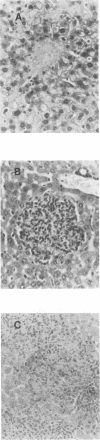
Abstract
The experimental system constructed with the medically significant yersiniae provides a powerful basic model for comparative study of factors required for expression of acute versus chronic disease. The system exploits the close genetic similarity between Yersinia pestis, the etiological agent of bubonic plague, and enteropathogenic Yersinia pseudotuberculosis and Yersinia enterocolitica. Y. pestis possesses three plasmids, of which one, shared by the enteropathogenic species, mediates a number of virulence factors that directly or indirectly promote survival within macrophages and immunosuppression. The two remaining plasmids are unique and encode functions that promote acute disease by enhancing bacterial dissemination in tissues and resistance to phagocytosis by neutrophils and monocytes. These properties are replaced in the enteropathogenic yersiniae by host cell invasins and an adhesin which promote chronic disease; the latter are cryptic in Y. pestis. Additional distinctions include specific mutational losses in Y. pestis which result in loss of fitness in natural environments plus gain of properties that facilitate transmission and infection via fleabite.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G., Wartenberg K., Knapp W. Zuckerzusammensetzung des Lipopolysaccharids und Feinstruktur der äusseren Membran (Zellwand) bei Yersinia enterocolitica. Zentralbl Bakteriol A. 1980;247(2):229–240. [PubMed] [Google Scholar]
- Albizo J. M., Surgalla M. J. Isolation and Biological Characterization of Pasteurella pestis Endotoxin. Infect Immun. 1970 Sep;2(3):229–236. doi: 10.1128/iai.2.3.229-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER E. E., SOMMER H., FOSTER L. E., MEYER E., MEYER K. F. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J Immunol. 1952 Feb;68(2):131–145. [PubMed] [Google Scholar]
- BAUGH C. L., LANHAM J. W., SURGALLA M. J. EFFECTS OF BICARBONATE ON GROWTH OF PASTEURELLA PESTIS. II. CARBON DIOXIDE FIXATION INTO OXALACETATE BY CELL-FREE EXTRACTS. J Bacteriol. 1964 Sep;88:553–558. doi: 10.1128/jb.88.3.553-558.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BICHOWSKY-SLOMNICKI L., BEN-EFRAIM S. BIOLOGICAL ACTIVITIES IN EXTRACTS OF PASTEURELLA PESTIS AND THEIR RELATION TO THE "PH 6 ANTIGEN". J Bacteriol. 1963 Jul;86:101–111. doi: 10.1128/jb.86.1.101-111.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W. An antigen determining virulence in Pasteurella pestis. Nature. 1956 Mar 3;177(4505):426–427. doi: 10.1038/177426b0. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. VIRULENCE OF PASTEURELLA PESTIS AND IMMUNITY TO PLAGUE. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1963;37:59–113. doi: 10.1007/978-3-662-36742-1_2. [DOI] [PubMed] [Google Scholar]
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve S. S., Straley S. C. lcrR, a low-Ca2(+)-response locus with dual Ca2(+)-dependent functions in Yersinia pestis. J Bacteriol. 1990 Aug;172(8):4661–4671. doi: 10.1128/jb.172.8.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Gurion R., Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981 Mar;5(2):183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985 Nov;50(2):392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Håkansson S., Forsberg A., Norlander L., Macellaro A., Bäckman A., Bölin I., Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991 Mar;173(5):1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Guan K. L., Dixon J. E., Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet C., Bruneteau M., Michel G. Lipopolysaccharides d'une souche à virulence atténuée de Yersinia pestis. Eur J Biochem. 1977 Oct 3;79(2):443–449. doi: 10.1111/j.1432-1033.1977.tb11826.x. [DOI] [PubMed] [Google Scholar]
- Brown S. D., Montie T. C. Beta-adrenergic blocking activity of Yersinia pestis murine toxin. Infect Immun. 1977 Oct;18(1):85–93. doi: 10.1128/iai.18.1.85-93.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J Infect Dis. 1967 Dec;117(5):403–417. doi: 10.1093/infdis/117.5.403. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Sample A. K., Yu D. Z., Zahorchak R. J., Hu P. C., Fowler J. M. Proteolysis of V antigen from Yersinia pestis. Microb Pathog. 1987 Jan;2(1):49–62. doi: 10.1016/0882-4010(87)90114-8. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R., Sulen A. Mutations Influencing the Assimilation of Nitrogen by Yersinia pestis. Infect Immun. 1971 Apr;3(4):580–588. doi: 10.1128/iai.3.4.580-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows T. W., Gillett W. A. The nutritional requirements of some Pasteurella species. J Gen Microbiol. 1966 Nov;45(2):333–345. doi: 10.1099/00221287-45-2-333. [DOI] [PubMed] [Google Scholar]
