Abstract
Understanding dental development in chimpanzees, our closest living relatives, is of fundamental importance for reconstructing the evolution of human development. Most early hominin species are believed to show rapid ape-like patterns of development, implying that a prolonged modern human childhood evolved quite recently. However, chimpanzee developmental standards are uncertain because they have never been based on living wild individuals. Furthermore, although it is well established that first molar tooth emergence (movement into the mouth) is correlated with the scheduling of growth and reproduction across primates broadly, its precise relation to solid food consumption, nursing behavior, or maternal life history is unknown. To address these concerns we conducted a photographic study of subadult chimpanzees (Pan troglodytes schweinfurthii) in Kanyawara, Kibale National Park, Uganda. Five healthy infants emerged their lower first molars (M1s) by or before 3.3 y of age, nearly identical to captive chimpanzee mean ages (∼3.2 y, n = 53). First molar emergence in these chimpanzees does not directly or consistently predict the introduction of solid foods, resumption of maternal estrous cycling, cessation of nursing, or maternal interbirth intervals. Kanyawara chimpanzees showed adult patterns of solid food consumption by the time M1 reached functional occlusion, spent a greater amount of time on the nipple while M1 was erupting than in the preceding year, and continued to suckle during the following year. Estimates of M1 emergence age in australopiths are remarkably similar to the Kanyawara chimpanzees, and recent reconstructions of their life histories should be reconsidered in light of these findings.
Keywords: human evolution, hominin life history, feeding ontogeny, primate nursing
The study of how primates and other mammals allocate energy to growth, reproduction, and maintenance over the course of life is of paramount interest to evolutionary anthropologists and biologists (1). Anatomical features such as molar tooth emergence, cranial capacity, and body mass are known to correlate with life history variables including gestation length, age at weaning, interbirth interval, and age at first reproduction across a taxonomically broad sample of primates (2–4). These correlations have stimulated numerous attempts to reconstruct the evolution of human development from dental remains of juvenile fossil hominins. Early hominin species show somewhat rapid African ape-like patterns of dental development, in contrast to a more extended period in early and archaic members of the genus Homo, and an even longer period in fossil Homo sapiens, implying that the extended modern human childhood evolved quite recently (1, 5, 6). However, these interpretations have recently been called into question for two primary reasons that we address in the current study.
Some have challenged the utility of tooth developmental data derived from captive animals for the reconstruction of hominin life history (7–9). Zihlman and colleagues (7) estimated maxillary tooth emergence ages from wild chimpanzee skeletons, which appeared to show later ages than living captive individuals. However, a reassessment of the Taï Forest chimpanzee skeletons (Pan troglodytes verus) found that ages at death in half of the subadults were not known with accuracy sufficient for precise comparisons with captive chimpanzees (10). Notably, one key individual in the former study was misidentified at field recovery, leading to overestimated ages for incisor and second molar emergence. These results have been extended to suggest that an important source of bias in estimating the timing of developmental events may be the use of deceased wild individuals, who are more likely to have experienced developmental delay due to stress or pathology (11). Thus, tooth emergence times must be established from living wild individuals for comparison with living captive individuals.
In addition, others have questioned the correlation between tooth development and life history traits within apes and humans (12–14). Whereas the emergence of the mandibular first molar (M1) into the oral cavity has been suggested to be coincident with weaning in many primates (2, 3), including the lesser apes (4), this relationship may not hold in great apes or humans (12, 14). First molars emerge some time before weaning in living apes, but several years after weaning in humans (12). It is important to note that both tooth eruption and weaning are processes rather than events, and precise developmental comparisons require the identification of discrete events during extended processes (15). Here we consider the process of M1 eruption from the initial event of gingival (gumline) emergence into the oral cavity to the attainment of functional occlusion. Primate weaning encompasses transitions from suckling to solid food intake; events may include the initial incorporation of solid food, the resumption of maternal estrous cycling, or the cessation of suckling (15).
