Abstract
Purpose
To develop a new bioassay method using human lung epithelial cells (CCL-185) to assess activity of transforming growth factor beta (TGF-β) in human tear fluid from normal subjects and patients with dry eye.
Methods
Two epithelial cell lines: mink lung cells (CCL-64) and human lung cells (CCL-185) were compared to detect the active form of TGF-β by BrdU incorporation (quantitation of cell DNA synthesis) and WST assay (metabolic activity of viable cells). The effect of TGF-β on the growth of CCL-185 cells was observed microscopically. Human tears from normal control subjects and patients with dry eye (DE) with and without Sjögren syndrome were evaluated for TGF-β concentration by Luminex microbead assay, and TGF-β activity by the CCL-185 cell growth inhibition bioassay.
Results
The metabolic activity of viable CCL-185 cells, measured by WST, was shown to be proportional to the TGF-β1 concentration (R2 = 0.919) and confirmed by BrdU assay (R2 = 0.969). Compared with CCL-185, metabolic activity of viable cells and DNA synthesis, measured by WST and BrdU incorporation assays, were shown to be less proportional to the TGF-β1 concentration in the CCL-64 line (R2 = 0.42 and 0.17, respectively). Coincubation with human anti–TGF-β1 antibody (MAB-240) yielded a dose-dependent inhibition of TGF-β1 (0.3 ng/mL) activity. CCL-185 cell growth observed microscopically was noted to decrease in response to increasing TGF-β1 concentrations. Levels of immuodetectable TGF-β1 and TGF-β2 were similar in normal and DE tears. TGF-β bioactivity in DE human tears measured by the CCL-185 cells assay was found to be higher (9777.5 ± 10481.9 pg/ml) than those in normal controls (4129.3 ± 1342.9 pg/mL) (P < 0.05). Among patients with DE, TGF-β bioactivity was highest in those with Sjögren syndrome. Approximately, 79.1% of TGF-β in DE tears and 37.6% TGF-β in normal tears were found to be biologically active.
Conclusions
The CCL-185 cell assay was found to be a suitable tool for assessing TGF-β activity in human tears. Tear TGF-β bioactivity increases in DE, particularly in Sjögren syndrome, where elevated levels of TGF-β1 transcripts in the conjunctival epithelium have been previously detected.
Keywords: TGF-β, bioassay, cornea, dry eye, tear
Transforming growth factor beta (TGF-β), a multifunctional cytokine, exists in 3 highly conserved isoforms (TGF-β1, TGF-β2, and TGF-β3) in mammals.1 TGF-β is synthesized as a large precursor protein with a pro- and mature regions.2 During intracellular processing, the preprotein, also known as the latency-associated peptide 1 (LAP-1), is cleaved by the intracellular protease furin.1,2 The mature region of TGF-β1 is secreted with the LAP noncovalently attached to latent TGF-β1 (L-TGF-β1) complex that does not become biologically active until the LAP-1 is removed or the complex is conformationally altered to yield active TGF-β1.1,2 TGF-β is known to inhibit or stimulate cell growth, regulate the immune system, and enhance the synthesis and deposition of extracellular matrix, during wound repair.1,3 Because of its wide ranging biological functions, it is not surprising that TGF-β has been implicated in multiple disease states.
Dry eye (DE) is a common ocular surface disease that can cause irritation and can degrade visual function by decreasing contrast sensitivity and functional visual acuity.4,5 DE has been demonstrated to cause inflammation on the ocular surface, evidenced by increased levels of inflammatory cytokines [interleukin (IL) 1, IL-6, and tumor necrosis factor α] in the tear fluid and corneal and conjunctival epithelium.6,7 TGF-β is a key immunoregulatory factor. Our group previously found increased levels of TGF-β1 messenger RNA within the conjunctival epithelium of patients with Sjögren syndrome compared with non-DE controls.8 Furthermore, experimental desiccating stress increased levels of Th-17–associated genes, including TGF-β1 and β2, in mouse cornea and conjunctiva.9 These previously reported studies have only evaluated TGF-β expression and the total amount of TGF-β protein, including latent and mature protein. The role of TGF-β in DE disease remains to be completely elucidated.
