Abstract
A 39-year-old male reported fevers, weight loss, watery loose stools, and decreased visual acuity in his right eye over the prior five years. He was pancytopenic, had an elevated American council on exercise level, total bilirubin, and alkaline phosphatase. Computed tomography revealed massive hepatosplenomegaly and emphysematous lung changes. Liver biopsy showed non caseating granulomas. The patient was diagnosed with extrapulmonary sarcoidosis and was treated with prednisone. The patient symptomatically improved but 5 mo later presented with abdominal pain caused by perforation of the cecum. He underwent a cecectomy and pathology revealed pneumatosis cystoides intestinalis. This represents the first reported association between pneumatosis cystoides intestinalis and sarcoidosis. The etiology of pneumatosis cystoides intestinalis in this case was likely multifactorial and involved both effects of the corticosteroids as well as the advanced nature of the gastrointestinal sarcoidosis. Furthermore this case has the unique features of emphysematous lung changes and pancytopenia which are uncommon with sarcoidosis.
Keywords: Sarcoidosis, Pneumatosis cystoides intestinalis, Pancytopenia, Emphysema, Corticosteroids
INTRODUCTION
Sarcoidosis is an idiopathic multisystem non-caseating granulomatous disease that has been reported to impact almost any organ. It is more common in African Americans and typically patients present with pulmonary manifestations associated with bilateral hilar adenopathy on chest X-ray. Very rarely does it present with predominantly gastrointestinal (GI) symptoms[1]. Pneumatosis intestinalis cystoides is a rare disorder defined as gaseous cysts in the bowel wall. While the etiology remains unclear, it has been associated with multiple conditions including, connective tissue disease, various drugs, colonoscopies, ileal surgeries, and chronic pulmonary disease[2,3]. Here we present a case of an individual with GI sarcoidosis who 5 mo after initial presentation was diagnosed with pneumatosis cystoides intestinalis.
CASE REPORT
A 39-year-old male presented with right lower quadrant abdominal pain that ranged between 5-9/10 severity and was diffusely located in both the right upper and lower quadrants of the abdomen. The pain begin approximately five years ago and has waxed and waned since. The week prior to admission the pain was significantly worsening. It was particularly aggravated by movement and coughing. Since the onset of symptoms five years ago, he reported watery loose bowel movements after meals, fevers up to 102F, night sweats, a 50lb unintentional weight loss, decreased visual acuity in his right eye and associated photophobia. He also described a chronic cough with occasional dark brown phlegm. He reported waking in the middle of the night coughing. He was initially evaluated multiple times at an outside hospital, but could not remember his specific diagnosis. He has a 15 pack year smoking history and denies any illicit drug use. His prior occupation involved working with copper products. His physical exam revealed severe tenderness over the right side of the abdomen. Liverspan was approximately 25 cm and spleen was easily palpable. The right eye showed an irregular opacity and photophobia.
On presentation he was pancytopenic, with an elevated alkaline phosphatase, gamma glutamyl transpeptidase, total bilirubin and angiotensin converting enzyme (ACE) level. Hemoglobin (Hgb) 13.1 g/dL [normal range (NR) 14.1-17.7 g/dL], platelet count 75 thousand/µL (NR 140-440 thousand/µL), white blood cell (WBC) 2.1 thousand/µL (NR 4.0-10 thousand/µL), alkaline phosphatase 383 IU/µL (NR 45-115 IU/µL), total bilirubin 1.7 mg/dL (NR 0.1-1.3 mg/dL), ACE was 97 U/L (NR 9-67 U/L). Anti-mitochondrial antibody, hepatitis panel, anti-nuclear antibody, human immunodeficiency virus, epstein-barr, and rapid plasma reagin serologies were negative. Tuberculin purified protein derivative and sputum acid fast smear were negative.
Abdominal computed tomography (CT) revealed a markedly enlarged liver and spleen each measuring 24 cm with distended portal and splenic veins (Figure 1A). Shotty retroperitoneal, upper abdominal and mesenteric lymphadenopathy was noted. CT chest revealed a subcentimeter enlargement of mediastinal lymphnodes that were pathological in number. Extensive bilateral centrilobular emphysematous changes were noted (Figure 1B). Bone marrow biopsy was normocellular. Immunophenotyping did not show diagnostic abnormalities of the B- or T-cells. Co-expression of abnormal antigens, antigen deletion, or light-chain clonality was not detected. Outside hospital records from 5 years prior to current admission were obtained. Full body positron emission tomography (PET) scan revealed no foci of abnormal uptake. Hgb was 13.9 g/dL, platelet count 220 thousand/µL, and WBC 4.5 thousand/µL. Alkaline phosphatase 388 IU/µL. Total bilirubin 1.2 mg/dL. Bone marrow biopsy showed hypocellular marrow with mild megaloblastic changes but no evidence of infiltrative changes.
