Abstract
B cells are known to play an important role in pathogenesis of human chronic graft vs. host disease (cGVHD). Our group has previously shown that IgG allo-antibodies recognize Y chromosome-encoded proteins (H–Y) and a dominant H–Y epitope, DEAD box protein (DBY-2) detectable 6–12 mo after transplant in male patients who receive grafts from female donors (F→M) hematopoietic cell transplantation (HCT). Here we present FACS studies of peripheral blood mononuclear cells collected 6 mo after transplant showing that 16 of 28 (57%) F→M HCT patients have circulating donor B cells that express B-cell receptor (mainly IgM and Igλ) specific for DBY-2. The detection of these DBY-2 B cells 6 mo after HCT are associated with cGVHD development (P = 0.004). Specifically, 15 of 16 F→M with DBY-2 B cells developed cGVHD. In contrast, cGVHD developed in only 5 of the 12 who did not have DBY-2 B cells detected. This demonstrates circulating human B cells binding an alloantigen (DBY-2) and that these DBY-2–specific B cells appear before development of cGVHD in roughly half of the F→M patients. Our study suggests that detection of anti–DBY-2 B cells may predict cGVHD and that this prediction may have clinical utility. Validation of this hypothesis will require larger prospective studies.
Keywords: allogeneic, antigen-specific B cells, transplantation, DDX3Y
Allogeneic hemtopoietic cell transplantation (allo-HCT) is potentially curative therapy for patients with leukemia or lymphoma. However, chronic graft vs. host disease (cGVHD) remains a significant cause of late morbidity and mortality (1–4).
Several studies indicate that donor-derived alloreactive B and T cells are involved in pathogenesis of cGVHD. In support of a B-cell role (4–9), the presence of circulating autoantibody (10) and alloantibody (11, 12) have been associated with development of cGVHD. Specifically, predominant B-cell subsets have been demonstrated in patients with cGVHD and identified in different studies as naïve (6) and postgerminal center B cells (7, 8, 10). In addition, B-cell related markers and antibodies have been recognized as biomarkers for characterization and scoring cGVHD (13, 14). Finally, Rituximab, which depletes B cells, has been successfully used as cGVHD therapy (8, 15–19).
Previous studies by our group have shown alloantibody responses occur in male HCT patients with female donors (F→M). These responses include donor-derived alloreactive IgG that recognizes one or more Y chromosome encoded proteins (H–Y antigens), including the DDX3Y protein (refer to hereafter as DBY) and its immunodominant DBY-2 peptide, which we use in studies here. In addition, donor-derived anti-DBY antibodies appear in serum in association with cGVHD in F→M patients, implicating alloreactive B cells in cGVHD pathogenesis (11, 20). To test the hypothesis that H–Y-specific B cells contribute to cGVHD pathogenesis, we have developed H–Y-specific FACS stain for their isolation and characterization.
Here, we demonstrate that, 6 mo after F→M transplant, more than half of 28 male patients with female donors develop circulating B cells whose surface IgM and IgG receptors specifically bind DBY-2 and hence are poised to undergo class switch and differentiate to plasma cells that produce IgG anti–DBY-2 antibodies. Furthermore, we show that their presence in circulation is strongly associated with the development of cGVHD (P = 0.004), i.e., the overwhelming majority (15 of 16) of patients who have DBY-2–specific B cells either have or will develop cGVHD within 1–3 mo. In contrast, only about half (5 of 12) of patients who do not have these B cells go on to develop cGVHD. As would be expected, we detected IgM and IgG anti–DBY-2 B cells in all but 2 of the patients who later developed circulating IgG anti–DBY-2 (P = 0.002).
The phenotype of the DBY-2–specific B cells that develop in the F→M patients is surprising. As is usual in studies with antigen-binding B cells in the mouse (21, 22), the amount of the antigen bound to the B cells is strongly correlated with the amount of surface Ig on the cells, which at the time point we examined is exclusively IgM and IgD associated mainly with Igλ light chains. However, even though these cells have most likely arisen in response to antigenic stimulation (DBY-2 on the male patient’s cells stimulating female donor B cells), they express a phenotype (CD19+IgM+IgD+CD38+ and CD27ˉ) commonly taken as characteristic of transitional B cells that have recently entered circulation from bone marrow.
Results
Retrospective Study Design.
This study characterized a series of 28 consecutive F→M HCT who consented to research blood sample collection before transplant and had samples cryopreserved 6 and 12 mo after HCT. Blood research samples were tested without knowledge of patient disease status, GVHD development, or other clinical characteristics. Patient characteristics are described in Table 1.
Table 1.
F→M HCT patient characteristics
| Characteristic | n |
| Total subjects | 28 |
| Primary disease | |
| Acute myelogenous leukemia | 10 |
| Non-Hodgkin lymphoma | 4 |
| Acute lymphoblastic leukemia | 3 |
| Chronic lymphocytic leukemia | 3 |
| Chronic myelogenous leukemia | 3 |
| Other | 5 |
| Age, median (range), y | 54 (21–65) |
| Conditioning regimen | |
| Myeloablative | 13 (46%) |
| Nonmyeloablative | 15 (54%) |
| Donor | |
| Related | 16 (57%) |
| Unrelated | 12 (43%) |
| cGVHD | |
| None | 8 (29%) |
| Mild | 3 (11%) |
| Moderate | 12 (43%) |
| Severe | 5 (17%) |
| aGVHD | |
| Grade II–IV | 9 (32%) |
| None–grade I | 19 (68%) |
| Survival, median (range), y | 3.2 y (1–5.9) |
B Cells Circulating in F→M HCT Patients Express Ig Receptors Specific for DBY-2, an Immuno-Dominant Epitope in the DBY Protein.
