Abstract
Numerous studies have characterized the cytokine modulation observed in human immunodeficiency virus (HIV) infected individuals, from initial infection through chronic disease. Progressive and non-progressive HIV infection models show the cytokine milieu differs in terms of production and responsiveness in these two groups, suggesting an understanding of the role cytokines play during infection is necessary for directing the immune response toward viral control. This review will cover cytokine induction and dysfunction during HIV pathogenesis, with a focus on the interplay between cytokines and transcription factors, T cell activation, and exhaustion. We highlight cytokines that have either vaccine adjuvant or therapeutic potential and discuss the need to identify key factors required for prevention of progression, clearance of infection, or protection from acquisition.
Keywords: HIV, T cells, Transcription factors, Cytokines, Vaccines
1. Introduction
Global statistics from UNAIDS indicate nearly 35 million people are currently infected with human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS). Around 2 million more people are infected each year and over 25 million people have died from AIDS since the disease was first identified in the early 1980s [1]. While therapeutic interventions and prevention education efforts are ongoing, there remains a pressing need for an in-depth understanding of true correlates of protection and the development of an effective vaccine in order to control this global pandemic.
Extensive research efforts have led to an exponential expansion of our knowledge of HIV disease progression. Numerous studies show that HIV can alter cytokine production and responsiveness, particularly in T cells, in order to increase virus production and hinder the immune response. Comparing subject populations, such as elite controllers (EC) and chronic progressors (CP), has helped to identify some of the key factors that may contribute to progression of HIV disease. HIV progression results in loss of T cell function and, eventually, leads to T cell exhaustion. CP progressively lose IL-2 and T-bet expression, develop IL-7 resistance, and lose cytotoxic and proliferative capability [2–4]. EC, by mechanisms still under study, are capable of maintaining highly functional and responsive T cells, which in turn help to prevent disease progression [5,6].
Our laboratory and others seek to define and understand T cell-mediated correlates of immune protection in HIV disease, as we believe this information will be essential for the development of effective therapeutic and vaccine strategies. The partial success or the lack thereof in recent vaccine trials, such as Thai RV144 trial and the Merck STEP trial, highlight the deficiencies in our ability to predict vaccine efficacy [7,8]. The IFN-γ ELISPOT benchmark standard needs to be replaced by true measures of HIV control and we need to look in the right places, with the correct technology, to identify key factors that drive a high quality, functional immune response. Standard PBMC studies will need to be augmented by investigation of lymphatic and mucosal tissues, as these compartments respond differently to HIV infection, therapeutic interventions, and vaccination. Most importantly, we still need to understand the optimal measures on which to infer proper vaccine efficacy.
This review will cover T cell cytokine production and responsiveness during HIV progression, the role of cytokines in vaccine development, and lessons we have learned from recent vaccine trials. We will not attempt to discuss the myriad cytokines produced in HIV infection by the innate immune response, which arise very shortly after acute exposure to HIV. Innate cytokine responses, especially type I interferon, play a key role in orchestrating the entire immune response to HIV and other pathogens, and have direct effects on T cells to alter their functional properties. Understanding these complex cytokine interactions may help to identify useful targets for development of therapeutics or vaccines.
2. CD4+ T cell cytokine production
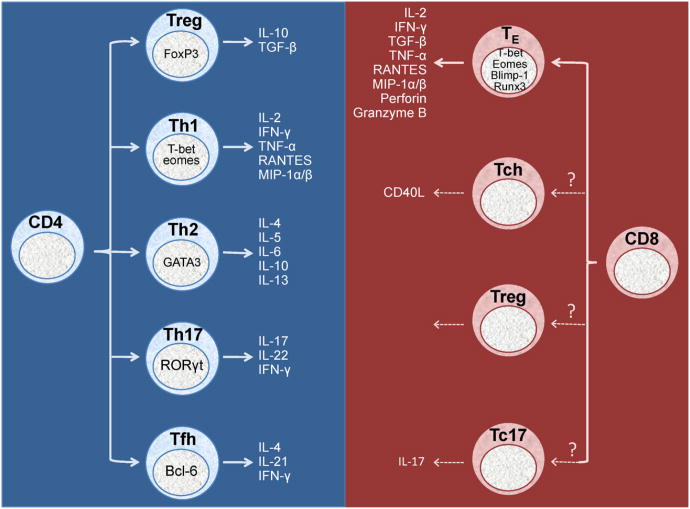
CD4+ T cell lineages are classically divided into either Th1 cells, which initiate a cellular immune response, or Th2 cells, which initiate a humoral immune response. However, CD4+ T cells can also become IL-17 producing Th17 cells, immunosuppressive T regulatory cells (Treg), or T follicular helper cells (Tfh). Each of these subsets likely plays an important role in nearly every normal immune response, and in the context of HIV, each of these subsets has probably been shown at some level to be protective, defective, or pathogenic. As follows we will briefly review these major response lineages, and then discuss their controlling elements (Fig. 1). Additional lineages have been described beyond those mentioned below, such as Th22, but we will not discuss these here.
Fig. 1.
Transcription factor-induced T cell subset formation and cytokine production. Transcription factors push CD4+ and CD8+ T cells toward distinct functional phenotypes, thereby influencing the cytokine milieu and direction of the immune response. Th = helper T cell, Tc = cytotoxic T cell, Treg = regulatory T cell, Tfh = T follicular helper cell, TE = effector T cell, IL = interleukin, IFN = interferon.
2.1. Th1/Th2
The first CD4+ T helper subsets defined by Mosmann and Coffman were the Th1 and Th2 subsets, defined primarily by production of IFN-γ vs. IL-4, respectively [9]. CD4+ T cell exposure to IL-12 and IFN-γ produces a Th1 cellular immune response [2,10]. Th1 cells primarily make IL-2, IFN-γ, TNF-α, and β-chemokines, and are thought to provide protective responses against intracellular pathogens. Increased IFN-γ levels activate cytotoxic CD8+ T cells and initiate killing of infected cells. These cytokines also have autoregulatory functions. Th1 production of IFN-γ restricts Th2 activity and drives Th1 responses [11]. HIV infection has been associated with reduced production of all Th1 cytokines, especially IL-2 [12].
The presence of IL-4 pushes CD4+ T cells toward a Th2 humoral response, which is thought to provide optimal protection against extracellular pathogens such as bacteria and parasites [9]. Th2 cells produce a number of cytokines, including IL-4, IL-5, IL-6, IL-10 and IL-13. These cytokines, particularly IL-4, result in B cell activation and antibody production. Th2 production of IL-4 forms a positive-feedback loop to further drive the Th2 response, while IL-4 and IL-10 act together to restrict Th1 activity [13]. Increases in Th2 cytokine production are associated with HIV infection, as is a skewing of the CD4+ T cell population toward a Th2 phenotype [14].
