Abstract
Ulcerative colitis (UC) is an inflammation-associated disease of the colon and rectum. The onset and progress of the disease are directly influenced by the nature of the intestinal microflora, the intestinal barrier function, and the immunological responses of the host. The epithelial invasion of pathogenic bacteria due to excess contact and/or barrier dysfunction is related to inflammation mediated by intestinal immune responses. Although the etiology of UC is not clearly understood, recent studies have shown a rising incidence of UC worldwide, and this phenomenon is more prominent in Asian countries and in Asian immigrants in Western countries. The increased prevalence of UC also contributes to an increased risk of developing colorectal cancer. Environmental factors, including changes in dietary habits, have been suggested as major risk factors of UC. A systematic review showed a negative association between UC risk and vegetable intake, whereas total fat, omega-6 fatty acids and meat intake were positively associated with an increased risk of UC. Individual dietary factors and energy balance have been suggested as having important roles in inducing changes in the microbial population and intestinal barrier integrity and in regulating inflammatory immune responses, directly or indirectly. Excess energy intake is now known to increase pathogenic microbial populations. Likewise, the application of appropriate probiotics may reverse the pathogenic progression of the disease. In the meantime, dietary anti-inflammatory compounds, including omega-3 fatty acids and other phytochemicals, may directly suppress inflammatory responses in the course of UC development. In this review, the increased prevalence of UC and its management are interpreted from the standpoint of nutritional modulation to regulate the intestinal microflora population, intestinal epithelium permeability, and inflammatory responses.
Keywords: Ulcerative colitis, Intestinal microflora, Immunity, Inflammation, Clinical, Obesity, Probiotics, Omega-3 fatty acids, Antioxidants
INTRODUCTION
Ulcerative colitis (UC) is a major type of inflammatory bowel disease (IBD) characterized by chronic inflammation in the colon and rectum. It progresses by extensive epithelial apoptosis and ulceration due to chronic inflammation induced by T-helper (Th) 2 cytokines[1]. Recent studies have also indicated that a balance between proinflammatory Th17 cells and immunsuppressive Treg cells play a crucial role in the development of UC[2]. Although the etiology of UC has not been clearly determined, environmental factors are thought to stimulate overt immune responses to bacterial components in individuals with high genetic susceptibility. A recent report on the incidence of IBD in Asia indicated that the prevalence of UC is growing rapidly in Japan, Hong Kong, and South Korea[3-5], countries where IBD used to be rare. The most recent reports on UC prevalence in Japan and South Korea provided figures of 63.6 and 30.9 per 100 000 people, respectively[4,5]. UC reportedly affects 0.24% of the United States population[6], and the prevalence in Northern European countries ranges from 40 to 240 per 100 000 people[7].
Lifestyle changes, as well as increased awareness of the disease and improved diagnosis, may have contributed to the increased incidence. Dietary habits in Asian countries have changed, resulting in a Western-style diet with fewer plant-based and more processed foods. A recent systematic review of 19 studies reported a negative association between UC risk and vegetable intake, whereas total fat, omega-6 fatty acids, and meat intake were positively associated with increased UC risk[8]. Information is limited, however, on the role of individual dietary components in UC development, and most nutritional modulation studies have focused on delaying relapses of UC, efforts that involve secondary rather than primary prevention. The cumulative incidence of relapse in UC are 30%, 72%, and 88% after 1, 5, and 10 years following the initial diagnosis[9]. Patients younger than 40 years have been shown to present with more severe disease at the time of diagnosis compared with older patients[10]. Importantly, the increased incidence of UC may be closely related to an increase in the prevalence of colorectal cancer (CRC). A subset study population with UC from the Kaiser Permanente Medical Care Program was analyzed for CRC incidence and mortality, and the standardized mortality ratio for CRC among UC patients was 2.0 (95%CI 1.3-2.7)[11]. Chronic inflammation mediates a wide range of signaling cascades that possibly facilitate colorectal carcinogenesis, and UC remission may reduce the risk of CRC. In this review, the pathophysiology of UC is summarized to allow understanding of the molecular mechanisms involved in the action of dietary components. Major dietary components reported to regulate, directly or indirectly, inflammatory responses in UC are discussed, with a focus on human studies where available. Genetic factors and therapeutic measures, while important to consider, fall outside the scope of this review.
INTESTINAL BARRIER FUNCTIONS AND INFLAMMATION IN UC
The human large intestine contains a concentration of approximately 1011-1012 microorganisms per gram of luminal content. These microbes can be either beneficial or harmful to the intestinal epithelium. Under normal circumstances, multiple mechanisms protect the intestinal epithelium from microbial invasion, but environmental stimuli in combination with genetic factors can facilitate overgrowth of harmful microflora and induce abnormal immune responses that disrupt the mucosal barrier, causing inflammation (Figure 1).
Figure 1.
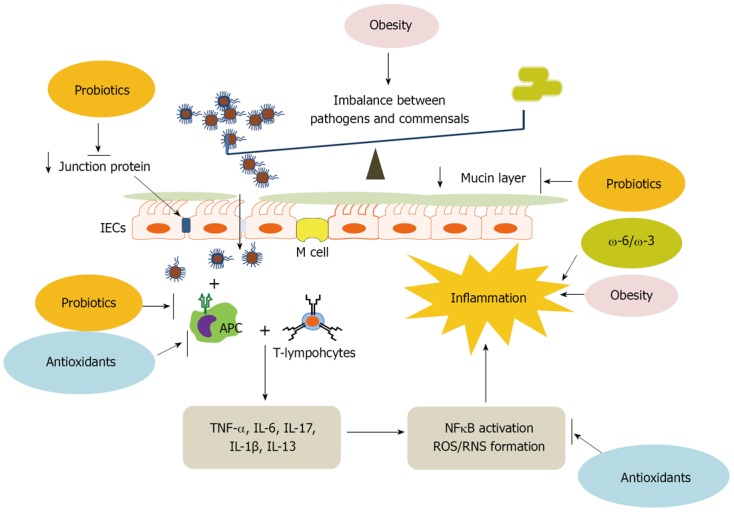
Disrupted intestinal homeostasis in ulcerative colitis and the role of nutritional factors. Ulcerative colitis is a chronic inflammatory disease of the colon and rectum. Inflammatory responses are induced by the penetration of excessive pathogenic bacteria due to the increased population of pathogenic bacteria, the loss of junction proteins and thin mucin layer. Once pathogens are recognized by antigen-presenting cells (APC), T-lymphocytes produce pro-inflammatory cytokines activating inflammation-inducing nuclear transcription factor, nuclear factor (NF)-κB and generating reactive oxygen species (ROS) and reactive nitrogen species (RNS) which result in the inflamed intestine. Obesity is known to cause imbalances between pathogens and commensals as well as chronic inflammation. The increased ω-6 to ω-3 fatty acid ratio in the diet also accelerates inflammatory responses. Probiotics supplementation helps to maintain gut health by retaining tight junctions and mucin layer. Probiotics and antioxidants suppress immoderate immune responses, and ROS-induced inflammatory responses are moderated by antioxidants. TNF-α: Tumor necrosis factor-α; IL-1β: Interleukin-1β; IECs: Intestinal epithelial cells.