Bioassays can provide useful information about TGF-β. Moreover, with the numerous reports of TGF-β involvement in a variety of diseases, bioassay methods available for measuring the biological activity of this cytokine have widespread application. Several bioassay methods for measuring TGF-β activity have been published. These include the TGF-β–induced proliferation of normal rat kidney fibroblasts in soft agar,10 the inhibition of Mv1Lu mink lung epithelial cell proliferation,11 and the use of mink lung cells transfected with a reporter vector, such as the plasminogen activator inhibitor, PAI-1.12 The A549 (CCL-185) cell line was previously shown to be a TGF-β–responsive cell line.13,14 Building upon this observation, the purpose of this study was to develop a simple and reproducible bioassay method to assess the activity of TGF-β in human lung epithelial cells (CCL-185) and to evaluate TGF-β activity of tear fluid obtained from normal subjects and patients with DE with and without Sjögren syndrome.
MATERIAL AND METHODS
TGF-β Bioassay
The human lung epithelial cells (CCL-185) and the mink lung epithelial cells (CCL-64) were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in Dulbecco Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 50 μg/mL of gentamicin, 1.25 μg/mL amphotericin B and 2 mM l-glutamine in a 100 cm2 plate at 37°C in 5% CO2 using a previously published method.15 To perform the assay, wells were rinsed with DMEM, then 50 μL of each sample including tears, recombinant human TGF-β1 or TGF-β2, anti-human TGF-β1 immunoglobulin G 1 (IgG1) (MAB 240) or anti-human TGF-β1,2,3 (all from R & D Systems, Minneapolis, MN) were added to each well of a 96-well cell culture plate. To each sample well, 6 × 103 human lung epithelial cells (CCL-185) or 12 × 103 mink lung epithelial cells (CCL-64), obtained from the trypsinzation of 80% confluent cultures, in 50 μL of DMEM were added. The bioassay plates were incubated at 37°C. After 24 or 48 hours, the plates were placed on a rotating platform set at approximately 100 rpm to gently mix the well contents for 20–30 minutes at room temperature before performing the BrdU or WST assays.
Growth inhibition standard curves were obtained by incubating CCL-185 or CCL-64 cells with recombinant human TGF-β1 or TGF-β2 (concentrations ranging from 0, 0.313, 0.625, 1.25, 2.5, and 5 to 10 ng/mL). The growth inhibitory effect of TGF-β1 (0.3 ng/mL) in CCL-185 was confirmed by neutralization with anti-human TGF-β1 IgG1 (concentrations ranging from 0, 0.16, 0.8, 4, and 20 to 100 μg/mL). To determine the specificity of the inhibitory effect of tear samples was because of TGF-β and to determine the relative inhibitory effects of TGF-β1 or TGF-β2 and TGF-β3, anti-human TGF-β1 IgG1 (20 μg/mL) or anti-human TGF-β1,2,3 (20 μg/mL) were added to the 3 tear samples before the addition of CCL-185 cells. The activity of TGF-β in tear samples obtained from patients with DE or normal control subjects was determined.
WST Assay
Proliferation of CCL-185 and CCL-64 cells was assessed using the WST-1 assay (Roche Molecular Biochemicals, Mannheim, Germany). Details of the WST-1 assay have been previously described.16 In brief, after incubation of the cells in a 96-well plate with 100 μL of cell culture medium for 48 hours, 10 μL of cell proliferation agent WST-1 was added to each well. The cells were then incubated for another 4 hours at 37°C in a 5% CO2 atmosphere. After 1 minute of shaking, the multiplate was placed in an enzyme-linked immunosorbent assay (ELISA) multiplate reader (Versamax; Molecular Devices, Sunnyvale, CA) and measured against a background control consisting of 100 μL of culture medium and 10 μL of cell proliferation reagent WST-1 at a wavelength of 450 nm (reference wavelength: 690 nm). The inhibition of cultured cells was then expressed by normalizing the units of absorbance of cells incubated at different TGF-β1 concentrations to the units of absorbance of cells incubated without TGF-β1 in the culture medium. All cell culture assays were repeated 5 times. The growth inhibition of the cells at a given TGF-β1 concentration was expressed as the median of the 5 measurements.
