Abstract
The majority of breast cancers are diagnosed in postmenopausal women. Competing comorbidities, particularly cardiovascular disease (CVD), should be considered when individualizing adjuvant therapies for these women. We compared the 10-year predicted breast cancer recurrence risk with CVD risk among postmenopausal women with hormone receptor-positive (HR+), non-metastatic breast cancer. CVD risk factor data were prospectively collected from postmenopausal women with stage I-III, HR+ breast cancer initiating adjuvant aromatase inhibitor therapy. We compared predicted 10-year CVD risk, including the composite index heart age, computed from modified Framingham risk score, with predicted 10-year risk of breast cancer recurrence using Adjuvant! Online. We created multivariable logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI) for greater CVD risk than breast cancer recurrence risk. Among 415 women, mean age and heart age were 60 and 67 years, respectively. Overall, 43% of women had a predicted 10-year CVD risk equivalent to breast cancer recurrence risk and 37% had CVD risk higher than breast cancer recurrence risk. Predicted CVD risk was higher than breast cancer recurrence risk for stage I disease (OR: 6.1, 95% CI: 3.4–11.2) or heart age >65 (OR: 12.4, 95% CI: 7.0–22.6). The majority of postmenopausal women with HR+ early breast cancer had a predicted 10-year CVD risk that was equivalent to or higher than breast cancer recurrence risk. Physicians should weigh competing risks and offer early screening and cardiac prevention strategies for women at a greater risk for CVD.
Keywords: Breast cancer risk, Cardiovascular disease risk, Adjuvant! Online, Modified Framingham risk score, Cancer survivorship
Introduction
Most women diagnosed with breast cancer in the United States are expected to survive their disease [1]. It is estimated that approximately 2 million breast cancer survivors currently reside in the United States [2], and this trend is expected to increase further with improved cancer detection and treatment in an aging population [3]. The 10-year survival from breast cancer currently approaches 90% for women with hormone receptor positive (HR+) disease, including older women (i.e., >65 years) who comprise about 40% of all new breast cancer diagnoses [4]. Treatment decisions in older women with breast cancer are particularly complex, given a higher likelihood of concomitant illnesses and competing comorbidities that must be carefully considered in their overall health management, especially in the adjuvant setting [5–8].
One of the most important comorbidities to consider in the elderly is cardiovascular disease (CVD), the leading cause of death among women in the United States [9]. Approximately 500,000 women die annually from CVD, with CVD-related deaths exceeding the next 7 causes of death combined (including lung cancer, breast cancer, colon cancer, and endometrial cancer). In a recent systematic review comparing the overall survival of individuals diagnosed with cancer, heart failure, and stroke, investigators demonstrated that the long term survival and prognosis associated with cancer were not necessarily worse and in some instances were better than those of heart failure and stroke [10]. Other authors have reported similar findings [11, 12].
According to Centers for Disease Control and Prevention (CDC, United States), life expectancy in the United States would increase by about 7 years if all major types of CVD were eliminated [9]. Consequently, national guidelines recommend that all adults undergo an office-based assessment to evaluate their risk of cardiac events based on various validated CVD risk prediction algorithms [13]. These widely used prediction algorithms incorporate age, gender, concentrations of total cholesterol and high-density lipoprotein cholesterol (HDL-C), smoking status, systolic blood pressure, and antihypertensive therapy to estimate a 10-year risk for developing cardiac disease. However, a 2006 American Heart Association survey reported that 30% of the female respondents significantly underestimated their risk of CVD and a large number did not undergo screening. It is likely that breast cancer survivors may further underestimate their risk for developing CVD because of a focus on risk of breast cancer recurrence [14].
Given the excellent breast cancer prognosis in most elderly women diagnosed with early breast cancer and the high prevalence of CVD risk, we sought to estimate and compare 10-year CVD risk and breast cancer recurrence risk in a cohort of postmenopausal women with stages I–III HR+ breast cancer enrolled in a prospective multi-center adjuvant aromatase inhibitor trial. We also sought to identify a subset of women for whom the predicted 10-year of CVD risk would exceed the 10-year risk estimate for breast cancer recurrence.