Weaning has been of considerable interest due to the putative contraceptive effect of lactation (16), which may impact lifetime reproductive success and population growth rates. Nursing may actually continue beyond the conception of a subsequent sibling, as documented in humans (17), chimpanzees (18, 19), and other anthropoids (15, 20), but ceases before the birth of the subsequent offspring. Therefore, the duration of nursing is shorter than the interbirth interval (IBI). Kelley and Schwartz (8) suggest that new M1 emergence ages derived from small samples of wild apes are consistent with patterns of IBI, age at first reproduction, and mean survivorship ages across great apes and humans. However, effectively nothing is known about variation in M1 emergence ages within populations or among species or subspecies of wild great apes (8, 9). Moreover, most assessments of the predictive value of M1 emergence fail to consider normal variation in life history characters such as weaning age or interbirth intervals (20), which are influenced by ecology and maternal energetics (15, 16, 19, 21–23).
To address these deficiencies, we present a photographic assessment of M1 eruption in a population of living wild chimpanzees (Pan troglodytes schweinfurthii) from Kanyawara (Kibale National Park, Uganda), and compare events during this process with behavioral data on infant dietary transitions and maternal life history. Conventional assessments of tooth eruption in skeletal remains are time consuming and often semidestructive, and frequently necessitate broad estimation to approximate gingival emergence ages. Our noninvasive approach avoids these pitfalls, and may be applied to other habituated wild primates living in terrestrial environments. Longitudinal observations of living subjects also provide critical information on somatic development, reproductive investment, and the interaction between dental development, life history, and ecology. These data allow for more informed assessments of the comparative evidence that has played a critical role in understanding human life history evolution.
Results
All five infant chimpanzees between 2 and 4 y of age emerged their mandibular M1s by or before they were 3.3 y old (Table 1 and Fig. 1). Three individuals were first photographed with their M1s past the initial point of gingival emergence (Gola, Wallace, and Quiver), whereas two were observed before and during early emergence (Azania and Moon). All infants have continued to nurse after M1 appearance, including two individuals whose mothers resumed estrous cycling during their third year of life. The most dentally precocious individual, Gola (SI Appendix, Fig. S1), was the fifth infant of a mother (Outamba) with an especially rapid reproductive rate. Outamba’s average IBI is 3.6 y, and she became pregnant again when Gola was 2.5 y old, although this was not a viable pregnancy. The least precocious (Moon) was the fourth infant of a mother with an IBI of over 10 y. The third individual (Azania) born to a multiparous mother showed intermediate dental development (M1 emergence at ∼3 y; Fig. 1) and her mother showed an IBI of 4.8 y. Two male infants (Wallace and Quiver) born to first-time mothers within the same month showed a similar degree of M1 emergence, but substantial variation in body mass (SI Appendix, Fig. S2).
Table 1.
Chimpanzee first molar emergence (in years) and life histories
| ID | Sex | Birth | M1 status age (y)* | Suckling observed | Maternal cycling/pregnancy | IBI† |
| Azania | F | 5/1/09 | Initial emergence at 3.0 y | Yes as of December 2012 | Not cycling as of December 2012 | 4.8 (4.3–5.3) |
| Gola | F | 3/3/09 | @ 2.5 only mesial cusps emerged | Yes as of December 2012 | Cycling by April 2011, pregnant by September 2011 | 3.6 (3.2–4.2) |
| Moon | M | 12/26/08 | Initial emergence at 3.3 y | Yes as of December 2012 | Not cycling as of December 2012 | 10.9‡ |
| Wallace | M | 8/5/08 | @ 3.1 mesial cusps well emerged | Yes as of December 2012 | Cycling by December 2011 | NA |
| Quiver | M | 8/3/08 | @ 3.2 mesial cusps well emerged | Yes as of December 2012 | Not cycling as of December 2012 | NA |
Birthdates estimated to within a month, save for Gola (known to the day).
*Molar status preceded by @ indicates that the individual's M1 region was first clearly observed at these ages.
†IBI, mother’s average interbirth interval with range in parentheses.
‡The IBI for Moon’s mother is considerably longer than other IBIs in this community. Although she has had other offspring, this is the only IBI where the previous infant survived after the birth of the subsequent infant. If individuals who died are included, her average IBI is 6.4 y.
Fig. 1.
