Abstract
The multiple memory systems hypothesis posits that dorsal striatum and hippocampus are central nodes in independent memory systems, supporting response-based and place-based learning, respectively. Although our understanding of the function of hippocampus within this framework is relatively well established, the contribution of dorsal striatum is less clear. This in part seems to be due to the heterogeneous nature of dorsal striatum, which receives extensive topographically organized projections from higher cortical areas. Here we quantified neural activity in the intact brain while mice and humans acquired analogous versions of the Morris water maze. We found that dorsomedial striatum and medial prefrontal cortex support the initial acquisition of what is typically considered a hippocampus-dependent spatial learning task. We suggest that the circuit involving dorsomedial striatum and medial prefrontal cortex identified here plays a more task-independent role in early learning than currently thought. Furthermore, our results demonstrate that dorsomedial and dorsolateral striatum serve fundamentally different roles during place learning. The remarkably high degree of anatomical overlap in brain function between mouse and human observed in our study emphasizes the extent of convergence achievable with a well-matched multilevel approach.
Keywords: functional MRI, immediate early gene, navigation
The multiple memory systems hypothesis posits that hippocampus and dorsal striatum are central nodes in independent memory systems, each supporting different aspects of learning and memory formation (1–4). In the context of spatial learning, the hippocampus supports place-based behavioral strategies relying on learning the general layout of the environment, whereas the dorsal striatum supports response-based behavioral strategies driven by task-specific stimuli (5–12). Although the dorsal striatum is often referred to as a unitary structure within the multiple memory systems framework, there is considerable evidence in rodents and humans that it is composed of functional subdivisions that support different aspects of learning (13–20).
Here we conducted parallel experiments in mouse and human to test whether dorsomedial and dorsolateral striatum make distinct contributions during early (initial acquisition) and late (overtraining) phases of place learning in the intact brain. Subjects performed the classic hippocampus-dependent hidden platform version of the Morris water maze, which was matched between species with respect to behavioral processing demands (9). This widely used paradigm has served a critical role in the study of neurobiological aspects of learning and memory over the past 25 years (21, 22).
Although it is well established that hippocampus supports the spatial processing demands of the water maze, much less is known about the precise contribution of dorsomedial and dorsolateral striatum during the early and late phases of learning in the hidden-platform version of the task. If dorsomedial striatum plays a general role in goal-directed learning, we predict that this region will support the nonspatial cognitive processing component of water maze learning important during initial task acquisition. We also targeted medial prefrontal cortex, with the expectation that its contribution to water maze learning is concomitant with dorsomedial striatum, because together these structures form a corticostriatal network that has been implicated in goal-directed learning (16, 23). Although it remains controversial as to whether rodents possess prefrontal cortical regions similar to humans and other primates (24, 25), few studies have directly examined whether corresponding behavioral processes in rodent and human are served by functionally homologous regions within prefrontal cortex (13). We also expected functional differences between dorsal striatum subdivisions because dorsolateral striatum plays an important role in habit learning, leading to the prediction that it will support water maze performance once the task has been overtrained. To focus on nonspatial cognitive processes, we compared water maze learning with a free-swimming control condition known to also include a spatial processing component (26). To ensure that the comparison between place learning and free-swimming isolated these processes, we included hippocampus (CA1 in mouse, posterior hippocampus in human) as a control region where differences were not expected.
Complementary imaging techniques were used in each species to identify the involvement of target structures during learning. In mouse, expression of immediate early gene (IEG) zif268 provided a molecular marker of learning-related neuronal activation. zif268 plays a critical role in synaptic plasticity and the consolidation of long-term memories (27–29) and has been frequently used to visualize brain activation in rodents after behavioral training, including spatial learning tasks (30). In human, functional magnetic resonance imaging (fMRI) was used to measure the hemodynamic response to brain activation (31). Both techniques enable the quantification of distributed patterns of cortical and subcortical brain activity, while preserving the integrity of neural circuits and neuronal functioning.
Results
Behavioral Learning Profile in Mouse.
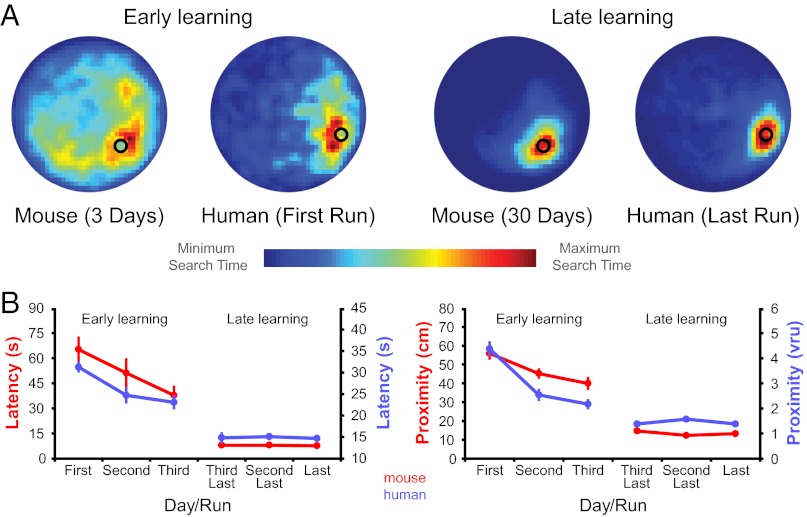
Two experimental groups of mice were trained on the hidden-platform version of the Morris water maze for 3 d (n = 7) and 30 d (n = 8). Behavioral performance after 3 d of training was characterized by a search pattern that was goal-directed but variable. In contrast, after 30 d of training the search pattern was highly focused on the hidden platform location (Fig. 1A). Both groups significantly decreased latency and search proximity over the course of training (3-d group: F2,12 ≥ 4.06, P < 0.05; 30-d group: F29,203 ≥ 33.8, P < 0.001; Fig. 1B). A direct comparison between experimental groups indicates that the 30-d group performed significantly better than the 3-d group on the final training day (t1,13 ≥ 7.7, P < 0.001). Furthermore, performance of the 30-d group plateaued after 10–15 d (Fig. S1A and B). These results confirm that the 3-d group and 30-d group represent early and late learning phases, respectively.
