detects Penicillin–Albumin in Vivo.
To learn whether penicillin binds albumin in vivo, we injected Lewis rats i.p. with penicillin (50 mg/rat) and 2 h later tested various tissue lysates for reactivity with Pen 9 antibody in Western blot. The results are shown in Fig. 5C. The 67-kDa band representing penicilloylated albumin appeared in all tissues examined and, as expected, was most abundant in serum. We also tested lysates of various cell lines raised in vitro. Fig. 5D shows that the penicillin-modified albumin band could be detected in mesenchymal stem cells, dendritic cells, and Jurkat, MOLT4, FAO, and CEM lines. Albumin was absent in the FAO rat hepatoma cell line, which is known to have dedifferentiated and to have lost its expression of albumin (16).
Albumin mRNA and Protein Is Expressed by T Cells.
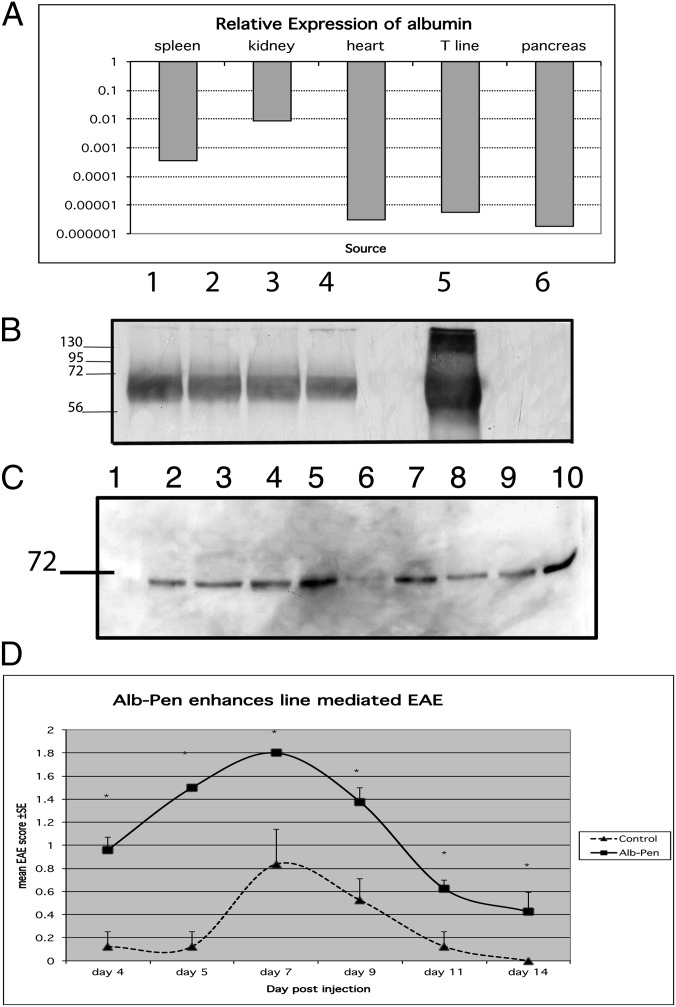
To test whether T cells might indeed produce albumin, we analyzed by RT-PCR the expression of albumin mRNA in rat T cells compared with other tissues. Fig. 6A shows that albumin mRNA could be detected in rat T cells, as well as in spleen, kidney, heart, and pancreas. A similar level of expression was detected in human CD4 T cells. To test the production of albumin protein by T cells, we used a monoclonal antibody that binds to human albumin and not to BSA (Sigma catalog no. A6684). Human CD3 T cells were cultured in 10% (vol/vol) FCS; after 3 d in culture, the T cells were stimulated with anti-CD3 (OKT3, 50 ng/mL) and IL-2 (100 U/mL). The cells were collected after 1, 2, and 4 d of stimulation. As seen in Fig. 6B, the human cells expressed significant amounts of human albumin in culture. Note that the antibody did not recognize BSA; therefore, the albumin expressed is of human origin.
Fig. 6.