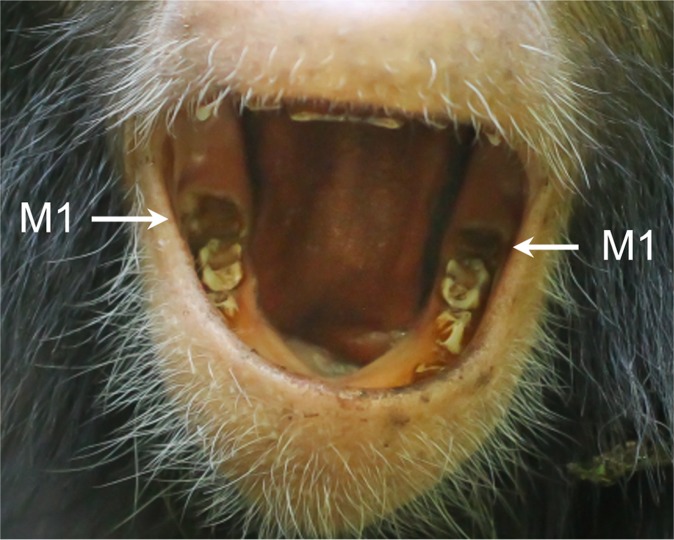
Azania showing eruption of both mandibular first molars (M1s). The left M1 (Right) was observed to be unerupted at 2.8 y of age, but a small dark spot was apparent by 3.0 y. It is unknown when the right M1 may have emerged, but it may have been earlier as it appears to be more advanced than the left M1. This photograph was taken at 3.1 y of age.
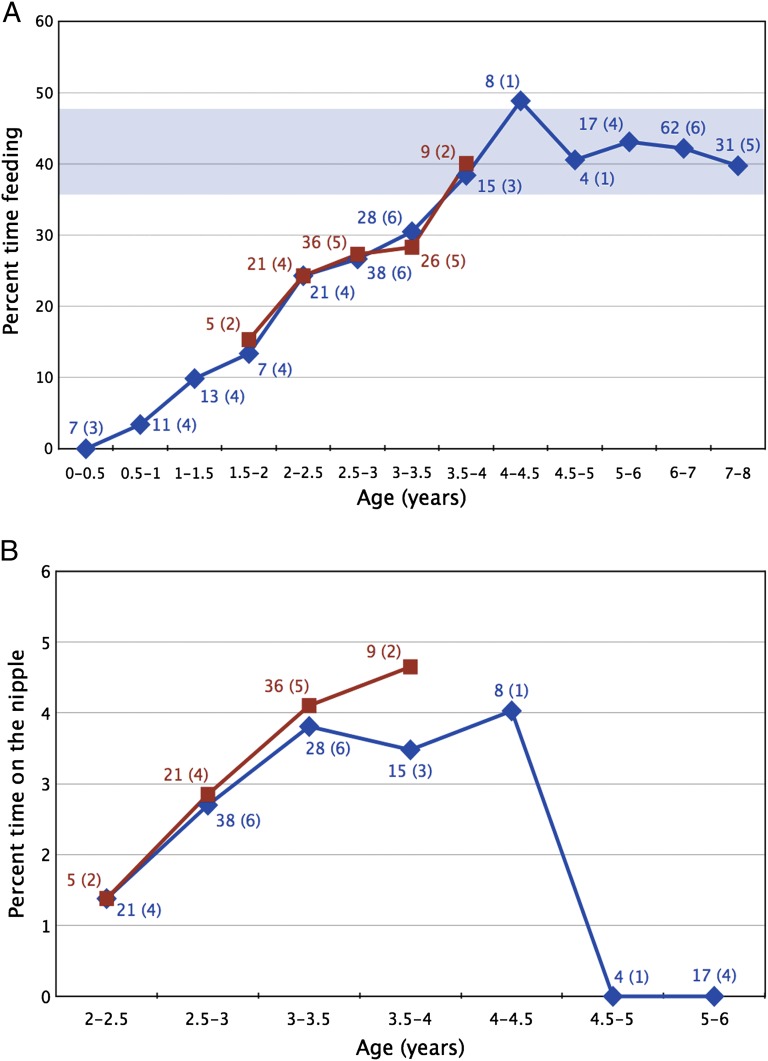
At Kanyawara, subadults showed a steady increase in feeding time for the first 4 y of life (Fig. 2), reaching adult levels by 3.5–4.0 y of age. Between 0.5 and 1.0 y of age, when infants feed on solid food, they primarily consume ripe fruit (SI Appendix, Fig. S3). Surprisingly, infants began eating the same percentage of fibrous foods as adults early in the second year of life, well before the emergence of any permanent teeth. The percentage of time infants spent on the nipple also increased from 2.0 to 3.5 y of age (Fig. 2). As of December 2012, the five individuals known to have emerged M1s by or before 3.3 y of age continued to nurse. Five older subadults were not observed nursing between the ages of 5 and 6 y, with the latest bout recorded for the female Wenka at 4.4 y of age. Our earliest photograph of her at 4.3 y of age reveals upper and lower molars in full occlusion (SI Appendix, Fig. S4).