Fig. 1.
Behavioral performance on mouse and human versions of the Morris water maze is closely matched. (A) Graphical representation of mouse and human search patterns during early and late learning (hidden platform displayed as back circle). (B) Reduction in latency and search proximity during initial acquisition of the water maze reflects an early learning phase in both species. Stable performance during overtraining is indicative of a late learning phase. Error bars in B represent SEM. Fig. S1 shows complete learning curves.
Dorsomedial Striatum and Medial Prefrontal Cortex Are Involved in Early Place Learning in Mouse.
To identify learning-specific changes in zif268 expression, experimental groups were compared with free-swimming control groups (3 d, n = 8; 30 d, n = 8) who explored the same environment except that the hidden platform and distal cues were not present. Experimental and free-swimming control groups were matched with respect to the overall amount of time spent swimming on each day. A nonswimming caged control group (n = 8) was also included to provide a baseline measure of zif268 expression. In all brain regions experimental and control groups displayed significantly higher zif268 expression compared with the caged control group (one-way between-groups ANOVA; main effect of group: F19,124 = 69.5, P < 0.001; post hoc tests: P ≤ 0.05).
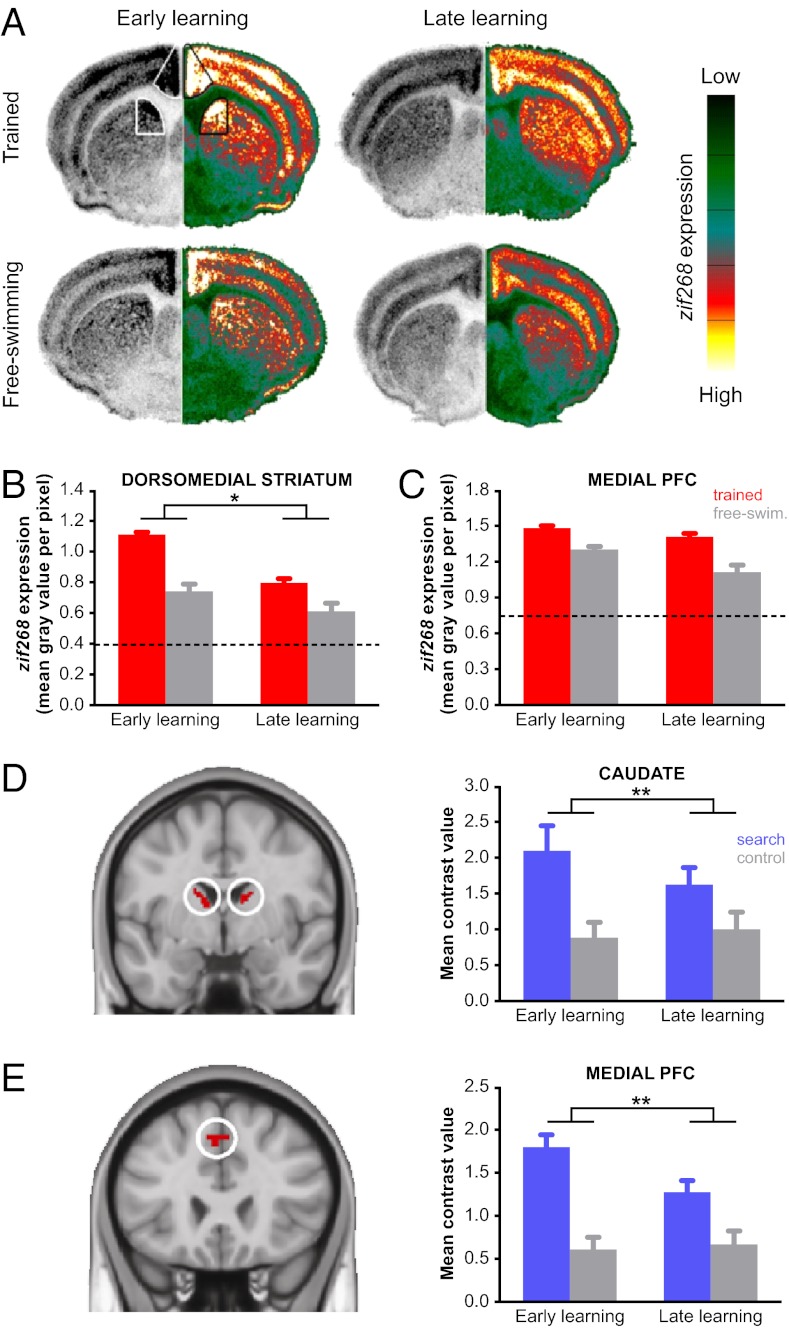
The strongest support for a phase-dependent contribution to place learning was observed in dorsomedial striatum. zif268 expression decreased between early and late learning to a greater extent in the experimental groups than in the free-swimming control groups (condition × learning phase interaction: F1,27 = 5.3, P < 0.05; Fig. 2 A and B), indicating a specific contribution to early place learning. Additionally, zif268 expression in the experimental groups was significantly higher than in the free-swimming control groups in both learning phases (post hoc tests: P ≤ 0.05), suggesting dorsomedial striatum remained involved in more general aspects of task performance. Although dorsolateral striatum did not exhibit learning-specific changes, zif268 expression was significantly higher in the experimental groups during both phases of learning (main effect of condition: F1,27 = 36.0, P < 0.001; Fig. 3A). Direct comparison between dorsomedial and dorsolateral striatum in the experimental groups confirmed that the pattern of learning-related changes in zif268 expression was different between dorsal striatum subdivisions (region × learning phase interaction: F1,26 = 53.5, P < 0.001).