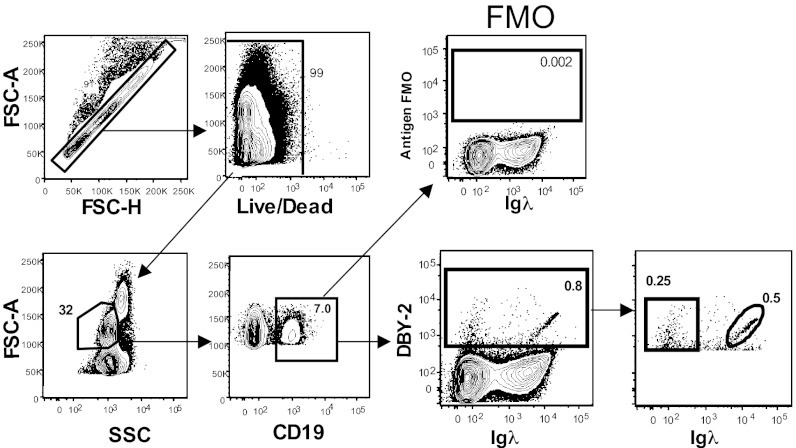
The DBY-2 peptide (KNDPERLDQQLANLDLNSEK) contains the DBY-2 epitope frequently recognized in allogeneic F→M antibody responses that occur following HCT (11, 20). Previous studies showed that 35% of F→M patients develop circulating IgG anti–DBY-2 antibodies detectable by ELISA 6–12 mo following HCT (11). Extending this work, we used FACS analyses to reveal circulating live B cells expressing Ig receptors that specifically bind DBY-2, defined as those cells whose DBY-2–binding level is above a threshold defined by the Fluorescence Minus One (FMO) control. i.e., a sample stained with all reagents except DBY-2 peptide (Fig. 1). Cells expressing either anti–DBY-2 associated with Igκ or Igλ light chains by definition fall within this FMO gate. Fig. 1 shows the gating scheme and data for a representative patient sample.
Fig. 1.
DBY-2–binding B cells express Igκ or Igλ light chains 180 d following F→M HCT. Gated FACS data for a representative 6-mo sample containing 0.8% DBY-2–binding B cells are shown staining CD19 and DBY-2 positive. The FMO gate excludes cells that nonspecifically bound the fluoro-chrome coupled DBY-2 peptide (y axis) and define the DBY-2–binding threshold (28).
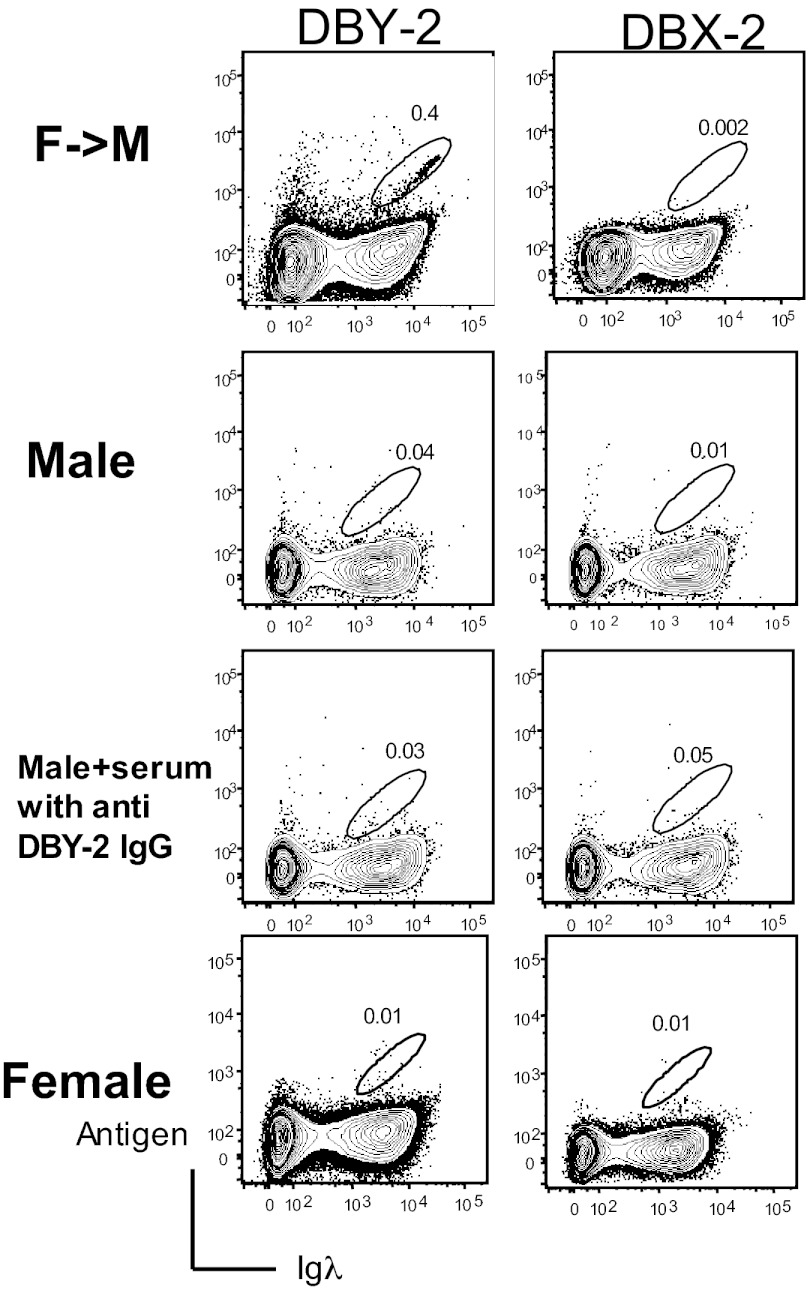
DBY-2–binding B cells (Fig. 1) were detected in 16 of 28 (57%) peripheral blood mononuclear cell (PBMC) samples collected 6 mo following F→M HCT (Fig. 2). As expected, these DBY-2 B cells were not detected in PBMC from 15 healthy males where H–Y antigens are “self” antigens. DBY-2 B cells were not detected in healthy female HCT donor PBMC samples (Fig. 3). Importantly, DBY-2–specific B cells were not detected following pre incubation of high-titer anti–DBY-2 IgG with normal male donor PBMCs. We conclude that the DBY-2 staining B cells observed after F→M HCT does not result from indirect IgG binding but rather cell-specific IgM expression.