Fig. 2.
Average observed time spent feeding on (A) solid foods and (B) on the nipple by subadult eastern chimpanzees. Community-level data are indicated with blue diamonds, and the five individuals known to have erupted their first molars during this study are indicated with red squares. The number of focal days for each age category are indicated next to the corresponding point, with the number of individuals in each category given in parentheses. The range of monthly averages of adult feeding time is indicated by the blue horizontal bar in A.
Discussion
The current study does not support suggestions that molar emergence in wild chimpanzees is systematically delayed relative to captive individuals (7, 8); M1 emergence in living subjects from two captive studies ranges from 2.1 to 4.0 y with a mean age of ∼3.2 y (n = 53) (24–26). Four of our five wild infants erupted their M1s prior to the mean captive age. This is consistent with similarities in molar crown formation times in captive and wild chimpanzees (10). Previous estimates of wild chimpanzee M1 eruption derive from a single deceased individual from the Taï Forest (7). This 3.76-y-old skeleton includes a cranium with unerupted maxillary M1s (illustrated in ref. 7, figure 1, p. 10542) and a mandible that is missing both M1s (illustrated in ref. 10, figure 6, p. 371). Although it is likely that the mandibular M1s had emerged before death (10, 11), it is difficult to precisely compare this individual with the living eastern African juveniles from Kanyawara. Future assessments of chimpanzee dental development may benefit from consideration of variation between subspecies (8, 9, 27), as western chimpanzees (Pan troglodytes verus), including the Taï Forest communities and most captive individuals (28), appear to have diverged from central and eastern chimpanzees ∼500,000 y ago (29).
Far less is known about molar emergence in other great apes. Kelley and Schwartz (8) estimated M1 emergence ages in one wild gorilla and two wild orangutan skeletons, which appeared to be later than published estimates for living captive individuals. They argue that their results lend support to captive–wild differences in chimpanzees (7), but note that estimates for M1 eruption age in captive orangutans and gorillas are “questionable.” In fact the birthdates of two lowland gorillas used for comparison are unknown as they were imported from Africa (30, 31), and the age reported for M1 emergence in a single captive orangutan is not considered to be reliable (32). Additional evidence is necessary to determine if and how environmental variation impacts primate dental development, as well as whether comparing deceased wild individuals with living captive individuals exaggerates differences (11).
For subadult chimpanzees at Kanyawara, adult patterns of feeding are evident by the time that maxillary and mandibular M1s come into functional occlusion (typically ∼3.5–4.0 y of age). Patterns of solid food consumption are similar to eastern chimpanzees at Mahale (33, 34), where infants supplement milk with solid foods by about 6 mo of age, and begin to sample most items that make up an adult diet by 3 y of age. Our results are consistent with those of Godfrey and colleagues (35), who examined a diverse primate sample and found that diet has a strong influence on rates of dental development that is independent of body size, brain size, or age at first reproduction. We suggest that M1 emergence in eastern chimpanzees may relate to a key dietary transition around age 3, before which infants are fundamentally dependent on their mothers for support as they develop the requisite anatomy and ecological knowledge to survive on their own if necessary. This is supported by general demographic patterns of wild chimpanzees, which show elevated mortality rates during the first 2 y of life (36). Eastern and western chimpanzee infants who lose their mothers before 2.5–3.0 y of age are highly unlikely to survive, even when adopted by other members of the community (37, 38). Moreover, confiscated orphan chimpanzees are rarely recovered and brought to sanctuaries before ∼2–3 y of age (according to normative standards of dental development and weight) (39), suggesting that younger individuals require direct maternal provisioning to survive until age 3.