Fig. 2.
Dorsomedial striatum and medial prefrontal cortex support early place learning in human and mouse. (A) Coronal sections from mouse displaying zif268 expression during early and late learning in experimental and free-swimming control groups. The left hemisphere shows the original autoradiogram in gray scale and is matched on the right by its pseudocolor counterpart. The color scale bar ranges from no signal (0, dark green) to maximum signal (255, white). Striatal and prefrontal subdivisions in mouse were based on known anatomical connectivity (Fig. S4 A and B). (B) A larger reduction in zif268 expression between early and late learning was observed in the experimental groups than in the free-swimming controls, suggesting a specific role for this region during early learning. (C) zif268 expression in medial prefrontal cortex decreased from early to late in both experimental and free-swimming control groups. (D) Posterior dorsomedial striatum in human (image displayed at Montreal Neurological Institute coordinate y = −3) responded more strongly to search trials than control trials (P < 0.05, FWE corrected). Subdivisions within human striatum were based on prior knowledge regarding functional differences (Fig. S4 C and D). (E) Medial prefrontal cortex in human (y = 24) responded more strongly to search trials than control trials (P < 0.05, FWE corrected) and was functionally connected to the dorsomedial striatum at rest (P < 0.05, FDR corrected). Mean contrast values were extracted from the activations shown in the left of D and E and are plotted to the right of each image. Error bars represent SEM. *P < 0.05; **P < 0.01.
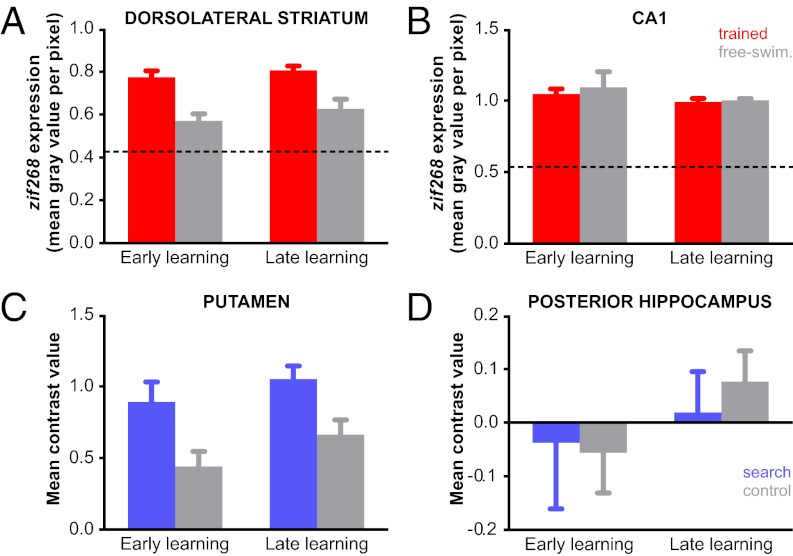
Fig. 3.
Dorsolateral striatum (ventral anterior subdivision displayed in human; Fig. S3 C and D show other subdivisions) and hippocampus did not show learning specific changes in mouse (A and B) or human (C and D). (A and C) In dorsolateral striatum, activity during water maze learning and the free-swimming control condition was greater in comparison with baseline in both species (caged controls in mouse and rest in humans). Furthermore, a generalized increase in water maze learning compared with free-swimming was observed in both species across early and late phases of training. See SI Discussion. (B and D) No differences were observed between experimental and free-swimming control groups in CA1 (mouse) or posterior hippocampus (human). Error bars represent SEM.
In medial prefrontal cortex zif268 expression was significantly higher in the experimental groups compared with the free-swimming control groups (main effect of condition: F1,27 = 40.3, P < 0.001) and decreased in both conditions from early to late learning (main effect of learning phase: F1,27 = 12.3, P < 0.01; condition × learning phase interaction not significant: F1,27 = 2.5, P = 0.13; Fig. S2 A and C). Although this pattern of results only indicates a general role in task performance, medial prefrontal cortex zif268 expression was negatively correlated with search proximity during the early phase of learning in the experimental mice (r = −0.84, P < 0.05; Fig. S2). That is, higher levels of zif268 corresponded to lower search proximity values (indicating better performance). Search proximity was not positively correlated with zif268 late in learning (r = 0.03, P = 0.94). These findings suggest that in addition to dorsomedial striatum, involvement of the medial prefrontal cortex was also important during early place learning. In the CA1 region of hippocampus we did not find differences between experimental and free-swimming control groups (main effect of condition: F1,18 = 0.3, P = 0.59) or evidence of learning-related changes (condition × learning phase interaction: F1,18 = 0.1, P = 0.74; Fig. 3B), confirming that dorsomedial striatum together with medial prefrontal cortex subserve nonspatial cognitive aspects of place-based learning in the water maze.
