Abstract
Purpose
to compare tear cytokine and chemokine concentrations in asymptomatic control and dysfunctional tear syndrome (DTS) patients and determine the correlations between tear inflammatory mediators and clinical severity.
Design
Prospective observational cohort study
Methods
Concentrations of epidermal growth factor (EGF), interleukin (IL) - 1α, 1β, 6, 10, 12 and 13, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α) and chemokines: IL-8 (CXC), MIP-1α (CCL3) and RANTES (CCL5) were measured by a multiplex immunobead assay in an asymptomatic control group and DTS patients with and without MGD. Spearman correlations between tear cytokines and severity of irritation symptoms and ocular surface signs were calculated.
Results
Tear concentrations of IL-6, IL-8 and TNF-α were significantly higher in DTS with and without MGD and EGF was significantly reduced in the DTS without MGD group compared to the control group. MIP-1α was greater in entire DTS and DTS without MGD groups than the control group and RANTES was greater in DTS with MGD than the control and DTS without MGD groups. IL-12 was significantly higher in the DTS with MGD than the DTS without MGD subgroup. Significant correlations were observed between IL-6 and irritation symptoms and between a number of cytokines and chemokines and clinical parameters.
Conclusions
As predicted, patients with DTS have higher levels of inflammatory mediators in their tears that show correlation with clinical disease parameters. Furthermore, different tear cytokine/chemokine profiles were observed in DTS patients with and without MGD groups.
Introduction
Dysfunctional Tear Syndrome (DTS) was proposed by the Delphi Dry Eye Panel Report in 2006 as a more encompassing term for dry eye disease based on evidence that inflammatory mechanisms were involved in the pathophysiology of the disease.1 It is now recognized that an imbalance or alteration of the tear film components that stabilize the tear film, regardless of cause, leads to increased tear osmolarity.2,3 Exposure of the ocular surface epithelia to osmotic stress activates stress pathways that signal increased production and release of inflammatory cytokines and other mediators that are capable of initiating a cascade of inflammatory events.4 The Dry Eye Workshop (DEWS) held in 2006 and 2007 elected to retain the name dry eye because it is embedded in literature; however, the group embraced the concept espoused by the Delphi panel by stating in their operational definition of dry eye that “It is accompanied by increased osmolarity and inflammation on the ocular surface.” 3
There is mounting evidence that inflammation plays a key role in the pathogenesis of the ocular surface disease that develops in dry eye.5 Decreased tear clearance was found to correlate with an increased concentration of IL-1α in the tears.6 Increased levels of RNA transcripts encoding the inflammatory cytokines IL-1, IL-6, IL-8, TNF-α and TGF-β1 were detected in the conjunctival epithelium of patients with Sjögrens syndrome keratoconjunctivitis sicca.7–9 Other immunopathological changes have been detected in the conjunctiva in both Sjögrens syndrome and non-Sjögrens syndrome related dry eye, including increased production of HLA Class II and ICAM-1 antigens and the chemokine receptor CCR5 by the conjunctival epithelium and increased T cell infiltration of the conjunctival epithelium and stroma.8–14 Clinical evidence indicates that anti-inflammatory therapies, such as corticosteroids and cyclosporine reduce the signs and symptoms of DTS and cyclospoein has been noted to decrease inflammatory cells and markers in the conjunctiva.5,15–19
The Delphi panel stratified DTS into conditions with and without lid margin disease and four levels of clinical severity based on symptoms and clinical signs for the tear deficient group were proposed.1 The DEWS management and therapy subcommittee subsequently adopted the severity grading scheme in the Delphi panel report, but recommended that any of the 4 severity levels could have a component of meibomian gland disease because this condition is often observed in patients with aqueous tear deficiency.15 The purpose of our study was to determine if there were differences in levels of tear cytokines and chemokines between an asymptomatic control group and DTS patients with and without meibomian gland disease (MGD). Furthermore, the correlation between concentrations of inflammatory mediators in the tears and the severity of clinical parameters of DTS was determined.