The luminal side of the intestinal membrane is in constant contact with intestinal microflora. The most evident characteristic of pathogenic bacteria are their invasiveness and induction of inflammatory responses in the intestinal epithelium[12]. The host system, in other words, is able to recognize and differentiate pathogens from the commensals. To protect the host from pathogenic bacteria, the intestinal epithelium is equipped with multiple defense systems. Intestinal epithelial cells (IECs), which are layered by glycoproteins such as mucin, form the first line of immune defense. Tight junction (TJ) proteins seal the space between IECs. The layer below the IECs is the sub-epithelial dome, containing antigen-presenting cells, and underneath are Payer’s patches, where B-cell follicles and T-cells reside.
Bacterial invasion is recognized by receptors called pattern recognition receptors (PRRs) in the IEC membrane, and the primary PRRs are toll-like receptors (TLRs). There are 10 different classes of TLRs expressed throughout the whole human gastrointestinal tract. The recognition of bacteria is carried out by means of communication between PRRs and microbial components such as lipopolysaccharide (LPS), and this is followed by immune responses to destroy the invading pathogens. Microbiota and viral-associated ligands use different types of TLRs depending on molecular patterns. For example, TLR2 recognizes lipopeptides, TLR 3 recognizes viral-derived dsRNA, and TLR4 recognizes LPSs[13]. Constant immune responses provoke chronic inflammation, which is mediated by pro-inflammatory cytokines and chemokines. The inflammation not only contributes to clinical features of inflammatory disease, but also exacerbates the penetration of pathogenic bacteria by increasing the membrane permeability, creating a vicious cycle. The ability of the intestinal epithelium to distinguish commensal from pathogenic bacteria is important because the intestine requires a symbiotic relationship with commensals without immune responses. Several explanations exist for the intestinal epithelium’s ability to discriminate harmful bacteria from commensals[14]. In brief, pathogenic bacteria possesses virulence factors to stimulate the innate immune responses while commensals mutate their molecular patterns, escaping recognition by TLRs, and attenuate the nuclear factor (NF)-κB pathway. Also, the hyporesponsiveness to commensals has been explained by the anti-inflammatory nature of the gut mucosa including reduced expression of PRRs and reduced inflammatory nature of intestinal immune cells.
DIETARY FACTORS MODULATING INTESTINAL BARRIER FUNCTIONS IN UC
Excess energy (obesity)
Obesity is the most convincing risk factor in the development of many noncommunicable diseases, including type 2 diabetes, cardiovascular diseases, non-alcoholic fatty liver disease, and selected types of cancer. World Health Organization statistics stated that physical inactivity and being overweight or obese contributed, respectively, to 5% and 6% of deaths[15]. The estimated prevalence of overweight [body mass index (BMI) ≥ 25 kg/m2] and obese (BMI ≥ 30 kg/m2) males and females aged 15 or older was ≥ 80% in the United States, ≥ 65% in Canada, Mexico, Argentina, Australia, the United Kingdom, and Germany, and ≥ 35% in all other countries, except for several African countries, India, and South East Asian countries[16].
Obesity and abdominal obesity have been specifically identified as the most convincing risk factors for the development of CRC[17]. In many studies, obesity-related inflammatory biomarkers were shown to be associated with disease activity. Among adipokines, resistin was considered to have a positive relationship with disease activity[18,19]. In a multicenter registry of children with IBD, 1 in 3 children with UC were overweight or obese, which is comparable to the rate in the general population[20]. However, prior IBD-related surgery was positively associated with being overweight or obese, which implies that obesity may provoke a more severe disease course. CRP was found to be a marker predictive of disease progression in a population-based prospective study[21]. CRP concentrations above 23 mg/L at diagnosis showed an odds ratio (OR) of 4.8 (95%CI, 1.5-15.1) in the prediction of surgery in UC patients. These results suggest that systemic inflammation worsens disease activity, and therefore the suppression of inflammatory events may be critical for the management of UC.
Mechanistic explanations have not been provided regarding the relationship between obesity and intestinal inflammation in patients. However, a number of rodent model studies have indicated that intestinal inflammation is mediated through obesity-related factors. Leptin was shown to act as a critical mediator in colitis development[22]. Leptin-deficient (ob/ob) mice were shown to be resistant to chemically-induced colitis, indicating that leptin is a critical regulator. Adiponectin, on the other hand, was shown to be a negative regulator in the development of dextran sulfate sodium (DSS)-induced colitis through the suppression of pro-inflammatory cytokine and chemokine production[23]. In a recent study, the effects of high-fat diet-induced obesity and chemically-induced colitis were compared in the development of UC[24]. A high-fat diet alone did not induce characteristic histopathological features of UC, indicating UC development is accelerated through inflammatory infiltration in the colon tissue of chemically-induced colitis. It is also interesting to note that the inflamed intestines of mice with chemically-induced colitis exhibited higher inflammatory activities in the mesenteric fat compared with obese mice, which implies that intestinal inflammation may precede systemic inflammation[25]. These results suggest that obesity may not be an independent risk factor for UC development, although it may aggravate disease progression.