Figure 1.
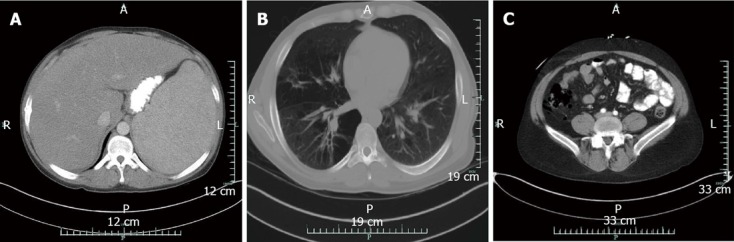
Computed tomography. A: Abdomen and pelvis showing massive hepatosplenomegaly at initial presentation; B: Chest at initial presentation demonstrating extensive bilateral emphysematous changes; C: Abdomen revealing pockets of free air consistent with perforation. No evidence of pneumatosis is seen on computed tomography scan. A: Anterior; P: Posterior; R: Right; L: Left.
The above findings were most consistent with a chronic inflammatory condition. Chronic inflammatory conditions that can cause hepatic noncaseating granulomas include sarcoidosis, Crohn’s disease, primary biliary cirrhosis, Whipple’s disease, copper poisoning, lymphomas, secondary syphilis. Given the negative full body PET scan, negative bone marrow and retroperitoneal lymph node biopsy, negative flow cytometry results, and long indolent course lymphoma was highly unlikely. While his previous occupation dealt with copper, symptoms started well before employment there and he did not demonstrate any other signs of copper poisoning. Whipple's disease is more common in European Caucasians and usually does not include ocular manifestations. However, it cannot be definitively excluded without a small bowel biopsy. Primary biliary cirrhosis and syphilis were ruled out through serologies. Crohn’s disease is possible but is unlikely to cause such marked hepatosplenomegaly or pancytopenia. An extrapulmonary manifestation of sarcoidosis is the most likely explanation.
The patient was started on a 40 mg prednisone and instructed to follow up in the clinic within a mo. At subsequent follow-ups at outpatient clinic 2 wk, 2 mo, and 3 mo after presentation he was having difficult to control steroid induced diabetes requiring increasing doses of metformin and glipizide. He reported home blood sugar measurements ranged from 100-250 mg/dL. He had two separate hospitalizations for hyperglycemia 1 mo and 4 mo after presentation. At each visit his prednisone dose was being tapered. At 4 mo follow up at outpatient clinic the dose was 25 mg. The second hospitalization occurred at an outside hospital 4.5 mo after initial presentation. On discharge the patient reported he was told to increase the dose of glipizide as well as to increase his prednisone dose from 25 mg to 40 mg daily. Overall he was reporting decreased distension and abdominal discomfort. ACE level measured at outside hospital after 4 mo of steroid therapy was down to 29 U/L (97 U/L at initial presentation).
Five mo after initial presentation he came to our institution with headache, dizziness, abdominal pain, and nonbloody diarrhea 1 d. He reported the abdominal pain and discomfort were approximately baseline for him. He was afebrile and normotensive. His abdomen was tender to palpation right greater than left with guarding and rebound. Fecal occult blood was negative. He was found to have blood glucose of 623 mg/dL. Admission labs demonstrated improved pancytopenia, decreased alkaline phosphatase, but an elevated lactate [lactate 5.6 mmol/L (normal 0.5-2.2 mmol/L), Hgb (17.6 g/dL), platelets (114 thousand/µL), WBC (6.2 thousand/µL), alkaline phosphatase (223 IU/L)]. CT of the abdomen revealed pockets of free air distributed circumferentially along the cecum consistent with perforation (Figure 1C). He was taken to the OR for exploratory laparotomy requiring cecectomy and creation of ileostomy and mucous fistula. In the operating room there was no identified perforation externally, but a 2 cm × 2 cm defect within the cecum that did not penetrate the serosal surface of the bowel. The mucosa was hemorrhagic and flattened. Pathology of the resected tissue revealed portions of large bowel with submucosal and mucosal hemorrhage and submucosa with irregular spaces lined with histiocytes (CD68 positive and CD31 negative) consistent with pneumatosis cystoides intestinalis (Figure 2).