2.2. Th17
As mentioned above, some CD4+ T cells do not fit the common Th1/Th2 classification system. One group is the Th17 CD4+ T cell, best know for its ability to produce IL-17, and is extremely important in mucosal tissues where they directly contribute to the maintenance of gut surface integrity [15]. Th17 cells are generated in response to IL-6 and TGF-β exposure in combination with RORγt transcriptional activation [16,17]. These cells are characterized by production of IL-17, a proinflammatory cytokine that is increased in HIV-infected subjects, as well as IFN-γ [18]. HIV infection is associated with preferential depletion of Th17 cells in the gut mucosa, where they play a key role in host defense against bacteria [19]. This selective gut Th17 depletion may contribute to the microbial translocation that is common in HIV disease progression [20].
2.3. Regulatory T cells
Regulatory T cells (Tregs), identified by expression of CD25 and FOXP3, are important for maintaining homeostasis in the immune system [21–23]. They help to prevent autoimmunity and limit tissue damage during infection by suppressing activation and effector functions, mainly through expression of IL-10 and TGF-β [24]. Tregs can be both helpful and harmful during HIV infection. Tregs have been shown to suppress general immune activation, which has been closely linked to HIV disease progression [25,26]. However, strong Treg responses may contribute to HIV pathogenesis by suppressing HIV-specific immune responses, particularly effector T cells [27–29]. Tregs may also contribute to HIV-related gut damage by inhibiting Th17 cell recovery [30]. Examination of T cell populations in the rectal mucosa show increased percentages of Tregs in chronic progressors compared to EC, which positively correlate with viral loads and immune activation [31]. Treg CD4+ T cell suppression can be blocked by inhibition of IL-13 [32].
2.4. T follicular helper cells
T follicular helper cells (Tfh) are found in lymphoid germinal centers and are responsible for driving B cell memory differentiation and plasma cell formation [33]. Tfh are classified by their expression, amongst other things, of the transcription factor Bcl-6, CXCR5, ICOS, PD-1 and production of large amounts of IL-21, which is necessary for B cell differentiation [34–36]. These cells are typically not found in circulation, and it remains debatable whether circulating CXCR5+ CD4+ T cells represent a cell population related to Tfh. These helper T cells are likely targets for HIV infection given their location in a tissue that undergoes significant remodeling during HIV infection [37,38]. During HIV infection, IL-21 producing CD4+ T cells are upregulated in the PBMC population and have been associated with maintenance of the CD8+ T cell pool and control of viremia, but whether these cells are related to Tfh is unclear [39].
3. CD8+ T cells and cytokine production
CD8+ T cells are essential for control of viremia during HIV infection. During acute infection, plasma viral loads initially increase followed by a period of control. This control directly coincides with the initiation of an HIV-specific CD8+ T cell response [40]. Non-human primate models confirm the critical role CD8+ T cells play in controlling HIV disease progression. SIV-infected rhesus macaques were CD8+ T cell depleted and immediately experience uncontrolled viremia. Upon the reappearance of CD8+ T cells, viral load decreased. This pattern was consistent in both acute and chronic SIV infected animals [41,42]. The CD8+ T cell functional properties responsible for control of HIV remain unclear, especially whether the cytokines produced from CD8+ T cells play an active role in limiting HIV replication.
3.1. CD8+ T cell subsets
While the existence and importance of various CD4+ T helper cell subsets has been made clear, whether the same types of T cell subsets exist within the CD8+ T cell pool has not been so forthcoming. The primary known function of CD8+ T cells is to recognize and kill infected cells through the targeted release of perforin and granzymes or expression of Fas ligand [43]. They can also produce cytokines such as IL-2, IFN-γ, TGF-β, and TNF-α, as well as a broad array of chemokines, including RANTES, MIP-1α, MIP-1β, etc., as we have discussed elsewhere [44].
There is evidence from mouse models that a subset of CD8+ T cells can express CD40 ligand (CD40L) and express cytokines typically associated with B cell help, such as IL-5 and IL-13 [45]. Work from our lab and others show CD40L+ CD8+ T cells can be detected in humans after polyclonal stimulation; however, what role these “CD8+ Th” cells might play in vivo is at this time unclear [46]. In relation to HIV, CD8+ Tc17 and CD8+ Treg cells have also been described, but the importance of these subsets still needs to be evaluated [32,47].
3.2. Drivers of the cytokine profile: lineage-defining transcription factors in T cells
Work over the past decade on the different CD4+ T cell subsets has revealed that differentiation of the known T helper subsets derives from the expression of so-called ‘lineage-defining transcription factors’. These include T-box expressed in T-cells (T-bet, also known as TBX21), eomesodermin (eomes), GATA-3, RORγt, and Bcl-6. These transcription factors exact their effects on the various CD4+ (and CD8+) T cell subsets through direct expression and repression of genes characteristic of their respective lineages (Fig. 1) [17,48,49].
A great deal of research has been directed toward the underlying mechanisms driving the Th1/Th2 paradigm. Recent data has indicated that these lineages are largely defined by the dual actions of T-bet and GATA-3 [50]. T-bet drives the Th1 programming cassette, and represses GATA-3. When T-bet becomes limiting, the effects of GATA-3 drive the cell toward a Th2 phenotype [50].
The transcriptional regulators T-bet and eomes drive CD8+ T cell differentiation, effector function, and memory formation and are critical factors in driving highly effective Th1 responses [48]. T-bet has a distinct expression profile that can be linked to CD8+ T cell function. T-bethi cells contain cytotoxic molecules, such as perforin and granzymes, and T-bet expression has been linked to decreased IL-2 production, although our lab has shown that IL-2 producing cells can be T-betlo[51,52]. Additionally, studies have shown T-bet can bind to the perforin promoter and T-bet loss has been linked to defective cytolytic function [53–55]. Eomes is functionally important for establishing long-term CD8+ T cell memory [56,57].
Interestingly, the functions of RORγt to drive Th17 development also appear to be dependent upon the expression of T-bet [58]. Much like GATA-3, T-bet indirectly represses RORγt, thereby causing the T cell to default toward a Th1 phenotype [58]. The conditions necessary to allow RORγt to drive Th17 at the expense of T-bet function are likely related to the particular cytokine milieu. Without T-bet and eomes, naïve CD4+ T cells preferentially differentiate into Th17 cells rather than Th1 cells capable of driving a cytotoxic CD8+ T cell response [59].
During HIV infection, CD8+ T cells lose the ability to express T-bet and eomes, and this correlates to CD8+ T cell cytolytic dysfunction. While T-bet deficient cells can still secrete IFN-γ and kill target cells, eomes deficiency prevents cytotoxic function [60,61]. Cohort studies show elite controllers maintain higher levels of T-bet expression than chronic progressors, further supporting the key role T-bet plays during HIV pathogenesis [4]. The cytokine IL-12, produced by dendritic cells, is known to directly drive T-bet and eomes expression [9,62]. Linked with this, IL-12 also enhances effector phenotype and memory formation [63]. IL-12 has been explored as a therapeutic option and may help to preserve T-bet and eomes expression, leading to maintenance of a cytotoxic CD8+ T cell pool.