Patients and Methods
Patients
This study was approved by the Baylor College of Medicine Institutional Review Board. Thirty patients with DTS (22 females, 8 males) meeting the inclusion and exclusion criteria were enrolled at the Ocular Surface Center of Baylor College of Medicine, Houston, TX. Patients completed a 12 question symptom questionnaire and had an ocular surface and tear evaluation performed by one of the investigators (SCP) that consisted (in the following order) of biomicroscopic examination of the face, lid margins and meibomian glands, fluorescein tear break-up time (TBUT), corneal fluorescein staining and conjunctival lissamine green staining. These tests were performed as previously described.20 Criteria for diagnosis of DTS included a symptom severity score > 20 and TBUT ≤ 7 seconds. Meibomian gland disease was diagnosed by evidence of dysfunction (lack of expressible meibum from ≥ 75% of glands) and the presence of two or more morphologic changes of the meibomian glands, including acinar atrophy, orifice metaplasia and vascular dilation on the posterior lid margin.21 Inclusion criteria for DTS include the presence of irritation symptoms with a symptom severity score >20 and tear break-up time ≤ 7 seconds. Patients were excluded if they were using any topical medications other than non-preserved artificial tears which could not be instilled on the day of the exam, contact lens use, ocular surgery in the past year or other ocular surface diseases. The United States European study group concensus criteria were used for diagnosis of Sjögren’s syndrome and Stevens-Johnson syndrome was diagnosed in patients with a history of acute vesiculobullous cutaneous eruption with involvement of 2 or more mucus membranes, including the conjunctiva.21
The patients were stratified into four levels of clinical severity based on signs and symptoms using the DEWS criteria (Table 1).15 Fourteen age and gender-matched normal control subjects (7 females and 7 males) were recruited. Inclusion criteria for these normal subjects were absence of corneal and conjunctival dye staining and a symptom severity score ≤ 20.
Table 1.
Severity Grading Criteria for Dysfunctional Tear Syndrome
| Group | Symptom Severity Score¥ | Tear Breakup Time (seconds) | Conjunctival Staining Score | Corneal Staining Score |
|---|---|---|---|---|
| No DTS | ≤ 20 | > 7 | 0 | 0 |
| DTS 1 | > 20 | ≤ 7 | ≤ 3 | ≤ 2 |
| DTS 2 | > 20 | ≤ 7 | ≥ 3 | ≤ 8 |
| DTS 3 | > 20 | ≤ 7 | ≥ 3 | > 8, including central cornea or filaments |
| DTS 4 | > 20 | ≤ 7 secs | ≥ 3 | ≥ 12 |
DTS = dysfunctional tear syndrome; DTS 1–4 = DTS severity levels 1–4
symptom severity score measured by ocular surface disease index (OSDI) questionnaire
Tear Collection and Multiplex bead analysis
Unstimulated tear fluid was collected from the inferior tear meniscus of each eye using a 0.5 μl glass capillary micro-pipette (Drummond, Broomall, PA). The tear samples from both eyes were eluted into one Eppendorf tube containing 9 μl of assay buffer for a final dilution of 1:10, centrifuged for 2 minutes and immediately transported in an insulated cooler to a −80°C freezer where they remained frozen until they were used for the immunoassay. Cytokines and chemokines in these samples were analyzed using a Luminex Beadlyte multicytokine array immunobead assay (Upstate Biotechnology, Lake Placid, NY). The cytokines and chemokines analyzed included: epidermal growth factor (EGF), interleukin (IL)- 1α, 1β, 6, 8 (CXCL8), 10, 12 (p70) and 13, TNF-α, IFN-γ,MIP-1α (CCL3), and RANTES (regulated upon activation, normal T cell expressed and secreted, CCL5). The concentrations of these factors in tears were calculated from standard curves of known concentrations of recombinant human cytokines. The lowest cytokine concentration in the linear portion of the standard curve was used for statistical comparison of tear samples with concentrations below this level.
Statistical Analysis
The sample size was calculated to show a 95% chance of detecting a 30% difference in tear IL-6 concentration between the control and DTS groups. Clinical parameters and cytokine concentrations in the 4 study groups (no DTS, entire DTS group, DTS with MGD subgroup and DTS without MGD) were compared by ANOVA with Tukey post-hoc testing (GraphPad Prism, La Jolla, CA). The mean value for both eyes was used for comparison of clinical parameters. Correlations between clinical parameters (symptom severity, Schirmer 1, corneal fluorescein staining) and tear cytokine concentrations were determined using rank (Spearman) correlations.
