Small GTP-binding proteins in the ADP ribosylation factor (Arf) family are master regulators of vesicle-mediated protein transport in the secretory and endocytic pathways (1). There are multiple members of this family in eukaryotic cells, with the lineage starting with Arf1 and its siblings (Arf2–Arf5) and extending to the more distant relatives Arf6, Sar1, and Arf-like (Arl) proteins. All these proteins cycle between GTP-bound and GDP-bound states with the assistance of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins. The best known function of GTP-bound Arfs is to recruit vesicle coat proteins from the cytosol onto the appropriate membrane for the purpose of budding a transport vesicle. However, a few links between Arfs and lipid modifying events have been reported (1). In PNAS, Tsai et al. report a unique function for Arl1, arguably the least understood member of the Arf family, in stimulating the activity of a phospholipid flippase in the trans-Golgi network (TGN) of budding yeast (2). Remarkably, this function of Arl1 also requires interaction with an ArfGEF (not an ArlGEF), infusing a bit of intrigue into the Arf family tree. These interactions are shown to be important for protein transport from the Golgi and the establishment of membrane asymmetry (2) (Fig. 1).
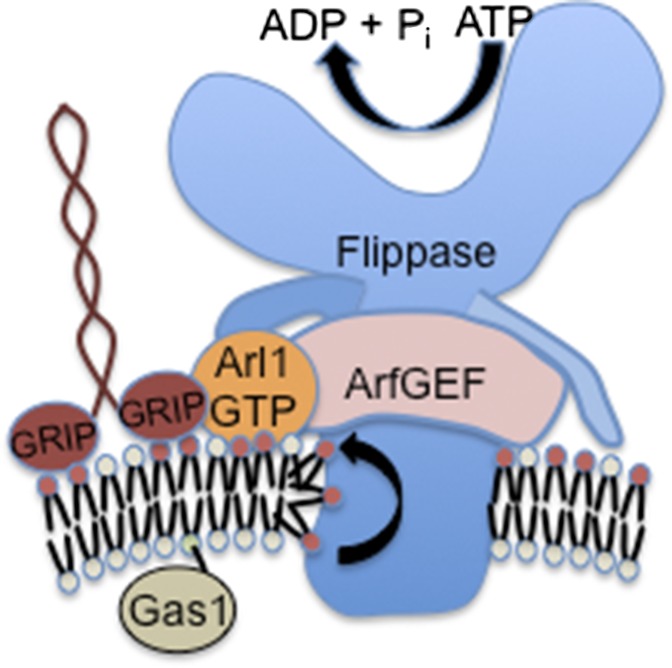
Fig. 1.
Interaction of Arl1 with the ArfGEF Gea2 and flippase Drs2 stimulates PS translocation at the TGN. Arl1 must be in its GTP-bound conformation to bind the N-terminal cytosolic tail of Drs2 and the N-terminal region of Gea2. These interactions stabilize Gea2 interaction with the C-terminal tail of Drs2 and stimulate flippase activity. PS is flipped to the cytosolic leaflet to generate an asymmetric membrane structure. Arl1–GTP and Drs2 flippase activity is required for recruitment of Imh1 via its GRIP domain. These events likely contribute to the budding of a vesicle that transports Gas1 to the plasma membrane.
The phospholipid flippase at the center of this regulatory circuit is Drs2, a founding member of another large protein family called type IV P-type ATPases (P4-ATPases) (3). P4-ATPases are distant relatives of cation pumps that establish ion gradients across cell membranes, but these flippases are a eukaryotic-specific lineage that evolved the ability to pump specific phospholipid molecules across the membrane bilayer rather than cations. Drs2, for example, flips phosphatidylserine (PS) and phosphatidylethanolamine unidirectionally from the luminal leaflet of the TGN to the cytosolic leaflet. This flippase activity creates a phospholipid gradient intrinsic to the membrane bilayer with PS and phosphatidylethanolamine enriched in the cytosolic leaflet, a structural organization that is maintained as membrane moves via vesicular (or tubular) transport intermediates to the plasma membrane.