Behavioral Learning Profile in Human.
Human subjects (n = 18) performed a virtual version of the Morris water maze task designed to closely match the processing demands of the rodent version to test whether target brain areas in human display a similar phase-dependent function in place learning. Subjects learned the location of a hidden platform over the course of three training sessions that were 3–4 d apart. Task-related fMRI data were acquired in session 1 to capture early place learning and in session 3 to measure the late learning phase after subjects had been overtrained outside the scanner in session 2 (six time series or “runs” were acquired in each session). During “search” trials subjects intercepted the hidden platform to learn its location, similar to the trials performed by the experimental mouse groups. The behavioral performance of subjects in session 1 was characterized by a search pattern similar to that observed during early learning in mouse (i.e., goal-directed but variable) (Fig. 1A). Latency and search proximity decreased significantly over the course of the first scan session (F5,85 ≥ 15.6, P < 0.001; Fig. 1B and Fig. S1 C and D). Significant reductions were also found for both measures during the behavioral training session (F5,85 ≥ 2.7, P < 0.05; Fig. S1 C and D), suggesting that learning had not plateaued in the first scan session. The search pattern during the final scan session (session 3) was highly focused on the hidden platform location, again similar to that observed in mouse. Neither latency nor search proximity decreased further during this scan session (F5,85 ≤ 0.92, P > 0.47), indicating stable performance. A direct comparison between scan sessions revealed that performance was significantly better during the second scan session compared with the first (F1,17 ≥ 35.5, P < 0.001). These results confirm that the first and second scan sessions represent early and late learning phases, respectively.
Phase-Dependent Contribution to Place Learning in Dorsomedial Striatum and Medial Prefrontal Cortex Is Similar in Mouse and Human.
We first identified brain areas contributing to virtual Morris water maze performance by testing which areas responded more strongly to search trials than control trials. During control trials subjects freely explored the water maze environment in the absence of the hidden platform and distal cues. Task performance was associated with significant activations [statistical threshold: family-wise error (FWE) corrected, P < 0.05] in bilateral dorsomedial striatum (caudate), bilateral dorsolateral striatum (putamen), and bilateral prefrontal cortex.
On the basis of prior knowledge of functional subdivisions within dorsomedial and dorsolateral striatum (32), these activations were subdivided into dorsal/ventral and anterior/posterior regions of interest. The resulting subdivisions were then subjected to further analysis to identify learning-specific activations. The most striking finding in human was a phase-dependent contribution of the dorsal posterior subdivision of dorsomedial striatum to early learning, consistent with what was observed in mouse (Fig. 2D). Activation in this region decreased significantly from early to late learning on search trials, but not on control trials (trial type × learning phase interaction: F1,17 = 10.06, P < 0.01; Fig. S3 A and B show anterior and ventral subdivisions). In contrast to dorsomedial striatum, activation in dorsolateral striatum did not exhibit learning-specific changes but remained significantly higher on search trials compared with control trials across both learning phases (trial type × learning phase interaction: F1,17 ≤ 2.06, P ≥ 0.17; Fig. 3C and Fig. S2 C and D). Direct comparison between posterior dorsomedial and dorsolateral striatum on search trials confirmed that there was a difference in the evolution of learning-related changes in activation between dorsal striatum subdivisions (region × learning phase interaction: F1,17 = 5.0, P < 0.05).
Because the dorsal posterior subdivision of dorsomedial striatum was the only area that exhibited a clear pattern of learning-specific activation, we tested whether a functionally connected subregion of prefrontal cortex was activated in a similar manner. First we determined which voxels in prefrontal cortex were (i) functionally connected to dorsomedial striatum (resting state fMRI data acquired at the start of session 1 served as an independent measurement), and (ii) responded more strongly to search than control trials. On the basis of these criteria we identified a single region of interest in medial prefrontal cortex (40 voxels extending from presupplementary motor area into dorsal anterior cingulate cortex; Fig. 2E). In this region we found a significant decrease in activation on search trials but not control trials (trial type × learning phase interaction: F1,17 = 14.7, P < 0.01). This pattern of activation is indeed similar to that observed in the dorsal posterior subdivision of dorsomedial striatum and indicates that both areas support early place learning, a finding that is consistent across human and mouse experiments.
Finally, we tested whether search trials were different to freely exploring the environment in the control condition with respect to hippocampus-dependent spatial processing demands. At the whole-brain level we did not find any voxels in the hippocampus that responded more strongly to search trials than control trials (even at a more lenient statistical threshold of P < 0.001 uncorrected). To further increase our sensitivity to detect differences in activation we restricted our analysis to the posterior subdivision of hippocampus, the region most likely to be engaged in spatial processing tasks in human (5, 33). We defined hippocampal subdivisions by performing a cluster analysis on resting state data, a technique that parcellates a brain area according to its profile of spontaneous activity (34). Within the posterior hippocampus subdivision we did not observe a difference in activation between search and control trials (main effect of trial type: F1,17 = 2.28, P = 0.15) or find any evidence of learning specific activity (trial type × learning phase interaction: F1,17 = 0.22, P = 0.64; Fig. 3D).