Results
The demographic and clinical findings in DTS patients and normal subjects are presented in Table 2.
Table 2.
Clinical Features of Dysfunctional Tear Syndrome and Control Patients
| Group | N | % Female | Mean Age ±SD | Symptom Severity Score¥ | Schirmer 1 score (mm) | Corneal Staining score | Conj LG Staining |
|---|---|---|---|---|---|---|---|
| No DTS | 14 | 50 | 45 ± 17.3 | 13 ± 6.1 | 26.8± 9.2 | 0.14±0.4 | 0± 0.0 |
| DTS | 30 | 73 | 55.2±16.4 | 37.8±11.2 P<0.001 |
14.2±9.1 P<0.001 |
4.8±4.4 P<0.0008 |
2.2±2.3 P<0.01 |
| DTS w/MGD | 9 | 100 | 62.2±14.7 | 31.6±10.8 P<0.001 |
15.4±8.3 P<0.05 |
2.9±3.4 | 1.3±1.8 |
| DTS w/o MGD | 21 | 62 | 52.2±16.5 | 40.5±10.4 P<0.001 |
13.7±9.6 P<0.001 |
5.6±4.6 P < 0.001 |
2.6±2.4 P<0.01 |
Statistical comparisons are between DTS groups and the controls.
DTS = dysfunctional tear syndrome; MGD = meibomian gland disease Conj = conjunctiva; LG = lissamine green
There was no difference in mean age between groups. There was no significant difference between eyes for any clinical severity parameter; therefore, the mean value of the two eyes was used for statistical comparison. Compared to the no DTS control group, symptom severity scores were significantly greater and Schirmer test scores were significantly less in the DTS group as a whole, as well as the 2 subgroups with and without MGD. Corneal fluorescein and conjunctival lissamine green staining scores were greater in the entire DTS and the DTS without MGD groups than the no DTS control group.
Demographic data and severity parameters for each of the 4 levels of DTS severity are presented in Table 3. Symptom severity scores were greater in level 3 than level 1 disease. There was no difference in Schirmer test scores between severity levels. Corneal fluorescein staining scores were significantly greater in level 3 than level 1 and conjunctival lissamine green scores were significantly greater in level 3 than levels 1 and 2.
Table 3.
Clinical Features of Dysfunctional Tear Syndrome Patients Stratified by Severity
| Severity Level | N | % Female | Mean Age ±SD | Symptom Severity Score | Schirmer 1 score (mm) | Corneal Staining score | Conj LG Staining |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 80 | 60±13.4 | 36.8± 8.4 | 16.6 ± 5.2 | 0.9 ± 2.0 | 0± 0.0 |
| 2 | 11 | 64 | 57.4±15.7 | 32.3±8.9 | 16.3±8.0 | 3.6±4.1 | 1±0.9 |
| 3 | 13 | 77 | 49.8±17.2 | 42.9±11.7 P< 0.05 ¶ |
12.8±10.9 | 7.1±3.6 P < 0.01¥ |
3.7±2.2 P < 0.001¥¶ |
| 4 | 1 | 100 | 78 | 23.0 | 0.00 | 13.5 | 6.0 |
The mean score of both eyes was used for the Schirmer 1 test, corneal fluorescein staining and conjunctival lissamine green (LG) staining scores; SD = standard deviation; Conj = conjunctiva
vs normal control,
vs Level 1;
vs Level 2
The sub classification of DTS conditions by disease severity is provided in Table 4.
Table 4.
Subclassification of Dysfunctional Tear Syndrome
| DTS Level | All DTS | MGD | SS | SJS |
|---|---|---|---|---|
| 1 | 5 | 1 | 0 | 0 |
| 2 | 11 | 7 | 0 | 0 |
| 3 | 13 | 1 | 6 | 1 |
| 4 | 1 | 0 | 0 | 1 |
| Total | 30 | 9 | 6 | 2 |
DTS = Dysfunctional Tear Syndrome; MGD = meibomian gland disease; SS = Sjögren’s syndrome; SJS = Stevens Johnson Syndrome
Tear Growth Factor and Cytokine Concentrations
The concentrations of all measured growth factors and cytokines in the tears were in the linear portions of their standard curves. Epidermal growth factor was used as a measure of lacrimal gland function. EGF was significantly lower in the DTS without MGD groups than the no DTS control group (Table 5).