Figure 2.
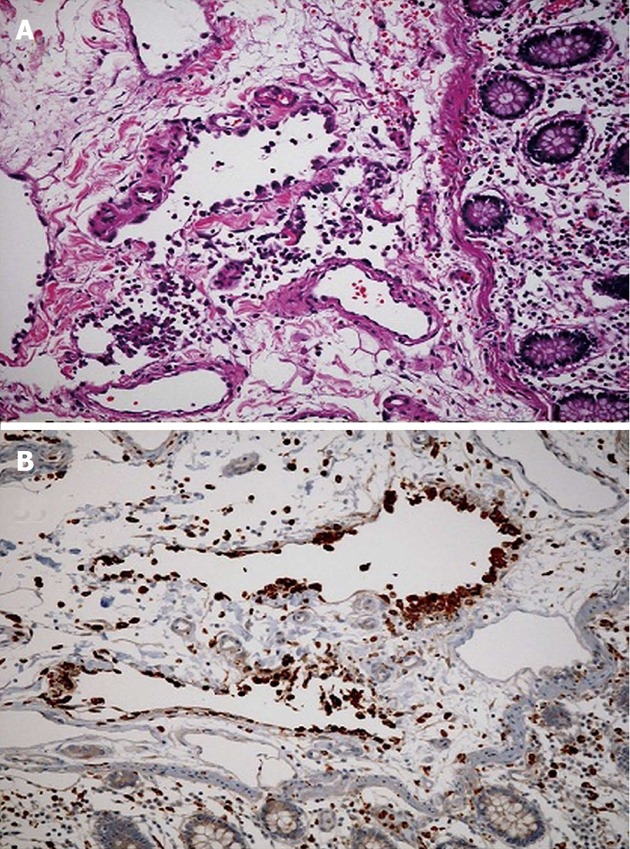
Pathology of the resected tissue revealed portions of large bowel with submucosal and mucosal hemorrhage and submucosa with irregular spaces lined with histiocytes (CD68 positive and CD31 negative) consistent with pneumatosis cystoides intestinalis. A: Cecum specimen stained with hematoxylin and eosin demonstrates partially endotheliolized cystic spaces in the submucosa lined by eosinophilic cells characteristic of pneumatosis intestinalis; B: Immunohistochemical staining for CD 68 which is found in cytoplasmic granules of cells within the monocyte/macrophage lineage confirms presents of histiocytes lining cystic spaces.
DISCUSSION
Sarcoidosis is an idiopathic multisystem non-caseating granulomatous disease that has been reported to impact almost any organ. It is more common in African Americans and typically patients present with pulmonary manifestations associated with bilateral hilar adenopathy on chest X-ray[1]. Other organs frequently involved include skin, lymph nodes, and ocular manifestations. The diagnosis is established by exclusion of other possibilities and can be supported by characteristic physical findings and biopsy showing noncaseating granulomas. ACE levels are elevated in 10% of chronic sarcoidosis and 60% of acute sarcoidosis[1].
The patient’s chief complaint related to the abdominal distension and pain secondary to hepatosplenomegaly. Abdominal pain is an infrequent chief complaint in sarcoidosis however, both the liver and spleen have been reported to be involved[4]. Hepatomegaly has been reported to be as high as 21% of patients clinically and as many as 13% of patients with liver involvement had involvement independent of any typical pulmonary manifestations[4]. Splenomegaly has been reported by physical exam in 5%-14% with hypersplenism seen in 15% of those with splenic involvement[4]. While biopsy specimens from the patient presented are not available given the degree of splenomegaly and pancytopenia one could hypothesize involvement.
The patient described presented with pancytopenia likely secondary to hypersplenism. He was severely thrombocytopenic and leukopenic and mildly anemic. Cytopenias have been frequently described in sarcoidosis. However, to our knowledge significantly fewer reports describing pancytopenia. One report describes thrombocytopenia and leukopenia secondary to hypersplenism a second report notes pancytopenia with splenomegaly both were resolved with splenectomy[5,6]. This indicates a potential therapeutic role for the patient described here, but repeat CT after 5 mo on steroids revealed dramatic improvement in spleen size (Figure 3).
Figure 3.