The phospholipid gradient in the plasma membrane can be used as a signaling platform much as ion gradients are used in signaling. Breaking of membrane asymmetry to expose PS in the outer leaflet, through activation of a “scramblase,” is critical in blood clotting cascades and is a signature feature of apoptotic cells (4). Another important consideration is that PS is a major anionic lipid in the plasma membrane. Concentration of PS in the cytosolic leaflet greatly impacts recruitment of peripheral membrane proteins to this surface and influences integral membrane protein function (5).
The physiological importance of the P4-ATPases is also coming into focus. There are at least 14 P4-ATPases in mammals and Drs2 orthologues, ATP8A1 and ATP8A2, are critically important for neuronal function. Mutations in ATP8A2 have recently been linked to cerebellar ataxia, mental retardation, and disequilibrium syndrome in humans and the axonal neurodegenerative and early death phenotypes of the wabbler-lethal mouse (6, 7). Loss of Atp8a1 in mice also causes neurological deficits, and the double mutant (Atp8a1−/− Atp8a2−/−) is reported to die shortly after birth (7, 8). Loss of ATP8B1 function is linked to severe liver disease in humans whereas other P4-ATPases are linked to B-cell development, hearing, male fertility, obesity, and type 2 diabetes in mice. Budding yeast express five P4-ATPases, with one member individually essential for viability (Neo1) and the other four (Drs2, Dnf1, Dnf2, Dnf3) collectively essential for viability and establishing membrane asymmetry (3).
Membrane asymmetry is only half of the P4-ATPase story as these proteins, like Arfs and Arls, play crucial roles in vesicle-mediated protein transport (3). It is thought that the unidirectional flip of phospholipid produces curvature in the membrane by expanding the cytosolic leaflet surface area at the expense of the luminal leaflet. This stress on the membrane would induce bending into the cytosol, making it easier for coat proteins to deform the membrane into a tightly curved vesicle (9). Arfs can also induce curvature in the membrane as their N-terminal amphipathic helices insert into the cytosolic leaflet upon GTP binding, further expanding this leaflet. Intrinsically curved proteins recruited to the membrane by Arf family members, such as coat proteins (e.g., COPI, COPII, and clathrin) and coat accessory proteins with BAR domains or amphipathic helices may also contribute to curvature and select cargo for incorporation into the forming vesicles (1).
Arl proteins are known to contribute to protein trafficking events at the TGN, although the precise role of Arl1 has been elusive (1). A few effectors are known to require Arl1-GTP for recruitment to the yeast TGN, including the coiled-coil protein Imh1 (through its GRIP domain) and a clathrin adaptor (GGA) (10, 11). However, these effectors do not seem to explain the full spectrum of Arl1 function at the TGN. Therefore, Tsai et al. predict that there must be other effectors of Arl1, and they perform a yeast two-hybrid screen using a “GTP-locked” mutant form of Arl1 (Q72L mutation) as bait (2). An N-terminal fragment of the ArfGEF Gea2 was identified as a potential Arl1 binding protein. This binding is shown to be direct, GTP-dependent, and confined to a site within the first 250 aa of Gea2 containing its dimerization and cyclophilin binding domain (2).
The piece of this puzzle linking Drs2 to the ArfGEF Gea2 was provided a few years ago, again through a two-hybrid screen, but starting with the Sec7 domain of Gea2 (which catalyzes GEF activity with Arf) as bait. This screen identified an interaction between Gea2 and the C-terminal tail of Drs2 (12). Residues important for this interaction were mapped on both partners, and mutational studies implied that the interaction was important, but for what? The picture started to clear up with the discovery that the C-terminal tail of Drs2 is a self-regulatory domain and that binding of this tail to phosphoinositides in the membrane and to ArfGEF actually stimulates the flippase activity of Drs2 (13).
Tsai et al. then ask if Arl1 is part of this Drs2–Gea2 complex and helps to stimulate flippase activity (2). The answer to these questions was yes. A ternary complex was defined with Arl1 showing GTP-dependent binding to the N-terminal cytosolic tail of Drs2 and the N-terminal domain of Gea2. Interestingly, none of these interactions were important for the TGN localization of the interacting partners (2). This was a surprising observation because another group had recently reported a similar binding interaction between Drosophila and mammalian homologues of Arl1 and ArfGEF (BIG1 and BIG2) (14). In this case, Arl1 binding contributed to the TGN localization of the metazoan ArfGEFs (14). For the yeast proteins, Gea2 remains membrane associated in the absence of Arl1, but its ability to bind Drs2 is markedly perturbed. Similarly, the absence of Drs2 disrupts the Arl1–Gea2 interaction. Importantly, Arl1 stimulated Drs2 flippase activity as measured in purified TGN membranes by using a fluorescent derivative of PS (nitrobenz-2-oxa-1,3-diazol-4-yl-PS) as the substrate. TGN membranes from the arl1∆ or gea2∆ cells displayed a comparable decrease in Drs2 flippase activity, and the GTP-locked recombinant Arl1
Tsai et al. report a unique function for Arl1, arguably the least understood member of the Arf family.