(A) Relative expression of albumin mRNA in various tissues by RT-PCR. Total RNA was extracted from various tissues and cDNA was prepared. Real-time PCR was performed and the quantities relative to liver (level of expression considered as 1) were depicted. (B) Human albumin expressed by human CD3 T cells. CD3 T cells were maintained in culture in 10% FCS in RPMI medium. Cells were stimulated with OKT3 antibody (50 ng/mL) and recombinant IL-2 (100 U/mL) for 1–4 d. Cells were lysed and 15 μg protein was run in SDS gels. Control lanes were purified human albumin (lane 5) and BSA (lane 6). A positive band was detected in cells before stimulation (lane 1) and at 1, 2, and 4 d (lanes 2–4) of stimulation. (C) Western blot of human CD4 T cells incubated with penicillin-modified albumin. The T cells were harvested after 1, 2, or 3 h. Cytoplasmic and nuclear fractions were run on SDS transferred to nitrocellulose and tested with Pen 9 antibody. Cytoplasmic and nuclear penicillin-labeled-albumin was seen after 1 h, and peaked at 3 h. Lane 1, NC indicates negative control; lanes 2–4, cytoplasmic lysate after 1-, 2-, and 3-h incubation with penicillin–albumin complex. Lane 5, positive control cytoplasmic lysate of cells incubated with penicillin. Lane 6, nuclear lysate; lanes 7–9, nuclear lysates after 1-, 2-, and 3-h incubation with penicillin-modified albumin. Lane 10, positive control nuclear lysate of cells incubated with penicillin. (D) Penicillin-modified albumin augments the pathogenicity of BP10 line. Human albumin was incubated or not with penicillin (100 mg/mL for 2 h at 37 °C), dialyzed against PBS, and then added at 5 mg/mL to stimulation medium of BP10 line. Following 3 d of stimulation, cells were washed and injected i.p. into naïve Lewis rats, 107 cells per rat. A control group was injected with the untreated line.
Penicillin-Modified Albumin Is Taken Up By T Cells.
To test whether penicillin-modified albumin can enter T cells, we incubated human serum albumin with penicillin, and then dialyzed it extensively. The resulting penicillin-modified albumin was incubated with purified human CD4 T cells for 1, 2, or 3 h, and the cells were lysed and tested by Western blot with monoclonal Pen 9 antibody. Fig. 6C shows the results. The penicillin-modified albumin detected by Pen 9 entered the cells and was detectable in the nuclei within 1 h. Thus, T cells can take up penicillin-modified albumin and transport it to the nucleus. Is the penicillin-modified albumin functional in T-cells?
Penicillin-Modified Albumin Augments the Pathogenicity of the BP10 Line.
To test whether the penicillin-modified albumin moiety itself could enhance the effector functions of a T-cell line, we stimulated the encephalitogenic BP10 line with penicillin-modified albumin (5 mg/mL) for 3 d in stimulation medium and tested in vivo the capacity of the line to mediate EAE. Fig. 6D shows that similar to penicillin alone, penicillin-modified albumin enhanced the pathogenicity of the T cells. In additional experiments, unmodified human albumin alone had no significant effect on the severity of the EAE mediated by the line.
Discussion
The present research uncovered three unexpected findings that bear important clinical and fundamental implications: (i) beta-lactam antibiotics in common use can act as modulators of T-cell behavior; (ii) different beta-lactam molecules can up-regulate proinflammatory T-cell phenotypes or down-regulate them specifically; and (iii) the immune modulation appears to be mediated by interaction of the beta-lactam molecule with albumin produced by the T cells.
Let us start with the third finding. Albumin is widely known to be a blood protein produced by the liver and active in maintaining osmotic pressure in the vascular system and as a carrier for a variety of body molecules and drugs (17). Nevertheless, we detected albumin expression in several tissues and cells, such as mesenchymal stem cells, dendritic cells, and Jurkat, MOLT4, FAO, and CEM lines (Fig. 6D). Moreover, “ectopic” albumin expression has been previously described in healing bone (18); skin (19); granulosa cells (20); kidney and pancreas (21); and mammary gland (22). Furthermore, albumin was described to affect several biological processes: secretion of TGF-β1 by kidney tubular cells (23), and prevention of apoptosis in neuroblastoma cells (24) in neuronal cells (25) and in chronic lymphocytic leukemia lymphocytes (26). In endothelial cells, albumin was found to activate the TGF-β receptor II, including the phosphorylation and nuclear translocation of SMAD proteins (27). Other studies have found albumin to interact with DNA (28), transfer RNA (29), and tumor-associated peptides and proteins (30). Many pharmacological studies of albumin have identified two major binding pockets of the molecule with specific endogenous and exogenous ligand binding-specific sites (31). There are earlier reports that penicillin binds covalently to albumin (32), and such binding affects the properties of albumin (33). However, we did not expect albumin to be produced by immune cells or to acquire immune functions following an interaction with beta-lactams.