Table 5.
Tear Epidermal Growth Factor Concentration in Dysfunctional Tear Syndrome
| Group | EGF pg/ml |
|---|---|
| No DTS | 1277±619 |
| DTS | 926±930 |
| DTS w/MGD | 1310±935 |
| DTS w/o MGD | 572±379. P < 0.05* |
vs normal control
EGF=epidermal growth factor; DTS = Dysfunctional Tear Syndrome; MGD = meibomian gland disease
The tear concentrations of the Th1 inducing cytokine IL-12 and cytokines produced by Th1 (IFN-γ and Th2 (IL-13) lymphocytes were evaluated. The concentration of IL-12 was significantly lower in the DTS without MGD group compared to the DTS with MGD group (Table 6). There was no difference between groups in the concentrations of the other T cell associated cytokines. The ratio of the Th1 cytokine IFN-γ to the Th-2 cytokine IL-13 was calculated for all study groups. Compared to the no DTS control group (0.11 ± 0.13), the mean IFN-γ/IL-13 ratio was greater in all 3 DTS groups (DTS = 1.1 ±2.6, DTS with MGD = 0.8 ±2.1 and DTS without MGD = 1.14 ±2.8, P = 0.05 vs no DTS)
Table 6.
T Cell Associated Cytokines in Dysfunctional Tear Syndrome
| Group | IL-12 | IFN-γ | IL-10 | IL-13 |
|---|---|---|---|---|
| Normal | 117.5±118.5 | 6.0±0.0 | 1.0±0.0 | 111.4±64.1 |
| DTS | 147.5±230.5 | 95.5±392.8 | 12.4±37.6 | 152.6±167.8 |
| DTS w/MGD | 301.6±355.3§ | 36.6±86.6 | 5.6±9.2 | 219.6±222.5 |
| DTS w/o MGD | 75.1±107.8 P<0.05¶ |
116.0±454.2 | 14.7±43.4 | 129.3±143 |
vs normal control;
vs DTS;
vs DTS with MGD;
vs DTS without MGD
DTS = Dysfunctional Tear Syndrome; MGD = meibomian gland disease
Levels of 3 chemokines (IL-8, MIP-1α, and RANTES) were compared between groups (Table 7). The concentration of IL-8 was higher in all 3 DTS groups than the no DTS group. MIP-1α was higher in the entire DTS and DTS without MGD groups than the no DTS group. In contrast, RANTES was significantly higher in the DTS with MGD group than the no DTS and the DTS without MGD groups.
Table 7.
Tear Chemokines in Dysfunctional Tear Syndrome
| Group | IL-8 | MIP-1α | Rantes |
|---|---|---|---|
| Normal | 176±72 | 1.5±0.5 | 371±209.9 |
| DTS | 1510±1671 P = 0.005* |
3035±5510 P=0.03* |
445.8±343.6 |
| DTS w/MGD | 1303±661 P<0.001* |
800±0.0 | 700.7±477.9 P=0.03*§ |
| DTS w/o MGD | 1657±2393 P<0.03* |
3504±6036 P=0.02* |
334.5±237.9 P=0.01¶ |
vs normal control;
vs DTS;
vs DTS with MGD;
vs DTS without MGD
DTS = Dysfunctional Tear Syndrome; MGD = meibomian gland disease
The levels of the inflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α) are presented in Table 8. Significantly elevated levels of IL-6 and TNF-α were found in the tear fluid of all DTS groups compared to the no DTS group.
Table 8.