3.3. Polyfunctionality
T cells are multi-functional; they can proliferate and secrete cytokines, influence these functions in other cells, and mediate cytolysis of infected cells. The ability to perform more than one function is referred to as polyfunctionality and it is assessed on a single cell basis. Multiparameter flow cytometry is currently the best tool for simultaneously quantifying multiple functions on a per cell basis [64].
Comparing T cell cytokine production profiles in elite controllers (EC) with profiles from chronic progressors (CP) helped to identify several key players in HIV control. In a study measuring CD8+ T cell IL-2, IFN-γ, andTNF-α expression, the overall number of responding cells did not differ but CD8+ T cells from EC were able to produce more cytokines per cell than CP [65,66]. Additionally, EC cells preferentially produced IL-2 [66]. This data suggests that the quality of the CD8+ T cell response, not the quantity, is correlated with immune protection [65,67–70]. It is becoming increasingly clear, however, that other functional populations can also be important, and that ‘monofunctional’ responses are likely only monofunctional because of the choice of responses to measure. Thus, especially in the area of HIV vaccines, all responses should be considered important when measuring immunogenicity of a vaccine, and subsequent challenge studies(if in model systems), or efficacy studies, should be the only measure of protective capacity.
While polyfunctionality has been linked to successful immune responses, it is important to ensure the “right” functions are measured. Recently, IL-2, IFN-γ, and TNF-α have been used as key markers and these cytokines do seem to correlate with viral control in HIV infection. However, expression of transcription factors like T-bet and T cell-mediated cytotoxicity, through granzymes or perforin, are also likely essential for control of HIV pathogenesis [4,62,71]. Polyfunctionality alone cannot control infection if the required functions are not present. Thus, it is necessary to identify and define correlates of immune protection. This ongoing process will be essential for testing new vaccine candidates to ensure they will elicit an effective response.
4. Cytokine effects on HIV disease progression and viral replication
During acute infection, HIV rapidly induces short-term production of IL-15 and type I interferons (including IFN-α), and long-term production of IL-18, IL-22, IFN-γ, and TNF. Shortly thereafter, HIV induces production of IL-10 [72]. HIV also causes defects in dendritic cell activation, which reduces production of IL-12, akey cytokine for initiating T-bet expression and Th1 responses [62].
The Th1 → Th2 switch hypothesis first suggested by Clerici and Shearer in 1993 further emphasizes the importance of understanding T cell cytokine production in HIV infection [14]. During early stages of HIV infection, Th1 responses dominate Th2 responses. Production of IL-2 and IFN-γ drive CD8+ T cell cytotoxic activity and viremia is controlled. At some point during the chronic stage of HIV infection, Th2 responses begin to dominate Th1 responses, favoring humoral immunity induced by IL-4 and IL-10 production. This cytokine switch strongly coincides with disease progression. Moreover, individuals with resistance to HIV infection despite repeated exposure tend to have very strong HIV-specific Th1 responses. Ongoing efforts seek to identify the exact stimuli that induce this switch with the hope of finding a way to either prevent or reverse it.
4.1. Effect of T cell produced cytokines on HIV replication
Many of the cytokines produced by T cells during the immune response can have a significant impact on HIV replication. Some cytokines, particularly proinflammatory cytokines like TNF-α, can increase HIV replication [73,74]. Other cytokines both stimulate and inhibit HIV replication. For example, IL-4 can induce HIV replication, but it also suppresses the HIV-replication driving cytokines TNF-α and IL-1β [75]. Alternatively, cytokines can repress HIV replication. The type I (IFN-α and IFN-β) and type II (IFN-γ) interferons are typically considered “antiviral”. IFN-α can block HIV reverse transcription at several levels, leading to reduced virus production [19]. Thus, it can be useful to assess many cytokines in the context of HIV infection as a means to detect the presence of an immune response. However, one should not infer a protective effect of these cytokines in the case of HIV. We will discuss some of these cytokines and their effects below in more depth.
4.2. Tumor necrosis factor
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine that can help control viral infections. TNF can bind to two different receptors on the cell surface, TNFRI and TNFRII. There is evidence that binding to either of these receptors on the surface of HIV infected cells can have differential effects on the cell [76]. After binding to the TNFRI receptor, TNF-α can initiate apoptosis in infected cells, thereby eliminating them from the body [77]. However, inflammation caused by TNF-α expression can also initiate the NF-κB signaling and result in increased production of virus, which enhances HIV progression [73,74]. Addition of IL-6 exacerbates the TNF-α effect [78]. IL-1α and IL-1β are also able to activate NF-κB [62].
4.3. Interleukin-2
IL-2 initiates Th1 CD4+ T cell responses and drives production of cytokines such as IFN-γ and TNF-α [79]. Studies comparing elite controllers to chronic progressors suggest IL-2 expressing CD8+ T cells correlate with the highest levels of protection during HIV infection [66]. HIV viremia also correlates with the inability to develop functional HIV-specific CD4+ T cells that can produce IL-2 [80]. However, IL-2 can induce HIV replication in T cells, and it has been shown that administration of IL-2 in vivo can cause ‘blips’ in plasma viral load [81]. IL-2 also induces CD8+ T cell-mediated HIV suppression, which likely counterbalances the increase in HIV replication [82]. Like IL-2, both IL-7 and IL-15 induce HIV replication but also drive CD8+ T cell control, which may lead to an overall beneficial effect [83–85].
4.4. Interferon-γ
IFN-γ is a well known antiviral cytokine, which acts by inhibiting viral replication [86]. IFN-γ can induce an antiviral state by initiating Fas-mediated cell killing and increasing sensitivity to TNF-α [87,88]. However, in the context of HIV infection, IFN-γ has been shown to both enhance HIV replication as well as to inhibit viral replication [55,89]. As a result, inducing T cell production of IFN-γ may have both beneficial and detrimental effects on HIV progression.
IFN-γ production has long been associated with T cell activation and is the most common readout for antigen-specific T cell responses (ELISPOT and flow cytometry). Assuch, IFN-γ expression has become the standard measure of efficacy for vaccine and therapy trials designed to elicit T cell responses [90]. However, vaccine strategies that can elicit strong IFN-γ responses do not always result in a protective immune response [91,92]. This is likely because overstimulation of CD4+ T cells drives terminal differentiation and consequent IFN-γ focused responses, rather than IL-2 producing central memory cells [93].