Further research must be done to learn how albumin modified by beta-lactam antibiotics is taken up by T cells and how the modified albumin affects T-cell gene expression and behavioral phenotype. The chemical modification of BSA by N-acetylglucosamine was previously described as a signal for nuclear translocation (34). Indeed, proteomic studies have identified albumin within nuclei (35). Interestingly, six of the genes that were modified by beta-lactam treatment of human CD4 T cells are situated in the TGF-β pathway (Table S2), similar to the documented effect of albumin on endothelial cells (27). The modification of TGF-β–related genes is likely to be important, because recent work implicates TGF-β signaling at the crossroads of T-cell differentiation into both Th17 effector cells (36) and Treg cells (37). The half-life of penicillin-modified albumin is prolonged to 7 d (32), compared with the half-life of free penicillin of 42 min; thus the biological effects of modified albumin are likely to be prolonged.
However, the implications of the observed effects of beta-lactams on T-cell immune functions are clinically important, irrespective of the molecular mechanisms responsible for them. In the medical literature, there are many publications linking exacerbation of multiple sclerosis, a Th1-type human autoimmune disease, to infections. These infections were of suspected viral origin of the respiratory tract (38) or bacterial origin of the sinuses or urinary tract (39, 40). Though the suggested mechanism implicated the Th1 cytokines released during the infection as stimulatory to the pathogenic autoreactive T cells, our data point to the use of penicillins during such infections as potential exacerbating factors.
In summary, the present work documents unique and significant effects of beta-lactam antibiotics on T-cell functions. The molecular mechanism appears to involve chemical modification of albumin, leading to widespread changes in cellular genes leading to a change in T-cell behavior. Beyond pointing out a unique function of albumin, these results suggest that the antibiotic-modified protein deserves testing in diseases where immune modulation might have therapeutic benefits. The in vivo autoimmune disease modulation seen with antibiotics raises the questions of how the antibiotics would affect additional important T-cell functions, such as Th2-mediated autoimmune diseases, viral infections, tumor cell rejection, and graft rejection. In preliminary experiments in C57BL mice inoculated with the syngeneic RMA tumor, the beta-lactam molecule 6-aminopenicillanic acid was found to suppress tumor growth, possibly by inducing an augmented Th1 immune response. The beta-lactam molecule 6-aminopenicillanic acid is essentially free of antibacterial activity, suggesting that it might be possible to develop beta-lactam immune modulators free of untoward effects on commensal bacteria and without selecting resistant bacteria.
Methods
Animals.
Inbred female Lewis rats and NOD mice were supplied by the animal breeding center of the Weizmann Institute of Science under the supervision of Harlan Laboratories and were used at 2–3 mo of age. Experiments were approved by the Weizmann Institute of Science Institutional Animal Care and Use Committee. Human peripheral blood lymphocytes from healthy donors were obtained from the blood bank of Sheba Medical Center, Tel Hashomer, Israel.
Reagents, Antigens, and Antibodies.
Mycobacterium tuberculosis H37Ra was purchased from Difco. Guinea-pig MBP and ConA were purchased from Sigma. Antibiotics were purchased from a local pharmacy. Anti-human CD3 (OKT3; eBioscience) was used to coat 24-well plates at 2 μg/mL in PBS. Rabbit polyclonal anti-human serum albumin was purchased from Sigma (catalog no. A0433). Mouse monoclonal against human serum albumin that does not bind BSA was from Sigma (catalog no. A6684). Mouse monoclonal anti-penicillin (Pen 9) was from AbD Serotec. CaptAvidin was from Invitrogen, and Sulfo-NHS-LC-Biotin was from Pierce.
Real-time PCR primers for rat albumin were forward primer CCCGATTACTCCGTGT; reverse primer TGGCGTTTTGGAATCCATA. Human primers for albumin were forward ATGCGCTATTAGTTCGTTAC; reverse primer CATGGTCGCCTGTTCA. Radioactive 3[H]benzylpenicillin was purchased from Amersham (250 μCi, 1 mCi/mL). Human albumin was from Calbiochem (Merck).
T-Cell Lines.
Antigen-specific T-cell lines were established from lymph node cells that had been stimulated with MBP (10 μg/mL) for 3 d in stimulation medium as described above. Following stimulation, the T-cell blasts were isolated on Lymphoprep (Nycomed Pharma) and seeded in propagation medium (41). Animals were injected i.p. with 107 MBP-stimulated T cells, following 6–8 cycles of in vitro stimulations. In our experience, and that of other investigators, MBP-reactive lines undergo a reduction in pathogenicity after six or more in vitro stimulations. In some experiments the BP10 line was stimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for 3 d in stimulation medium, without antigen-presenting cells (42).