BrdU Assay
The effect of TGF-β1 on CCL-185 and CCL-64 cell proliferations was also determined using a BrdU labeling assay performed as recommended by the manufacturer (Roche Diagnostics, Corp, Indianapolis, IN) and a previously published report.17 Briefly, after incubation of the cells in a 96-well plate with 100 μL cell culture medium for 24 hours, 10 μL of BrdU labeling solution was added and incubated for 2 hours at 37°C. The supernatants were removed to stop the reaction, and cells were fixed by incubating with 200 μL per well of FixDenat solution for 30 minutes. The supernatant was removed and anti–BrdU-POD solution (100 μL per well of 1:100 dilutions) was added and incubated for 90 minutes at room temperature. The antibody was removed, and cells were washed 3 times and substrate solution was added. The reaction was stopped by adding 25 μL of 1 M HCl, and the absorbance of the developed color was measured at 370 nm with reference measured at 492 nm. The absorbance were compared with untreated cells and expressed as percentage of inhibition of cell proliferation. All cell culture assays were repeated 5 times. The growth inhibition of cells exposed to a given TGF-β1 concentration was expressed as the median of the 5 measurements.
Patient Selection
The clinical protocol was approved by the Baylor College of Medicine Institutional Review Board. After receiving informed consent, 16 patients with newly diagnosed DE by 1 investigator (S.C.P.) and 10 asymptomatic control subjects were enrolled. Criteria for diagnosis of DE included an OSDI score >20 with one or more of the following signs: tear film breakup time <7 seconds, punctate corneal fluorescent staining, or Schirmer I score <10 mm. Exclusion criteria included use of any topical medications other than preservative-free artificial tears that could not be used on the day of tear collection, presence of another ocular surface ocular disease including allergic conjunctivitis or anterior blepharitis, contact lens wear, prior laser in situ keratomileusis surgery, cataract surgery in the past year, and use of systemic drugs that may cause dryness, such as antihistamines and antidepressants in the past 2 months. Patients were stratified into 3 subgroups: meibomian gland disease, non-Sjögren aqueous tear deficiency, and Sjögren syndrome–associated aqueous tear deficiency. Meibomian gland disease was diagnosed by the presence of 2 or more morphologic changes of the meibomian glands, including acinar atrophy, orifice metaplasia, and vascular dilation on the posterior lid margin.18 The United States European study group consensus criteria were used for diagnosis of Sjögren syndrome.19 The patients were segregated into 4 levels of severity according to reported criteria of the DEWS report.20,21 Asymptomatic control subjects were recruited from employees of Baylor College of Medicine, and patients presenting for routine eye examinations.
Tear Fluid Collection
Minimally stimulated tear fluid (0.5 μL) was atraumatically collected with a 0.5 μL volume glass capillary tube (Drummond Scientific, Broomall, PA) by capillary action from the inferior tear meniscus of each eye within 1 second. Tear samples from both eyes (1 μL total) were fully eluted into a sterile tube containing 9 μL of phosphate-buffered saline and 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO). Tubes were sealed with a cap containing a rubber O-ring to prevent evaporation and immediately stored at −80°C until activity assays were performed.
TGF-β Luminex Immunobead Cell Signaling Assay
The levels of total TGF-β1 and TGF-β2 in human tears were measured using Luminex bead assay (Upstate-Millipore) with HCl activation. Tear samples were activated by adding 1 N HCl to each tube. Then, tubes were incubated for 1 hour at room temperature, and samples were neutralized by addition of 1 N NaOH. Tear samples were pipetted into wells of a 96-well plate and incubated overnight with 25 μL of 1× beads coupled to TGF-β1 and TGF-β2 protected from light. Controls included assay buffer and recombinant human TGF-β1 and TGF-β2. Serial dilutions of TGF-β1 and TGF-β2 were added to wells in the same plate to generate a standard curve. After overnight incubation, 25 μL of biotinylated secondary cytokine antibody mixture was applied for 1.5 hours in the dark at room temperature. The reactions were detected with streptavidin–phycoerythrin conjugate with a Luminex 100 IS 2.3 system (Austin, TX).