Discussion
Here we have demonstrated that dorsomedial striatum and medial prefrontal cortex support the initial acquisition of what is typically considered a hippocampus-dependent spatial learning task. In the past, work on animal and human memory systems has mostly evolved in separate domains. However, it is now apparent that converging evidence from different model systems is required for a comprehensive understanding of learning and memory processes.
The multiple memory systems hypothesis has often treated the dorsal striatum as a unitary structure that is central to response-based learning (see, e.g., ref. 1). Contrary to this view, we found that dorsomedial but not dorsolateral striatum makes a critical contribution to the early phase of place learning (SI Discussion). This result is consistent with previous reports of impaired expression of place learning after dorsomedial striatum lesions (18–20, 35–37). For example, when rats performed a water maze task designed to test for a preference between previously learned response-based and place-based strategies, dorsomedial striatum lesions resulted in a lower likelihood of choosing a place-based strategy (4, 19). Yin and Knowlton (20) showed that dorsomedial and dorsolateral striatum make distinct contributions to plus maze performance, linking each subdivision to place-based and response-based strategies, respectively. Here we extend this line of evidence by demonstrating in both mouse and human that dorsomedial striatum contributes to place-based learning processes in the intact brain. This is important because most rodent studies investigating the multiple memory systems hypothesis have used maze tasks that require a choice between behavioral strategies when part of the brain is lesioned. Although this approach is useful for identifying double dissociations, it is limited to the study of behavior that is produced by an impaired memory system. The IEG expression and fMRI activation we observed in dorsomedial striatum resulted from behavior produced by a fully intact memory system and provides a complementary form of evidence to the aforementioned lesion work. Furthermore, our data support Yin and Knowlton’s recommendation (20) that the multiple memory systems hypothesis should be revised to take into account functional subdivisions within the dorsal striatum.
It is worthwhile noting that dorsomedial striatum involvement during the early phase of place learning seems to contrast with the findings of other maze learning experiments in human (5, 6, 38, 39). Dorsomedial striatum activations have typically been associated with nonspatial behavioral strategies, such as landmark based navigation (5), route following (6, 39) and habit learning (38). This is somewhat surprising given that these behaviors are now generally more associated with the dorsolateral striatum in rodents (17, 20). Although the design of maze learning experiments in humans was often inspired by work in rodents, it remains a possibility that subtle task differences can lead to altered processing demands. Importantly, the experimental and control conditions in the present experiment were designed to be as similar as possible between species.
We also observed learning-specific activation in medial prefrontal cortex, suggesting that together with dorsomedial striatum these areas form a cortico-subcortical loop that supports early place learning. What then, is the specific role of dorsomedial striatum and medial prefrontal cortex? Our experiments were designed to specifically target nonspatial processing during place learning in the water maze. A difference in IEG expression and fMRI activation between water maze learning and free-swimming control conditions in hippocampus would likely reflect a difference is spatial processing demands between conditions. However, differences were not observed in CA1 (mouse) and posterior hippocampus (human), indicating that the increased IEG expression and fMRI activation in dorsomedial striatum and prefrontal cortex during early learning is unlikely to be related to spatial processing demands. Others have shown that neurons in the dorsomedial striatum are most active during maze navigation at decision-making locations, at reward locations, and at the location of cues predicting reward delivery (40, 41). This is in contrast to hippocampal neurons that usually represent a single location in space. Thus, dorsomedial striatum seems to encode environmental information relevant to the successful outcome of a task that results in immediate or delayed reward. Interestingly, this suggests a more generalized role for dorsomedial striatum in navigation tasks, regardless of whether a spatial or nonspatial strategy is used. In human, evidence from instrumental conditioning tasks highlighted a similar role for the dorsomedial striatum in learning actions and their reward consequences (42, 43).
In addition to dorsomedial striatum we observed concomitant activity in medial prefrontal cortex during early place learning. Medial prefrontal cortex projects extensively to dorsomedial striatum, and our data suggest these regions might interact to serve related processes during early place learning. In particular, imaging work in humans attempting to delineate the executive functions of medial prefrontal cortex has revealed its involvement in conflict monitoring, error detection, and processes driving reinforcement learning (44, 45). Brain activity reported in these studies was in close anatomical proximity to the location found in our place learning task. Recently, Alexander and Brown (46) proposed a new model to reconcile previous theories: they posit that medial prefrontal cortex encodes action-outcome associations in relation to environmental information processed in a specific task context. This general function exhibits remarkable similarities to the properties identified for neurons in dorsomedial striatum during maze navigation described above. Most importantly, evidence suggests that medial prefrontal cortex activity reflects surprise resulting either from the occurrence of unexpected events or the nonoccurrence of expected events (46). Even though experimental evidence for this theory has mainly been derived from classic reinforcement and decision-making paradigms, the occurrence of these types of events is ubiquitous in many learning tasks, including maze navigation. For example, consider a human or mouse approaching the hidden platform but suddenly realizing that it has been missed on the basis of environmental information, such as being too close to the pool wall. This would likely be an example of surprise resulting from not finding the platform (and the associated reward) at the expected location. Thus, our results in combination with recent findings give rise to the hypothesis that dorsomedial striatum in conjunction with medial prefrontal cortex process environmental information to detect deviations from an expected behavioral outcome. Consequently, the dorsomedial striatum–medial prefrontal cortex circuit identified here seems to support learning in many different task categories, including reinforcement learning, motor skill acquisition, and spatial learning.