Induction of EAE.
Active EAE was induced by s.c. injection of 25 μg guinea-pig MBP (GpMBP) in complete Freund’s adjuvant (CFA). CFA was prepared by adding 4 mg/mL Mycobacterium tuberculosis (Mt) H37Ra (Difco) to incomplete Freund’s adjuvant. Adoptive EAE was transferred by i.p. injection of GpMBP-activated cells of the BP10 line as described (6). Clinical EAE was observed 4–6 d following administration of T-cell line and 11–12 d following GpMBP/CFA injection. Clinical scoring was +1, paralysis of tail; +1.5, paresis of posterior paws and ataxia; +2, paraplegia; +3, paralysis extending to thoracic spine; +4, a moribund state.
AA Induction and Assessment.
Heat-killed Mt strain H37Ra (Difco) was finely ground using a pestle and mortar, and then suspended to a final concentration of 10 mg/mL in incomplete Freund's adjuvant. Test rats were injected at the base of the tail with 100 μL of Mt suspension. The day of AA induction was designated as day 0. Disease severity was assessed by direct observation of all four limbs in each animal. A relative score between 0 and 4 was assigned to each limb based on the degree of joint inflammation, redness, and deformity; thus, the maximum possible score for an individual animal was 16. The results are presented as the mean ± SE of total score (4).
Radioactive Penicillin-Binding Assay.
We obtained tritium-labeled benzylpenicillin from Amersham (250 μCi, 1 mCi/mL). Human CD4 or CD8 T cells were stimulated in 24-well plates, 5 × 106 cells/mL, with PMA and ionomycin for 72 h in the presence of 10 or 20 μCi of labeled penicillin. Following stimulation, the cells were collected, lysed, and separated by SDS/PAGE. The gels were fixed, treated with 1 M sodium salicylate, and dried. The dried gels were exposed to X-ray film (BioMax MS film) for 14 d, with intensifying screen (BioMax TranScreen; Eastman Kodak), and then developed.
Human T Cells.
T cells were purified from the peripheral bloods of healthy human donors (Blood Bank, Sheba Medical Center). The whole blood was incubated (20 min, 22 °C) with RosetteSep human T-cell enrichment mixture (StemCell Technologies). The remaining unsedimented cells were then loaded onto lymphocyte separation medium (ICN Biomedicals), isolated by density centrifugation, and washed with PBS. The purified cells were 95% CD3+ T cells. In a second round of purification, CD3+ T cells were labeled for selection with a magnetically coupled mAb against CD4 (Miltenyi Biotec). The purified cells obtained (usually 97% CD4+ T cells) were cultured in RPMI medium 1640 containing 10% heat-inactivated FCS.
Western Blot.
Rat tissues were ground with a tissue grinder in lysis buffer. The homogenate was centrifuged 14,000 × g for 15 min in 4 °C, and the supernatant was used for Western blotting. The protein concentration was determined using the Bio-Rad DC Protein Assay. Western blots were performed as described (43).
Immunoprecipitation.
For IP experiments, T cells were incubated with penicillin (50 μg/mL) for the times indicated and then lysed in lysis buffer. Lysates were incubated with rabbit polyclonal antibody to human serum albumin (Sigma; 1 h room temperature). Next, the mixture was incubated with Protein A Sepharose for 1 h, and after three washes in PBS the bound proteins were eluted with sample buffer by heating to 95 °C for 5 min and run in SDS gels. The 67-kDa band was excised, digested with trypsin, and subjected to mass spectrometry as described (44).
Statistical Analysis.
The animal disease scores were compared using the Mann–Whitney test.
Supplementary Material
Acknowledgments
We thank Drs. Meirav Pevsner-Fisher, Michal Cohen-Sfady, and Noam Cohen for helpful discussions. This work was supported in part by a grant from the Yeda CEO Fund.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215722110/-/DCSupplemental.