Statistical Analyses
Statistical analyses were performed with GraphPad Prism (La Jolla, CA) and Excel (Microsoft, Bellevue, WA) softwares. Data are expressed as mean ± SD. Statistical comparisons of tear TGF-β activity levels and total protein levels between groups were performed by an unpaired 2-tailed t test. A P value <0.05 was considered to be statistically significant.
RESULTS
Effects of TGF-β1 on Growth and Viability of CCL-185 and CCL-64 Cells
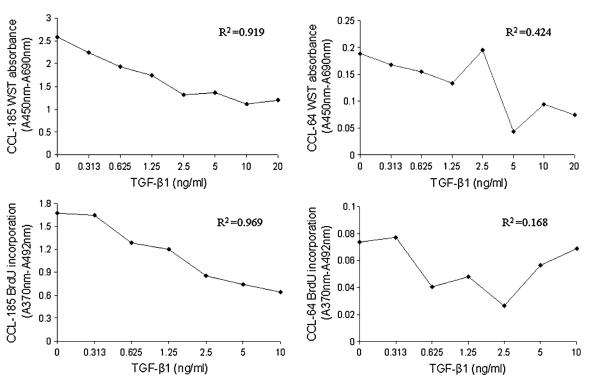
The effects of TGF-β1 on DNA synthesis during cell proliferation and metabolic activity of viable CCL-185 and CCL-64 cells were investigated with the BrdU and WST-1 assays, respectively. As shown in Figures 1 A–D, the number of viable CCL-185 cells, measured by WST, was shown to be proportional to the TGF-β1 concentration (R2 = 0.919). The finding was confirmed by the BrdU assay (R2 = 0.969). Compared with the CCL-185 cell line, metabolic activity of viable cells and level of cell DNA synthesis, measured by WST and BrdU incorporation assays, were shown to be less proportional to the TGF-β1 concentration in the CCL-64 cell line (R2 = 0.424, 0.168, respectively). Because TGF-β produced the same pattern in the WST-1 and BrdU assays using CCL-185 cells, indicating a decrease in metabolically active cells and DNA synthesis, WST-1 was selected to evaluate TGF-β bioactivity in the subsequent studies.
FIGURE 1.
Effects of TGF-β1 on growth and viability of CCL-185 and CCL-64 cells. A–D, The growth inhibition standard curves of CCL-185 or CCL-64 cells exposed to recombinant human TGF-β1 (concentrations ranging from 0, 0.313, 0.625, 1.25, 2.5, and 5 to 10 ng/mL) using BrdU and WST-1 assays, respectively.
Analysis of TGF-β1 and β2 Responses in CCL-185 Cells
The relative potency of 2 major isoforms of TGF-β, TGF-β1 and β2 was investigated in the bioassay. TGF-β1 was determined to have dose proportional response in CCL-185 cells when used in the range of 0.313–10 ng/mL. TGF-β2 also yielded similar dose–response curves in the assay but showed slightly less activity than observed for TGF-β1. This indicates that the CCL-185 cells are most sensitive to the TGF-β1 isoform (Fig. 2).
FIGURE 2.
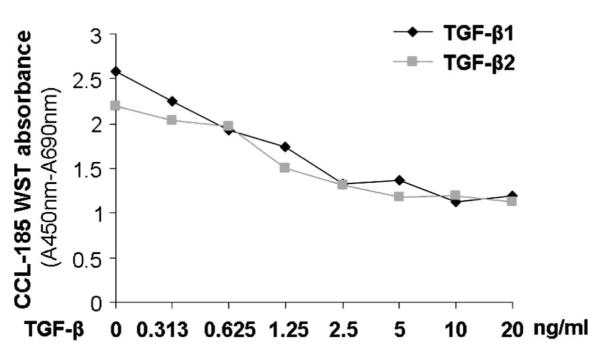
Growth inhibitory effects of TGF-β1 and TGF-β2 on CCL-185 cells. CCL-185 cells were exposed to recombinant human TGF-β1 and TGF-β2 in concentrations ranging from 0 to 10 ng/mL, and cell viability was assessed by the WST-1 assay.