- Bölin I., Forsberg A., Norlander L., Skurnik M., Wolf-Watz H. Identification and mapping of the temperature-inducible, plasmid-encoded proteins of Yersinia spp. Infect Immun. 1988 Feb;56(2):343–348. doi: 10.1128/iai.56.2.343-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984 Jan;43(1):72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- CRUMPTON M. J., DAVIES D. A. An antigenic analysis of Pasteurella pestis by diffusion of antigens and antibodies in agar. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):109–134. doi: 10.1098/rspb.1956.0021. [DOI] [PubMed] [Google Scholar]
- Carniel E., Antoine J. C., Guiyoule A., Guiso N., Mollaret H. H. Purification, location, and immunological characterization of the iron-regulated high-molecular-weight proteins of the highly pathogenic yersiniae. Infect Immun. 1989 Feb;57(2):540–545. doi: 10.1128/iai.57.2.540-545.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mercereau-Puijalon O., Bonnefoy S. The gene coding for the 190,000-dalton iron-regulated protein of Yersinia species is present only in the highly pathogenic strains. Infect Immun. 1989 Apr;57(4):1211–1217. doi: 10.1128/iai.57.4.1211-1217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Charnetzky W. T., Brubaker R. R. RNA synthesis in Yersinia pestis during growth restriction in calcium-deficient medium. J Bacteriol. 1982 Mar;149(3):1089–1095. doi: 10.1128/jb.149.3.1089-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchas R. F., Carniel E. A highly efficient electroporation system for transformation of Yersinia. Gene. 1990 Mar 1;87(1):133–137. doi: 10.1016/0378-1119(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sluiters C., de Rouvroit C. L., Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989 Jan;171(1):254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Sory M. P., Laroche Y., Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica 0:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986 Aug;1(4):349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Vanootegem J. C., Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987 May;2(5):367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- Cowart R. E., Foster B. G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985 Apr;151(4):721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- DAVIES D. A. Dideoxysugars of Pasteurella pseudotuberculosis-specific polysaccharides, and the occurrence of ascarylose. Nature. 1961 Jul 1;191:43–44. doi: 10.1038/191043a0. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A., Brubaker R. R. Consequences of aspartase deficiency in Yersinia pestis. J Bacteriol. 1978 Nov;136(2):757–764. doi: 10.1128/jb.136.2.757-764.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Mutation to rhamnose utilization in Pasteurella pestis. J Bacteriol. 1957 May;73(5):641–648. doi: 10.1128/jb.73.5.641-648.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. The irreversibility of methionine synthesis from cysteine in pasteurella pestis. J Bacteriol. 1952 May;63(5):675–680. doi: 10.1128/jb.63.5.675-680.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgat M., Ben-Gurion R. Mode of action of pesticin. J Bacteriol. 1969 May;98(2):359–367. doi: 10.1128/jb.98.2.359-367.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Emödy L., Heesemann J., Wolf-Watz H., Skurnik M., Kapperud G., O'Toole P., Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989 Dec;171(12):6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Mode of action of pesticin: N-acetylglucosaminidase activity. J Bacteriol. 1979 Aug;139(2):495–501. doi: 10.1128/jb.139.2.495-501.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Fowler J. M., Brubaker R. R. Mutations to tolerance and resistance to pesticin and colicins in Escherichia coli phi. J Bacteriol. 1981 May;146(2):506–511. doi: 10.1128/jb.146.2.506-511.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. Genetic analysis of the yopE region of Yersinia spp.: identification of a novel conserved locus, yerA, regulating yopE expression. J Bacteriol. 1990 Mar;172(3):1547–1555. doi: 10.1128/jb.172.3.1547-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Goguen J. D., Walker W. S., Hatch T. P., Yother J. Plasmid-determined cytotoxicity in Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun. 1986 Mar;51(3):788–794. doi: 10.1128/iai.51.3.788-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985 Aug;49(2):291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Głosnicka R., Gruszkiewicz E. Chemical composition and biological activity of the Yersinia pestis envelope substance. Infect Immun. 1980 Nov;30(2):506–512. doi: 10.1128/iai.30.2.506-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., CARLIN C. E. Studies on the nutrition and physiology of Pasteurella pestis. I. A casein hydrolyzate medium for the growth of Pasteurella pestis. J Bacteriol. 1957 Jan;73(1):122–129. doi: 10.1128/jb.73.1.122-129.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. N., Laird W., Robinson D. M., Cavanaugh D. C. Commonality of a virulence factor among Yersinia species. J Infect Dis. 1980 Mar;141(3):413–413. doi: 10.1093/infdis/141.3.413. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Gross U., Schmidt N., Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986 Nov;54(2):561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C. B., Niesel D. W., Holmgren J., Jonson G., Klimpel G. R. Interferon production by Shigella flexneri-infected fibroblasts depends upon intracellular bacterial metabolism. Infect Immun. 1990 Feb;58(2):399–405. doi: 10.1128/iai.58.2.399-405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier S., Charnetzky W. T. Glyoxylate bypass enzymes in Yersinia species and multiple forms of isocitrate lyase in Yersinia pestis. J Bacteriol. 1981 Jan;145(1):452–458. doi: 10.1128/jb.145.1.452-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J., Lindberg B., Brubaker R. R. Structural studies of the O-specific side-chains of the lipopolysaccharide from Yersinia enterocolitica Ye 128. Carbohydr Res. 1980 Jan 1;78(1):212–214. doi: 10.1016/s0008-6215(00)83675-7. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Brubaker R. R. Characterization of pesticin. Separation of antibacterial activities. J Biol Chem. 1974 Aug 10;249(15):4749–4753. [PubMed] [Google Scholar]
- Hu P. C., Yang G. C., Brubaker R. R. Specificity, induction, and absorption of pesticin. J Bacteriol. 1972 Oct;112(1):212–219. doi: 10.1128/jb.112.1.212-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Mammalian cell adhesion functions and cellular penetration of enteropathogenic Yersinia species. Mol Microbiol. 1989 Oct;3(10):1449–1453. doi: 10.1111/j.1365-2958.1989.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Janssen W. A., Surgalla M. J. Plague bacillus: survival within host phagocytes. Science. 1969 Feb 28;163(3870):950–952. doi: 10.1126/science.163.3870.950. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skarpeid H. J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985 Feb;47(2):561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- Lawton W. D., Molnar D. M. Lysogenic conversion of Pasteurella by Escherichia coli bacteriophage P1 CM. J Virol. 1972 Apr;9(4):708–709. doi: 10.1128/jvi.9.4.708-709.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton W. D., Stull H. B. Chromoome mapping of asteurella pseudotuberculosis by interrupted mating. J Bacteriol. 1971 Mar;105(3):855–863. doi: 10.1128/jb.105.3.855-863.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L. E., Klempner M. S., Straley S. C. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect Immun. 1990 Aug;58(8):2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Genetic determinants of Shigella pathogenicity. Annu Rev Microbiol. 1988;42:127–150. doi: 10.1146/annurev.mi.42.100188.001015. [DOI] [PubMed] [Google Scholar]
- Mazza G., Karu A. E., Kingsbury D. T. Immune response to plasmid- and chromosome-encoded Yersinia antigens. Infect Immun. 1985 Jun;48(3):676–685. doi: 10.1128/iai.48.3.676-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehigh R. J., Sample A. K., Brubaker R. R. Expression of the low calcium response in Yersinia pestis. Microb Pathog. 1989 Mar;6(3):203–217. doi: 10.1016/0882-4010(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesell P., Ivins B. E., Ristroph J. D., Dreier T. M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983 Jan;39(1):371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Montie D. B. Protein toxins of Pasteurella pestis. Subunit composition and acid binding. Biochemistry. 1971 May 25;10(11):2094–2100. doi: 10.1021/bi00787a021. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Mortlock R. P., Brubaker R. R. GLUCOSE-6-PHOSPHATE DEHYDROGENASE AND 6-PHOSPHOGLUCONATE DEHYDROGENASE ACTIVITIES OF PASTEURELLA PESTIS AND PASTEURELLA PSEUDOTUBERCULOSIS. J Bacteriol. 1962 Nov;84(5):1122–1123. doi: 10.1128/jb.84.5.1122-1123.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder B., Michiels T., Simonet M., Sory M. P., Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989 Aug;57(8):2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendrak M. L., Perry R. D. Characterization of a hemin-storage locus of Yersinia pestis. Biol Met. 1991;4(1):41–47. doi: 10.1007/BF01135556. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Harmon P. A., Bowmer W. S., Straley S. C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986 Nov;54(2):428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Pendrak M. L., Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990 Oct;172(10):5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack C., Straley S. C., Klempner M. S. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. 1986 Aug 28-Sep 3Nature. 322(6082):834–836. doi: 10.1038/322834a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Martinez R. J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Cowan C., Perry R. D., Straley S. C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2(+)-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991 Apr;173(8):2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Leung K. Y., Barve S. S., Straley S. C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989 Oct;171(10):5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protsenko O. A., Anisimov P. I., Mozharov O. T., Konnov N. P., Popov Iu A. Vyiavlenie i kharakteristika plazmid chumnogo mikroba, determiniruiushchikh sintez pestitsina I, antigena fraktsiia I i ékzotoksina "myshinogo" toksina. Genetika. 