There are several reports showing evidence of obesity-associated changes in the intestinal microbiota. However, there is no clear explanation if this is due to adiposity or dietary composition[26]. Therefore, the altered microbial population is either a cause or a consequences of obesity. A recent report suggested that diets high in saturated fat promote taurine-conjugated bile acid formation, altering microbial composition towards the overgrowth of a sulfite-reducing pathobiont, Bilophilla wadsworthia. This accelerated the development of colitis in interleukin (IL)-10 -/- mice[27], providing evidence of a direct contribution from increased dietary fat. High-saturated fat diets altered microbial composition, resulting in a significantly higher ratio of Firmicutes to Bacteroidetes[28]. A decreased proportion of Bacteroidetes[29] was also observed in IBD, suggesting a possible mediating effect of these bacteria in obesity-related pathogenic changes in the intestinal epithelium. Others have reported that changes in the gut microbiota directly contribute to the development of obesity and related metabolic disturbances[30,31], which requires further investigation.
Fatty acids
The intakes of pro-inflammatory omega-6 fatty acids and anti-inflammatory omega-3 fatty acids have been suggested as important regulators in UC disease activity. A recent systematic review concluded that available randomized controlled trials do not indicate that omega-3 fatty acids are useful to alleviate IBD[32].
Epidemiological studies have been conducted to investigate the association between UC and nutrients. A total of 139 UC patients were identified as a subgroup of the population participating in a large prospective cohort study, the European Prospective Investigation into Cancer and Nutrition (EPIC)[33]. Results indicated that there is a marginally significant association between UC and an increasing percentage intake of energy from total polyunsaturated fatty acids (OR, 1.19; 95%CI, 0.99-1.14, P = 0.07). In another EPIC sub-cohort report, the highest quartile of intake of linoleic acid was positively associated with UC risk (OR, 2.49; 95%CI, 1.23-5.07)[34]. A nested United Kingdom cohort study analyzed the effect of total and specific dietary omega-3 fatty acids intake on the risk of UC[35]. Docosahexaenoic acid (DHA) was found to have a statistically significant protective OR of 0.43 (95%CI, 0.22-0.86), while total omega-3 fatty acids and eicosapentaenoic acid (EPA) showed marginally negative values.
Few studies have reported fatty acid composition markers of UC in human biospecimens. In accordance with the above mentioned epidemiological evidence, one study showed that the erythrocyte membrane content of linoleic acid, the most abundant dietary polyunsaturated acid present in plant seed oil was significantly higher in IBD patients (29 UC and 20 CD patients) compared with control subjects (n = 31)[36]. In the same study, UC patients showed a significantly higher ratio of arachidonic acid (AA) to EPA compared with control subjects. Leukotrienes (LTs) are pro-inflammatory lipid mediators involved in the progression of UC. The 5-lipoxygenase pathway (5-LOX) catalyzes the formation of LTs from AA, and the deletion of the gene encoding 5-LOX in an animal model prevented the development of chemically-induced colitis[37]. In a prospective case-control study, UC patients had significantly higher urinary LTE4 excretion compared with controls[38]. A subpopulation of the EPIC prospective cohort study showed that subjects in the highest quartile for adipose tissue AA concentration had a relative risk for UC of 4.16 (95%CI, 1.38-2.27)[39].
Experimental animal model studies have investigated the effects on UC development of omega-3 fatty acid supplementation or an increased ratio of omega-3 to omega-6 fatty acid in the diet[41-43]. Possible mechanistic explanations include: (1) the restoration of membrane TJ protein expression and distribution; or (2) the activation of peroxisome proliferator-activated receptor-γ, possibly inhibiting NFκB transcriptional activity. However, Varnalidis et al[42] suggested that the ameliorating effects of omega-3 fatty acids are accompanied by increased colonic neutrophil infiltration, and needs further clarification. Matsunaga et al[41] reported that feeding fish oil (8% w/w) exacerbates DSS-induced colitis in mice by suppressing the expression of adiponectin in myofibroblasts of the intestinal epithelium.
Apart from omega-6 and omega-3 fatty acids, short chain fatty acids have been suggested to suppress intestinal inflammation through G-protein-coupled receptor 43 (GPR-43)[44]. Acetate supplementation suppressed DSS-induced colonic inflammation in wild-type mice, while GPR-43 -/- mice did not respond to acetate. These results explain the role that intestinal microbiota play in producing short chain fatty acids from dietary fiber in the maintenance of a healthy gut.
The efficacy and safety of fish oil in the remission of IBD [Crohn’s disease (CD) or UC] have been investigated in clinical trials. A recent meta-analysis retrieved 9 randomized, placebo-controlled trial of fish oil administered for at least 6 mo[45]. A total of 1.8-6.2 g/d of DHA + EPA or total omega-3 fatty acids were provided during the study period, and the primary outcome was relapse rate. A meta-analysis indicated that there was no significant reduction in the relative risk of UC, while the pooled relative risk of CD relapse was 0.77 (95%CI, 0.61-0.98). A clinical trial based on n-3 polyunsaturated diet therapy (n-6 to n-3 fatty acid ratio of approximately 1) reported that subjects exhibiting disease remission after a n-3 PUFA diet had a higher ratio of n-3/n-6 fatty acids in the red cell membrane compared with the relapse group[46].
Probiotics
Probiotics are live, nonpathogenic microorganisms that contribute to the improvement of epithelial and mucosal barrier function through various mechanisms, including reduced intestinal pH, inhibition of pathogenic bacteria, and modulation of the intestinal mucosa immune responses[47-49]. Although there is a growing body of evidence from in vivo studies on the beneficial effects of probiotics in UC, reliable clinical trials are limited in number. The aim of this chapter is to review randomized, double-blind, placebo-controlled clinical trials which suggest that probiotics are effective in UC.
The probiotic Escherichia Coli strain Nissle 1917 (EcN) is non-pathogenic and one of the best characterized strains used as a probiotic drug[50]. The clinical effectiveness of EcN in adult UC patients seems to be promising[51]. Patients with moderate distal active UC were assigned to treatment with either 10, 20, or 40 mL of EcN enema (n = 23, 23, 24) containing 108 CFU/mL or placebo once a day for at least 2 wk. If there was no disease activity index (DAI) improvement after 2 wk, patients were classified as non-responders and discontinued therapy. Those classified as responders could receive the treatment for 4 or 8 wk. Although the number of responders was not significantly higher in the EcN group than in the placebo group, the efficacy of rectal EcN treatment was dose-dependent and significant in the per-protocol analysis. This Phase II study showed that EcN is an effective and well-tolerated alternative or supplementary treatment in mild-to-moderate distal UC patients.