Our five primary subjects showed an unexpected trend for increased time on the nipple around M1 emergence. Nursing behavior in 2- to 3-y-old individuals is similar to infants at Gombe (40) and Mahale (34), and the increase in suckling after this age was also found at Mahale. Indices of insulin levels in lactating Kanyawara females suggest that energy transfer to nursing offspring is greatly reduced after 2 y of age (19), implying that the peak in nursing time around M1 emergence does not necessarily mean infants are receiving substantial nutritional supplementation. Nursing data are particularly difficult to collect from wild primates, and suckling behavior may continue beyond the point where infants are energetically independent, a transition influenced by food availability and metabolic needs of infants and mothers (15). Lee (20) suggests that weaning is more facultatively variable than anatomical traits such as body mass, to which we might add M1 emergence age as well. For example, 11 Gombe infants ceased suckling between 4.2 and 7.2 y of age (38), and nonindustrial human populations show even greater age ranges (41), exceeding the known ranges of M1 emergence ages for chimpanzees (26) and humans (42).
The results of our study do not support the 1:1 correlation between M1 emergence and weaning age found across 14 primate species (2, 3), nor the observation that the duration of lactation corresponds “reasonably well” with molar emergence age in hominoids (20). Although intraspecific trends may not necessarily follow interspecific patterns (43), others have noted that the correlation between M1 emergence and weaning age found across primates does not appear to hold for great apes or humans (12, 14). First molar emergence in this chimpanzee population is not coincident with weaning events such as the introduction of solid foods, resumption of maternal estrous cycling, or the termination of suckling. Nor is M1 emergence age consistent with Lee and colleagues’ (44) “duration of lactation” metric (IBI minus gestation length) among our infant–mother dyads. The mothers of our focal animals evince IBIs that nearly span the mean ages of all great apes and humans (8) as well as most other chimpanzee communities (21–23), which would not be predicted from the relatively narrow range of our focal animals’ M1 emergence ages.
Compared with other sites, Kanyawara appears to be representative of chimpanzees in general. The site exhibits intermediate food abundance, and both the community size (currently 57 individuals) and population density (1–2 individuals per kilometer squared) are average compared with other long-term field studies (23). There has been modest population growth since the initiation of behavioral observations in 1988, suggesting that this community is not being negatively affected by anthropogenic effects of human encroachment, disease, or severe food shortages. Infant mortality also appears to be comparable to other sites (save for Mahale) (22). Limited data suggest that Kanyawara females may cease nursing infants earlier than other eastern African chimpanzee populations (34, 38). Recent studies on lactation in humans and other primates have underscored the importance of considering maternal energetics on reproductive transitions (22, 45–47), revealing complex relationships between metabolism, reproductive cycling, and lactation. Others have highlighted the impacts of social and ecological factors on reproductive rates both within and among populations (21–23). One of the more noteworthy characteristics of the Kanyawara community is that females appear to have the lowest rates of reproduction of any major study site, although the absolute differences are small. In contrast to the situation at Mahale where infanticide is common, female reproductive success is low due to long interbirth intervals (22, 23), although, as noted above, this varies substantially among individuals.
Knowledge of chimpanzee growth and development is crucial for understanding the evolution of human life history. Until recently, extant reference taxa such as humans and captive apes had been used to model the evolution of human development, presuming that extinct hominins followed similar developmental trajectories (1). Recent studies of dental development in samples of wild apes and diverse human populations have revealed greater developmental variation than was previously known (7, 8, 27, 42). Kelley and Schwartz (9) highlight their earlier finding of wild great ape M1 emergence ages between ∼3.8 and 4.6 y of age, noting that M1 emergence in six Plio-Pleistocene hominins is estimated to have occurred between ∼2.7 and 3.9 y of age (with a mode of ∼3 y). They suggest that these differences imply one of the three scenarios: Plio-Pleistocene hominins had faster life histories than wild great apes as suggested by their younger M1 emergence ages; rapid early dental development may have facilitated early weaning, but other aspects of their life histories were more prolonged as suggested by their enlarged cranial capacities; or M1 emergence ages have been systematically underestimated in fossil hominins and may be more similar to wild great apes.