Patients and methods
Study population
Data were collected from participants in a prospective NIH-supported clinical trial entitled “A multi-center randomized clinical trial correlating the effects of 24 months of aromatase inhibitors (exemestane or letrozole) on surrogate markers of response with aromatase polymorphisms” (exemestane or letrozole pharmacogenomics [ELPh] trial) (clinicaltrials.gov identifier #: NCT00228956). Details regarding the ELPh trial were previously published [15]. In brief, participants were recruited at one of three sites: Indiana University Melvin and Bren Simon Cancer Center, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, and the University of Michigan Comprehensive Cancer Center. Subjects must have met the following criteria: female gender, postmenopausal status, histologically-proven ductal carcinoma in situ (DCIS) or stages I–III invasive carcinoma of the breast that was HR+ by immunohistochemical staining, and ECOG (eastern cooperative oncology group) performance status 0–2. The women must have completed recommended local therapy and (neo) adjuvant chemotherapy at the time of enrollment. Women were excluded from the study if they met any of the following criteria: history of bilateral mastectomy, history of radiation to the contralateral breast, prior use of an aromatase inhibitor, personal history of ovarian, endometrial, fallopian tube, and/or primary peritoneal carcinomatosis.
Risk models
A planned substudy of the ELPh trial, and the primary objective of this report, was a comparison of predicted 10-year risk of CVD and breast cancer. ELPh participants completed questionnaires that solicited sufficient data to calculate a traditional Framingham 10-year risk of coronary heart disease (CHD) and a “Modified Framingham” 10-year risk for CVD [16]. The traditional Framingham risk score (FRS) utilizes the cardiac risk factors (i.e., age, smoking, hypertension and whether treated or not, HDL cholesterol, and total cholesterol) to provide a 10-year estimate of the absolute risk for an initial hard CHD event (i.e., myocardial infarction, CHD, or death) [17]. This risk score is recommended in national consensus guidelines to estimate CHD risk and is referred to as the traditional FRS in our study [13]. Recently, D’Agostino, et al. developed a new CVD risk assessment tool that broadens the focus from only CHD to any CVD and, therefore, assesses risk of developing any major atherosclerotic CVD event, i.e., all CHD events, plus stroke, intermittent claudication, peripheral arterial disease, and heart failure [16]. This risk score is referred to as a modified Framingham risk score in our study. The risk score includes age, family history of heart disease, smoking, Body Mass Index (BMI), diabetes status, systolic blood pressure and whether on anti-hypertensive medications, HDL cholesterol, and total cholesterol. This new tool also provides a calculation of a “heart age” (vascular age), as a composite index of the individual CVD risk.
Breast cancer 10-year recurrence risk estimate was computed from Adjuvant! Online, a widely used online breast cancer prognostic calculator [18]. In Adjuvant! Online the recurrence risk is based on variables including demographic variables (age and comorbidity), tumor variables (grade, size of tumor, and number of positive axillary lymph nodes), and treatment variables (use of hormonal therapy such as aromatase inhibitors, and/or chemotherapy). The actual values for each of these variables for an individual patient was manually entered in the prognostic calculator and the corresponding breast cancer 10-year recurrence risk estimate was obtained. Comorbidity was set as “average for age” as that is the default option in Adjuvant! Online. Women with DCIS and those who received neo-adjuvant chemotherapy were excluded from the study analyses because the breast cancer recurrence risk cannot be calculated using Adjuvant! Online.
Statistical analysis
Based on the predicted 10-year risk for CVD and 10-year breast cancer recurrence risk calculated from the modified Framingham risk score and Adjuvant! Online, respectively, we defined three probability-based risk categories: low risk defined as <10%, moderate risk defined as 10–25%, and high risk defined as >25%. We chose the CVD cut-offs because the American College of Cardiology recommends using <6% = low, 6–20% moderate, and >20% high for the CHD calculation [19], and generally one’s CVD risk is about 1.5× higher than one’s CHD risk.
We compared risk categories of CVD and breast cancer recurrence and calculated percentages of women who had estimated CVD risk < breast cancer risk, CVD risk = breast cancer risk, and CVD risk > breast cancer risk. We considered risk factors associated with breast cancer recurrence (lymph node involvement, tumor grade, tumor size, stage) and CVD (composite index, heart age) in the assessment of prediction for having CVD risk > breast cancer risk. We used univariate and multivariable logistic regression models to investigate the effect of the risk factors associated with higher predicted risk for CVD incidence than breast cancer recurrence at 10 years. Odds ratios (OR) that indicate the strength of the association were estimated with the 95% confidence intervals (CI). Since breast cancer stage is a composite index of lymph node status and tumor size, stage was not simultaneously included in the multivariable model that included lymph node status or tumor size, and vice versa. We performed statistical analyses using SAS (v 9.2, SAS Institute, Cary, NC). All tests were two-sided with P < 0.05 considered statistically significant.