The Neutralization of the Growth Inhibitory Effect of TGF-β1
The specificity of the growth inhibitory activity of TGF-β1 on CCL-185 cells was confirmed by neutralization with anti–TGF-β1 antibody. Based on a previously reported study evaluating the ED80 concentration for TGF-β1 in CCL-185 cells,22 0.3 ng/mL was used to establish the dose–response curve for anti–TGF-β1 antibody. The anti–TGF-β1 antibody concentrations chosen ranged from 0.16 to 100 μg/mL. This range yielded adequately defined upper and lower asymptotes of the resulting dose–response curve and providing at least 3 points on the linear portion of the curve (Fig. 3).
FIGURE 3.
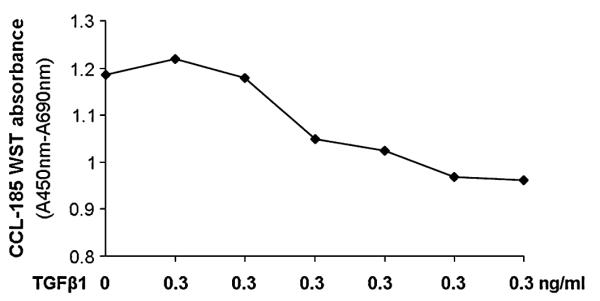
Neutralization of the growth inhibitory effect of TGF-β1 (0.3 ng/mL) in CCL-185 by anti-human TGF-β1 IgG1 (concentrations ranging from 0 to 100 μg/mL) measured with the WST-1 assay.
Effects of TGF-β1 and Anti–TGF-β1 Antibody on Growth of CCL-185 Cells
To confirm the WST and BrdU results, the effects of TGF-β1 and anti-TGF-β1 on the growth of CCL-185 cells was observed by microscopy. As shown in Figure 4, increasing growth arrest was observed as the TGF-β1 concentration in the culture media increased. Anti–TGF-β1 antibody was seemed to neutralize the growth inhibitory effect of TGF-β1.
FIGURE 4.
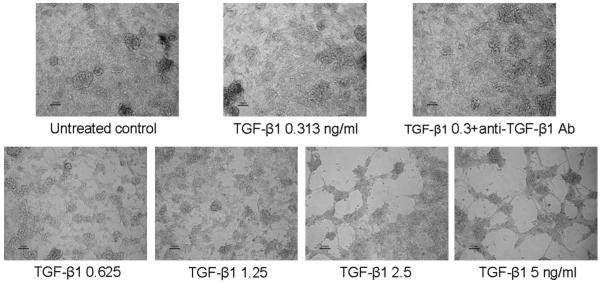
Effects of TGF-β1 and anti–TGF-β1 antibody on growth of CCL-185 cells. Microscopic images showing growth inhibitory effects of TGF-β1 (concentrations ranging from 0 to 10 ng/mL) and effect of anti–TGF-β1 (20 μg/mL) antibody on the growth of CCL-185 cells treated with 0.3 ng/mL TGF-β1. Magnification: ×10.
Increased TGF-β Activity in DE Tears
Tears from patients with DE and normal controls were initially evaluated by Luminex immunobead assay to determine the presence and concentration of TGF-β isoforms. As shown in Figure 5A, there is no difference between the 2 groups in the concentration of TGF-β1 (concentrations in NL and DE groups were 5480 ± 721.2 and 5413.3 ± 573.9 pg/mL, respectively). There was a slight but nonsignificant increase in TGF-β2 concentration in DE tears compared with control tears (concentrations in NL and DE groups were 5502.5 ± 2964.4 and 6952.2 ± 3186.8 pg/mL, respectively). The concentration of TGF-β3 was below the level of detection in both groups.
FIGURE 5.
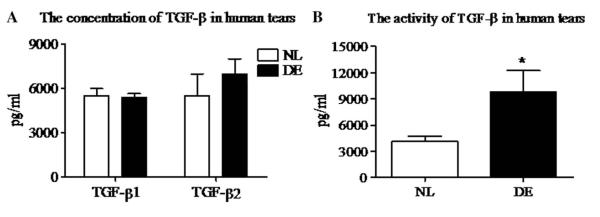
Concentration and activity of TGF-β in human tears. A, The concentration of TGF-β1 and μ2 in DE tears and normal subjects (NL, n = 10) and patients with DE (DE, n = 16) measured by Luminex immunobead assay. B, Activity of TGF-β in tears from normal human subjects and patients with DE detected by CCL-185 cells bioassay. Results shown are mean ± SD. *P < 0.05 DE versus control group.