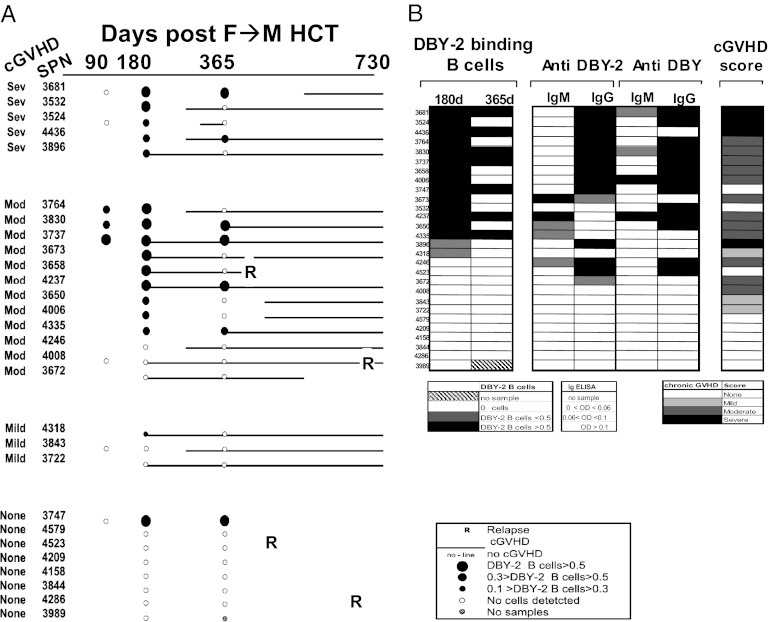
Fig. 2.
Anti–DBY-2 B-cell development, anti–DBY-2 Ig, and severity of cGVHD in 28 F→M HCT patients. (A) The temporal development of anti–DBY-2 B cells and cGVHD is schematically depicted for 28 F→M patients with clinical follow-up through 730 d on the x-axis. Filled circles indicate presence of DBY-2–specific B cells; the size of the circle reflects the percentage of these B cells among PBMC lymphocytes. Open circles indicate that anti–DBY-2 B-cell frequencies were below detectability (<0.1%) of PBMC lymphocytes. Solid line indicates cGVHD onset and duration. R marks time of hematologic malignancy relapse. (B) The frequency of DBY-2 B cells detected at 180 and 365 d are related to Ig development against DBY-2 peptide epitope, full-length DBY protein, and cGVHD development. cGVHD patients were ranked by frequency of DBY-2 B cells detected 180 d after transplant and secondarily by presence of IgG anti–DBY-2 in serum. Other columns include the unique Stanford Patient Number (SPN), each patient’s National Institutes of Health cGVHD score, the maximum IgM and IgG anti-DBY or anti–DBY-2 levels measured by ELISA within 1 y after HCT. The legends indicate the relative intensity of each value.
Fig. 3.
DBY-2–binding B cells are detected in some F→M patients after HCT but not in healthy male and female donors. Data for a representative F→M HCT patient collected 180 d after HCT (Top) and for health controls (Upper Middle, Lower Middle, and Bottom). Less than 0.1% DBY-2–specific B cells were detected in 15 normal male and 8 female samples. The third row shows no DBY-2–specific B cells were detected following preincubation of serum collected from F→M HCT containing high-titer anti–DBY-2 IgG with normal male donor PBMCs and suggests DBY-2 staining observed after F→M HCT is not an indirect IgG-mediated binding but rather cell-specific IgM-dependent binding.