Tear Inflammatory Cytokines in Dysfunctional Tear Syndrome
| Group | IL-1α | IL-1β | IL-6 | TNF-α |
|---|---|---|---|---|
| Normal | 1.0±0.0 | 3.0±5.8 | 26.5±21.8 | 126.8±44.5 |
| DTS | 73.8±239.8 | 57.7±207.1 | 238.0±278.2 P<0.05* |
464.4±392 P=0.02* |
| DTS w/MGD | 21.4±57.6 | 13.5±24 | 289.0±272.2 P =0.001* |
323.2±251.9 P=0.04* |
| DTS w/o MGD | 92.1±275.7 | 73.0±239.5 | 210.0±282.9 P=0.02* |
542.9±445.6 P=0.01* |
vs normal control
DTS = Dysfunctional Tear Syndrome; MGD = meibomian gland disease
To determine whether tear cytokine concentrations were influenced by age, the concentrations of EGF, IL-6 and IL-8 were compared in normal subjects of 3 age groups (20–40, 41–60, 61–80). No difference was observed between the 3 age groups for any of these cytokines.
Correlation Analyses
The rank (Spearman) correlation coefficients between tear fluid cytokine/chemokine concentrations and parameters of ocular surface disease are presented in Table 9. The severity of irritation symptoms was positively correlated with IL-6 (Figure 1). Schirmer 1 scores were positively correlated with EGF and inversely correlated with IL-10, IL-8, MIP-1α, IL-1α, IL-1β and IL-6 concentrations. Corneal fluorescein and conjunctival lissamine green staining showed negative correlation with EGF, while they were positively correlated with IFNγ, IL-8, MIP1α, IL-1α, IL-1β and IL-6 concentrations (Figure 2).
Table 9.
Correlation Between Tear Cytokines and Clinical Severity Parameters in Dysfunctional Tear Syndrome
| Cytokine | Symptom Severity Scores | Schirmer 1 Scores | Corneal Fluorescein Staining Scores | Conjunctival Lissamine Staining Scores |
|---|---|---|---|---|
|
| ||||
| EGF | − 0.25 | 0.44 | − 0.38 | − 0.46 |
| NS | P = 0.003 | P = 0.01 | P = 0.002 | |
|
| ||||
| IL-12 | 0.03 | − 0.05 | − 0.16 | − 0.14 |
| NS | NS | NS | NS | |
|
| ||||
| IFN-γ | 0.19 | − 0.26 | 0.34 | 0.29 |
| NS | NS | P = 0.02 | P = 0.06 | |
|
| ||||
| IL-10 | 0.18 | − 0.29 | 0.22 | 0.43 |
| NS | P = 0.06 | NS | P = 0.004 | |
|
| ||||
| IL-13 | 0.15 | − 0.05 | − 0.12 | − 0.1 |
| NS | NS | NS | NS | |
|
| ||||
| IL-8 | 0.17 | − 0.28 | 0.39 | 0.34 |
| NS | P = 0.01 | P < 0.01 | P = 0.02 | |
|
| ||||
| MIP-1α | 0.18 | − 0.3 | 0.40 | 0.38 |
| NS | P = 0.05 | P < 0.01 | P = 0.01 | |
|
| ||||
| Rantes | −0.05 | 0.1 | −0.24 | −0. 14 |
| NS | NS | NS | NS | |
|
| ||||
| IL-1α | 0.20 | −0.3 | 0.33 | 0.49 |
| NS | P =0.05 | P = 0.03 | P = 0.0008 | |
|
| ||||
| IL-1β | 0.19 | −0.32 | 0.39 | 0.38 |
| NS | P = 0.04 | P < 0.01 | P= 0.01 | |
|
| ||||
| IL-6 | 0.32 | −0.32 | 0.41 | 0.38 |
| P = 0.04 | P = 0.04 | P < 0.01 | P = 0.01 | |
|
| ||||
| TNF-α | −0.04 | − 0.12 | 0.21 | 0.11 |
| NS | P = NS | NS | NS | |
Numbers are Spearman correlation coefficients; Significant correlations are in bold font; NS = non-significant correlation
Figure 1. Tear IL-6 vs Irritation Symptoms.