We then compared TGF-β activity in DE and normal human tears using the CCL-185 cell bioassay. As shown in Figure 5B, the mean level of total TGF-β activity was significantly higher in the tears of patients with DE (9777.5 ± 10,481.9 pg/mL) than in the tears of normal control subjects (4129.3 ± 1342.9 pg/mL) (P < 0.05). Table 1 presents the clinical parameters and mean tear TGF-β activity in the 3 subsets of patients with DE. TGF-β activity was higher in all 3 DE subgroups than the control group, reaching statistical significance for the Sjogren syndrome group. Approximately, 79.1% TGF-β was biologically active in DE tears compared with 37.6% in normal control tears.
TABLE 1.
Comparison of Dry Eye Subgroups
| MGD | Non-SS ATD | SS ATD | |
|---|---|---|---|
| Number (n) | 6 | 4 | 8 |
| Mean age ± SD (yrs) | 43.4 ± 10.6 | 65.7 ± 10.2 | 60.3 ± 7.7 |
| TBUT (s) | 4.1 ± 0.8 | 3.9 ± 2.2 | 2.6 ± 0.9 |
| Schirmer 1 score (mm) | 30.2 ± 8.5 | 8.1 ± 1.9 | 8.3 ± 7.3 |
| Corneal FL staining score | 3 ± 2.7 | 3.5 ± 3.1 | 6.5 ± 5.7 |
| Cong LG score | 0.3 ± 0.4 | 0.5 ± 0.6 | 4.9 ± 2.1 |
| Conjunctivochalasis† | 0.3 ± 0.5 | 0.3 ± 0.5 | 0 |
| Severity level‡ | 1.5 ± 0.5 | 1.5 ± 0.6 | 2.6 ± 0.9 |
| TGF-β activity (pg/mL) | 4821.6 ± 3917.2 | 7938.9 ± 12217.6 | 14413.8 ± 12014.3 (P < 0.05)* |
Versus normal control group.
0, not present; >0, present.
based on criteria proposed by the DE Workshop.20
FL, fluorescein; LG, lissamine green; TBUT, tear breakup time in seconds.
To determine whether the antiproliferative effects of human tears in the CCL-185 cells were because of TGF-β, tears samples from 3 subjects were preincubated with anti-TGF-β1,2,3. The antiproliferative effects of tears could be completely inhibited by 20 μg/mL of anti-TGF-β1,2,3. Furthermore, to determine the relative contribution of the TGF-β1 isoform on this growth inhibitory effect, tears from 3 subjects were preincubated with anti–TGF-β1 (20 μg/mL) antibody. The TGF-β1–specific antibody neutralized 56% of total tear bioactivity.
DISCUSSION
The goal of this project was to develop a sensitive bioassay to detect TGF-β activity in human tears. We found the CCL-185 cell line to be very sensitive to the growth inhibitory effect of TGF-β, and this cell line was subsequently used to compare TGF-β activity in tears obtained from healthy control and DE groups. Several methods for measuring TGF-β activity have been described previously.10–12 The standard assay is growth inhibition of CCL-64 mink lungs epithelial cells measured by [3H]-thymidine incorporation. The A549 cell line was also previously shown to be a TGF-β–responsive cell line.13,14
In our study, we compared 2 cell lines: CCL-185 and CCL-64 using the BrdU and WST-1 assays. BrdU incorporation was used as a parameter for cell proliferation by measuring its incorporation into newly synthesized DNA. Metabolic activity in these cells was measured by incubation with the tetrazolium salt, WST-1, that is cleaved into a colored formazan product by metabolically active cells. We found that TGF-β1 produced better dose-dependent growth inhibition in CCL-185 cells by either the WST-1 assay or the BrdU assay (R2 = 0.919 and 0.969, respectively) than in CCL-64 cells (R2 = 0.424 for WST-1 and R2 = 0.168 for BrdU). These findings indicate that the CCL-185 cells are more sensitive and accurate than CCL-64 for measuring TGF-β activity. Consequently, we chose CCL-185 cells for additional studies. Furthermore, a decrease in both WST-1 formation and BrdU incorporation were observed in CCL-185 cells indicating that TGF-β1 inhibits DNA synthesis and the number of metabolically active CCL-185 cells. Between these 2 assays, the BrdU incorporation assay is more complex and time consuming than the WST-1 assay. So, we elected to use the WST-1 assay to measure tear TGF-β bioactivity in CCL-185 cells.