Because immune reconstitution after myeloablative and nonmyeloablative conditioning may differ, we included a similar number of 15 myeloablative and 13 nonmyeloablative conditioned F→M patients, and their detection of DBY-2–specific B cells did not statistically differ (Table 2). As shown in Fig. 4A, the median absolute number of B cells detected 6 mo after allo-HCT was 136 per μL and ranged between 18 and 400 per μL. The absolute number of CD19+ B cells did not statistically differ in relation to conditioning intensity or donor relationship (data not shown). The DBY-2–binding B cells collected from transplant patients’ blood are donor derived because both whole blood and CD19+ B cells showed greater than 95% donor origin as measured by short tandem repeat (STR) 3 mo after transplantation.
Table 2.
Univariate analyses of DBY-2 B cells and DBY-2 IgG development
| Anti–DBY-2 B cells (day180) | IgG anti–DBY-2 within 1 y after HCT | |||
| Total | 16/28* (57%) | 14/28 (50%) | ||
| Conditioning | ||||
| Myeloablative | 8/13 (62%) | ns | 6/13 (43%) | ns |
| Nonmyeloablative | 7/15 (47%) | 9/15 (60%) | ||
| Donor | ||||
| Related | 11/16 (67%) | ns | 9/16 (56%) | ns |
| Unrelated | 6/12 (50%) | 5/12 (42%) | ||
| cGVHD | ||||
| None | 1/8 (13%) | P < 0.004 | 2/8 (25%) | P < 0.01 |
| Mild | 1/3 (33%) | 0/3 (0) | ||
| Moderate | 9/12 (75%) | 8/12 (67%) | ||
| Severe | 5/5 (100%) | 4/5 (80%) | ||
*Positive subjects/total subjects in the group.
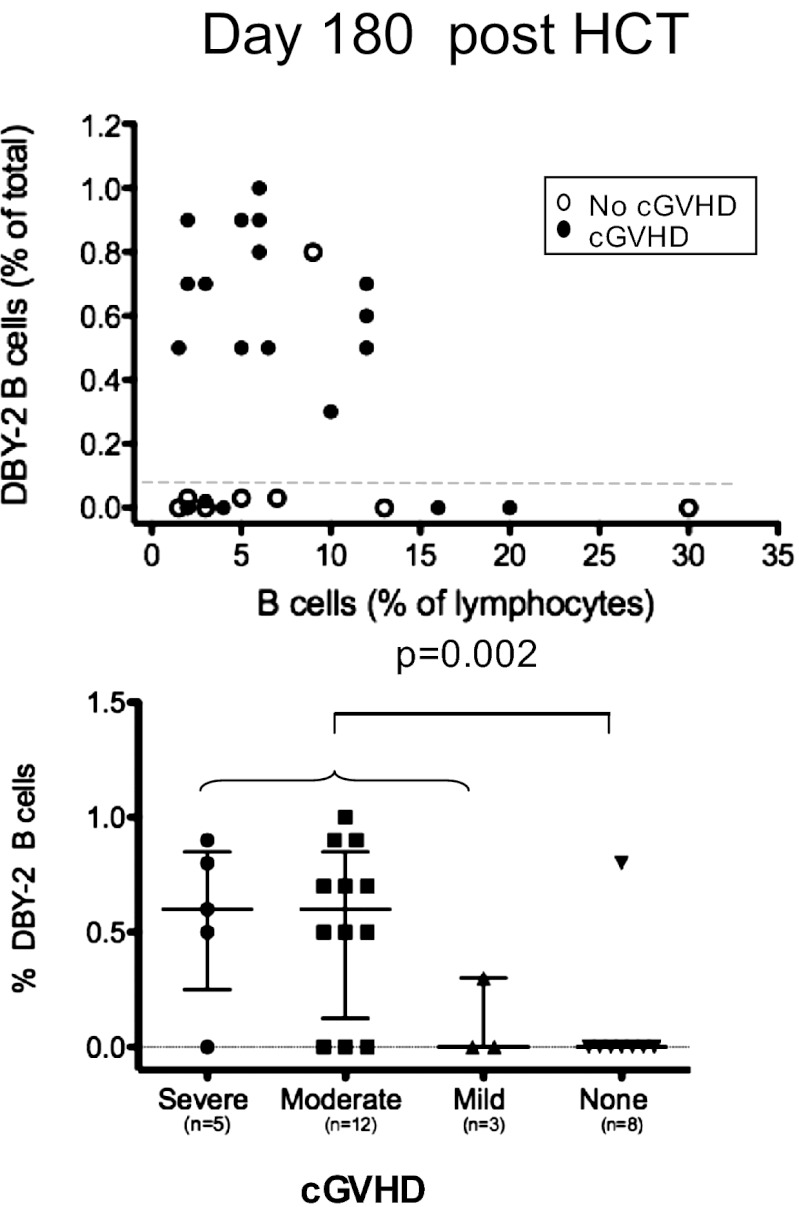
Fig. 4.