In conclusion, we have demonstrated that dorsomedial striatum and medial prefrontal cortex play an important role during early place learning in a task typically thought to be hippocampus-dependent. Most strikingly, the pattern of activation observed in our target regions was remarkably similar between mouse and human, providing converging evidence from two model systems that are mostly studied independently. Our results provide further evidence that dorsomedial and dorsolateral striatum serve fundamentally different functions during place learning. On the basis of our findings and related work in humans and rodents using other learning tasks, we hypothesize that the identified dorsomedial striatum–medial prefrontal cortex circuit might play a much more task-independent role in early learning than currently thought.
Methods
Mouse Experiment.
Subjects.
Eight-week-old female C57BL/6J mice (Centre D'Elevage Janvier) were group housed (five to seven mice per cage) in standard cages with wood-shaving bedding. Food and water were available ad libitum, and mice were handled for 1 wk (tail coloring) before the start of behavioral testing. The housing environment was temperature and humidity controlled, with a 12-h light–dark cycle (lights on at 8:00 AM). Behavioral testing was performed during the light phase. All procedures were approved by the ethical research committee of KU Leuven in accordance with the Declaration of Helsinki.
Behavioral procedures.
Mice were trained on the hidden platform version of the Morris water maze (SI Methods, Mouse Experiment, Behavioral procedures provides details on apparatus; complete methods are included in SI Methods). Each trial began at one of four starting locations by placing the mouse at the edge of the pool facing toward the center. During trials the experimenter remained seated at a fixed location. When a trial was not completed in 2 min the mouse was guided to the platform and remained there for 15 s.
All mice arrived in the laboratory at the same time and were handled daily. From the start of the experiment all cages were transferred to the training room each day. Experimental mice were trained to find the hidden platform for 3 d (one session of four trials per day) and 30 d (two sessions of four trials per day for the first 25 d of training, then one session of four trials per day for the remaining 5 d; 5 consecutive training days were followed by 2 rest days). Trials in each session were separated by a 15-min break, and when two sessions were performed on a single day they were separated by 2 h. Free-swimming control mice (3 d, 30 d) explored the same environment except that the hidden platform and distal cues were removed. With distal cues present in the free-swimming condition goal-directed navigation and learning remains possible (albeit not learning of an escape platform location). Therefore, the likelihood of achieving true free-swimming performance (i.e., not goal-directed) was optimized by the removal of distal cues. Nonswimming caged control mice did not receive any water maze training but were always transferred between housing and training rooms together with the other four groups during the 30-d testing period. All mice were 15 wk old on the final day of training.
Behavior was recorded using Ethovision video tracking equipment and software (Noldus). Overall task performance was evaluated by calculating the time taken to find the hidden platform (latency). Spatial performance was evaluated by calculating the average distance between the mouse and the hidden platform (search proximity). A repeated-measures one-way ANOVA was used to test for learning-related changes in the experimental groups. The α-level was set to 0.05.
Quantitative in situ hybridization to determine zif268 expression.
zif268 in situ hybridization was performed using previously established methods in our laboratory (47). Briefly, animals where killed at the age of 15 wk by cervical dislocation 45 min after the final training trial, and brains were immediately frozen in 2-methylbutane (Merck) at a temperature of −40 °C. Coronal sections (25 µm) were cut on a cryostat (Microm HM 500 OM) and mounted onto 0.1% poly-L-lysine coated slides (Sigma-Aldrich). A series of brain sections covering the entire rostrocaudal extent of the striatum/anterior cingulate (medial prefrontal cortex) and hippocampus were collected (48) and kept at −30 °C. Tissue was postfixed in 4% (vol/vol) paraformaldehyde in 0.12 M phosphoric acid in PBS (0.1 M, pH 7.4, 30 min, 4 °C; 0.9% NaCl), dehydrated [50% (vol/vol), 70% (vol/vol), 98% (vol/vol), 100% (vol/vol), 5 min], and delipidated [100% (vol/vol) chloroform, 10 min). The mouse-specific synthetic zif268 probe (NM_007913.5, sequence: 5′-ccgttgctcagcagcatcatctcctccagyttrgggtagttgtcc-3′) was end-labeled with 33P-dATP (New England Nuclear) using terminal deoxynucleotidyl transferase (Invitrogen). Unincorporated nucleotides were removed using mini Quick Spin columns (Roche Diagnostics). The radioactive labeled probe was mixed with a hybridization mixture [50% (vol/vol) formamide, 4× standard saline citrate, 1× Denhardt’s solution, 10% (wt/vol) dextran sulfate, 100 μg/mL Herring sperm DNA, 250 μg/mL tRNA, 60 mM DTT, 1% (wt/vol) N-lauryl-sarcosine, and 26 mM NaHPO4 (pH 7.4)] and applied to a series of dehydrated sections with overnight incubation at a temperature of 37 °C. The next day the sections were rinsed in 1× standard saline citrate buffer at 42 °C, air-dried, and apposed to an autoradiographic film (Kodak) together with a [14C] microscale (GE Healthcare). Films were developed 3 wk later in Kodak D19 developing solution and fixed in Rapid fixer (Ilford Hypam).