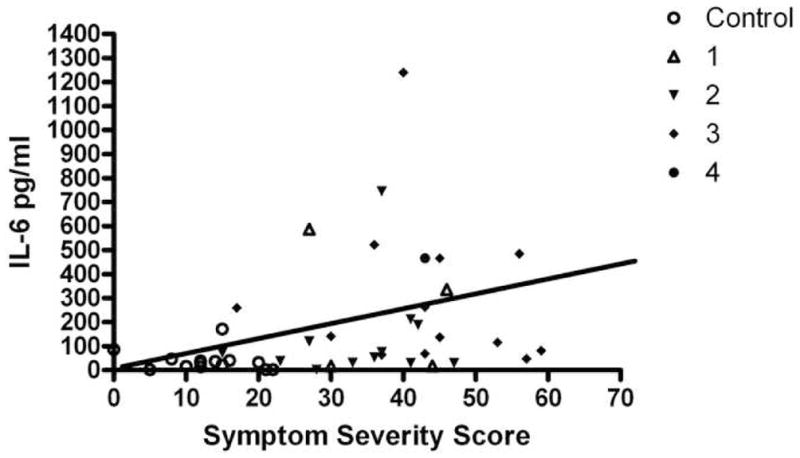
Correlation between tear IL-6 concentration in pg/ml and ocular irritation symptoms measured by a 12 question questionnaire. Symbols designate control or dysfunctional tear syndrome (DTS) severity level 1–4.
Figure 2. Tear IL-6 vs Corneal Fluorescein Staining.
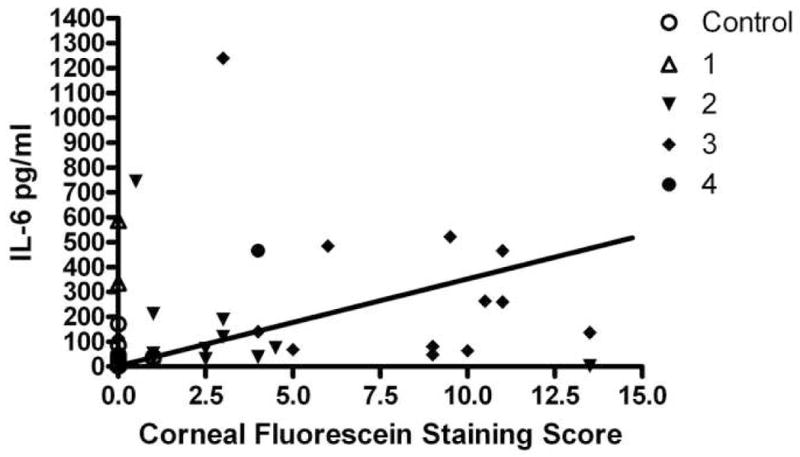
Correlation between tear IL-6 concentration in pg/ml and the severity of corneal fluorescein staining. Symbols designate control or dysfunctional tear syndrome (DTS) severity level 1–4.
Discussion
This study found significant differences in the concentrations of certain cytokines and chemokines in eyes with DTS compared to asymptomatic control eyes. Furthermore, differences were noted between the DTS groups with and without MGD. Clinical severity parameters of DTS, such as irritation symptoms, Schirmer test scores and corneal and conjunctival dye staining scores showed a significant correlation with the concentration of certain cytokines in tears.
The DTS was proposed by the Delphi panel under the premise that changes in tear composition, specifically increases in inflammatory cytokine concentrations, are responsible for the irritation symptoms and ocular surface disease that develops in this condition.1 DTS is currently diagnosed by the presence of irritation symptom and disease severity is classified by certain objective signs.1,15 Tear cytokine/chemokine assays provide direct evidence of ocular surface inflammation in this condition. Furthermore, elevated levels of tear cytokines might prove to be a better indicator of disease severity than currently utilized clinical tests, such as corneal fluorescein staining or Schirmer testing. It is also possible that elevated tear cytokines may be a more sensitive disease marker than traditional signs of dry eye in patients with mild Level 1 disease who complain of ocular irritation symptoms, yet lack objective signs, such as punctate corneal fluorescein staining.
This study had a relatively small sample size that was powered to detect differences in IL-6 between DTS and control eyes. Despite this design, significant differences were observed in the concentrations of a number of cytokine/chemokines in eyes with DTS compared to controls and our findings provides clues as to which cytokines/chemokines have the greatest potential to be used for diagnosis. The groups in this study were not sex matched and it is possible this could have influenced the study results; however, no significant difference was noted in the tear concentration of IL-6, the cytokine showing the greatest correlation with clinical severity, between females and males. This suggests that the small difference in sex between groups did not bias the results. It is possible that a panel containing a combination of cytokines/chemokines may provide greater discrimination between groups than any single factor. For example, the DTS with MGD subgroup had significantly greater levels of tear EGF and IL-12 than those without MGD.