4.5. T cell produced cytokines in elite controllers
HIV elite controllers are able to maintain undetectable viral loads for many years without therapeutic intervention and do not exhibit signs of disease progression [94]. EC typically maintain strong HIV-specific T cell responses. Unlike chronic progressors, HIV-specific CD4+ T cells in EC are polyfunctional, retain the ability to produce IL-2, and maintain proliferative ability [5,6]. The direct relevance of polyfunctional HIV-specific CD4+ T cells in elite control has remained unclear, specifically because of early observations that showed HIV-specific CD4+ T cells were highly likely to be directly infected and (presumably) eliminated due to targeted infection by HIV due to their specificity [95]. Are these cells present because they play a direct role in control of viremia, or is their presence simply a reflection of successful viral control? The presumption is that HIV-specific CD4+ T cells, and their resultant cytokine production, are necessary to help maintain effective B cell antibody responses and HIV-specific CD8+ T cell effector activity.
Perhaps more importantly, a great deal of evidence indicates that HIV-specific CD8+ T cells are essential for controlling HIV viral replication and disease progression in elite controllers [96,97]. EC have CD8+ T cells that are more polyfunctional, better able to proliferate, and have more cytolytic function than their chronic progressor counterparts [65,98]. Most notably, chronic progressors rapidly lose the ability to express perforin in HIV-specific CD8+ T cells while EC maintain this cytolytic function [62]. This is likely a result of decreased T-bet expression in chronic progressors compared to EC [86]. EC are also known for having more abundant, more polyfunctional HIV-specific T cell responses in mucosal sites, such as the gut and rectum, than chronic progressors [99,100].
5. Cytokines in HIV-induced immune exhaustion and chronic immune activation
In an effort to better understand the correlates of protection against HIV, many groups have concentrated on understanding the differences between pathogenic HIV and SIV infections and non-pathogenic SIV infection of natural hosts. Both pathogenic and non-pathogenic infections are characterized by high viral loads, depletion of CD4+ T cells in gut associated lymphoid tissues (GALT), robust IFN-γ responses during acute infection and a switch from a Th1 to a Th2 cytokine profile during chronic infection, measured by an increase in IL-4 and a decrease of IFN-γ production [101–103]. During pathogenic infection, CD4+ T cell depletion is progressive, extends to peripheral blood and affects the memory T cell pool, contributing to immunodeficiency and disease progression. In contrast, non-pathogenic infection primarily depletes effector T cells, allowing the memory compartment to remain intact. In fact, natural hosts are able to maintain the naïve T cell population and replenish the lymphocyte compartment, preventing disease progression to AIDS [101,102,104]. The subsequent impact on CD4+ T cell cytokine production after the loss of the central memory pool (primarily IL-2) or the effector memory pool (primarily IFN-γ) remains to be understood, as well as the ramifications that these differential losses may have on the immune response to HIV in general. Furthermore, non-pathogenic infections have limited generalized immune activation, unlike the state in pathogenic disease. One proposed explanation for the low levels of systemic activation in non-pathogenic infection is the preservation of IL-17 producing Th17 helper T cells in GALT, which help preserve mucosal integrity and prevent translocation of microbial products into systemic circulation [102,104,105].
5.1. Effects of regulatory responses and IL-10
The role of IL-10 as a regulatory cytokine in chronic viral infections was first discovered in studies by Oldstone and colleagues [106,107]. IL-10, produced by monocytes and CD4+ Tregs, overall has a dampening effect on Th1 type responses and reduces antigen presentation by downregulating MHC class II from the cell surface of antigen-presenting cells [108]. Furthermore, IL-10 produced by monocytes is tightly regulated by Tregs through the production and secretion of IL-27 [108]. Blocking the IL-10R results in increased proliferation, IFN-γ secretion and, to a lesser extent, IL-2 secretion by antigen-specific T cells [108]. During chronic HIV infection, levels of IL-10 in the plasma are significantly higher compared to uninfected individuals and HIV controllers [108] reducing the capacity of the immune system to clear the virus. In addition to regulating cytokine production, IL-10 also induces expression of PDL-1 on macrophages [109], which can inhibit T cell function by engaging its receptor PD-1 on T cells during antigen presentation. During primary HIV and SIV infection, there is a decrease of Treg cells in GALT tissues. This T cell subset expresses CCR5, making them susceptible to infection and cytotoxic killing [110]. Loss of Tregs may lead to a decrease in IL-10 secretion in the gut and lack of control of the immune response at this initial site of viral replication, which contributes to immune activation and destruction of the gut barrier.
5.2. Loss of T cell cytokine production during HIV infection
Cytokines produced during HIV infection contribute directly to the pronounced chronic immune activation state present in chronic HIV infection. This begins during acute HIV infection, and has broad effects on lymphocytes in the circulation and in the various tissues that harbor HIV infected cells. Several studies have shown that viral replication in gut-associated lymphoid tissues (GALT) leads to severe CD4+ T cell depletion in pathogenic HIV/SIV infections. This depletion is in part due to direct killing of infected CD4+ T cells, but it is exacerbated by production of pro-inflammatory cytokines such as IFN-γ and TNF-α that induce activation and death of uninfected bystander CD4+ T cells [111]. Additionally, depletion of Th17 helper CD4+ T cells involved in mucosal host defense against extracellular bacteria leads to structural damage of GALT tissues and translocation of microbial products from the gut lumen into systemic circulation, suggested by increased LPS and sCD14 levels in the plasma [105,112–115]. It has also been suggested that low APC frequencies in GALT tissues of HIV infected individuals playarole in the altered cytokine milieu that favors CD4+ T cell differentiation into Th1 rather than Th17 T cells [115]. Moreover, there is data suggesting that soluble HIV-Nef protein induces macrophages to produce pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, MIP-1α and MIP-1β by activating the MAPK and NF-κB pathways, contributing to sustained activation of the immune system [116].
As a result of prolonged antigen stimulation and systemic immune activation, T cells gradually lose polyfunctionality. Proliferative capacity, IL-2 production and cytotoxicity are diminished first, followed by TNF-α and, in severe cases, IFN-γ, a process referred to as T cell exhaustion [12,117–119]. Studies have shown that T cell exhaustion is antigen specific, meaning that during HIV infection, individuals have exhausted HIV-specific T cells, but EBV-specific, CMV-specific or Flu-specific T cells largely appear unchanged compared to HIV-negative donors [120,121]. Furthermore, there is evidence suggesting that CD8+ T cell production of MIP-1β, TNF-α, CD107a and IFN-γ is determined by the duration and intensity of exposure to antigen, indicating that exhaustion could be a consequence of failure to control viremia [122,123]. Another consequence of persistent antigen and immune activation is the down regulation of the IL-7 receptor α-chain (CD127), and the IL-2 and IL-15 receptor β-chain (CD122) driving defective memory differentiation and down regulation of anti-apoptotic proteins [117,124].