The induction of remission in patients with mild-to-moderate UC was also demonstrated with VSL3™ treatment. VSL3 is a high-concentration probiotic mixture, which combines 8 different bacterial species including Bifidobacterium (B.) longum, B. breve, B. infantis, Lactobacillus (L.) casei, L. plantarum, L. acidophilus, L. Bulgaricus, and Streptococcus thermophilus[52]. In a multicenter, randomized, double-blind, placebo-controlled trial, subjects received either VSL3 (n = 77) containing 3.6 × 1012 CFU or placebo (n = 70) twice per day for 12 wk[53]. VSL3 led to a 50% improvement in DAI at 6 wk and clinical remission was shown in 33 patients given VSL3 (42.9%) compared with 11 patients given placebo (15.7%) at 12 wk.
A single-center, randomized, double-blind, placebo-controlled trial of the efficacy of symbiotic treatment was performed using 18 patients with active UC[54]. Participants were given either 2 × 1011 B. longum and 6 g of prebiotic fructo-oligosaccharide/inulin mix (n = 9) or placebo (n = 9) twice a day for 4 wk. The results showed that consumption of symbiotic treatment over 4 wk significantly ameliorated mucosal mRNA levels of human beta-defensins 2, 3, and 4, which are strongly upregulated in active UC. In addition, mucosal mRNA levels of tumor necrosis factor-α (TNF-α) and IL-1α were significantly decreased in the symbiotic group, with remission of sigmoidoscopy scores. This small trial provided the potential efficacy of symbiotic therapy for patients with active UC. The effect of L. acidophilus La-5 and B. lactis BB-12 (Probio-Tec AB-25) was investigated in a randomized, double-blind, placebo-controlled trial[55]. Patients with left-side UC were given either Probio-Tec AB-25 (n = 20) or placebo (n = 12) for 52 wk, and clinical, endoscopic, and histological parameters were analyzed at entry and in case of relapse. In this study, there was no statistical difference in remission rate and the median time to relapse between the two groups after 1 year of treatment. The authors concluded that Probio-Tec AB-25 was well tolerated by study participants and that a failure to prove efficacy in remission rate may be due to the small sample size.
The use of probiotics as maintenance therapy in UC patients seems promising. Two randomized, double blind, double-dummy clinical trials to determine the efficacy of EcN were conducted. In the preliminary study, 120 patients with inactive UC were treated with either an oral preparation of EcN containing 25 × 109 bacteria daily or mesalazine 1500 mg daily for 12 wk. The start and end DAI scores demonstrated no significant difference between the two groups. The relapse rate was 16.0% in EcN group and 11.3% among those taking mesalazine[56]. The same authors investigated the effectiveness of EcN in 327 patients with UC. Subjects received either probiotics containing 5 × 1010 bacteria or mesalazine 1500 mg daily (n = 162, 165) for 12 mo. According to the per protocol analysis, 36.4% of patients in the EcN group experienced a relapse, compared with 33.9% of patients in the mesalazine group. The investigators concluded that EcN is efficient and safe in maintaining remission, and equivalent to the standard mesalazine[57]. Another study also confirmed that the administration of EcN was as effective as mesalazine in the maintenance of remission in patients with UC[58]. Patients with active UC were randomized to EcN treatment of 5 × 1010 probiotics/d (n = 57) or mesalazine treatment (1200 mg/d, n = 59) for 12 mo. In the intention-to-treat analysis, the health conditions were improved in 39 (68.4%) subjects in the EcN group and 44 (74.5%) subjects in the mesalazine group. The mean time of remission was 42 d (median 37 d) in the EcN group and 44 d (mean 42 d) in the mesalazine group. These results support the conclusion that EcN treatment has an equivalent effect to mesalazine in maintaining remission of UC.
The efficacy of VSL3 on induction and maintenance of remission in patients with active UC was studied in a long-term, randomized, double-blind, placebo-controlled trial[59]. In this study, subjects were children (mean age: 9.8 years; range: 1.7-16.1 years) and they were received either VSL3 containing 4.5-18 × 1011 bacteria/d (n = 14) or placebo (n = 15) for 1 year. All of the 29 patients were also treated with mesalazine during this trial period. Three of the 14 (21.4%) subjects in the VSL3 group and 11 of the 15 (73.3%) subjects in the placebo group relapsed within 1 year of follow-up. At 6 mo, 12 mo, or at time of relapse, endoscopic and histological scores were significantly lower in the VSL3 group compared with the patients in the placebo group. No adverse effect related to VSL3 was observed. Although the sample size of this study was small, it provided evidence for the efficacy and safety of VSL3 in pediatric UC patients receiving conventional IBD therapy.
Another study evaluated the efficacy of VSL3 supplementation in patients with mild-to-moderate UC who were already being treated with 5-aminosalicylic acid (5-ASA) and/or immunosuppressants[60]. The patients were randomly treated with either VSL3 (n = 65) including 3600 × 109 CFU/d or placebo (n = 66) for 8 wk. After 8 wk, decreases in DAI scores of 50% or more were significantly higher in the VSL3 group than in the placebo group. However, there was no significant difference in stool frequency, physician rating of disease, and endoscopic scores between the two groups. Although the mechanism(s) of VSL3 treatment in IBD therapy is unclear, these two randomized, double-blind, placebo-controlled clinical trials showed the potential synergistic activity of probiotics.
A large number of animal model studies have reported protective effects of probiotics, and these results are summarized elsewhere[61]. Most of the studies used DSS mouse[62-64], trinitrobenzene sulfonic acid (TNBS) mouse[65-67] and IL-10-/- mouse[68-70]. The most frequently used probiotics were VSL3, Lactobacillus, Bifidobacterium, and Saccharomyces. The proposed mechanisms of action were as follows: (1) reduction of inflammatory cytokine and chemokine levels; (2) downregulation of TLR signaling followed by immune suppression; (3) increase in epithelium gap junction protein expressions; (4) prevention of pathogen growth and/or attachment; (5) increase in mucin production; (6) decrease in leukotriene B4 production; and (7) increase in short-chain fatty acid production. The reduced level of inflammatory cytokines and chemokines were thought to be mediated through signal transducer and activator of transcriptions-3 signaling[71,72] and/or NF-κB activation[73,74].