Autoradiographic images were scanned (CanoScan LiDE 600F; Canon), and optical densities (mean gray value per pixel) were quantified with ImageJ (image processing and analysis in Java; National Institutes of Health). Optical density was measured in three brain sections per mouse along the rostrocaudal axis for each target region. Striatum and medial prefrontal cortex slices were taken from +1.10 mm to +0.38 mm relative to bregma (Fig. S4A) and CA1 slices from −1.58 mm to −2.54 mm relative to bregma (48). Within striatum we targeted dorsolateral and superior dorsomedial subdivisions (Fig. S4B). The template of the striatal and medial prefrontal cortex compartments was drawn bilaterally over brain sections. Mean gray values were averaged across hemispheres and brain slices, resulting in a single data point for each region per animal. A one-way between-groups ANOVA was use to test differences in IEG expression between the caged control group and all experimental and control groups. To test for learning-related changes in IEG expression, mean gray values were entered into an ANOVA (2 conditions × 2 learning phases). For all analyses the α-level was set to 0.05 and Bonferroni correction applied to post hoc tests. Statistical analyses were performed in Statistica 9 (StatSoft).
Corticosterone levels.
Comparison of corticosterone levels ensured that between-group differences in IEG expression were not confounded by stress (SI Methods, Mouse Experiment, Corticosterone levels). A one-way between-groups ANOVA revealed that corticosterone levels did not differ significantly between groups (F4,26 = 1.462, P = 0.24; Fig. S5). Furthermore, previous work has demonstrated that zif268 expression is generally not influenced by stress (49).
Human Experiment.
Subjects.
Eighteen female subjects (aged 20–28 y, mean age 23.1 y) participated in the fMRI study. All were right-handed with no history of neurological disease. Before testing, subjects were required to provide written informed consent to the procedures, which were approved by the Ethics Committee of KU Leuven in accordance with the Declaration of Helsinki.
Task.
A custom virtual environment analogous to the Morris water maze was constructed in Blender (www.blender.org) and rendered in MATLAB (2007b; The Mathworks) (SI Methods, Human Experiment, Task provides details on the virtual environment; complete methods are included in SI Methods). Subjects viewed the room from a first-person perspective and moved around by pressing buttons on an MRI-compatible button box (Current Designs Inc.).
Trial procedures.
Over the course of the experiment subjects performed “search” and “control” trials, which were designed to be compatible with our mouse water maze experiment. All trials began from one of four starting zones (separated by 90°) located at the perimeter of the pool, with the exact position within a given starting zone varying by ±10° from trial to trial. Subjects always faced the center of the pool at the beginning of the trial.
The goal of search trials was to navigate to the hidden platform as quickly and directly as possible. When the goal location was successfully intercepted the walls of the room turned green for 1 s, after which the subject remained at the same location for a further 3 s. During this 4-s period forward movement and orienting were not possible. The maximum time limit for search trials was 45 s. If a trial reached the maximum time limit the walls of the room turned red for 1 s, after which the subject remained in their final unsuccessful location for a further 3 s (forward movement and orienting were again not possible during this 4-s period).
During control trials subjects moved freely within the pool. No distinguishing features were present on the walls, preventing any goal-directed navigation. Control trials were matched to the average duration of search trials (between 10 and 20 s) and finished in a similar manner, with the only difference being that the color of the walls always turned blue (which did not relate to feedback provided during other trials). Fig. S6B shows screenshots.
A third trial type, “prediction” trials, required the subject to explicitly indicate where they thought the hidden platform was located via a button press. Analysis of prediction trials is not presented here because we focused on the conditions closest to the mouse experiment.
Experimental protocol.
Four testing sessions were completed, each on a separate day. The first session familiarized subjects with the experimental procedures and trial order before scanning. During this session a limited number of trials were performed in a different environment from that used in the main experiment.
One or two days later subjects returned for the first scan session. From this session onward the environment and the location of the hidden platform was unchanged. Subjects performed six runs of trials, with each run lasting at least 8 min. SI Methods, Human Experiment, Experimental protocol gives the order of trial presentation.
A second identical scan session was performed 6–8 d after the first. Between scan sessions subjects performed a training session during which only behavioral data were acquired. The behavioral training session also consisted of six runs of trials each lasting 8 min.
Resting-state protocol.
In addition to acquiring task-related fMRI data, subjects were also scanned for 7 min in a resting state before the onset of task performance. Subjects were required to fixate on a white cross in the center of a black screen and were instructed to relax and think of nothing in particular.
Behavioral analysis.
The same behavioral measures as those previously described in mouse (i.e., latency and search proximity) were also used to quantify performance on the virtual water maze. To test for learning within each session we conducted a one-way repeated measures ANOVA (runs 1–6). Statistical analyses were performed in Statistica 9. The α-level was set to 0.05.
Statistical analysis of FMRI data.
SI Methods, Human Experiment, Image acquisition and SI Methods, Human Experiment, Image preprocessing describe scan parameters and preprocessing procedures. Search trials, control trials, and rest after control trials were modeled for each subject as boxcar functions convolved with the canonical hemodynamic response function within a first-level general linear model. The time series in each voxel was high-pass filtered at 1/160 Hz to remove low-frequency drifts. The contrasts search > rest and control > rest were specified separately for each run.
Contrasts were entered into a second-level random-effects ANOVA model with the factors trial type (search > rest and control > rest) and run (runs 1–6 and 13–18). The model was estimated under the assumption of dependent measurements and unequal variances. The t-contrast identifying areas responding more strongly to search than control trials was thresholded at P < 0.05, FWE corrected for multiple comparisons within the whole brain, and only included clusters above 30 voxels.