5.3. Exhaustion receptors and cytokines in HIV infection
Upregulation of the inhibitory receptor programmed death 1 (PD-1) is widely associated with T cell exhaustion during HIV infection [125–128].PD-1 is inducibly expressed on activated T cells, and its ligands (PDL-1 andPDL-2)are broadly expressed on APCs and non-APCs. Binding of PD-1 to its ligands activates an immunosuppressive pathway, attenuating TCR-medicated CD4+ and CD8+ T cell proliferation and production of cytokines suchasIFN-γ,IL-10 and IL-2 [129]. In vitro experiments with CD8+-depleted peripheral blood mononuclear cells (PBMCs) from HIV infected subjects demonstrate that blocking the interaction between PD-1 and its ligand, PDL-1, results in increased protein secretion of IL-2 and IFNγ from CD4+ T cells. However, blocking PD-1/PDL-1 interactions can only partially rescue the exhausted phenotype. Work using a mouse model of chronic infection has identified other inhibitory receptors involved in exhaustion [130]. One of these receptors, lymphocyte-activation gene 3 (Lag-3) plays a negative regulatory role in T cells through intracellular signaling, resulting in decreased proliferation and decreased production of Th1 cytokines, IFN-γ, IL-2 and TNF-α, but not Th2 cytokines, IL-4, IL-5 or IL-10 [3]. Likewise, CD160 binding to herpes virus entry mediator (HVEM) on the surface of CD4+ T cells has been shown to decrease secretion of Th1 cytokines IL-2, TNF-α and IFN-γ as well as Th2 cytokines IL-4, IL-5, IL-10 and IL-13 in a concentration dependent manner [131]. Another receptor, 2B4, has dual function depending on levels of surface expression, co-stimulatory or inhibitory, and regulates cytotoxicity and IFN-γ secretion [132–134].
Recent studies looking at expression of the previously mentioned inhibitory receptors on human PBMCs from HIV infected patients confirmed the upregulation of PD-1, CD160 and 2B4 but not Lag-3. This study also showed differences in inhibitory receptor expression between CD4+ and CD8+ T cells as well as different stages of differentiation [135]. Of note, expression levels are reduced with ART treatment indicating that virus replication plays a role in upregulation of these receptors. Experiments utilizing mice to look at effects of blocking multiple inhibitory receptors have shown that these receptors act synergistically. Blocking PD-1 and Lag-3 interactions with their ligands results in greater CD8+ T cell responses compared to single receptor blockade [130]. In humans, in vitro blockade of PD-1 increased production of IL-2, IFN-γ, IL-13 and IL-21 by HIV-specific CD4+ T cells [127,136] and increased proliferation and IFN-γ production by CD8+ T cells [127].
6. What we have learned from recent vaccine trials
HIV vaccines have had limited success in preventing acquisition, decreasing viral loads, or resolving infection. The two most promising and notable clinical vaccine trials were the Merck STEP trial, which elicited strong cellular responses, and the RV144 Thai vaccine trial, which induced humoral responses.
Despite being able to elicit promising CD8+ T cell responses, as measured by IFN-γ ELISPOT in PBMC during phase I trials, the Merck STEP trial Ad5 vaccine was unable to control or prevent HIV infection [7]. While IFN-γ ELISPOT has long been considered the standard readout for an effective immune response, this trial strongly suggests IFN-γ production alone is not a suitable measure for vaccine efficacy. The recent RV144 Thai vaccine trial was moderately successful and indicated a protective vaccine for HIV may be possible. The vaccine offered some protection against heterosexual transmission, but did not reduce disease progression. The vaccine induced modest results, eliciting some protective HIV-specific IgG humoral immunity, but was generally poor at inducing HIV-specific neutralizing antibodies and T cells. Additionally, vaccine-induced responses were very short-lived [8], possibly due to the relative paucity of vaccine-induced T cell responses. Future trials are planned that will incorporate modalities that will ideally stimulate a more balanced immune response containing both an antibody and T cell component.
7. Therapeutic interventions and vaccine strategies
It is clear that our current vaccine approaches are not eliciting the required response to either prevent infection or to therapeutically limit viral replication. New strategies will need to be developed that can induce an effective immune response. A successful prophylactic HIV vaccine strategy will likely need to stimulate effector T cells to home to mucosal tissues, induce polyfunctional memory T cell responses, and elicit a humoral antibody response [91]. However, in the setting of therapeutic vaccination, simply expanding the previously ineffective CD8+ T cell response has proven insufficient to enable control of viremia. As such, therapeutic interventions must focus on qualitatively changing the HIV-specific immune responses in order to be efficacious.
Preliminary data suggest cytokine adjuvants for therapeutic vaccines may boost immune responses. Many cytokine adjuvants have been tested, such as IL-2, IL-12, IL-15, and IFN-γ (reviewed in [137]) in the hopes of eliciting a Th1 response or boosting existing HIV-specific CD8+ T cells. These cytokine-adjuvanted vaccine studies show a wide range of often conflicting results. Overall, it appears that DNA vaccine immunogenicity has been most improved by co-expression of either IL-12 or IL-15 with HIV plasmids [138,139].
Alternatively, Berzofsky has suggested a “push–pull” approach for therapy in which cytokine-adjuvanted vaccination (push) would be followed by inhibitory signal blockade (pull) will enhance vaccine efficacy [140]. The goal of this approach is to use cytokines to “steer” the immune response in a particular direction, such as Th1, while at the same time inhibiting the factors that contribute to reduced cellular function and exhaustion, such as PD-1, CTLA-4 or IL-10.
A number of cytokine-adjuvanted therapeutic vaccine studies have been performed in order to attempt rectify the lack of CD4+ T cell help or other deficiencies in the cellular immune response to HIV. For the most part these have met with extremely limited success.
7.1. Interleukin-2
In an effort to drive Th1 CD4+ T cell responses, IL-2 has been tested as a therapeutic option. Initial studies in mice and rhesus macaques showed IL-2 adjuvanted HIV vaccines induced both humoral as well as cellular immunity [63,141]. However, large-scale clinical trials show IL-2 treatment does not increase CD127 expression on T cells or decrease plasma IL-7 levels [142]. IL-2 was found to have no effect on clinical outcome in HIV-infected subjects [57].
7.2. Interleukin-7
IL-7 plays a role in early T cell development and proliferation [71,143]. It can regulate Bcl-2 expression and has been shown to promote both CD4+ and CD8+ T cell survival during HIV infection [144]. In addition, IL-7 has been linked to enhanced CXCR4, but not CCR5, expression and isbelieved to aidin the R5 to X4 switch [145]. Through interaction with the IL-7 receptor (CD127) and NFAT, IL-7 can regulate HIV transcription and induce reactivation of latent virus [146]. Decreased CD127 expression is observed on both CD4+ and CD8+ T cells during chronic HIV infection, resulting in decreased IL-7 responsiveness, cell survival and cytotoxic activity [147–152]. Plasma IL-7 levels are increased in HIV-infected persons, correlate with and may be a result of decreased CD127 expression during chronic infection [153].