Results
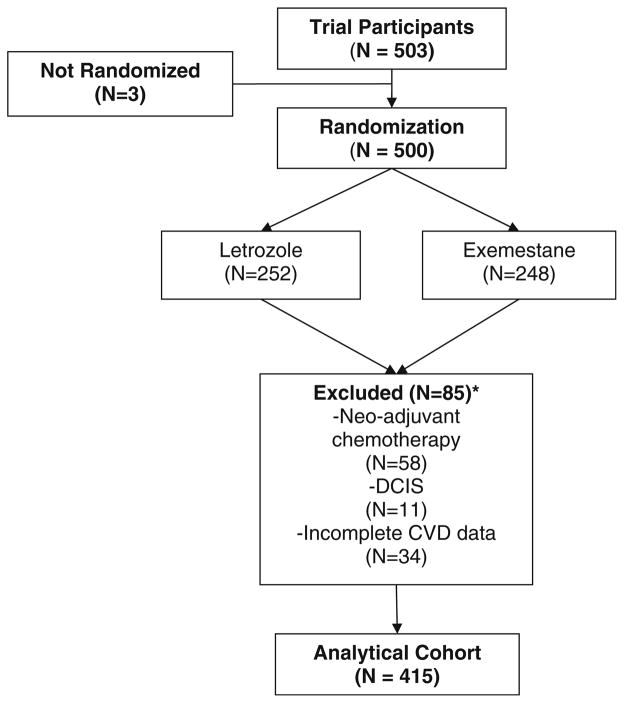
A total of 503 women participated in the ELPh trial. While women were randomized to one of two aromatase inhibitors, the cohort was combined for the purpose of this analysis as the agent choice would not influence the study endpoint. Of these, 58 women received neo-adjuvant chemotherapy, 11 had DCIS, and 34 had missing or incomplete CVD data (numbers not mutually exclusive) and were excluded from the study analyses (Fig. 1). Thus, a total of 415 women were included in the analytical cohort for this study. Patient characteristics are included in Table 1. The mean age of breast cancer diagnosis was 60 years. The majority of women had stage I disease (67.5%) and grade 2 tumors (54.3%). In the cohort, the mean cholesterol concentration was 200 mg/dl, mean BMI was 30 kg/m2, 11.8% had a personal history of diabetes, and 23.6% family history of heart disease. There were no statistically significant differences in the distribution of the breast cancer risk factors when stratified by CVD risk (Table 2).
Fig 1.
Consort diagram. Abbreviations: * Numbers not mutually exclusive, DCIS ductal carcinoma in situ, CVD cardiovascular disease
Table 1.
Distribution of age, breast cancer and CVD risk factors at baseline in the analytical cohort
| Variable | Summary statistic (N = 415) |
|---|---|
| Age (years)* | 60 (±9) |
| Breast cancer risk factors* | |
| Tumor size (cm) | 1.6 (±1.1) |
| Positive lymph nodes, n (%) | 74 (17.8%) |
| Grade, n (%) | |
| 1 | 115 (28.9%) |
| 2 | 216 (54.3%) |
| 3 | 67 (16.8%) |
| Stage, n (%) | |
| I | 253 (67.5%) |
| II | 114 (30.4%) |
| III | 8 (2.1%) |
| CVD risk factors* | |
| Total cholesterol (mg/dl) | 200 (±36) |
| HDL cholesterol (mg/dl) | 58 (±17) |
| SBP (mm Hg) | 131 (±19) |
| DBP (mm Hg) | 74 (±11) |
| BMI (kg/m2) | 30.0 (±6.5) |
| Heart age (years) | 67 (±17) |
| Current smoking status, n (%) | 32 (7.7%) |
| Diabetes, n (%) | 49 (11.8%) |
| Family h/o heart disease, n (%) | 98 (23.6%) |
Continuous variables are reported as mean and standard deviation (in parenthesis)
BMI body mass index, CVD cardiovascular disease, DBP diastolic blood pressure, HDL high-density lipoprotein, h/o history of, SBP systolic blood pressure
Table 2.