Our five Kanyawara chimpanzees more than double the known range of M1 emergence ages in wild great apes and are similar to the estimates for Plio-Pleistocene hominins derived by Kelley and Schwartz (9). However, the complex interplay between ecology, energy balance, somatic development, and life history within chimpanzees and humans suggests that caution is warranted when inferring particular life history traits from M1 emergence in juvenile hominins. Reconstructions of hominin life history that use M1 emergence age as a proxy for weaning (20) or for comparison with wild great apes (9) would benefit from additional information on covariation in dental development, ecology, and life history within and among populations, subspecies, and species of living great apes. The approach outlined here may further our understanding of the evolution of human growth and development, as well as the ontogeny of feeding behavior and nutritional independence.
Methods
High-resolution photographs were taken between August 2011 and December 2012 in the Kanyawara chimpanzee community using a digital single-lens reflex (SLR) camera with high sensitivity (ISO speed at least 3,200), high frames per second (at least 6), and a wide aperture lens (focal ratio f/4 at 200 mm). First molar emergence was assessed from exposed cusp tips at or above the gumline (Fig. 1), the standard used in laboratory settings and for living humans. Subsequent images confirmed protein-stained cusps were closely followed by the mesial and distal aspects of the tooth (SI Appendix, Fig. S1). First molar emergence ages for captive animals derive from three primary sources: Nissen and Reisen (24), Kraemer and colleagues (48), and Conroy and Mahoney (25). The 15 individuals in Nissen and Reisen’s study were born in captivity from parents imported from Guinea (49), with a mean lower M1 emergence age of 3.26 (range = 2.67–3.75 y, weighted average of both sexes). We chose to exclude Kraemer’s individuals because a number of these animals were imported from the wild and were of unknown age (50). The majority of the 38 individuals from Conroy and Mahoney’s study (25) are of western African origin (51). This population was reported to show a mean mandibular M1 emergence age of 3.19 (range = 2.14–3.99 y) (26).
Behavioral data collected on 20 subadults from July 2010 to May 2012 included 1-min scans of focal behavior (solid food feeding, resting, walking, climbing, being carried, time on nipple, and play). When focal activity could not be determined, the behavior was recorded as “out of view.” For all scans where the focal individual was observed feeding, the species and part of the food was recorded. Fibrous foods included leaves, wood, bark, and pith. Focal data on 33 adults were collected from July 2009 to September 2011 as above. Urine collected daily from adult females was tested for hormonal indications of pregnancy. Interbirth intervals were calculated for mothers with at least two offspring when the infant’s birth was known to the month, and the individual survived at least until the birth of a sibling. Activity budgets were calculated by dividing the number of 1-min scans of the respective behaviors for each day by the total number of 1-min scans that the focal animal was in view on that day. Time spent feeding on ripe fruit and fibrous foods was calculated by taking the number of 1-min feeding scans on a particular food item and dividing it by the total number of feeding scans by individuals in that age category. Only days with at least 6 h of data for an individual in view were used. Photographic and behavioral data collection protocols were reviewed and approved by the Harvard University Faculty of Arts and Sciences Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
Katie Hinde, Jay Kelley, David Pilbeam, Holly Smith, Nancy Tang, and Melissa Emery Thompson provided helpful comments on this manuscript along with Chris Dean and three anonymous reviewers. We acknowledge Jay Kelley for a helpful discussion of the initial project. Nick Brazeau, Helena Kraemer, James Mahoney, William McGrew, Emily Otali, Nancy Tang, and field assistants Sunday John, James Kyomuhendo, Francis Mugurusi, Solomon Musana, and Wilberforce Tweheyo provided research assistance. Funding was provided by the Leakey Foundation, Harvard University, Wenner Gren Hunt Fellowship, and National Science Foundation Grants 0849380 (to R.W.) and 1126470 (to T.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218746110/-/DCSupplemental.