Although the specific mechanisms of action of probiotics are unknown, their therapeutic effects may be related to modulation of antigen-presenting cells. Local T cell immunity is an important factor involved in the specific intestinal immune system and its activation is programmed by dendritic cells (DCs) that help maintain mucosal tolerance and contribute to the development of chronic intestinal inflammation as key initiators of innate and adaptive immune responses in the gut[75-77]. However, the immunological mechanisms of certain probiotics are still poorly understood while several studies have demonstrated that anti-inflammatory activity of probiotics is due to modulation of DC and regulatory T cell (Treg) phenotype function. It was reported that the therapeutic effect of VSL3 was due to regulation mucosal CD4+ T cell responses in a colitis-associated CRC model[78]. VSL3 administration in UC patients significantly decreased TLR-2 expression on colonic DC[79]. Also, VSL3 supplementation increased IL-10 production and decreased IL-12p40 production by colonic DC[76]. VSL3 administration induced a significant expansion of mucosal Treg cells in UC patients[80]. L. rhamnosus induced maturation of monocyte-derived DC and induced both lower IL-12 and IL-18 production and development of T cells without a typical Th phenotype[81]. A probiotic mixture containing L. helveticus and L. rhamnosus increased follicular Treg cells in Citrobacter rodentium-induced colitis[82]. L. casei inhibited TNF-induced secretion of the T-cell chemokine interferon-inducible protein 10 (IP-10) in IECs by blocking IP-10 protein secretion and IP-10-mediated T-cell transmigration[83]. L. casei lysate DN-114 001 treatment increased the numbers of CD4(+)FoxP3(+)Treg cells in mesenteric lymph nodes, decreased the production of TNF-α, interferon-γ, and IL-10 in Peyer’s patches and large intestine, and changed the gut microbiota composition in a DSS-induced colitis model[84]. L-peptidoglycan purified from Ls33 also ameliorated TNBS-induced colitis by development of CD103(+) DCs and CD4(+)Foxp3(+) Treg cells[85]. Thomas et al[86] showed that Saccharomyces bourladii, a probiotic yeast preparation, reduced IL-6 and TNF-α and decreased the expression of co-stimulated surface markers CD40, CD80, and the migration marker CD197 (CCR7) on LPS-stimulated human 92 induced apoptosis of antigen-stimulated T cells. These data suggest that each strain exhibits a different ability to modulate DC and Treg functions and explain various potential mechanism(s) of bacterial strains.
Current evidence suggests that probiotics are good complementary and alternative medicine candidates to maintain remission and prevent relapse of UC. However, the clinical efficacy results are limited, and strain-specific mechanistic explanations are insufficient. Additional large-scale clinical trials are required to shed light on optimal doses and treatment periods.
Antioxidant vitamins and phytochemicals
Oxidative stress is known as a potential etiological and/or triggering factor in the initiation and preservation of UC[47,87-89]. Inflammation augments oxidative stress by activating reactive oxygen and/or reactive nitrogen, generating enzymes such as NAD(P)H oxidase, inducible nitric oxide synthase, and myeloperoxidase[89,90]. A group of antioxidants has been used to ameliorate the clinical condition of UC patients. In addition, several studies have shown that patients with UC often have antioxidant nutrient deficiencies at the time of diagnosis[91-93]. Although the important role of antioxidants in UC has been seen in several studies, few clinical trials have been conducted. A randomized, controlled trial evaluated the efficacy of a nutritionally balanced oral supplement including fish oil, fructo-oligosaccharides, gum arabic, vitamin E, vitamin C, and selenium in 121 patients with mild-to-moderate UC[93]. Patients consumed either 510.3 g of the oral supplement or placebo each day for 6 mo. Compared with the placebo group, both intent-to-treat and completer patients given the oral supplement showed a significant decrease in the dose of prednisone required to control clinical symptoms over 6 mo. Mirbagheri et al[94] performed an open-label, preliminary trial for the efficacy of D-α-tocopherol using 14 patients who were receiving concomitant therapy with 5-ASA and/or immunosuppressants. Patients received a D-α-tocopherol enema (8000 U/d) for 12 wk. At the end of 12 wk, the DAI score had statistically decreased from the beginning of the study, all 14 patients responded clinically to the therapy, and remission was induced in 9 patients (64%) without adverse events.
In recent years, several studies have investigated the efficacy of curcumin, a polyphenolic antioxidant in Curcuma longa L., in experimental models of UC. These studies have showed a strong antioxidant effect, as well as an anti-inflammatory effect, of curcumin in this model. A small, open-label, pilot clinical trial of the effect of curcumin in patients with IBD (5 UC, 5 CD) receiving IBD medication (5-ASA or corticosteroids) was conducted[95]. The patients were given curcumin 1100 mg/d for 1 mo and 1650 mg/d for an additional 2 mo. All 5 patients with UC had significant improvement in their medication, as follows: two patients stopped taking 5-ASA, two reduced 5-ASA dosage, and one stopped corticosteroids entirely. This encouraging pilot study showed the strong potential efficacy of curcumin in UC patients.
In a multicenter, randomized, double-blind, placebo-controlled trial, the efficacy of curcumin in 89 patients with quiescent UC was evaluated[96]. In addition to their usual medication (sulfasalazine or mesalamine), participants received either curcumin (2 g/d) or placebo (n = 45, 44) for 6 mo. DAI and endoscopic scores were determined at entry, every 2 mo (DAI), at the conclusion of the 6-mo trial, and after a 6-mo follow-up. The relapse rate was significantly lower in the curcumin group than in the placebo group and the recurrence rate was significantly reduced in the curcumin group compared with the placebo group. Both the DAI and endoscopic score were also improved in the curcumin group without any adverse effects. Although only two clinical trials have been performed, the potential therapeutic capability of curcumin in UC patients has been shown, possibly resulting from its antioxidant and anti-inflammatory effects. However, large-scale, randomized, double-blind, placebo-controlled clinical trials are required to achieve convincing evidence for the routine use of antioxidants in patients with UC.