Anti–DBY-2 B-cell frequencies quantified 180 d after 28 F→M HCT. y-axis in both the Upper and Lower shows the relative frequency of DBY-2–binding B cells in relation to total PBMC lymphocytes shown on the x-axis (above) or in relation to cGVHD severity (below). (Upper) Open circles indicate cGVHD patients; closed circles show similar data for patients who did not develop cGVHD. (Lower) Anti–DBY-2 B-cell staining associates with any cGVHD development in comparison with none (P = 0.004; Fisher exact test).
The DBY-2–binding B cells in the transplant patients generally expressed both Igκ and Igλ DBY-2 receptors. In Fig. 1 (Lower Right), the Igκ expressing anti–DBY-2 cells are bounded by the square insert on the left side of the figure and the Igλ expressing anti–DBY-2 cells are bounded by the oval on the right. The amount of DBY-2 bound to cells with Igλ-containing receptors tends to be proportional to the level of receptor expression on the cells, resulting in the “diagonal” distribution for DBY-2–binding cells that is visible when DBY-2–binding is plotted against Igλ expression (Fig. 1, Lower Right). The “tightness” of this diagonal suggests that the Igλ-containing receptors for DBY-2 are expressed at varied levels but that the binding avidity of the receptors is fairly similar. This diagonal pattern is expected for monoclonal antibodies or for a group of cells in which single or closely related Ig sequences are responsible for the antigen binding. Diagonal patterns were not observed with Igκ DBY-2–binding cells in any of the subjects tested.
Detection of DBY-2-Specific B Cells at Day 180 Precedes Development of cGVHD in the Majority of F→M Transplant Patients.
Fig. 2A schematically presents each patient’s temporal development of cGVHD in relation to their 6 and 12 mo DBY-2 B-cell measurements. The detection of DBY-2 B cells is highly associated with cGVHD (P = 0.004; Fig. 2A). Considering the 16 patients in whom DBY-2–binding B cells were detected 180 d following F→M HCT, 15 ultimately developed cGVHD. For six patients, the “day 180” clinic visit was also their cGVHD diagnosis date (ranging 155–182 d following HCT). The nine others with DBY-2 B cells detected were diagnosed with cGVHD at later clinic visits. Interestingly, the absolute and relative number of DBY-2–specific B cells was significantly higher 6 mo following HCT in patients who developed moderate or severe cGVHD compared with those with mild cGVHD or none (P = 0.02; Fig. 4B). In these 28 F→M HCT, none had cGVHD diagnosed before their 6-mo sample collection. It may be important that our earliest diagnosis of cGVHD was 155 d after HCT, because cGVHD can sometimes develop as early as 90 d after HCT, but our limited patients sample did not happen to include such early cGVHD. Although samples collected at 90 d after HCT were available for only 8 patients, we have included this limited day 90 data in Fig. 2A because 3 of 9 had detectable DBY-2 B cells and suggests that follow-up studies should include samples collected as early as 90 d after HCT.
Detection of DBY-2–Specific B Cells Precedes the Development of Circulating Anti-DBY Antibodies.
As expected, the majority (11 of 14) of the F→M transplant patients who had anti–DBY-2 IgG develop within 1 y after HCT also had DBY-2–specific B cells detected 180 d following HCT (Fig. 2B). Interestingly, the number of patients who had anti–DBY-2 B cells detected from PBMC collected 1 y after transplant was lower than the day 180 frequency (Fig. 2B). This may be due to the migration of cells from blood into lymphoid organs. Additionally, the cells may have been eliminated by treatment for cGVHD.
Detection of Anti–DBY-2 IgG Within 1 Year After F→M HCT Associates with cGVHD Development.
Circulating anti–DBY-2 IgG was detected in plasma collected from 14 of 28 (50%) patients within one year of transplant. As we previously reported for allogeneic IgG developing against any of five full-length H–Y antigens (20), the detection of anti–DBY-2 IgG associated with the development of cGVHD (P = 0.002) and did not associate with patient’s primary disease, conditioning regimen, or donor relationship (Table 2). None of these 28 F→M HCT patients had DBY-2 IgG detected in their 6-mo samples when already 16 of 28 (57%) had detectable DBY-2 B cells. Our demonstration that these DBY-2–specific B cells appear before development of both their corresponding IgG and cGVHD suggests that detection of anti–DBY-2 B cells may predict cGVHD with clinical utility.