To analyze the growth inhibitory activity of different isoforms of TGF-β on CCL-185 cells, the potency of the 2 major isoforms of TGF-β, TGF-β1 and μ2, was investigated using the WST-1 assay. We found that TGF-β2 yielded similar dose–response curves but showed slightly less activity than observed for TGF-β1. This indicates these cells are most sensitive to the TGF-β1 isoform. Additionally, to verify the specificity of TGF-β1 in growth-inhibiting CCL-185 cells, the ability of anti-TGF-β1 to neutralize TGF-β1 was assessed in the assay. Using the ED80 concentration (0.3 ng/mL) for TGF-β1, anti–TGF-β1 antibody yielded a dose-dependent inhibition of TGF-β1 activity. To confirm these data, CCL-185 cell growth observed microscopically was noted to decrease in response to increasing TGF-β1 concentrations.
DE is a common ocular surface disease that can impact productivity and quality of life and is characterized by eye irritation symptoms and blurred vision. Disrupted corneal epithelial barrier and accelerated corneal epithelial loss in DE lead to corneal surface irregularity, which degrades visual function by decreasing contrast sensitivity and functional visual acuity. Sight threatening corneal ulceration can occur in severe DE conditions such as Sjögren syndrome or Steven–Johnson syndrome.
DE has been demonstrated to cause inflammation on the ocular surface, evidenced by increased levels of inflammatory cytokines (IL-1, IL-6, and tumor necrosis factor a) in the tear fluid and corneal and conjunctival epithelium.6,7 TGF-β is a key immunoregulatory factor, and 2 isoforms, TGF-β1 and TGF-β2, have been detected in human tears.15 Both the lacrimal gland and conjunctival goblet cells all have been found to produce and secrete TGF-β.23,24 Our group has previously reported increased expression of messenger RNA encoding several different inflammatory cytokines, including TGF-β1 within the conjunctival epithelium of patients with Sjögren syndrome and aqueous tear deficiency compared with non-DE controls.8 We have recently reported that experimental desiccating stress increases levels of Th-17–associated genes (IL-6, IL-23, TGF-β1 and μ2, IL-23R, IL-17R, IL-17A, RORγt, and CCL20) in the cornea and conjunctiva of mice. Furthermore, IL-17 was found to contribute to the disruption of corneal epithelial barrier function in response to desiccating stress by stimulating production of MMP-3 and MMP-9.9 Th-17 differentiation is driven by IL-6 and TGF-β.25,26 These findings suggest that TGF-β may play a crucial role in regulating the ocular surface immune response in DE. TGF-β is generally released in a biologically latent form. Immunoassays (such as ELISA or Luminex) can only measure the total quantity of TGF-β, not the biologically active form.
Using the Luminex microbead assay to measure total TGF-β1 and μ2 (including latent and active forms) concentrations in human tears, we found a slight but nonsignificant increase in TGF-β2 concentration in DE tear compared with control tear. However, no difference in TGF-β1 concentration was noted. In our previous report, TGF-β1 was found to be the predominant isoform when measured by ELISA.15 In the present study, we found nearly equal concentration of TGF-β1 and TGF-β2 measured by a Luminex immunobead assay. The relative differences observed between these assays may be because of the tear collection method or the format (plate vs. solution), sensitivity, or antibody specificity of the immunodetection method. The manufacturer of the Luminex kit used to detect both TGF-β isoforms in the current study reported no cross-reactivity of the TGF-β1 antibodies for TGF-β2 and < 5% cross-reactivity of the TGF-β2 antibodies for TGF-β1. We found TGF-β1 and TGF-β2 to be below the level of detection when assayed with the kit for the other isoform (ie, TGF-β2 and TGF-β1, respectively). In contrast to the immunoassay, we found a higher levels of TGF-β activity in tears from patients with DE (9777.5 ± 10481.9 pg/mL) than in the control tears (4129.3 ± 1342.9 pg/mL) (P < 0.05), with 79.1% of TGF-β in DE tears being biologically active compared with 37.6% in the normal control tears. The highest tear TGF-β activity was found in tear samples obtained from patients with Sjögren syndrome, the subgroup that also had the most severe ocular surface disease. This is consistent with our previous finding of increased levels of TGF-β1 transcripts in conjunctival epithelium obtained from patients with Sjögren syndrome.8 Additional studies are warranted to determine if this increase in bioactive TGF-β is a marker for Sjögren syndrome.