Many studies have reported that antioxidant supplementation has therapeutic efficacy in animal models, and these results were summarized in a recent review[97]. Mechanisms of action include: (1) direct scavenging of reactive oxygen species; (2) suppression of proinflammatory protein expression by downregulating related enzymes or transcription factors; and (3) alteration of leukocyte cell surface molecules.
UC is associated with increased gastrointestinal permeability[98,99]. Although the causes of increased permeability are not completely understood, an inflammation-associated increase in permeability has been shown to result in bacterial translocation into the lamina propria, which exacerbates the loss of barrier function. We have previously reported that aloe anthraquinones and chromone ameliorate colonic inflammatory responses in a DSS-induced UC model; aloesin, an aloe chromone, showed the most drastic results[100]. Additionally, we studied whether aloesin modulates mucosal permeability and found that this phytochemical recovered the gene expression levels of apical junctional complex proteins, which regulate gut permeability and barrier function (unpublished data). These results suggest that the anti-inflammatory property of aloesin is partly based on regulation of gut permeability, although human studies of clinical use are required.
Over the last few decades, phytochemical-based complementary and alternative treatments have emerged as relatively effective and safe therapeutic strategies for UC. Although a significant number of animal studies have established the potential beneficial effects on UC of phytochemicals and bioactive components of other natural sources, the exact mechanisms and/or molecular targets of their anti-inflammatory actions are not fully understood. Further studies are needed to evaluate their safety, targets, phytochemical-drug interactions, and pharmacokinetic information. Understanding these issues through well-designed clinical trials will enable us to choose reliable complementary and alternative therapies for patients with UC.
CONCLUSION
Because the increased prevalence of UC is potentially due to changes in dietary habits, especially in Eastern countries, it is now considered a lifestyle-related disease. It has been proposed that UC onset and progress are modulated by dietary factors such as excess energy intake, saturated fat intake, fatty acid ratio in the diet, and antioxidant intake. However, well-controlled human intervention trials are required to clarify the effect of nutritional factors on UC development. As understanding of the mechanisms involved in UC improves, a target-oriented search for compounds possessing therapeutic or complementary value becomes possible (Table 1). Nutritional modulation that aims to not only prevent the onset and relapse of the disease but also maintain remission will contribute to the successful management of UC.
Table 1.
Action targets of major beneficial dietary components in ulcerative colitis
| Omega-3 fatty acids | Probiotics | Anti-oxidants | Aloe | |
| Beneficial bacteria growth | + | |||
| Short chain fatty acid production | + | |||
| Mucin repletion | + | + | ||
| Junction protein restoration | + | |||
| Suppression of inflammatory eicosanoids | + | |||
| Blockade of inflammatory signals | + | + | + | |
| Suppression of immune response | + | + | + | |
| Reduction in oxidative stress | + |
Footnotes
Supported by Mid-career Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A2A2A01046228)
P- Reviewers Meyer N, Yamakawa M, Odes S S- Editor Song XX L- Editor Cant MR E- Editor Xiong L
References
- 1.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Sabatino A, Biancheri P, Rovedatti L, Macdonald TT, Corazza GR. Recent advances in understanding ulcerative colitis. Intern Emerg Med. 2012;7:103–111. doi: 10.1007/s11739-011-0719-z. [DOI] [PubMed] [Google Scholar]
- 3.Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 4.Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659–665. doi: 10.1007/s00535-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 5.Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 6.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn‘s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 9.Park JB, Yang SK, Byeon JS, Park ER, Moon G, Myung SJ, Park WK, Yoon SG, Kim HS, Lee JG, et al. Familial occurrence of inflammatory bowel disease in Korea. Inflamm Bowel Dis. 2006;12:1146–1151. doi: 10.1097/01.mib.0000235094.01608.59. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Cheon JH, Moon CM, Park JJ, Hong SP, Kim TI, Kim WH. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion. 2010;81:237–243. doi: 10.1159/000253850. [DOI] [PubMed] [Google Scholar]
- 11.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–389. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Sansonetti PJ. War and peace at the intestinal epithelial surface: an integrated view of bacterial commensalism versus bacterial pathogenicity. J Pediatr Gastroenterol Nutr. 2008;46 Suppl 1:E6–E7. doi: 10.1097/01.mpg.0000313819.96520.27. [DOI] [PubMed] [Google Scholar]
- 13.Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis. 2010;16:1583–1597. doi: 10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan N. Telling apart friend from foe: discriminating between commensals and pathogens at mucosal sites. Innate Immun. 2010;16:391–404. doi: 10.1177/1753425909357577. [DOI] [PubMed] [Google Scholar]
- 15. Available from: http: //www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.
- 16. Available from: http: //apps.who.int/infobase.