Together, this suggests IL-7 acts as a double-edged sword during HIV infection and illustrates how T cell count maintenance can be both positive and negative. While T cells help to fight viral infection, they are also the primary targets of HIV. Although IL-7 helps to maintain T cell numbers, it greatly increases the likelihood of infection in the target cell pool by enhancing co-receptor expression and viral replication. By facilitating survival of infected cells, IL-7 helps maintain an actively replicating viral pool, which aids in further viral dissemination.
IL-7 has been used as a vaccine adjuvant with mixed results [64,67]. Since IL-7 can provide support for IL-15 in terms of memory cell preservation, it may be beneficial to explore utilizing these two adjuvants together [71,154].
7.3. Interleukin-12
IL-12 stimulates T cells, pushing them toward a Th1 phenotype, and increases IFN-γ production and cytotoxicity [155]. When used as a vaccine adjuvant, IL-12 increases HIV-specific T cell responses and cytotoxic cell functions [156]. IL-12 administration increases both cellular and humoral responses and decreases viral load. Co-administering IL-12 with IL-15 gave a similar outcome [139]. Adjuvanting DNA vaccines with IL-12 has proven both safe and immunogenic in chimpanzees, although the effect is only short-term [157]. Although mouse and primate studies showed detectable cellular responses to IL-12 adjuvanted vaccines, a recent human clinical trial suggests IL-12 has low immunogenic capacity [157–159].
7.4. Interleukin-15
IL-15 induces T cell activation and proliferation. IL-15 exposure enhances memory CD8+ T cell function in HIV-infected subjects and has been shown to promote HIV-specific CD8+ T cell survival [160,161]. Additionally, ex vivo experiments indicate IL-15 activity is enhanced by IL-21, suggesting a possible avenue for therapeutic intervention [162]. IL-15 adjuvanted HIV vaccination results in increased IFN-γ production by HIV-specific T cells, increased CD8+ T cell polyfunctionality, and rapid control of viremia [163–165]. IL-15 has not been linked to decreases in viral load [139]. Preliminary studies show IL-15 may function more efficiently if administered after vaccination with an HIV-specific plasmid, when compared to co-administration [166].
Also of note, like IL-7, IL-15 plays a strong role in homeostatic proliferation of T cells. As such, it can have both positive and negative effects in terms of HIV progression. Several studies have linked IL-15 with increased viral set point and poor clinical outcomes, so care must be used when developing vaccines strategies including this cytokine [85].
7.5. Interleukin-21
IL-21 increases T cell function. IL-21, much like IL-15, enhances memory CD8+ T cell activity during HIV infection [37]. Exposure to IL-21 increases CD8+ T cell production of IFN-γ and perforin, making them more functional. IL-21 and IL-15 can act synergistically to increase the level of the CD8+ T cell functional response [37,167,168]. In a mouse challenge model using a vaccinia virus expressing a modified HIV-gp140, immunization with IL-21 alone reduced viral aquisition rates and co-immunization with IL-15 increased both the magnitude and duration of HIV-gp140-specific T cell responses [169]. Vaccines adjuvanted with IL-21 increase HIV-specific CD8+ T cell cytotoxic function (granzyme and perforin) [170,171].
7.6. Interferon-α
IFN-α might be an important cytokine for therapeutic intervention. It is important for the innate immune response to viral infection but IFN-α levels are not elevated during chronic HIV infection [100,101]. Plasmacytoid DCs (pDC) are the main source of IFN-α in the body and are rapidly depleted in the blood during HIV infection [102–104]. Preliminary data from a recent clinical trial with Peg-IFN-α (Dr. L J Montaner, Wistar Institute) suggests IFN-α may be able to suppress HIV replication, even in the absence of highly active antiretroviral therapy (HAART), likely through enhanced innate immune effectors or HIV-specific CD8+ T cells. IFN-α enhances expression if IFN-γ, granzyme, perforin, and cytolytic function in vitro [105]. Additionally, administration of IFN-α in humans increases perforin and granzyme levels in CD8+ T cells and is associated with decreased viremia [106,107]. Together, this suggests IFN-α therapy may enhance or reconstitute HIV-specific CD8+ T cell effector responses in chronic HIV infection, leading to better control of viremia.
8. Summary
The partial success of recent and ongoing cytokine adjuvant and vaccine trials suggests we are drawing closer to the goal of designing effective therapeutics and preventatives for HIV infection. However, these strategies are still unable to produce a fully protective immune response, stressing the importance of identifying true correlates of protection from infection or pathogenesis. A comprehensive understanding of the intricacies of the cytokine and cellular response to infection, as well as HIV-induced changes, will allow us to design mechanisms to drive the immune response toward protective phenotype. More importantly, identifying novel factors like transcription factors or unique cell subsets that influence protection will enable us to establish techniques to accurately measure the effectiveness of newly developed therapies. We believe the successful employment of this strategy will help to significantly advance the field of HIV therapy and prevention, and hopefully aid in reducing the global burden of this disease.
Acknowledgments
This research was supported by NIH R01 AI076066. M.A. Reuter was supported by NIH 5T32 AI007632.
Biographies

Morgan A. Reuter, PhD, is a postdoctoral research fellow at the University of Pennsylvania Perelman School of Medicine in the laboratory of Dr. Michael Betts. She earned her PhD in microbiology at Case Western Reserve University under the mentorship of Dr. David McDonald. Her research interests include HIV immunology, T cell activation markers, and vaccine adjuvants.

Carolina Pombo, BSc, is a graduate student at the University of Pennsylvania Perelman School of Medicine in the laboratory of Dr. Michael Betts. She earned her BSc in biology at Barry University. Her research interests include T cell exhaustion during chronic viral infections, specifically during HIV infection.

Michael R. Betts, PhD, is an associate professor at the University of Pennsylvania Perelman School of Medicine. He received his doctorate from the University of North Carolina in the laboratory of Dr. Jeffrey A. Frelinger. His dissertation examined the role of HIV-1 specific cytotoxic T lymphocytes in non-progressive HIV infection. His postdoctoral research was conducted with Richard A. Koup, MD at the University of Texas Southwestern Medical Center, where he advanced the use of intracellular cytokine staining for measuring HIV-specificTcell responses. His work redefined the breadth, magnitude, and immunodominance patterns of HIV-specific T cells in chronic HIV infection. In 2001 he became a research fellow at the National Institutes of Health Vaccine Research Center. He continued to develop flow cytometry-based assays for measuring T cell responses, including initial development of the CD107a degranulation assay and polyfunctional T cell analysis using multiparametric flow cytometry. He joined the University of Pennsylvania Department of Microbiology faculty in 2005. He is an editor for AIDS Research and Human Retroviruses, a section editor for the Journal of Immunology, and an associate editor for Journal of Virology. His current research interests are T cell responses in human viral infections, including HIV, cytomegalovirus, Epstein–Barr virus, adenovirus, and influenza, and development of novel methodology to identify and characterize antigen-specific T cells.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
Contributor Information
Morgan A. Reuter, Email: mreuter@mail.med.upenn.edu.