Distribution of breast cancer characteristics among the various CVD risk categories
| Breast cancer characteristics | CVD risk
|
P value | ||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Median tumor size | 1.5 cm | 1.4 cm | 1.4 cm | 0.93 |
| Negative lymph node | 76.9% | 81.8% | 84.1% | 0.44 |
| Grade 1 or 2 | 80.1% | 86.6% | 82.2% | 0.39 |
| Stage I | 67.6% | 64.9% | 75.6% | 0.39 |
Medians were compared using nonparametric Kruskal–Wallis test for continuous variable and proportions were compared using exact χ2 test
CVD cardiovascular disease
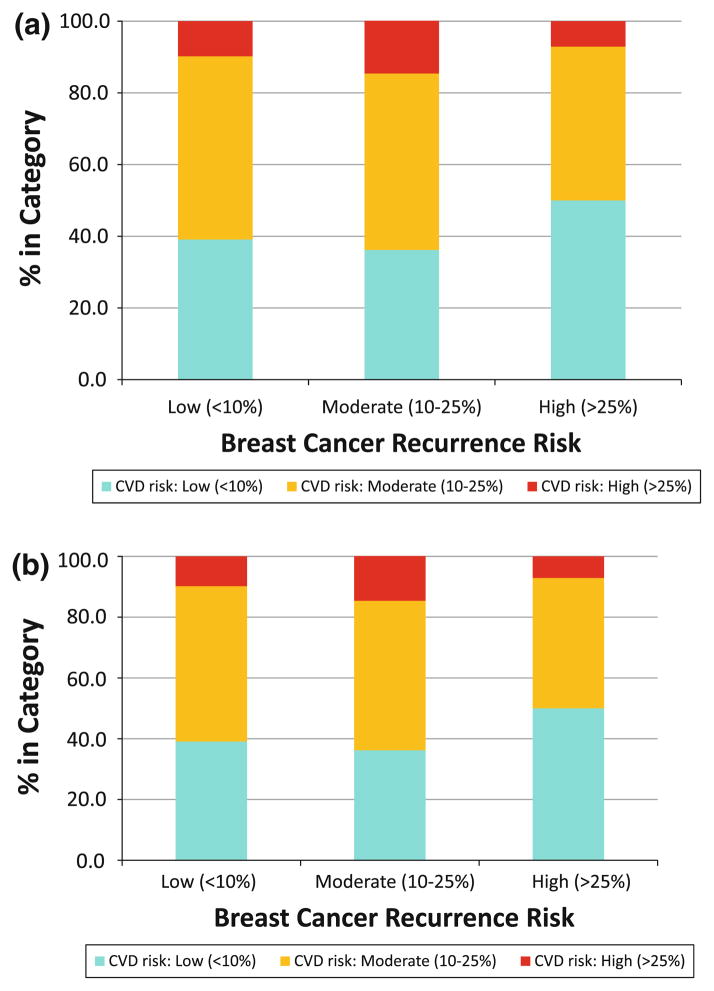
The individual distributions of breast cancer and CVD predicted risk in the cohort are illustrated in Fig. 2. Overall, 49% of the participants had low (<10%), 47% had moderate (10–25%), and 4% had high (>25%) 10-year predicted risk of breast cancer recurrence, while 38% of women had low (<10%), 50% had moderate (10–25%), and 12% had high (>25%) 10-year predicted risk of CVD (Fig. 2a). We then evaluated the distribution of CVD risk within the three breast cancer recurrence risk categories (Fig. 2b), and essentially did not observe any major difference in the distribution (P = 0.6). Approximately 50% of women in the cohort had moderate CVD risk including those with low breast cancer recurrence risk. Similarly, about 10% of women in the cohort had high CVD risk, including those with low or moderate breast cancer recurrence risk.
Fig 2.