1983 Jul;19(7):1081–1090. [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Neilands J. B., Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983 Apr;154(1):324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Skurnik M., Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988 Aug 11;334(6182):522–524. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- Sample A. K., Brubaker R. R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987 Oct;3(4):239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- Sample A. K., Fowler J. M., Brubaker R. R. Modulation of the low-calcium response in Yersinia pestis via plasmid-plasmid interaction. Microb Pathog. 1987 Jun;2(6):443–453. doi: 10.1016/0882-4010(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Samuelsson K., Lindberg B., Brubaker R. R. Structure of O-specific side chains of lipopolysaccharides from Yersinia pseudotuberculosis. J Bacteriol. 1974 Mar;117(3):1010–1016. doi: 10.1128/jb.117.3.1010-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema D. J., Brubaker R. R. Outer membrane peptides of Yersinia pestis mediating siderophore-independent assimilation of iron. Biol Met. 1989;2(3):174–184. doi: 10.1007/BF01142557. [DOI] [PubMed] [Google Scholar]
- Sikkema D. J., Brubaker R. R. Resistance to pesticin, storage of iron, and invasion of HeLa cells by Yersiniae. Infect Immun. 1987 Mar;55(3):572–578. doi: 10.1128/iai.55.3.572-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990 Mar;58(3):841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Bölin I., Heikkinen H., Piha S., Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984 Jun;158(3):1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Goguen J. D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988 Oct;56(10):2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Goguen J. D. Nucleotide sequence of the plasminogen activator gene of Yersinia pestis: relationship to ompT of Escherichia coli and gene E of Salmonella typhimurium. Infect Immun. 1989 May;57(5):1517–1523. doi: 10.1128/iai.57.5.1517-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Sample A. K., Brubaker R. R., Goguen J. D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988 Oct;56(10):2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982 Apr;36(1):129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Cibull M. L. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989 Apr;57(4):1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984 Sep;45(3):649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Brubaker R. R. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984 Mar;43(3):895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Brubaker R. R. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984 Oct;133(4):2226–2230. [PubMed] [Google Scholar]
- Une T., Nakajima R., Brubaker R. R. Roles of V antigen in promoting virulence in Yersiniae. Contrib Microbiol Immunol. 1987;9:179–185. [PubMed] [Google Scholar]
- Viitanen A. M., Toivanen P., Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990 Jun;172(6):3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S., Neilands J. B., Bittner M. L., Hemming B. C., Haymore B. L., Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Yang G. C., Brubaker R. R. Effect of ca on the synthesis of deoxyribonucleic Acid in virulent and avirulent yersinia. Infect Immun. 1971 Jan;3(1):59–65. doi: 10.1128/iai.3.1.59-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Chamness T. W., Goguen J. D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986 Feb;165(2):443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Goguen J. D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985 Nov;164(2):704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. B., Miller V. L., Falkow S., Schoolnik G. K. Sequence, localization and function of the invasin protein of Yersinia enterocolitica. Mol Microbiol. 1990 Jul;4(7):1119–1128. doi: 10.1111/j.1365-2958.1990.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Zahorchak R. J., Brubaker R. R. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect Immun. 1982 Dec;38(3):953–959. doi: 10.1128/iai.38.3.953-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitman D., Ben-Gurion R. Transduction of Pasteurella pestis. Virology. 1972 Feb;47(2):513–516. doi: 10.1016/0042-6822(72)90291-7. [DOI] [PubMed] [Google Scholar]
- Zsigray R. M., Hopper J. B., Zukowski K., Chesbro W. R. Integration of the Vwa plasmid into the chromosome of Yersinia pestis strains harboring F' plasmids of Escherichia coli. Infect Immun. 1985 Mar;47(3):670–673. doi: 10.1128/iai.47.3.670-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck B. S., Verhoef J. Iron and host defence. Eur J Clin Microbiol. 1983 Feb;2(1):6–10. doi: 10.1007/BF02019915. [DOI] [PubMed] [Google Scholar]