References
- 1.Smith BH, Tompkins RL. Toward a life history of the hominidae. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 2.Smith BH. Dental development as a measure of life history in primates. Evolution. 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith BH. Life history and the evolution of human maturation. Evol Anthropol. 1992;1:134–142. [Google Scholar]
- 4.Dirks W, Bowman JE. Life history theory and dental development in four species of catarrhine primates. J Hum Evol. 2007;53(3):309–320. doi: 10.1016/j.jhevol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Dean C, et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature. 2001;414(6864):628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- 6.Smith TM, et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc Natl Acad Sci USA. 2010;107(49):20923–20928. doi: 10.1073/pnas.1010906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zihlman A, Bolter D, Boesch C. Wild chimpanzee dentition and its implications for assessing life history in immature hominin fossils. Proc Natl Acad Sci USA. 2004;101(29):10541–10543. doi: 10.1073/pnas.0402635101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley J, Schwartz GT. Dental development and life history in living African and Asian apes. Proc Natl Acad Sci USA. 2010;107(3):1035–1040. doi: 10.1073/pnas.0906206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley J, Schwartz GT (2012) Life-history inference in the early hominins Australopithecus and Paranthropus. Int J Primatol 33(6):1332–1363.
- 10.Smith TM, et al. Dental development of the Taï Forest chimpanzees revisited. J Hum Evol. 2010;58(5):363–373. doi: 10.1016/j.jhevol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Smith BH, Boesch C. Mortality and the magnitude of the “wild effect” in chimpanzee tooth emergence. J Hum Evol. 2011;60(1):34–46. doi: 10.1016/j.jhevol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Robson SL, Wood B. Hominin life history: Reconstruction and evolution. J Anat. 2008;212(4):394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guatelli-Steinberg D. Recent studies of dental development in Neandertals: Implications for Neandertal life histories. Evol Anthropol. 2009;18:9–20. [Google Scholar]
- 14.Humphrey LT. Weaning behaviour in human evolution. Semin Cell Dev Biol. 2010;21(4):453–461. doi: 10.1016/j.semcdb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Lee PC. The meanings of weaning: Growth, lactation, and life history. Evol Anthropol. 1996;5:87–96. [Google Scholar]
- 16.Valeggia C, Ellison PT. Lactational amenorrhoea in well-nourished Toba women of Formosa, Argentina. J Biosoc Sci. 2004;36(5):573–595. doi: 10.1017/s0021932003006382. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster JB, Kaplan HS. In: Endocrinology of Social Relationships. Ellison PT, Gray PG, editors. Cambridge: Harvard Univ Press; 2009. pp. 95–119. [Google Scholar]
- 18.Pusey AE. 1978. The physical and social development of wild adolescent chimpanzees (Pan troglodytes schweinfurthii). PhD dissertation (Stanford University, Stanford, CA)
- 19. Emery Thompson M, Muller M, Wrangham R (2012) The energetics of lactation and the return to fecundity in wild chimpanzees. Behav Ecol 23(6):1234–1241.
- 20. Lee PC (2012) Growth and investment in hominin life history evolution: patterns, processes, and outcomes. Int J Primatol 33(6):1309–1331.
- 21.Knott C. 2001. in Reproductive Ecology and Human Evolution, ed Ellison PT (Aldine de Gruyter, Hawthorne, NY), pp 429–463.
- 22. Emery Thompson M Reproductive ecology of female chimpanzees. Am J Primatol, 10.1002/ajp.22084. [DOI] [PubMed]
- 23.Potts KB. In: Building Babies: Primate Development in Proximate and Ultimate Perspective. Clancy KBH, Hinde K, Rutherford JN, editors. New York: Springer; 2013. pp. 83–100. [Google Scholar]
- 24.Nissen HW, Riesen AH. The eruption of the permanent dentition of chimpanzee. Am J Phys Anthropol. 1964;22:285–294. doi: 10.1002/ajpa.1330220315. [DOI] [PubMed] [Google Scholar]
- 25.Conroy GC, Mahoney CJ. Mixed longitudinal study of dental emergence in the chimpanzee, Pan troglodytes (Primates, Pongidae) Am J Phys Anthropol. 1991;86:243–254. [Google Scholar]
- 26.Kuykendall KL, Mahoney CJ, Conroy GC. Probit and survival analysis of tooth emergence ages in a mixed-longitudinal sample of chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1992;89(3):379–399. doi: 10.1002/ajpa.1330890310. [DOI] [PubMed] [Google Scholar]