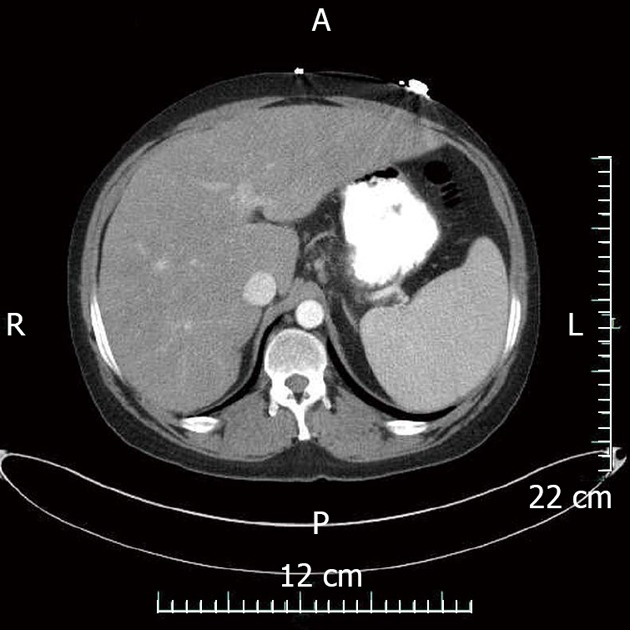
Computed tomography abdomen with contrast demonstrating marked reduction in spleen size and moderate reduction in hepatomegaly after 5 mo of steroid treatment. A: Anterior; P: Posterior; R: Right; L: Left.
Pneumatosis cystoides intestinalis is a rare disorder defined as gas within the bowel wall. The presentation can range from being asymptomatic to acute abdominal pain. The mechanism of pneumatosis cystoides intestinalis is multifactorial. Current theories propose luminal gas which could be pulmonary or bacterial in origin dissect through mucosa which has been compromised. Mucosa may be compromised by a number of mechanisms including immunologically or physically[7].
While typically diagnosed via CT scan[2] certain cases where the pneumatosis is very subtle the finding may be missed and air contrast enema may be useful[8,9]. Furthermore, when diagnosed via CT, the majority of patients can be managed nonoperatively as exploratory laparotomy is likely to be of benefit to only a subset of patients. Those patients include those with abdominal distension, peritonitis, and lactic acidemia[10]. The patient described here represents one of these subtle cases as he was only found to have pneumatosis on pathology. He clearly was in need of operative management given the pneumoperitoneum, abdominal distension, and elevated lactic acid.
Pneumatosis cystoides intestinalis has been linked to a number of conditions in the past. One of which is chronic obstructive lung disease[2,3]. Pulmonary function tests obtained 4 mo after presentation at an outside institution revealed moderate obstructive lung disease with abnormal diffusion capacity [forced expiratory volume in 1 second (FEV1)/forced vital capacity = 70% predicted, FEV1 = 69% predicted, single breath diffusion Dsb 38% predicted]. Typically sarcoidosis most commonly affects the lungs, but the typical radiographic presentation was absent. On chest X-ray bihilar and mediastinal lymphadenopathy (75%) and pulmonary infiltrates (50%) are the most common presentations[1]. This usually occurs in an upper lobe and nodular predominance. On CT scan typical features include bilateral perihilar opacities, bilateral symmetric hilar or mediastinal lymphadenopathy, fibrotic changes such as reticular opacities, architectural distortion, or bronchiectasis[1]. The patient presented had severe emphysematous changes which are rarely associated with sarcoidosis. Prior case reports have documented emphysema in patients with sarcoidosis though the mechanism is unclear[11,12]. While he does have a 15 pack year hx it would be unlikely all lung abnormalities are due to past tobacco at only the age of 39, though this does confound the finding.
Pneumatosis cystoides intestinalis has been very frequently linked to medications. Corticosteroids have been reported most often. Many of the other conditions, pneumatosis cystoides intestinalis has classically been linked to are treated with steroids as well, including connective tissue disease and some autoimmune conditions[2,3,13,14]. The hypothesized mechanism suggests the immunosuppressive effects of steroids results in depletion of the lymphoid tissue within the Peyer’s patches, this leads to mucosal disruption allowing intraluminal gas diffusion[15,16]. One review indicates that 33% of patients with pneumatosis intestinalis had received prior steroid treatment. Another 32% had received either chemotherapy or methotrexate[15,17]. This hypothesis is supported by the fact that other immunosuppressive or cytotoxic drugs have also been associated with pneumatosis cystoides intestinalis. This includes but is not limited to sunitinib, cisplatin, irinotecan, and docetaxel, methotrexate[17-20].