Distribution of the predicted breast cancer recurrence and cardiovascular disease (CVD) risk among the study participants, separately (a), and CVD risk within the breast cancer recurrence categories (b). Breast Cancer Risk = 10-year predicted risk of breast cancer recurrence using adjuvant! online CVD Risk = 10-year predicted risk of CVD incidence using the modified Framingham risk score
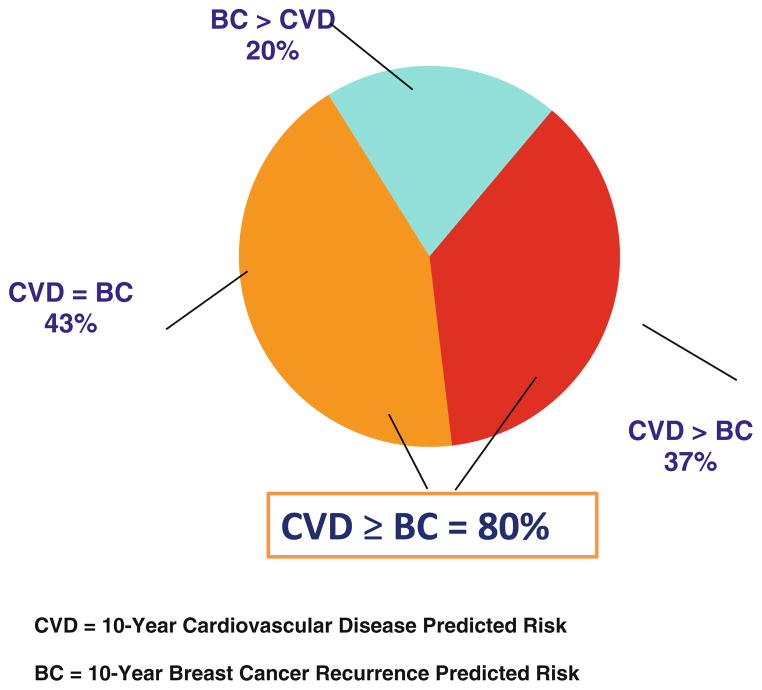
We then compared the relative distribution of the predicted CVD risk and breast cancer recurrence risk in the cohort as outlined in Fig. 3. Overall, 43% of women in the cohort had a 10-year predicted risk of a CVD event equivalent to that of breast cancer recurrence, and 37% had a predicted risk of a CVD event higher than the breast cancer recurrence. Overall, 80% of the cohort had a predicted CVD risk that was equal or greater than the predicted breast cancer recurrence risk.
Fig 3.
Relative distribution of the predicted breast cancer recurrence risk (BC) and cardiovascular disease (CVD) risk among the study participants. CVD = 10-year CVD predicted risk. BC = 10-year breast cancer recurrence predicted risk
Evaluation of individual breast cancer prognostic factors revealed that women with stage I disease (OR: 6.1, 95% CI: 3.4–11.2, P <0.0001), tumors <2 cm (OR: 6.7, 95% CI: 3.1–14.7, P <0.0001), grade 1 or 2 disease (OR: 2.4, 95% CI = 1.1–5.3, P <0.03), or negative lymph nodes (OR: 4.4, 95% CI: 2.0–9.6, P = 0.0002) were significantly more likely, as compared to the corresponding counterparts, to have a 10-year predicted CVD risk greater than the 10-year breast cancer recurrence risk (Table 3). Analysis of CVD risk factors revealed that women with heart age >65 years were significantly more likely (OR: 12.5, 95% CI: 6.9–22.6, P <0.0001), as compared to their counterparts, to have a 10-year predicted CVD risk greater than the 10-year breast cancer recurrence risk.
Table 3.
Univariate and multivariable adjusted odds ratio (ORs) for variables predicting estimated CVD risk greater than breast cancer risk
| Variable | Odds ratioa (95% CI) | P valuea | Adjusted odds ratiob (95% CI) | P valueb |
|---|---|---|---|---|
| Heart age | ||||
| >65 | 9.4 (5.5–12.1) | <0.0001 | 12.6 (7.0–22.6) | <0.0001 |
| ≤65 | Reference | Reference | ||
| Lymph node positive | ||||
| No | 3.7 (1.9–7.1) | 0.0001 | 4.3 (2.0–9.3) | 0.0002 |
| Yes | Reference | Reference | ||
| Tumor grade | ||||
| 1 or 2 | 2.4 (1.2–4.6) | 0.010 | 2.4 (1.1–5.3) | 0.030 |
| 3 | Reference | Reference | ||
| Tumor size | ||||
| ≤2cm | 5.6 (2.8–11.3) | <0.0001 | 6.8 (3.1–14.9) | <0.0001 |
| >2cm | Reference | Reference | ||
| Tumor stage | ||||
| I | 4.2 (2.4–7.1) | <0.0001 | 6.1 (3.4–11.2) | <0.0001 |
| II or III | Reference | Reference | ||
Univariate logistic regression model, where probability modeled was predicted CVD risk > predicted breast ca risk
Multivariable logistic regression model adjusted for age and risk factors associated with breast cancer recurrence (lymph node involvement, tumor grade, tumor size, stage) and CVD (the composite index, heart age) where probability modeled was predicted CVD risk > predicted breast cancer risk. Note as breast cancer stage is a composite index of lymph node and tumor size, stage was not simultaneously included in the multivariable model with lymph node or tumor size and vice versa
CVD cardiovascular disease
Discussion
This study highlights the relative importance of considering CVD risk among postmenopausal women with HR+ non-metastatic breast cancer. Our study findings suggest that 80% of postmenopausal women with HR+ breast cancer who are considered for adjuvant aromatase inhibitor therapy have a predicted 10-year CVD risk equivalent or higher than their 10-year breast cancer recurrence risk. Women with stage I breast cancer or heart age >65 years were particularly likely to have a higher predicted CVD risk than breast cancer recurrence risk at 10-years.