EGF, a cytokine secreted by the lacrimal glands, was noted to have a significant inverse correlation with the Schirmer test. The Schirmer test is recognized for its variability and poor correlation with parameters of ocular surface disease and there is controversy whether the test is more accurate without or with topical anesthesia. EGF may be a better indicator of lacrimal gland function than the Schirmer test. This and previous studies have found a correlation between EGF and the severity of OSD.7 Although EGF is typically considered to be an epithelial mitogen, these findings suggest that it also has a role in ocular surface homeostasis.
In addition to EGF, the severity of ocular surface epithelial disease was correlated with several cytokines and chemokines, including IFN-γ, IL-8, MIP-1α, IL-1α, IL-1β and IL-6. This may represent a primary effect of these factors on the surface epithelia, or it may be a secondary to their ability to amplify inflammation by recruiting bone marrow derived cells to the ocular surface, which in turn may produce cytokines. Certain factors, such as IL-1, IL-6, IL-8 and TNF-α are well recognized to be produced by inflamed epithelial cells and they may signal ocular surface stress. This may be the reason why these cytokines have been detected in the tears of patients with other inflammatory ocular surface diseases, such as seasonal allergic conjunctivitis and vernal and atopic keratoconjunctivitis.23,14 An alternative explanation is that patients with these inflammatory conditions often have secondary changes of the lids and Meibomian glands that cause DTS. Among the various factors evaluated, IL-6 was found to have significant correlation with the severity of both symptoms and signs.
There is increasing evidence of a treatable inflammatory component of DTS.5 Clinical trials of anti-inflammatory/immunomodulatory agents, such as cyclosporine A and corticosteroids have shown improvement in clinical signs.16,17 The Dephi panel had empirically recommended institution of anti-inflammatory therapy for level 2 and worse disease.1 Given our finding of significant correlation between certain cytokines with ocular surface disease, detection of tear cytokines above a certain threshold might provide an objective indicator of which patients will benefit from anti-inflammatory therapy.
Basic studies in human dry eye patients and in animal models of dry eye indicate there is recruitment of T cells to the conjunctiva in dry eye.10,25,26 In the current study, there was a predominance of the Th2 cytokine IL-13 compared to the Th1 cytokine IFN-γ in normal eyes. An increase in IFN-γ was observed in DTS as a whole and in the DTS without MGD group. This indicates that IFN-γ producing inflammatory cells may be recruited to the ocular surface in DTS. IFN-γ has been noted to promote goblet cell loss and stimulate production of cornified envelope precursors by the conjunctival epithelium in an experimental murine model of dry eye.27 Interestingly, another Th2 cytokine, IL-4 was below the level of detection in all groups.
Irritation symptoms are the primary complaint of most patients seeking medical consultation for DTS. While decreased lubrication of the ocular surface and disruption of corneal barrier function may be responsible for the irritation symptoms in some patients with DTS, the cause of the eye discomfort is not readily apparent in other patients. Inflammation is a well recognized cause for pain and certain inflammatory cytokines, such as IL-1, IL-6 and TNF-α, have been found to affect neural sensitivity and cause hyperalgesia.28,29 In this study, tear IL-6 concentration was found to positively correlate with the severity of irritation symptoms. It is possible that IL-6 directly or indirectly lowers sensory nerve thresholds in the ocular surface, rendering patients more susceptible to environmental stimuli such as air drafts. Detection of elevated tear IL-6 concentrations in patients with mild level 1 DTS may suggest inflammation induced hyperalgesia as the cause for irritation symptoms in these patients.
Acknowledgments
Funding/Support: NIH Grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, The William Stamps Farish Fund, The Hamill Foundation and an unrestricted grant from Allergan, Inc.
Biography

Helene Y. Lam, M.D. attended medical school at University of California, Los Angeles where she was elected to AOA. She did her Ophthalmology residency at Baylor College of Medicine, and completed a Corneafellowship at the Massachusetts Eye and Ear Infirmary, where she served as Chief Fellow. Dr. Lam currently works in Boston with Harvard Vanguard as a comprehensive ophthalmologist and cornea specialist. Her research interests include ocular surface pathology and corneal transplant rejection.