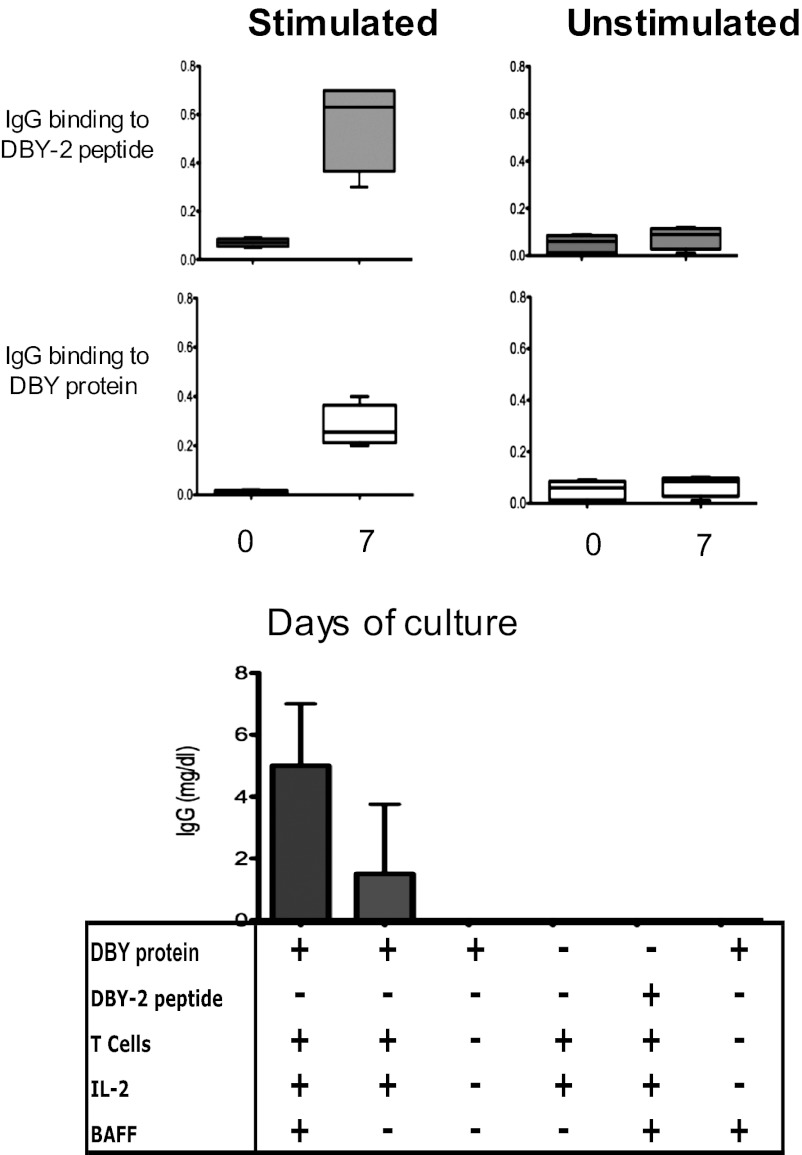
IgG That Binds DBY Protein and DBY-2 Peptide Is Secreted Following ex Vivo Stimulation of FACS-Sorted Anti–DBY-2–Specific B Cells.
FACS sorted DBY-2–binding B cells cultured with DBY protein plus IL-2, BAFF, and autologous T cells for seven days secreted ELISA-detectable IgG that specifically detected DBY-2 and the full-length DBY protein (Fig. 5, Upper). Both the intact DBY protein and autologous T cells were required for this stimulation because virtually no detectable antibody was produced if either was omitted from the culture. In contrast, BAFF was helpful but not necessary for antibody production (Fig. 5, Lower). The requirement for T cells and the full-length protein antigen suggests that this anti–DBY-2 response is T cell-dependent.
Fig. 5.
DBY-2–binding B cells secret IgG in presence of T cells and DBY-antigen. FACS sorted DBY-2–binding B cells are incubated in the absence (Upper Left) or in the presence (Upper Right) of autologous T cells, IL-2, DBY-protein and BAFF. Relative IgG antibody levels for DBY-2 and DBY proteins are shown after 7 d of incubation. Secreted IgG levels were determined in presence of the reagents as depicted below X-axis for n = 9 first column, n = 4 for second column, n = 2 for third and subsequent conditions.
FACS Phenotype of the DBY-2–Binding B Cells in F→M Patients.
The median frequency of DBY-2–binding B cells in the F→M patients is 0.7% (0.3–1.0%) of CD-19 B cells (Fig. 4, Upper). This relatively high frequency is in the range (>0.1%) frequently observed for antigen reactive cells in immunized hosts. Nevertheless, the phenotype of these cells surprisingly corresponds to the commonly accepted phenotype for naïve or transitional B cells in man, i.e., CD19+IgM+IgD+CD27−CD38+CD5− (Fig. 6). None of the DBY-2–binding B cells in the F→M HCT patients express CD27, which is typically expressed on memory B cells. However, occasional blood samples drawn when GVHD is present contained cells expressing the typical phenotype for isotype-switched plasmablasts (CD19+IgM+IgD+Igλ+CD27+CD38+CD5−).
Fig. 6.

Phenotype of the DBY-2–binding B cells. Singlet, live, lymphocyte, and B cells are gated for the presence of DBY-2 binding and Igλ. These cells are further shown to be IgD+, CD38+, and CD27−.
Discussion
This study reports the development of a FACS method pairing antigen-specific staining with paired B-cell receptor detection using lambda/kappa light chain detection to identify a high frequency alloreactive donor B-cell–binding DBY-2 that develops following allogeneic F→M HCT and is not detected in normal male or female donors. These cells were detected in fifteen of twenty F→M HCT recipients who developed chronic graft versus host disease (cGVHD). In contrast, they were only detected in one of eight patients who did not develop cGVHD. Thus, we conclude that the presence of B cells with receptors that recognize the DBY-2 is positively associated with development of cGVHD (P = 0.004, Fisher Exact Test).