Further analyses focused on the striatum, prefrontal cortex and hippocampus. Regions of interest (ROIs) were defined on the basis of a priori anatomical and functional criteria (SI Methods, Human Experiment, ROI definition). For each of the ROIs created in striatum (Fig. S4D), medial prefrontal cortex (Fig. 2E), and hippocampus (Fig. S7), the marsbar toolbox (50) was used to extract the mean contrast value of all voxels [i.e., an estimate of the hemodynamic response to either search or control trials (compared with rest) in the area of interest]. Unsmoothed images were used to avoid including signal from neighboring regions. To test for changes in activation over the course of learning, contrast values were entered into an ANOVA (2 trial types × 2 learning phases). Statistical analyses were performed in Statistica 9. The α-level was set to 0.05. Post hoc tests were Bonferroni corrected.
Supplementary Material
Acknowledgments
We thank Detlef Balschun, Victor Sabanov, and Johan Wagemans for helpful discussions. Funding for this project was provided by an interdisciplinary research grant from KU Leuven (IDO/06/004). D.G.W. was supported by Research Foundation–Flanders Grants G.0401.12 and G.0758.10. A.L. was supported by a PhD grant from the Agency for Innovation by Science and Technology Flanders.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217832110/-/DCSupplemental.
References
- 1.Ghiglieri V, Sgobio C, Costa C, Picconi B, Calabresi P. Striatum-hippocampus balance: From physiological behavior to interneuronal pathology. Prog Neurobiol. 2011;94(2):102–114. doi: 10.1016/j.pneurobio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 3.Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 5.Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA. 2008;105(15):5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37(5):877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 7.Marchette SA, Bakker A, Shelton AL. Cognitive mappers to creatures of habit: Differential engagement of place and response learning mechanisms predicts human navigational behavior. J Neurosci. 2011;31(43):15264–15268. doi: 10.1523/JNEUROSCI.3634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald RJ, White NM. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61(3):260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 9.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ Press; 1978. [Google Scholar]
- 11.Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci. 1992;106(3):439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 12.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 13.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29(11):2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27(15):4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22(2):513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 17.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 18.Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: Relation to thigmotaxis. Behav Brain Res. 1999;100(1-2):5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 19.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19(7):2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11(4):459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 22.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993;18(1):33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 23.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1-2):145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Preuss TM. Do rats have a prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1-2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Shires KL, Aggleton JP. Mapping immediate-early gene activity in the rat after place learning in a water-maze: the importance of matched control conditions. Eur J Neurosci. 2008;28(5):982–996. doi: 10.1111/j.1460-9568.2008.06402.x. [DOI] [PubMed] [Google Scholar]
- 27.Barry DN, Commins S. Imaging spatial learning in the brain using immediate early genes: Insights, opportunities and limitations. Rev Neurosci. 2011;22(2):131–142. doi: 10.1515/RNS.2011.019. [DOI] [PubMed] [Google Scholar]
- 28.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142(1-2):17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 29.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74(4):183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55(4):564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire EA, Burgess N, O’Keefe J. Human spatial navigation: Cognitive maps, sexual dimorphism, and neural substrates. Curr Opin Neurobiol. 1999;9(2):171–177. doi: 10.1016/s0959-4388(99)80023-3. [DOI] [PubMed] [Google Scholar]
- 32.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 33.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 34.Mantini D, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31(36):12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamoto T, Okaichi H. Use of win-stay and win-shift strategies in place and cue tasks by medial caudate putamen (MCPu) lesioned rats. Neurobiol Learn Mem. 2001;76(2):192–208. doi: 10.1006/nlme.2001.4006. [DOI] [PubMed] [Google Scholar]
- 36.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24(2):125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi E, et al. Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behav Brain Res. 2012;226(1):171–178. doi: 10.1016/j.bbr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Hirshhorn M. Grady C. Rosenbaum RS. Winocur G. Moscovitch M. Brain regions involved in the retrieval of spatial and episodic details associated with a familiar environment: An fMRI study. Neuropsychologia. 2012;50(13):3094–1306. doi: 10.1016/j.neuropsychologia.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci. 2003;23(13):5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berke JD, Breck JT, Eichenbaum H. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol. 2009;101(3):1575–1587. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Meer MA, Johnson A, Schmitzer-Torbert NC, Redish AD. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67(1):25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 43.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 44.Alexander WH, Brown JW. Competition between learned reward and error outcome predictions in anterior cingulate cortex. Neuroimage. 2010;49(4):3210–3218. doi: 10.1016/j.neuroimage.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forster SE, Brown JW. Medial prefrontal cortex predicts and evaluates the timing of action outcomes. Neuroimage. 2011;55(1):253–265. doi: 10.1016/j.neuroimage.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Brussel L, Gerits A, Arckens L. Evidence for cross-modal plasticity in adult mouse visual cortex following monocular enucleation. Cereb Cortex. 2011;21(9):2133–2146. doi: 10.1093/cercor/bhq286. [DOI] [PubMed] [Google Scholar]
- 48.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd Ed. San Diego, CA: Elsevier Academic; 2008. [Google Scholar]
- 49.Pace TW, et al. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22(7):1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- 50.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan. NeuroImage. 2002;16:497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.