could restore WT flippase activity to arl1∆ membranes. Moreover, the arl1∆ mutant, or cells expressing Drs2 mutants lacking the Arl1 binding site, exhibited a loss of PS asymmetry at the plasma membrane (2).
The influence of these interactions on specific Arl1 effectors and pathways was also impressive (2). Although complete loss of Drs2 or Gea2 had no effect on the Arl1-dependent recruitment of GGA to the Golgi, Imh1 recruitment to the TGN was lost. Even more subtle mutations eliminating Arl1 binding sites in Drs2 or Gea2 caused loss of Imh1 membrane association, as did inactivation of Drs2 flippase activity. These results imply a requirement for Arl1-GTP binding and perhaps PS in the cytosolic leaflet for Imh1 membrane association. Arl1 is required for transport of a cargo protein, Gas1, from the TGN to the plasma membrane. Gas1 transport was also strongly dependent on the formation of the ternary Drs2–Gea2–Arl1 complex. Thus, it appears that a critical role of Arl1 in these pathways is to stimulate Drs2 flippase activity. Active Drs2 and Arl–GTP recruits Imh1 to the TGN and somehow facilitates Gas1 transport from the TGN to the plasma membrane.
This paper by Tsai et al. (2) provides an important step forward in understanding the biology of Arl proteins by identifying effectors that drive membrane transformation events in the TGN important for protein transport and the establishment of membrane asymmetry. As with most good stories, there are as many interesting questions raised as answered. For example, why does the Golgi phospholipid flippase activity need to be regulated by such complex interactions with proteins that have other jobs to do? Is the ArfGEF in the ternary complex with Arl1 and Drs2 also capable of activating Arf, or are these two activities mutually exclusive? Most proteins can be transported from the TGN to the plasma membrane in the absence of Arl1 or Drs2, so why does Gas1 rely strongly on the Arl1–Drs2–Gea2 complex and Imh1 for export? How well conserved is this regulatory mechanism in animal cells? It will be interesting to see how closely the Arl, ArfGEF and P4-ATPase family trees are intertwined throughout eukaryotic evolution.
Footnotes
The author declares no conflict of interest.
See companion article on page E668.
References
- 1.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai P-C, Hsu J-W, Liu Y-W, Chen K-Y, Lee F-JS. Arl1 regulates spatial membrane organization at the trans-Golgi network through interaction with the Arf-GEF Gea2p and flippase Drs2p. Proc Natl Acad Sci USA. 2013;110:E668–E677. doi: 10.1073/pnas.1221484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: Building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821(8):1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevers EM, Williamson PL. Phospholipid scramblase: An update. FEBS Lett. 2010;584(13):2724–2730. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 6.Emre Onat O, et al. Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, et al. Mutations in a P-type ATPase gene cause axonal degeneration. PLoS Genet. 2012;8(8):e1002853. doi: 10.1371/journal.pgen.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levano K, et al. Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning. J Neurochem. 2012;120(2):302–313. doi: 10.1111/j.1471-4159.2011.07543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham TR. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14(12):670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Setty SR, Shin ME, Yoshino A, Marks MS, Burd CG. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr Biol. 2003;13(5):401–404. doi: 10.1016/s0960-9822(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 11.Singer-Krüger B, et al. Yeast and human Ysl2p/hMon2 interact with Gga adaptors and mediate their subcellular distribution. EMBO J. 2008;27(10):1423–1435. doi: 10.1038/emboj.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantalat S, et al. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117(Pt 5):711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan P, et al. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11(12):1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christis C, Munro S. The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2. J Cell Biol. 2012;196(3):327–335. doi: 10.1083/jcb.201107115. [DOI] [PMC free article] [PubMed] [Google Scholar]