- 17.Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. 2007. [Google Scholar]
- 18.Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, Dietz E, Norman K, Buning C, Winklhofer-Roob BM, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, Parhofer KG, Göke B, Broedl UC. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 20.Long MD, Crandall WV, Leibowitz IH, Duffy L, del Rosario F, Kim SC, Integlia MJ, Berman J, Grunow J, Colletti RB, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2162–2168. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 22.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 23.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, Ferreira AV, de Faria AM, Cara DC, Alvarez Leite JI. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011;10:204. doi: 10.1186/1476-511X-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Lelliott C, Håkansson P, Ploj K, Tuneld A, Verolin-Johansson M, Benthem L, Carlsson B, Storlien L, Michaëlsson E. Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metabolism. 2008;57:1704–1710. doi: 10.1016/j.metabol.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Bäckhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58 Suppl 2:44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- 27.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 30.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22:117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 32.Cabré E, Mañosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br J Nutr. 2012;107 Suppl 2:S240–S252. doi: 10.1017/S0007114512001626. [DOI] [PubMed] [Google Scholar]
- 33.Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57–64. doi: 10.1159/000121412. [DOI] [PubMed] [Google Scholar]
- 34.Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606–1611. doi: 10.1136/gut.2008.169078. [DOI] [PubMed] [Google Scholar]
- 35.John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602–606. doi: 10.1097/MEG.0b013e3283352d05. [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y, Kawakami Y, Kunii D, Okada H, Azuma M, Le DS, Yamamoto S. Elevated concentrations of linoleic acid in erythrocyte membrane phospholipids in patients with inflammatory bowel disease. Nutr Res. 2008;28:239–244. doi: 10.1016/j.nutres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Cuzzocrea S, Rossi A, Mazzon E, Di Paola R, Genovese T, Muià C, Caputi AP, Sautebin L. 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest. 2005;85:808–822. doi: 10.1038/labinvest.3700276. [DOI] [PubMed] [Google Scholar]
- 38.Stanke-Labesque F, Pofelski J, Moreau-Gaudry A, Bessard G, Bonaz B. Urinary leukotriene E4 excretion: a biomarker of inflammatory bowel disease activity. Inflamm Bowel Dis. 2008;14:769–774. doi: 10.1002/ibd.20403. [DOI] [PubMed] [Google Scholar]
- 39.de Silva PS, Olsen A, Christensen J, Schmidt EB, Overvaad K, Tjonneland A, Hart AR. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology. 2010;139:1912–1917. doi: 10.1053/j.gastro.2010.07.065. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, Li J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411–420. doi: 10.1111/j.1742-4658.2007.06210.x. [DOI] [PubMed] [Google Scholar]
- 41.Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, et al. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348–1357. doi: 10.1002/ibd.20491. [DOI] [PubMed] [Google Scholar]
- 42.Varnalidis I, Ioannidis O, Karamanavi E, Ampas Z, Poutahidis T, Taitzoglou I, Paraskevas G, Botsios D. Omega 3 fatty acids supplementation has an ameliorative effect in experimental ulcerative colitis despite increased colonic neutrophil infiltration. Rev Esp Enferm Dig. 2011;103:511–518. doi: 10.4321/s1130-01082011001000003. [DOI] [PubMed] [Google Scholar]
- 43.Grimstad T, Bjørndal B, Cacabelos D, Aasprong OG, Janssen EA, Omdal R, Svardal A, Hausken T, Bohov P, Portero-Otin M, et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand J Gastroenterol. 2012;47:49–58. doi: 10.3109/00365521.2011.634025. [DOI] [PubMed] [Google Scholar]
- 44.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336–345. doi: 10.1002/ibd.21374. [DOI] [PubMed] [Google Scholar]
- 46.Uchiyama K, Nakamura M, Odahara S, Koido S, Katahira K, Shiraishi H, Ohkusa T, Fujise K, Tajiri H. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1696–1707. doi: 10.1002/ibd.21251. [DOI] [PubMed] [Google Scholar]
- 47.Ioannidis O, Varnalidis I, Paraskevas G, Botsios D. Nutritional modulation of the inflammatory bowel response. Digestion. 2011;84:89–101. doi: 10.1159/000323456. [DOI] [PubMed] [Google Scholar]
- 48.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan MW, Kale AA, Bere P, Vajjala S, Gounaris E, Pakanati KC. Microbes, intestinal inflammation and probiotics. Expert Rev Gastroenterol Hepatol. 2012;6:81–94. doi: 10.1586/egh.11.94. [DOI] [PubMed] [Google Scholar]
- 50.Trebichavsky I, Splichal I, Rada V, Splichalova A. Modulation of natural immunity in the gut by Escherichia coli strain Nissle 1917. Nutr Rev. 2010;68:459–464. doi: 10.1111/j.1753-4887.2010.00305.x. [DOI] [PubMed] [Google Scholar]
- 51.Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement Altern Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman TM, Plosker GL, Figgitt DP. VSL3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371–1387. doi: 10.2165/00003495-200666100-00006. [DOI] [PubMed] [Google Scholar]
- 53.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–129, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J Crohns Colitis. 2011;5:115–121. doi: 10.1016/j.crohns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 57.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 59.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 60.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanau RM, Neuman MG. Nutritional and probiotic supplementation in colitis models. Dig Dis Sci. 2012;57:2786–2810. doi: 10.1007/s10620-012-2284-3. [DOI] [PubMed] [Google Scholar]
- 62.Wong CC, Zhang L, Li ZJ, Wu WK, Ren SX, Chen YC, Ng TB, Cho CH. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol. 2012;27:1205–1212. doi: 10.1111/j.1440-1746.2012.07158.x. [DOI] [PubMed] [Google Scholar]
- 63.Hudcovic T, Kolinska J, Klepetar J, Stepankova R, Rezanka T, Srutkova D, Schwarzer M, Erban V, Du Z, Wells JM, et al. Protective effect of Clostridium tyrobutyricum in acute dextran sodium sulphate-induced colitis: differential regulation of tumour necrosis factor-α and interleukin-18 in BALB/c and severe combined immunodeficiency mice. Clin Exp Immunol. 2012;167:356–365. doi: 10.1111/j.1365-2249.2011.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atkins HL, Geier MS, Prisciandaro LD, Pattanaik AK, Forder RE, Turner MS, Howarth GS. Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine-transport system in a rat model of inflammatory bowel disease. Dig Dis Sci. 2012;57:713–719. doi: 10.1007/s10620-011-1943-0. [DOI] [PubMed] [Google Scholar]