Our results suggest that CVD risk is an important potential health problem in postmenopausal breast cancer survivors. This is particularly worrisome as cancer survivors are less likely to receive cardiovascular preventive care as compared to the general population. For example, in a retrospective study involving about 4,000 breast cancer cases and matched non-cancer controls, breast cancer survivors were less likely to have cholesterol monitoring for detection of CVD as compared to controls. Since many individuals have subclinical CVD and are asymptomatic, screening and risk assessment are imperative to detect asymptomatic patients at risk for CVD. The use of effective medications and lifestyle modifications already available to modify cardiac risk factors such as elevated blood pressure, high cholesterol, and diabetes can lead to better patient outcomes and improve survival [20, 21]. The knowledge generated from the use of a CVD risk assessment tool, as in this study, can guide prevention strategies and may reduce the rates of morbidity and mortality because of CVD.
Estimates for CVD and breast cancer recurrence in the study represent predictions and not observed outcomes. Therefore, breast cancer therapies that increase CVD risk could further increase the risk for a CVD event. Several chemotherapy regimens are associated with a substantial decrease in ejection fraction and potentially increased risk for cardiomyopathy, especially in older breast cancer patients [22–24]. Newer monoclonal antibodies such as trastuzumab, and possibly bevacizumab, are also associated with increased cardiac toxicity [25–28]. Of particular relevance for our study population, results from one large adjuvant trial suggested that adjuvant aromatase inhibitor use was associated with a slight increase in CVD risk compared to tamoxifen, but other trials did not suggest an increased risk [29, 30]. Investigators have recently performed a meta analysis of 7 large adjuvant aromatase inhibitors trials with a sample size of 30,023 patients. Adjuvant aromatase inhibitor use was associated with a slightly increased CVD risk (OR: 1.26, 95% CI: 1.10–1.43, P <0.001; number needed to harm = 132) [30]. In light of these recent findings, the actual risk of CVD after adjuvant aromatase inhibitor therapy might be even higher than suggested by our study.
Our study is associated with a few limitations. First, the CVD tool used in this study does not take into account lifestyle practices of the participants, including diet and exercise, and adherence to CVD medications such as antihypertensives. Such data could give a better picture about whether those with identified increased CVD risk have or have not already incorporated lifestyle modifications in their health plan, the degree of compliance with CVD medications, and whether such practices are different among breast cancer survivors as compared to the general population. Second, as noted above, the effect of aromatase inhibitor therapy on CVD risk is currently not well defined and may modify the strength of association between breast cancer recurrence risk and CVD risk. Third, we compared breast cancer recurrence risk with CVD incidence risk and one could argue the morbidity and mortality associated with these risks are not identical, i.e., having breast cancer recurrence is not identical to having a CVD event. However, the CVD risk computed from the modified Framingham risk score is the risk of a major atherosclerotic CVD event, i.e., myocardial infarction, stroke, heart failure etc. and not just a minor CVD event such as rise in cholesterol. Regardless, our data suggest the importance of recognizing that a large proportion of postmenopausal women with HR+ breast cancer have a CVD risk that is greater than their risk of developing a breast cancer recurrence, and the proper identification and early intervention of these women is crucial. Finally, while our study did not evaluate deaths because of CVD versus breast cancer, two recent studies have suggested that CVD is indeed the most common cause of death for women diagnosed with DCIS or stage I disease and for women age ≥80 years with stage II disease and attention to this comorbidity should be prioritized [31–33].