References
- 1.Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 3.de Louvois J, Mulhall A, Hurley R. Cefuroxime in the treatment of neonates. Arch Dis Child. 1982;57(1):59–62. [PMC free article] [PubMed] [Google Scholar]
- 4.Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J Immunol. 2002;169(6):3422–3428. doi: 10.4049/jimmunol.169.6.3422. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwenhuis EE, et al. Oral antibiotics as a novel therapy for arthritis: Evidence for a beneficial effect of intestinal Escherichia coli. Arthritis Rheum. 2000;43(11):2583–2589. doi: 10.1002/1529-0131(200011)43:11<2583::AID-ANR28>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Mor F, Cohen IR. Shifts in the epitopes of myelin basic protein recognized by Lewis rat T cells before, during, and after the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 1993;92(5):2199–2206. doi: 10.1172/JCI116822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 8.Strauch UG, et al. Influence of intestinal bacteria on induction of regulatory T cells: Lessons from a transfer model of colitis. Gut. 2005;54(11):1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achiron A, Gurevich M, Friedman N, Kaminski N, Mandel M. Blood transcriptional signatures of multiple sclerosis: Unique gene expression of disease activity. Ann Neurol. 2004;55(3):410–417. doi: 10.1002/ana.20008. [DOI] [PubMed] [Google Scholar]
- 10.Satoh J, et al. T cell gene expression profiling identifies distinct subgroups of Japanese multiple sclerosis patients. J Neuroimmunol. 2006;174(1-2):108–118. doi: 10.1016/j.jneuroim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998;4(1):78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- 12.Garren H, et al. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15(1):15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, et al. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes. 1997;46(5):758–764. doi: 10.2337/diab.46.5.758. [DOI] [PubMed] [Google Scholar]
- 14.de Haan P, de Jonge AJ, Verbrugge T, Boorsma DM. Three epitope-specific monoclonal antibodies against the hapten penicillin. Int Arch Allergy Appl Immunol. 1985;76(1):42–46. doi: 10.1159/000233659. [DOI] [PubMed] [Google Scholar]
- 15.Yvon M, Anglade P, Wal JM. Identification of the binding sites of benzyl penicilloyl, the allergenic metabolite of penicillin, on the serum albumin molecule. FEBS Lett. 1990;263(2):237–240. doi: 10.1016/0014-5793(90)81382-x. [DOI] [PubMed] [Google Scholar]
- 16.Cairo G, Lucchini M. Molecular basis of reduced albumin gene expression in hepatoma cell lines with different growth rates. Exp Cell Res. 1993;206(2):255–260. doi: 10.1006/excr.1993.1145. [DOI] [PubMed] [Google Scholar]
- 17.Evans TW. Review article: Albumin as a drug—biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(Suppl 5):6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Igarashi A, Misawa H, Tsurusaki Y. Enhancement of albumin expression in bone tissues with healing rat fractures. J Cell Biochem. 2003;89(2):356–363. doi: 10.1002/jcb.10510. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YX, Lai R, Lee WH, Zhang Y. Frog albumin is expressed in skin and characterized as a novel potent trypsin inhibitor. Protein Sci. 2005;14(9):2469–2477. doi: 10.1110/ps.051551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karligiotou E, Kollia P, Kallitsaris A, Messinis IE. Expression of human serum albumin (HSA) mRNA in human granulosa cells: Potential correlation of the 95 amino acid long carboxyl terminal of HSA to gonadotrophin surge-attenuating factor. Hum Reprod. 2006;21(3):645–650. doi: 10.1093/humrep/dei374. [DOI] [PubMed] [Google Scholar]
- 21.Nahon JL, et al. Albumin and alpha-fetoprotein gene expression in various nonhepatic rat tissues. J Biol Chem. 1988;263(23):11436–11442. [PubMed] [Google Scholar]
- 22.Shamay A, et al. Expression of albumin in nonhepatic tissues and its synthesis by the bovine mammary gland. J Dairy Sci. 2005;88(2):569–576. doi: 10.3168/jds.S0022-0302(05)72719-3. [DOI] [PubMed] [Google Scholar]
- 23.Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME. The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292(5):F1464–F1470. doi: 10.1152/ajprenal.00069.2006. [DOI] [PubMed] [Google Scholar]