Carolina Pombo, Email: carolmar@mail.med.upenn.edu.
Michael R. Betts, Email: betts@mail.med.upenn.edu.
References
- 1.Ebrary Inc. AIDS epidemic update December 2009. Geneva, Switzerland: UNAIDS; 2009. [Google Scholar]
- 2.Koesters SA, Alimonti JB, Wachihi C, Matu L, Anzala O, Kimani J, et al. IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. European Journal of Immunology. 2006 Feb;36(2):336–44. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 3.Macon-Lemaitre L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005 Jun;115(2):170–8. doi: 10.1111/j.1365-2567.2005.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011 Apr 7;117(14):3799–808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer WB, Zaunders JJ, Yuan FF, Wang B, Learmont JC, Geczy AF, et al. Mechanisms of HIV non-progression; robust and sustained CD4+ T-cell proliferative responses to p24 antigen correlate with control of viraemia and lack of disease progression after long-term transfusion-acquired HIV-1 infection. Retrovirology. 2008;5:112. doi: 10.1186/1742-4690-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, et al. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. Journal of Virology. 2007 Mar;81(6):2713–25. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov;372(9653):1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New England Journal of Medicine. 2009 Dec;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 10.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine and Growth Factor Reviews. 1999 Mar;10(1):41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default tothe Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. Journal of Experimental Medicine. 1994 Apr;179(4):1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007 Oct;27(4):670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Shearer GM. A TH1 → TH2 switch is a critical step in the etiology of HIV infection. Immunology Today. 1993 Mar;14(3):107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Current Opinion in Immunology. 2007 Dec;19(6):652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. Journal of Experimental Medicine. 2002 Jun;195(12):1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006 Sep;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Maek ANW, Buranapraditkun S, Klaewsongkram J, Ruxrungtham K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-nega-tive T cells in human immunodeficiency virus infection. Viral Immunology. 2007 Spring;20(1):66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- 19.Shirazi Y, Pitha PM. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. Journal of Virology. 1992 Mar;66(3):1321–8. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhawan S, Wahl LM, Heredia A, Zhang Y, Epstein JS, Meltzer MS, et al. Interferon-gamma inhibits HIV-induced invasiveness of monocytes. Journal of Leukocyte Biology. 1995 Dec;58(6):713–6. doi: 10.1002/jlb.58.6.713. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nature Immunology. 2003 Apr;4(3):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology. 1995 Aug;155(3):1151–64. [PubMed] [Google Scholar]
- 23.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+ CD4+ regulatory T cells. International Immunology. 2004 Nov;16(11):1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 24.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunology. 2005 Apr;6(4):353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 25.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. Journal of Virology. 2008 Sep;82(17):8307–15. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao Y, Fu J, Xing S, Fu B, Zhang Z, Shi M, et al. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology. 2009 Sep;128(1 Suppl.):e366–75. doi: 10.1111/j.1365-2567.2008.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. Journal of Virology. 2004 Mar;78(5):2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proceedings of the National Academy of Sciences of the United States of America; 2007; Feb, pp. 3390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, et al. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and non-lytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Research and Human Retroviruses. 2007 Mar;23(3):438–50. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 30.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Science Translational Medicine. 2010 May;2(32):32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. Journal of Virology. 2011 Nov;85(21):11422–34. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nigam P, Velu V, Kannanganat S, Chennareddi L, Kwa S, Siddiqui M, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. Journal of Immunology. 2010 Feb;184(4):1690–701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- 33.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007 Aug;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends in Immunology. 2010 Oct;31(10):377–83. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunology. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008 Jul;29(1):127–37. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Hufert FT, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, et al. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997 Jun;11(7):849–57. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. Journal of Infectious Diseases. 2007 Feb;195(4):551–61. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 39.Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. Journal of Immunology. 2010 Jul;185(1):498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 40.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of Virology. 1994 Jul;68(7):4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 42.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. Journal of Experimental Medicine. 1999 Mar;189(6):991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Current Opinion in Immunology. 2003 Oct;15(5):522–7. doi: 10.1016/s0952-7915(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 44.Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunological Reviews. 2011 Jan;239(1):109–24. doi: 10.1111/j.1600-065X.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May;357(6373):80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 46.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. European Journal of Immunology. 1992 Oct;22(10):2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 47.Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-producing CD8 Tcells during late chronic stage of pathogenic simian immunodeficiency virus infection. Journal of Immunology. 2011 Jan;186(2):745–53. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- 48.Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunological Reviews. 2010 May;235(1):219–33. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 49.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunology. 2010 Feb;11(2):114–20. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proceedings of the National Academy of Sciences of the United States of America; 2009; Oct, pp. 17876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutboul F, Puthier D, Appay V, Pelle O, Ait-Mohand H, Combadiere B, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005 Nov;19(17):1981–6. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 52.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathogens. 2010 May;6(5):e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Jacod S, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients–effects of antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2006 Jul;42(3):277–85. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 54.Zaunders JJ, Moutouh-de Parseval L, Kitada S, Reed JC, Rought S, Genini D, et al. Polyclonal proliferation and apoptosis of CCR5+ T lymphocytes during primary human immunodeficiency virus type 1 infection: regulation by interleukin (IL)-2, IL-15, and Bcl-2. Journal of Infectious Diseases. 2003 Jun;187(11):1735–47. doi: 10.1086/375030. [DOI] [PubMed] [Google Scholar]
- 55.Vingerhoets J, Bisalinkumi E, Penne G, Colebunders R, Bosmans E, Kestens L, et al. Altered receptor expression and decreased sensitivity of T-cells to the stimulatory cytokines IL-2, IL-7 and IL-12 in HIV infection. Immunology Letters. 1998 Mar;61(1):53–61. doi: 10.1016/s0165-2478(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 56.Sasson SC, Zaunders JJ, Zanetti G, King EM, Merlin KM, Smith DE, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. Journal of Infectious Diseases. 2006 Feb;193(4):505–14. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 57.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. New England Journal of Medicine. 2009 Oct;361(16):1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nature Immunology. 2011 Jan;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. Journal of Immunology. 2008 Dec;181(12):8700–10. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008 Jul;321(5887):408–11. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002 Jan;295(5583):338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 62.Poli G, Kinter AL, Fauci AS. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America; 1994; Jan, pp. 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JJ, Yang JS, Manson KH, Weiner DB. Modulation of antigen-specific cellular immune responses to DNA vaccination in rhesus macaques through the use of IL-2, IFN-gamma, or IL-4 gene adjuvants. Vaccine. 2001 Mar;19(17-19):2496–505. doi: 10.1016/s0264-410x(00)00479-5. [DOI] [PubMed] [Google Scholar]