Alternative mechanisms have been described for other drugs that have been implicated in pneumatosis cystoides intestinalis. The chemotherapeutic agents described above have not only been suggested to contribute to the development of pneumatosis cystoides intestinalis via their immunosuppressive effects but also through their apoptotic effects on rapidly dividing cells resulting in compromise of mucosal integrity. Vascular endothelial growth factor and epidermal growth factor inhibitors are hypothesized to damage the microvasculature of the intestinal wall resulting in compromise of mucosal integrity[19,21]. Alpha glucosidase inhibitors, miglitol and acarbose, inhibit the absorption of carbohydrates. It has been suggested that digestion of these carbohydrates by intestinal flora result in gas production. The increased intraluminal pressure could potentially lead to dissection through the mucosa[15,22,23].
The patient was on a steroid taper, but was increased from 25 mg to 40 mg by an outside hospital for reasons that are unclear 1 mo prior to perforation. The 5 mo course of steroids coupled with the recent increased dosage thus could have played a significant role in the pathogenesis through immunosuppression as detailed above. However, given the patients improvement in symptoms and ACE level it seems the initial course of tapering the dosage as symptoms improved was the best course of action.
The patient described here likely had a number of factors that could have additively contributed to the development of pneumatosis cystoides intestinalis. The steroid treatment coupled with his baseline pancytopenia clearly placed him in an immunosuppressed state. The massive hepatomegaly and respiratory disease may have lead to increased intraabdominal pressure producing strain on the mucosal walls. We may also speculate that the 5 year history of untreated GI sarcoidosis may have further weakened the intestinal walls through granulomatosis inflammationa nd disruption of normal architecture. Cumulatively, this created an environment where pneumatosis intestinalis could readily develop.
The described case represents a unique presentation of the difficult to recognize and diagnose GI sarcoidosis. The initial findings of pancytopenia and emphysematous changes in the lungs are typically not found in sarcoidosis. Furthermore this is the first reported association between pneumatosis intestinalis and sarcoidosis though the exact mechanism of pneumatosis cystoides intestinalis is unclear and likely multifactorial as in many such cases.
Footnotes
P- Reviewer Tosetti C S- Editor Huang XZ L- Editor A E- Editor Xiong L
References
- 1.O’Regan A, Berman JS. Sarcoidosis. Ann Intern Med. 2012;156:ITC5–1, ITC5-2, ITC5-3, ITC5-4, ITC5-5, ITC5-6, ITC5-7, ITC5-8, ITC5-9, ITC5-10, ITC5-11, ITC5-12, ITC5-13, ITC5-14, ITC5-15, quiz ITC5-16. doi: 10.7326/0003-4819-156-9-201205010-01005. [DOI] [PubMed] [Google Scholar]
- 2.Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231–239. doi: 10.1186/2047-783X-14-6-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzaroli F, Turco L, Ceroni L, Galloni SS, Buonfiglioli F, Calvanese C, Mazzella G. Pneumatosis cystoides intestinalis. World J Gastroenterol. 2011;17:4932–4936. doi: 10.3748/wjg.v17.i44.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103:3184–392; quiz 3193. doi: 10.1111/j.1572-0241.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 5.Haran MZ, Feldberg E, Miller G, Berrebi A. Sarcoidosis presenting as massive splenomegaly and bicytopenia. Am J Hematol. 2000;63:232–233. doi: 10.1002/(sici)1096-8652(200004)63:4<232::aid-ajh15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Thadani U, Aber CP, Taylor JJ. Massive splenomegaly, pancytopenia and haemolytic anaemia in sarcoidosis. Acta Haematol. 1975;53:230–240. doi: 10.1159/000208188. [DOI] [PubMed] [Google Scholar]