In summary, the CCL-185 cell assay was found to be a suitable tool for assessing TGF-β activity in human tears. TGF-β1 activity in tears is increased in DE with the highest bioactivity in Sjögren syndrome.
Acknowledgments
Supported in part by National Institutes of Health grant EY11915 (S. C. Pflugfelder), Department of Defense CDMRP PRMRP grant FY06 PR064719 (D. Q. Li), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, the William Stamps Farish Fund, and Allergan, Inc.
Footnotes
Commercial Relationship: None.
REFERENCES
- 1.Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–374. [PubMed] [Google Scholar]
- 2.Khalil N. TGF-beta: from latent to active. Microbes Infect. 1999;1:1255–1263. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn JA, Roos A, de GB, et al. Transforming growth factor-beta and the glomerular extracellular matrix in renal pathology. J Lab Clin Med. 1994;123:34–47. [PubMed] [Google Scholar]
- 4.Miljanovic B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chotikavanich S, de Paiva CS, Li DQ, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 7.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 8.Jones DT, Monroy D, Ji Z, et al. Alterations of ocular surface gene expression in Sjogren’s syndrome. Adv Exp Med Biol. 1998;438:533–536. doi: 10.1007/978-1-4615-5359-5_75. [DOI] [PubMed] [Google Scholar]
- 9.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccation stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzino A. Behavior of transforming growth factors in serum-free media: an improved assay for transforming growth factors. In Vitro. 1984;20:815–822. doi: 10.1007/BF02618298. [DOI] [PubMed] [Google Scholar]
- 11.Danielpour D, Dart LL, Flanders KC, et al. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- 12.Abe M, Harpel JG, Metz CN, et al. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 13.Tang W, Yang L, Yang YC, et al. Transforming growth factor-beta stimulates interleukin-11 transcription via complex activating protein-1-dependent pathways. J Biol Chem. 1998;273:5506–5513. doi: 10.1074/jbc.273.10.5506. [DOI] [PubMed] [Google Scholar]
- 14.Elias JA, Zheng T, Einarsson O, et al. Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J Biol Chem. 1994;269:22261–22268. [PubMed] [Google Scholar]
- 15.Gupta A, Monroy D, Ji Z, et al. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996;15:605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 16.Peuster M, Fink C, von SC. Biocompatibility of corroding tungsten coils: in vitro assessment of degradation kinetics and cytotoxicity on human cells. Biomaterials. 2003;24:4057–4061. doi: 10.1016/s0142-9612(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 17.Ayalasomayajula SP, Ashton P, Kompella UB. Fluocinolone inhibits VEGF expression via glucocorticoid receptor in human retinal pigment epithelial (ARPE-19) cells and TNF-alpha-induced angiogenesis in chick chorioallantoic membrane (CAM) J Ocul Pharmacol Ther. 2009;25:97–103. doi: 10.1089/jop.2008.0090. [DOI] [PubMed] [Google Scholar]
- 18.Bron AJ, Abelson MB, Ousler G. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShopet al. (2007) Ocul Surf. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 19.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjögren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjögren’s Syndrome. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 21.Management and therapy of dry eye disease: report of the management and therapy subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 22.Rapoza ML, Fu D, Sendak RA. Development of an in vitro potency assay for therapeutic TGFbeta antagonists: the A549 cell bioassay. J Immunol Methods. 2006;316:18–26. doi: 10.1016/j.jim.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino K, Garg R, Monroy D, et al. Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr Eye Res. 1996;15:615–624. doi: 10.3109/02713689609008901. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JD, Farris RL. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988;106:484–487. doi: 10.1001/archopht.1988.01060130530029. [DOI] [PubMed] [Google Scholar]
- 25.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]