The population in this study is broadly representative of postmenopausal breast cancer observed in the community and thus has important implications for primary care providers and oncologists. While there is no doubt that living with a diagnosis of breast cancer and enduring treatment is a cause for fear and concern, it is important that women, particularly those with low breast cancer recurrence risk and high CVD risk, be educated on CVD risk factors and be properly counseled and treated for existing risk factors. The study findings should motivate primary care providers and oncologists to optimize medication management and advocate lifestyle modification to effectively minimize cardiovascular risk factors while addressing the breast cancer recurrence and treatment-related issues. Oncologists may wish to compare risk of developing CVD to risk of recurrence of breast cancer both to determine adjuvant aromatase inhibitor use and to more specifically tailor CVD preventive strategies. Patients’ knowledge of their heart age may affect her view of CVD risk and ideally enhance adherence to medications and to recommended lifestyle modifications. Indeed, women’s knowledge of their personal risk of heart disease is correlated with increased action to modify risk [34]. For example a 50 year old female with significant CVD risk factors may be better able to appreciate her CVD risk if she knew that her heart age is equivalent of a typical 70 year old woman rather than just knowing she carries a 20% risk of CVD over the next 10 years. Certain lifestyle interventions such as physical activity can potentially reduce the risk of both CVD and breast cancer recurrence and should be encouraged [35–37]. Similarly, certain cardiac medications, such as aspirin, have been reported to decrease the risk of breast cancer recurrence and improve survival [38–40]. Adopting a global view of health among breast cancer survivors may lead to minimizing risk for developing CVD and enhance the quality and quantity of healthy years.
In conclusion, in this study cohort, about two-thirds of postmenopausal women with early stage HR+ breast cancer had a predicted 10-year CVD risk equivalent or higher than their 10-year breast cancer recurrence risk. Oncologists should be aware of CVD risk among women recommended adjuvant breast cancer therapy especially for women with stage I breast cancer or heart age >65 years who are particularly likely to have higher CVD risk than breast cancer recurrence risk, offer early cardiac prevention strategies for patients at significant risk of CVD, and thus potentially help improve the overall survival for an individual.
Acknowledgments
Supported in part by pharmacogenetics research network Grant U-01 GM61373, which supports the consortium on breast cancer pharmacogenomics, and by investigator-initiated grants from Novartis and Pfizer. Dr. DeFilippis is supported by a national research service award (NRSA) training Grant (T32-HL-07227). Drs. Flockhart and Stearns received research Grants from Novartis and Pfizer. Dr. Stearns received honoraria from AstraZeneca. Dr. Henry receiver research funding from AstraZeneca. Dr. Hayes has been a consultant to Oncimmune, Chugai, and biomarker strategies, owns stocks in Oncimmune, and received research funding from Novartis and Pfizer, GlaxoSmithKline and Veridex. Dr. Storniolo received honoraria from Pfizer.
Contributor Information
Aditya Bardia, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA.
Erin T. Arieas, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
Zhe Zhang, Division of Biostatistics and Bioinformatics, Department of Oncology, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Andrew DeFilippis, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Karineh Tarpinian, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA.
Stacie Jeter, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA.
Anne Nguyen, Department of Medicine, Melvin and Bren Simon Cancer Center, Indiana University, Indianapolis, IN, USA.
N. Lynn Henry, Breast Oncology Program, University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA.
David A. Flockhart, Department of Medicine, Melvin and Bren Simon Cancer Center, Indiana University, Indianapolis, IN, USA
Daniel F. Hayes, Breast Oncology Program, University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA
Jill Hayden, Breast Oncology Program, University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA.
Anna Maria Storniolo, Department of Medicine, Melvin and Bren Simon Cancer Center, Indiana University, Indianapolis, IN, USA.
Deborah K. Armstrong, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA
Nancy E. Davidson, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA
John Fetting, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA.
Pamela Ouyang, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Antonio C. Wolff, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA
Roger S. Blumenthal, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
M. Dominique Ashen, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Vered Stearns, Email: vstearn1@jhmi.edu, Breast Cancer Program, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Bunting-Blaustein Cancer Research Building 1, Room 144, 1650 Orleans Street, Baltimore, MD 21231, USA.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed July 25, 2011];American cancer society: breast cancer overview. 2011 http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-overview-key-statistics.