Previous studies have shown that H–Y antibody develop following F→M HCT in association with cGVHD (20), and here we show immune dominant peptide epitope DBY-2 was similarly recognized by IgG in 50% of these 28 F→M HCT patients and associated with cGVHD (P = 0.002). However, these H–Y IgG antibodies are rarely detected before the onset of GVHD and thus are unlikely to have cGVHD predictive value. In contrast, this study identifies B cells that express IgM and IgG receptors specific for DBY-2 and show that these alloantigen-binding B cells often precede the onset of cGVHD. Thus, their presence in a patient may warrant pre-emptive cGVHD therapy. A prospective clinical study with a larger patient sample could appropriately test this hypothesis.
The role that DBY-2–binding B cells play in the cGVHD disease process is unclear. They may simply be bystanders that are induced by mechanisms that activate T cells that may actually mediate cGVHD. However, our observation that they commonly preceded cGVHD developments suggests that they may play an early pathogenic role. In fact, as recent findings with mouse GVHD models suggest, they may play a role in antigen presentation (23) necessary for stimulation of pathogenic T-cell clonal expansion and/or induction of inflammatory cytokine production and alloreactive antibody production (23, 24).
Importantly, the DBY-specific B cells are found in F→M transplant patients where, by virtue of the sex mismatch between donor and host, the donor B cells are extensively exposed to the host DBY protein and its component DBY-2 peptide. Thus, it is not surprising that we find an expanded population of donor B cells with receptors that recognize a host alloantigen such as DBY-2. Consistent with this argument, the DBY-2–binding B cells in the F→M patients are present at the relatively high frequencies common for antigen reactive cells generated in response to an antigenic stimulus.
Basically, the presence of DBY-2–binding B cells in F→M patients would lead us to believe that they are memory B cells that developed from naïve female donor B cells when they encountered the host DBY-2 antigen. However, quite surprisingly, the phenotype of these anti–DBY-2 B cells corresponds to the commonly accepted phenotype for human naïve or transitional B cells (CD19+IgD+IgM+CD27−CD38+CD5−) (6–8, 10, 23) that have recently emerged from bone marrow and are on their way to lymphoid organs. Although these B cells may ultimately give rise to the plasma cells that produce the IgG anti–DBY-2 found in circulation later in the disease, their current phenotype belies this fate. Future studies may help to resolve this paradox.
In summary, we show here that F→M HCT patients commonly develop donor B cells with Ig receptors that recognize host male antigens. These B cells, which develop before, or concurrent with the onset of cGVHD, precede the onset of antibodies production to male H–Y antigens. These findings may provide a mechanistic explanation for the moderate efficacy of in vivo B-cell depletion in treating cGVHD, and further suggests that more focused B-cell targeting, e.g., with DBY-2 in F→M HCT, might be more effective cGVHD therapy. In addition, the prospective monitoring of anti–DBY-2 B cells may direct a more effective schedule for alloreactive B-cell depletion therapy toward a goal of cGVHD prevention. Finally, DBY-2 B-cell monitoring will help elucidate whether current in vivo B-cell depletion therapy for cGVHD effectively depletes these alloreactive B cells or if they persist and proliferate when cGVHD recurs.
Materials and Methods
Human Subjects.
Since October 1, 2005, all allogeneic HCT patients were invited to participate in an ongoing Institutional Review Board approved research protocol that cryopreserves PBMC and plasma samples collected 3, 6, and 12 mo (±15%) following F→M HCT in the BMT tissue bank at Stanford Stem Cell Transplantation Laboratory. Because patients’ clinic schedules vary, a 15% leeway was allowed for each research sample collection time point. All samples were obtained from patients providing informed consent, and the research sample protocol was monitored by the Stanford University School of Medicine Institutional Review Board. All patients suspected of cGVHD development were evaluated in real-time by one of three dedicated Stanford cGVHD clinicians and further reviewed by cGVHD Scoring committee to uniformly apply National Institutes of Health consensus cGVHD criteria (25). For this retrospective study, we identified a consecutive series of 28 male patients with female donors who had enrolled on our research sample protocol with their transplant dates ranging from January 17, 2006 to May 12, 2010, and who had survived at least 1 y with research blood samples successfully collected 6 and 12 mo following HCT. All patients had undergone transplantation for lymphoid and blood malignancies and are described in Table 1.
Donor Chimerism Analyses.
Donor chimerism analyses were performed on whole blood and PBMCs separated into CD3 and CD19 populations using Dynal-coated immunomagnetic beads. Donor engraftment used DNA genotyping of simple sequence-length polymorphic markers that encode short tandem repeats, as described (26).
Detection of Serum Antibodies Reactive with DBY and DBY-2.