- 7.St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68–75. doi: 10.1001/archsurg.138.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto K, Ueyama T, Iwashita I, Utsunomiya T, Honda H, Onitsuka H, Haraguchi Y, Kojima N, Takano H, Masuda K. Colonic submucosal tumors: comparison of endoscopic US and target air-enema CT with barium enema study and colonoscopy. Radiology. 1994;192:697–702. doi: 10.1148/radiology.192.3.8058936. [DOI] [PubMed] [Google Scholar]
- 9.Ihara E, Harada N, Motomura S, Chijiiwa Y. A new approach to Pneumatosis cystoides intestinalis by target air-enema CT. Am J Gastroenterol. 1998;93:1163–1164. doi: 10.1111/j.1572-0241.1998.354_q.x. [DOI] [PubMed] [Google Scholar]
- 10.Duron VP, Rutigliano S, Machan JT, Dupuy DE, Mazzaglia PJ. Computed tomographic diagnosis of pneumatosis intestinalis: clinical measures predictive of the need for surgical intervention. Arch Surg. 2011;146:506–510. doi: 10.1001/archsurg.2011.95. [DOI] [PubMed] [Google Scholar]
- 11.Judson MA, Strange C. Bullous sarcoidosis: a report of three cases. Chest. 1998;114:1474–1478. doi: 10.1378/chest.114.5.1474. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman I, Mann N. Boeck’s sarcoid; a case of sarcoidosis complicated by pulmonary emphysema and cor pulmonale. Ann Intern Med. 1949;31:153–162. doi: 10.7326/0003-4819-31-1-153. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera GE, Scopelitis E, Cuellar ML, Silveira LH, Mena H, Espinoza LR. Pneumatosis cystoides intestinalis in systemic lupus erythematosus with intestinal vasculitis: treatment with high dose prednisone. Clin Rheumatol. 1994;13:312–316. doi: 10.1007/BF02249034. [DOI] [PubMed] [Google Scholar]
- 14.Ohara H, Kato Y, Nakano M, Ishii Y, Serizawa H, Watanabe N, Wakabayashi K, Tsunematsu S, Kumagai N, Tsuchimoto K, et al. [A case of pneumatosis cystoides intestinalis induced by steroid pulse therapy for severe acute hepatitis B] Nihon Shokakibyo Gakkai Zasshi. 2011;108:1237–1243. [PubMed] [Google Scholar]
- 15.Saito M, Tanikawa A, Nakasute K, Tanaka M, Nishikawa T. Additive contribution of multiple factors in the development of pneumatosis intestinalis: a case report and review of the literature. Clin Rheumatol. 2007;26:601–603. doi: 10.1007/s10067-005-0179-9. [DOI] [PubMed] [Google Scholar]
- 16.Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90:1747–1758. [PubMed] [Google Scholar]
- 17.Sequeira W. Pneumatosis cystoides intestinalis in systemic sclerosis and other diseases. Semin Arthritis Rheum. 1990;19:269–277. doi: 10.1016/0049-0172(90)90049-l. [DOI] [PubMed] [Google Scholar]
- 18.Candelaria M, Bourlon-Cuellar R, Zubieta JL, Noel-Ettiene LM, Sánchez-Sánchez JM. Gastrointestinal pneumatosis after docetaxel chemotherapy. J Clin Gastroenterol. 2002;34:444–445. doi: 10.1097/00004836-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Flaig TW, Kim FJ, La Rosa FG, Breaker K, Schoen J, Russ PD. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest New Drugs. 2009;27:83–87. doi: 10.1007/s10637-008-9146-z. [DOI] [PubMed] [Google Scholar]
- 20.Kung D, Ruan DT, Chan RK, Ericsson ML, Saund MS. Pneumatosis intestinalis and portal venous gas without bowel ischemia in a patient treated with irinotecan and cisplatin. Dig Dis Sci. 2008;53:217–219. doi: 10.1007/s10620-007-9846-9. [DOI] [PubMed] [Google Scholar]
- 21.Coriat R, Ropert S, Mir O, Billemont B, Chaussade S, Massault PP, Blanchet B, Vignaux O, Goldwasser F. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs. 2011;29:1090–1093. doi: 10.1007/s10637-010-9458-7. [DOI] [PubMed] [Google Scholar]
- 22.Kojima K, Tsujimoto T, Fujii H, Morimoto T, Yoshioka S, Kato S, Yasuhara Y, Aizawa S, Sawai M, Makutani S, et al. Pneumatosis cystoides intestinalis induced by the α-glucosidase inhibitor miglitol. Intern Med. 2010;49:1545–1548. doi: 10.2169/internalmedicine.49.3634. [DOI] [PubMed] [Google Scholar]
- 23.Yanaru R, Hizawa K, Nakamura S, Yoshimura R, Watanabe K, Nakamura U, Yoshinari M, Matsumoto T. Regression of pneumatosis cystoides intestinalis after discontinuing of alpha-glucosidase inhibitor administration. J Clin Gastroenterol. 2002;35:204–205. doi: 10.1097/00004836-200208000-00020. [DOI] [PubMed] [Google Scholar]