- 3.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 4.Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Biganzoli L, Sargent DJ, Aapro M. Adjuvant therapy in the elderly: making the right decision. J Clin Oncol. 2007;25(14):1870–1875. doi: 10.1200/JCO.2006.10.3457. [DOI] [PubMed] [Google Scholar]
- 6.Ahern TP, Lash TL, Thwin SS, Silliman RA. Impact of acquired comorbidities on all-cause mortality rates among older breast cancer survivors. Med Care. 2009;47(1):73–79. doi: 10.1097/MLR.0b013e318180913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin JS, Hunt WC, Same JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72(2):594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.CDC. [Accessed July 25, 2011];Heart disease and stroke prevention. 2011 www.cdc.gov/nccdphp/publications/AAG/pdf/dhdsp.pdf.
- 10.Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Katus HA, Debus J, Bischof M. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 12.Collins TC, Petersen NJ, Menke TJ, Souchek J, Foster W, Ashton CM. Short-term, intermediate-term, and long-term mortality in patients hospitalized for stroke. J Clin Epidemiol. 2003;56(1):81–87. doi: 10.1016/s0895-4356(02)00570-x. [DOI] [PubMed] [Google Scholar]
- 13.Third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 14.Mosca L, Mochari H, Christian A, Berra K, Taubert K, Mills T, Burdick KA, Simpson SL. National study of women’s awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113(4):525–534. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- 15.Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111(2):365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19(4):980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 19.Wickramasinghe SR, DeFilippis AP, Lloyd-Jones DM, Blumenthal RS. A convenient tool to profile patients for generalized cardiovascular disease risk in primary care. Am J Cardiol. 2009;103(8):1174–1177. doi: 10.1016/j.amjcard.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Eagle KA, Ginsburg GS, Musunuru K, Aird WC, Balaban RS, Bennett SK, Blumenthal RS, Coughlin SR, Davidson KW, Frohlich ED, et al. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation. 2010;121(12):1447–1454. doi: 10.1161/CIRCULATIONAHA.109.904029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger JS, Jordan CO, Lloyd-Jones D, Blumenthal RS. Screening for cardiovascular risk in asymptomatic patients. J Am Coll Cardiol. 2010;55(12):1169–1177. doi: 10.1016/j.jacc.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 22.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-over-expressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 23.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz PA, Hussey MA, Moinpour CM, Unger JM, Hutchins LF, Dakhil SR, Giguere JK, Goodwin JW, Martino S, Albain KS. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on southwest oncology group protocol s8897. J Clin Oncol. 2008;26(8):1223–1230. doi: 10.1200/JCO.2007.11.8877. [DOI] [PubMed] [Google Scholar]
- 25.Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, Bellmunt J, Burstein HJ, Schutz FA. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 26.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 27.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the national surgical adjuvant breast and bowel project B-31 and the north central cancer treatment group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 28.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 29.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, et al. American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 31.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, McCarthy EP. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29(12):1570–1577. doi: 10.1200/JCO.2010.33.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christian AH, Rosamond W, White AR, Mosca L. Nine-year trends and racial and ethnic disparities in women’s awareness of heart disease and stroke: an American heart association national study. J Womens Health (Larchmt) 2007;16(1):68–81. doi: 10.1089/jwh.2006.M072. [DOI] [PubMed] [Google Scholar]
- 35.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28(26):4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, Al-Delaimy WK, Thomson CA, Kealey S, Hajek R, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braithwaite D, Satariano WA, Sternfeld B, Hiatt RA, Ganz PA, Kerlikowske K, Moore DH, Slattery ML, Tammemagi M, Castillo A, et al. Long-term prognostic role of functional limitations among women with breast cancer. J Natl Cancer Inst. 2010;102(19):1468–1477. doi: 10.1093/jnci/djq344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardia A, Ebbert JO, Vierkant RA, Limburg PJ, Anderson K, Wang AH, Olson JE, Vachon CM, Cerhan JR. Association of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs with cancer incidence and mortality. J Natl Cancer Inst. 2007;99(11):881–889. doi: 10.1093/jnci/djk200. [DOI] [PubMed] [Google Scholar]
- 39.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]