The ELISA protocol that we use has been published (11, 20). Briefly, purified DBY, DBX, DBY-2 (KNDPERLDQQLANLDLNSEK) and DBX-2 (ENALGLDQQFAGLDLNSSD, disparate amino acids are bolded), and HIVp24 (negative control) proteins were coated onto 96-well ELISA plates. The ELISA plates were blocked with 2% nonfat dry milk powder in TBST for 2 h before the patient plasma and controls were added. The plates were incubated overnight at 4 °C, washed and goat anti-human IgG conjugated to alkaline phosphatase was added to detect bound IgG antibodies. Finally, a chromogenic alkaline phosphatase substrate was added and the absorbance at 405 and 450 nm determined 30 min later. Data for readings at 450 nm are reported to maintain continuity with previous assays. Values >3 SD above the mean for 32 healthy male donor samples were considered positive.
High-Dimensional Flow Cytometry Analysis.
Cryopreserved PBMC samples were thawed and washed in deficient RPMI media supplemented with 4% (vol/vol) FCS. Greater than 90% viable PBMC were detected after thawing. Biotin-coupled antigen (DBY-2 or DBX-2) was added to the cells and 20 min later, a “cocktail” of fluorochrome conjugated monoclonal antibodies detecting CD19, CD21, CD43, CD5, CD23, IgM and IgG, CD27, and dead cells (Table S1) was added. Following 20-min incubation, cells were spun and washed and incubated for 20 min with fluorochrome-conjugated streptavidin. Data were collected for 1–5 × 106 cells on LSRII (BD Biosciences). The data were analyzed using FlowJo (TreeStar) and further analyzed with Excel and Prism (GraphPad Software).
FACS-sort.
PBMC were thawed and stained as described above. Antigen-specific B cells were sorted into FCS a custom ARIAII (BD Biosciences) instrument. Approximately 70% of the sorted viable cells were recovered.
Culture Conditions.
DBY-2–specific CD19+ B cells and CD3+ T cells were separately sorted with FACS and cells were cocultured (106 per mL) in RPMI with 10% FCS and DBY protein (0.05 μg/mL). IL-2 (50 IU/mL) and BAFF (R&D Systems) were tested at 10 ng/mL. Cultures were maintained at 37 °C with 5% CO2 in 5% O2 incubators (27). Harvested supernatant was stored at −80 °C before IgG ELISAs.
Statistical Analyses.
Nonparametric Kruskal–Wallis, Mann–Whitney U tests, and Fisher Exact test were used as indicated. The tests were performed in Prism (GraphPad Software).
Supplementary Material
Acknowledgments
We thank the patients who participated in this study as well as all the BMT nurses, patient coordinators, and staff at Stanford University Medical Center who made this work possible. We gratefully acknowledge the technical assistance provided by Fang Wu, Joanne Otani, Mindy Ratra, Spenser Perloff, and Kelsi Schoenrock. We also thank John Mantovani for administrative assistance. This work was supported by National Heart Lung and Blood Institute Grant R21 HL084318, National Cancer Institute Grant P01 CA049605, and Stanford University Cancer Institute Support Grant 1P030CA124435-01.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222900110/-/DCSupplemental.
References
- 1.Socié G. Chronic GVHD: A new risk score? Blood. 2011;117(24):6408–6409. doi: 10.1182/blood-2011-04-350389. [DOI] [PubMed] [Google Scholar]
- 2.Socié G, et al. ATG-Fresenius Trial Group Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 3.Kohrt HE, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114(5):1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai S, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen JL, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120(12):2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzmina Z, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117(7):2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 7.Sarantopoulos S, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarantopoulos S, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117(7):2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.She K, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(4):386–397. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- 10.Sarantopoulos S, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miklos DB, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103(1):353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechalekar A, Cranfield T, Sinclair D, Ganzckowski M. Occurrence of autoantibodies in chronic graft vs. host disease after allogeneic stem cell transplantation. Clin Lab Haematol. 2005;27(4):247–249. doi: 10.1111/j.1365-2257.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 13.Schultz KR, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Socié G. Chronic GVHD: B cells come of age. Blood. 2011;117(7):2086–2087. doi: 10.1182/blood-2010-12-322297. [DOI] [PubMed] [Google Scholar]
- 15.Deneberg S, Lerner R, Ljungman P, Ringden O, Hägglund H. Relapse of preB-ALL after rituximab treatment for chronic graft versus host disease: implications for its use? Med Oncol. 2007;24(3):354–356. doi: 10.1007/s12032-007-0002-3. [DOI] [PubMed] [Google Scholar]
- 16.Kharfan-Dabaja MA, Bazarbachi A. Emerging role of CD20 blockade in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(10):1347–1354. doi: 10.1016/j.bbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Kharfan-Dabaja MA, et al. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: A systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15(9):1005–1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Zaja F, et al. GITMO (Gruppo Italiano Trapianto Midollo Osseo) Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 19.Cutler C, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miklos DB, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci USA. 2012;109(14):5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, et al. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc Natl Acad Sci USA. 2012;109(14):5382–5387. doi: 10.1073/pnas.1121631109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JS, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol. 2012;189(1):222–233. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan M, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipovich AH, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Millan MT, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73(9):1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Sahaf B, et al. Culturing of human peripheral blood cells reveals unsuspected lymphocyte responses relevant to HIV disease. Proc Natl Acad Sci USA. 2008;105(13):5111–5116. doi: 10.1073/pnas.0712363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: A guide for the perplexed. Nat Immunol. 2006;7(7):681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.