- 65.Mariman R, Kremer B, van Erk M, Lagerweij T, Koning F, Nagelkerken L. Gene expression profiling identifies mechanisms of protection to recurrent trinitrobenzene sulfonic acid colitis mediated by probiotics. Inflamm Bowel Dis. 2012;18:1424–1433. doi: 10.1002/ibd.22849. [DOI] [PubMed] [Google Scholar]
- 66.Mencarelli A, Distrutti E, Renga B, D’Amore C, Cipriani S, Palladino G, Donini A, Ricci P, Fiorucci S. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS One. 2011;6:e22978. doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uronis JM, Arthur JC, Keku T, Fodor A, Carroll IM, Cruz ML, Appleyard CB, Jobin C. Gut microbial diversity is reduced by the probiotic VSL3 and correlates with decreased TNBS-induced colitis. Inflamm Bowel Dis. 2011;17:289–297. doi: 10.1002/ibd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen HQ, Yang J, Zhang M, Zhou YK, Shen TY, Chu ZX, Zhang M, Hang XM, Jiang YQ, Qin HL. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1287–G1297. doi: 10.1152/ajpgi.00196.2010. [DOI] [PubMed] [Google Scholar]
- 69.Chu ZX, Chen HQ, Ma YL, Zhou YK, Zhang M, Zhang P, Qin HL. Lactobacillus plantarum prevents the upregulation of adhesion molecule expression in an experimental colitis model. Dig Dis Sci. 2010;55:2505–2513. doi: 10.1007/s10620-009-1063-2. [DOI] [PubMed] [Google Scholar]
- 70.Ohkusa T, Yoshida T, Sato N, Watanabe S, Tajiri H, Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J Med Microbiol. 2009;58:535–545. doi: 10.1099/jmm.0.005801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeganegi M, Leung CG, Martins A, Kim SO, Reid G, Challis JR, Bocking AD. Lactobacillus rhamnosus GR-1 stimulates colony-stimulating factor 3 (granulocyte) (CSF3) output in placental trophoblast cells in a fetal sex-dependent manner. Biol Reprod. 2011;84:18–25. doi: 10.1095/biolreprod.110.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, Suzuki A, Sata M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417–426. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim CH, Kim HG, Kim JY, Kim NR, Jung BJ, Jeong JH, Chung DK. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol Lett. 2012;328:13–19. doi: 10.1111/j.1574-6968.2011.02470.x. [DOI] [PubMed] [Google Scholar]
- 74.Thomas CM, Versalovic J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 2011;1:148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niess JH, Reinecker HC. Dendritic cells in the recognition of intestinal microbiota. Cell Microbiol. 2006;8:558–564. doi: 10.1111/j.1462-5822.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 76.Hespel C, Moser M. Role of inflammatory dendritic cells in innate and adaptive immunity. Eur J Immunol. 2012;42:2535–2543. doi: 10.1002/eji.201242480. [DOI] [PubMed] [Google Scholar]
- 77.Kayama H, Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity. Int Immunol. 2012;24:673–680. doi: 10.1093/intimm/dxs094. [DOI] [PubMed] [Google Scholar]
- 78.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Hontecillas R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 2012;7:e34676. doi: 10.1371/journal.pone.0034676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng SC, Plamondon S, Kamm MA, Hart AL, Al-Hassi HO, Guenther T, Stagg AJ, Knight SC. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm Bowel Dis. 2010;16:1286–1298. doi: 10.1002/ibd.21222. [DOI] [PubMed] [Google Scholar]
- 80.Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, Vitolo D, Boirivant M. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. 2008;14:662–668. doi: 10.1002/ibd.20369. [DOI] [PubMed] [Google Scholar]
- 81.Braat H, de Jong EC, van den Brande JM, Kapsenberg ML, Peppelenbosch MP, van Tol EA, van Deventer SJ. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J Mol Med (Berl) 2004;82:197–205. doi: 10.1007/s00109-003-0509-9. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigues DM, Sousa AJ, Johnson-Henry KC, Sherman PM, Gareau MG. Probiotics are effective for the prevention and treatment of Citrobacter rodentium-induced colitis in mice. J Infect Dis. 2012;206:99–109. doi: 10.1093/infdis/jis177. [DOI] [PubMed] [Google Scholar]
- 83.Hoermannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, Blaut M, Hölzlwimmer G, Laschinger M, Haller D. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One. 2009;4:e4365. doi: 10.1371/journal.pone.0004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, Hornova M, Srutkova D, Hudcovic T, Ridl J, et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 86.Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156:78–87. doi: 10.1111/j.1365-2249.2009.03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–1345. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 88.Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. 2012;27:1004–1010. doi: 10.1111/j.1440-1746.2012.07108.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood) 2012;237:474–480. doi: 10.1258/ebm.2011.011358. [DOI] [PubMed] [Google Scholar]
- 90.Yasukawa K, Tokuda H, Tun X, Utsumi H, Yamada K. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res. 2012;46:1427–1436. doi: 10.3109/10715762.2012.732698. [DOI] [PubMed] [Google Scholar]
- 91.Razack R, Seidner DL. Nutrition in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:400–405. doi: 10.1097/MOG.0b013e3281ddb2a3. [DOI] [PubMed] [Google Scholar]
- 92.Marx G, Seidman EG. Inflammatory bowel disease in pediatric patients. Curr Opin Gastroenterol. 1999;15:322–325. doi: 10.1097/00001574-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Seidner DL, Lashner BA, Brzezinski A, Banks PL, Goldblum J, Fiocchi C, Katz J, Lichtenstein GR, Anton PA, Kam LY, et al. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial. Clin Gastroenterol Hepatol. 2005;3:358–369. doi: 10.1016/s1542-3565(04)00672-x. [DOI] [PubMed] [Google Scholar]
- 94.Mirbagheri SA, Nezami BG, Assa S, Hajimahmoodi M. Rectal administration of d-alpha tocopherol for active ulcerative colitis: a preliminary report. World J Gastroenterol. 2008;14:5990–5995. doi: 10.3748/wjg.14.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 96.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Singh UP, Singh NP, Busbee B, Guan H, Singh B, Price RL, Taub DD, Mishra MK, Nagarkatti M, Nagarkatti PS. Alternative medicines as emerging therapies for inflammatory bowel diseases. Int Rev Immunol. 2012;31:66–84. doi: 10.3109/08830185.2011.642909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Büning C, Geissler N, Prager M, Sturm A, Baumgart DC, Büttner J, Bühner S, Haas V, Lochs H. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;18:1932–1939. doi: 10.1002/ibd.22909. [DOI] [PubMed] [Google Scholar]
- 99.Toedter G, Li K, Sague S, Ma K, Marano C, Macoritto M, Park J, Deehan R, Matthews A, Wu GD, et al. Genes associated with intestinal permeability in ulcerative colitis: changes in expression following infliximab therapy. Inflamm Bowel Dis. 2012;18:1399–1410. doi: 10.1002/ibd.22853. [DOI] [PubMed] [Google Scholar]
- 100.Park MY, Kwon HJ, Sung MK. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011;88:486–492. doi: 10.1016/j.lfs.2011.01.010. [DOI] [PubMed] [Google Scholar]