- 27.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Martin LB. Molar development in common chimpanzees (Pan troglodytes) J Hum Evol. 2007;52(2):201–216. doi: 10.1016/j.jhevol.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Ely JJ, et al. Subspecies composition and founder contribution of the captive U.S. chimpanzee (Pan troglodytes) population. Am J Primatol. 2005;67(2):223–241. doi: 10.1002/ajp.20179. [DOI] [PubMed] [Google Scholar]
- 29.Hey J. The divergence of chimpanzee species and subspecies as revealed in multipopulation isolation-with-migration analyses. Mol Biol Evol. 2010;27(4):921–933. doi: 10.1093/molbev/msp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz AH. Eruption and decay of the permanent teeth in primates. Am J Phys Anthropol. 1935;19:489–581. [Google Scholar]
- 31.Willoughby DP. All About Gorillas. South Brunswick, NJ: A. S. Barnes & Company; 1978. [Google Scholar]
- 32.Smith BH, Crummett TL, Brandt KL. Age of eruption of primate teeth: A compendium for aging individuals and comparing life histories. Yearb Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 33.Hiraiwa-Hasegawa M. In: The Chimpanzees of the Mahale Mountains. Nishida T, editor. Tokyo: University of Tokyo Press; 1990. pp. 277–283. [Google Scholar]
- 34.Nishida T. Chimpanzees of the Lakeshore. Cambridge, UK: Cambridge Univ Press; 2012. [Google Scholar]
- 35.Godfrey LR, Samonds KE, Jungers WL, Sutherland MR. Teeth, brains, and primate life histories. Am J Phys Anthropol. 2001;114(3):192–214. doi: 10.1002/1096-8644(200103)114:3<192::AID-AJPA1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Hill K, et al. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40(5):437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 37.Boesch C, Bolé C, Eckhardt N, Boesch H. Altruism in forest chimpanzees: The case of adoption. PLoS ONE. 2010;5(1):e8901. doi: 10.1371/journal.pone.0008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pusey AE. Mother-offspring relationships in chimpanzees after weaning. Anim Behav. 1983;31:363–377. [Google Scholar]
- 39.Wobber V, Hare B. Psychological health of orphan bonobos and chimpanzees in African sanctuaries. PLoS ONE. 2011;6(6):e17147. doi: 10.1371/journal.pone.0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark C. In: Primate Biosocial Development: Biological, Social, and Ecological Determinants. Chevalier-Skolnikoff S, Poirier FE, editors. New York: Garland Publishing; 1977. pp. 235–260. [Google Scholar]
- 41.Sellen DW. Comparison of infant feeding patterns reported for nonindustrial populations with current recommendations. J Nutr. 2001;131(10):2707–2715. doi: 10.1093/jn/131.10.2707. [DOI] [PubMed] [Google Scholar]
- 42.Liversidge HM. In: Patterns of Growth and Development in the Genus Homo. Thompson JL, Krovitz GE, Nelson AJ, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 73–113. [Google Scholar]
- 43.Steudel K. Patterns of intraspecific and interspecific allometry in Old World primates. Am J Phys Anthropol. 1982;59(4):419–430. doi: 10.1002/ajpa.1330590412. [DOI] [PubMed] [Google Scholar]
- 44.Lee PC, Majluf P, Gordon IJ. Growth, weaning and maternal investment from a comparative perspective. J Zool (Lond) 1991;225:99–114. [Google Scholar]
- 45.Valeggia C, Ellison PT. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. Am J Hum Biol. 2009;21(4):559–566. doi: 10.1002/ajhb.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21(4):512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- 47.Rosetta L, Lee PC, Garcia C. Energetics during reproduction: A doubly labeled water study of lactating baboons. Am J Phys Anthropol. 2011;144(4):661–668. doi: 10.1002/ajpa.21475. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer HC, Horvat JR, Doering C, McGinnis PR. Male chimpanzee development focusing on adolescence: Integration of behavioral with physiological changes. Primates. 1982;23:393–405. [Google Scholar]
- 49.Dewsbury DA. 2006. in Monkey Farm: A History of the Yerkes Laboratories of Primate Biology, Orange Park, Florida, 1930–1965 (Bucknell Univ Press, Lewisburg, PA)
- 50. Goodall J, Stanford Outdoor Primate Facility “Gombe West.” Kenneth M. Cuthbertson Papers (SC0582). Department of Special Collections and University Archives, Stanford University Libraries. Box 40, Folder 12. (ca. 1973)
- 51.Peterson D, Goodall J. Visions of Caliban: On Chimpanzees and People. Boston: Houghton Mifflin; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.