- 24.Gallego-Sandín S, et al. Albumin prevents mitochondrial depolarization and apoptosis elicited by endoplasmic reticulum calcium depletion of neuroblastoma cells. Eur J Pharmacol. 2005;520(1-3):1–11. doi: 10.1016/j.ejphar.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Gum ET, et al. Human serum albumin and its N-terminal tetrapeptide (DAHK) block oxidant-induced neuronal death. Stroke. 2004;35(2):590–595. doi: 10.1161/01.STR.0000110790.05859.DA. [DOI] [PubMed] [Google Scholar]
- 26.Jones DT, et al. Albumin activates the AKT signaling pathway and protects B-chronic lymphocytic leukemia cells from chlorambucil- and radiation-induced apoptosis. Blood. 2003;101(8):3174–3180. doi: 10.1182/blood-2002-07-2143. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui SS, Siddiqui ZK, Malik AB. Albumin endocytosis in endothelial cells induces TGF-beta receptor II signaling. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L1016–L1026. doi: 10.1152/ajplung.00356.2003. [DOI] [PubMed] [Google Scholar]
- 28.Malonga H, Neault JF, Arakawa H, Tajmir-Riahi HA. DNA interaction with human serum albumin studied by affinity capillary electrophoresis and FTIR spectroscopy. DNA Cell Biol. 2006;25(1):63–68. doi: 10.1089/dna.2006.25.63. [DOI] [PubMed] [Google Scholar]
- 29.Malonga H, Neault JF, Tajmir-Riahi HA. Transfer RNA binding to human serum albumin: A model for protein-RNA interaction. DNA Cell Biol. 2006;25(7):393–398. doi: 10.1089/dna.2006.25.393. [DOI] [PubMed] [Google Scholar]
- 30.Graner MW, et al. Cargo from tumor-expressed albumin inhibits T-cell activation and responses. Cancer Res. 2004;64(21):8085–8092. doi: 10.1158/0008-5472.CAN-04-1871. [DOI] [PubMed] [Google Scholar]
- 31.Ghuman J, et al. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353(1):38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 32.Christie G, Kitteringham NR, Park BK. Drug-protein conjugates—XIII. The disposition of the benzylpenicilloyl hapten conjugated to albumin. Biochem Pharmacol. 1987;36(20):3379–3385. doi: 10.1016/0006-2952(87)90314-5. [DOI] [PubMed] [Google Scholar]
- 33.Bertucci C, Barsotti MC, Raffaelli A, Salvadori P. Binding properties of human albumin modified by covalent binding of penicillin. Biochim Biophys Acta. 2001;1544(1-2):386–392. doi: 10.1016/s0167-4838(00)00253-3. [DOI] [PubMed] [Google Scholar]
- 34.Guinez C, Morelle W, Michalski JC, Lefebvre T. O-GlcNAc glycosylation: A signal for the nuclear transport of cytosolic proteins? Int J Biochem Cell Biol. 2005;37(4):765–774. doi: 10.1016/j.biocel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Desrivières S, et al. Comparison of the nuclear proteomes of mammary epithelial cells at different stages of functional differentiation. Proteomics. 2007;7(12):2019–2037. doi: 10.1002/pmic.200600994. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7(6):443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 38.Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(6):736–741. doi: 10.1136/jnnp.64.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metz LM, McGuinness SD, Harris C. Urinary tract infections may trigger relapse in multiple sclerosis. Axone. 1998;19(4):67–70. [PubMed] [Google Scholar]
- 40.Rapp NS, Gilroy J, Lerner AM. Role of bacterial infection in exacerbation of multiple sclerosis. Am J Phys Med Rehabil. 1995;74(6):415–418. doi: 10.1097/00002060-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Mor F, Lohse AW, Karin N, Cohen IR. Clinical modeling of T cell vaccination against autoimmune diseases in rats. Selection of antigen-specific T cells using a mitogen. J Clin Invest. 1990;85(5):1594–1598. doi: 10.1172/JCI114610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MD, McAllen K, Sharp BM. Regulation of delta opioid receptor expression by anti-CD3-epsilon, PMA, and ionomycin in murine splenocytes and T cells. J Leukoc Biol. 1999;65(5):707–714. doi: 10.1002/jlb.65.5.707. [DOI] [PubMed] [Google Scholar]
- 43.Mor F, Weinberger A, Cohen IR. Identification of alpha-tropomyosin as a target self-antigen in Behçet’s syndrome. Eur J Immunol. 2002;32(2):356–365. doi: 10.1002/1521-4141(200202)32:2<356::AID-IMMU356>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Mor F, Izak M, Cohen IR. Identification of aldolase as a target antigen in Alzheimer’s disease. J Immunol. 2005;175(5):3439–3445. doi: 10.4049/jimmunol.175.5.3439. [DOI] [PubMed] [Google Scholar]
- 45.Foord RD. Cefuroxime: Human pharmacokinetics. Antimicrob Agents Chemother. 1976;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.