Footnotes
Financial/Disclosure: The authors have no proprietary interest in any of the findings reported in this manuscript.
Contributions of Authors: Design of Study: Helene Lam, M.D., Cintia S. de Paiva, M.D.; Michael E. Stern, PhD, Stephen C. Pflugfelder, M.D.; Conduct of the Study: Helene Lam, M.D., Lauren Bleiden, M.D., Cintia S. de Paiva, M.D.; William Farley, MS, Michael E. Stern, PhD, Stephen C. Pflugfelder, M.D.; Interpretation of Data: Helene Lam, M.D., Cintia S. de Paiva, M.D.; Michael E. Stern, PhD.
Statement about conformity with author information: This study and the informed consent used was approved by the Baylor College of Medicine institutional review board (IRB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behrens A, Doyle JJ, MPH, Stern L, et al. Dysfunctional tear syndrome. A Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 2.Pflugfelder SC. Dry Eye, Focal Points. American Academy of Ophthalmology; San Francisco, CA: 2006. [Google Scholar]
- 3.Lemp MA, Baudouin C, Baum J, et al. The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) The Ocular Surface. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, de Paiva CS, Tong L, et al. Stress-activated protein kinase signaling pathways in dry eye and ocular surface disease. The Ocular Surface. 2005;3:5154–5157. doi: 10.1016/s1542-0124(12)70244-6. [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC. Anti inflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Afonso A, Sobrin L, Monroy DC, et al. Tear fluid gelatinase B activity correlates with IL-1α concentration and fluorescein tear clearance. Invest Ophthalmol Vis Sci. 1999;40:2506–12. [PubMed] [Google Scholar]
- 7.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Current Eye Research. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 8.Jones DT, Yen M, Monroy D, et al. Evaluation of cytokine expression in the conjunctival epithelia of Sjogren’s syndrome patients. Invest Ophthalmol Vis Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 9.Jones DT, Ji A, Monroy D, Pflugfelder SC. Evaluation of ocular surface cytokine, mucin, and cytokeratin expression in Sjögren’s Syndrome. Adv Exp Med Biol. 1998;438:533–536. doi: 10.1007/978-1-4615-5359-5_75. [DOI] [PubMed] [Google Scholar]
- 10.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 11.Rolando M, Barabino S, Mingari C, et al. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea. 2005;24:951–4. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- 12.Baudouin C, Haouat N, Brignole F, et al. Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br J Ophthalmol. 1992;76:545–9. doi: 10.1136/bjo.76.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006;124:710–6. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 14.Baudouin C, Liang H, Bremond-Gignac D, et al. CCR 4 and CCR 5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. J Allergy Clin Immunol. 2005;116:614–9. doi: 10.1016/j.jaci.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Pflugfelder SC, Geerling G, Kinoshita S, et al. Management and Therapy of Dry Eye Disease: Report of the Management and Therapy Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 16.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 17.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0. 5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–57. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–96. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 19.Brignole F, Pisella PJ, De Saint Jean M, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42:90–5. [PubMed] [Google Scholar]
- 20.De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141:438–45. doi: 10.1016/j.ajo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Bron AJ, Abelson MB, Ousler G, et al. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocular Surf. 2007;5:108–52. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjögren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjögren’s Syndrome. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–84. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 24.Sack R, Conradi L, Beaton A, et al. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85:528–38. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Pflugfelder SC, de Paiva CS, Li D-Q, Stern ME. Epithelial-Immune Interaction in Dry Eye. Cornea. 2008 doi: 10.1097/ICO.0b013e31817f4075. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogrens syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 27.De Paiva CS, Villarreal AL, Corrales RM, et al. Pflugfelder SC Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–60. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 28.Mika J, Korostynski M, Kaminska D, et al. Interleukin-1alpha has antiallodynic and antihyperalgesic activities in a rat neuropathic pain model. Pain. 2008 Mar 26; doi: 10.1016/j.pain.2008.02.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Zanjani TM, Sabetkasaei M, Mosaffa N, et al. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur J Pharmacol. 2006;538:66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]