- 64.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nature Reviews Immunology. 2004 Aug;4(8):648–55. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 65.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akinsiku OT, Bansal A, Sabbaj S, Heath SL, Goepfert PA. Interleukin-2 production by polyfunctional HIV-1-specific CD8 T cellsis associated with enhanced viral suppression. Journal of Acquired Immune Deficiency Syndromes. 2011 Oct;58(2):132–40. doi: 10.1097/QAI.0b013e318224d2e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature Medicine. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 68.Schellens IM, Borghans JA, Jansen CA, De Cuyper IM, Geskus RB, van Baarle D, et al. Abundance of early functional HIV-specific CD8+ T cells does not predict AIDS-free survival time. PLoS One. 2008;3(7):e2745. doi: 10.1371/journal.pone.0002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. Journal of Experimental Medicine. 2007 Jun;204(6):1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. Journal of Virology. 2001 Dec;75(24):11983–91. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carini C, Essex M. Interleukin 2-independent interleukin 7 activity enhances cytotoxic immune response of HIV-1-infected individuals. AIDS Research and Human Retroviruses. 1994 Feb;10(2):121–30. doi: 10.1089/aid.1994.10.121. [DOI] [PubMed] [Google Scholar]
- 72.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. Journal of Virology. 2009 Apr;83(8):3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type1through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proceedings of the National Academy of Sciences of the United States of America; 1989; Aug, pp. 5974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proceedings of the National Academy of Sciences of the United States of America; 1989; Apr, pp. 2365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foli A, Saville MW, Baseler MW, Yarchoan R. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood. 1995 Apr;85(8):2114–23. [PubMed] [Google Scholar]
- 76.Lazdins JK, Grell M, Walker MR, Woods-Cook K, Scheurich P, Pfizenmaier K. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. Journal of Experimental Medicine. 1997 Jan;185(1):81–90. doi: 10.1084/jem.185.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine and Growth Factor Reviews. 2003 Jun-Aug;14(3/4):185–91. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 78.Poli G, Kinter A, Justement JS, Kehrl JH, Bressler P, Stanley S, et al. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proceedings of the National Academy of Sciences of the United States of America; 1990; Jan, pp. 782–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinter A, Arthos J, Cicala C, Fauci AS. Chemokines, cytokines and HIV: a complex network of interactions that influence HIV pathogenesis. Immunological Reviews. 2000 Oct;177:88–98. doi: 10.1034/j.1600-065x.2000.17708.x. [DOI] [PubMed] [Google Scholar]
- 80.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. Journal of Experimental Medicine. 2003 Dec;198(12):1909–22. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davey RT, Jr, Chaitt DG, Piscitelli SC, Wells M, Kovacs JA, Walker RE, et al. Subcutaneous administration of interleukin-2 in human immunodeficiency virus type 1-infected persons. Journal of Infectious Diseases. 1997 Apr;175(4):781–9. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]
- 82.Kinter AL, Bende SM, Hardy EC, Jackson R, Fauci AS. Interleukin 2 induces CD8+ T cell-mediated suppression of human immunodeficiency virus replication in CD4+ T cells and this effect overrides its ability to stimulate virus expression. Proceedings of the National Academy of Sciences of the United States of America; 1995; Nov, pp. 10985–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrari G, King K, Rathbun K, Place CA, Packard MV, Bartlett JA, et al. IL-7 enhancement of antigen-driven activation/expansion of HIV-1-specific cytotoxic T lymphocyte precursors (CTLp) Clinical and Experimental Immunology. 1995 Aug;101(2):239–48. doi: 10.1111/j.1365-2249.1995.tb08345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moran PA, Diegel ML, Sias JC, Ledbetter JA, Zarling JM. Regulation of HIV production by blood mononuclear cells from HIV-infected donors: I. Lack of correlation between HIV-1 production and T cell activation. AIDS Research and Human Retroviruses. 1993 May;9(5):455–64. doi: 10.1089/aid.1993.9.455. [DOI] [PubMed] [Google Scholar]
- 85.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. Journal of Immunology. 2008 Jan;180(1):350–60. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004 Feb;75(2):163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 87.Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Research. 1998 Jul;58(13):2832–7. [PubMed] [Google Scholar]
- 88.Tsujimoto M, Yip YK, Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. Journal of Immunology. 1986 Apr;136(7):2441–4. [PubMed] [Google Scholar]
- 89.Koyanagi Y, O'Brien WA, Zhao JQ, Golde DW, Gasson JC, Chen IS. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep;241(4873):1673–5. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 90.Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural plasmodium falciparum infection and disease. Nature Medicine. 2004 Apr;10(4):406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 91.Elias D, Akuffo H, Britton S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005 May;99(5):363–8. doi: 10.1016/j.trstmh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Skinner MA, Ramsay AJ, Buchan GS, Keen DL, Ranasinghe C, Slobbe L, et al. A DNA prime-live vaccine boost strategy in mice can augment IFN-gamma responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis. Immunology. 2003 Apr;108(4):548–55. doi: 10.1046/j.1365-2567.2003.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nature Immunology. 2002 Sep;3(9):852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 94.Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opinion on Biological Therapy. 2009 Jan;9(1):55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002 May;417(6884):95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 96.Blankson JN. Control of HIV-1 replication in elite suppressors. Discovery Medicine. 2010 Mar;9(46):261–6. [PubMed] [Google Scholar]
- 97.Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Current Opinion in HIV and AIDS. 2011 May;6(3):169–73. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunology. 2002 Nov;3(11):1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 99.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, et al. Mucosal immune responses to HIV-1 in elite controllers:a potential correlate of immune control. Blood. 2009 Apr;113(17):3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schuitemaker H, Kootstra NA, Koppelman MH, Bruisten SM, Huisman HG, Tersmette M, et al. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. Journal of Clinical Investigation. 1992 Apr;89(4):1154–60. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003 Mar;18(3):441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 102.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. Journal of Clinical Investigation. 2007 Nov;117(11):3148–54. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ. etal.CD4+T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. Journal of Experimental Medicine. 2004 Sep;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nature Medicine. 2009 Aug;15(8):861–5. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 Oct;112(7):2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proceedings of the National Academy of Sciences of the United States of America; 2010; Feb, pp. 3018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature Medicine. 2006 Nov;12(11):1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kwon DS, Angin M, Hongo T, Law KM, Johnson J, Porichis F, et al. CD4+ CD25+ regulatory T cells impair HIV-specific CD4 T cell responses by upregulating IL-10 production in monocytes. Journal of Virology. 2012 Jun;86(12):6586–94. doi: 10.1128/JVI.06251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodriguez-Garcia M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, et al. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. Journal of Leukocyte Biology. 2011 Apr;89(4):507–15. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, et al. Suppressive activity of regulatory T cells correlates with high CD4(+) T-cell counts and low T-cell activation during chronic simian immunodeficiency virus infection. AIDS. 2011 Mar;25(5):585–93. doi: 10.1097/QAD.0b013e3283437c7b. [DOI] [PubMed] [Google Scholar]
- 111.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. Journal of Experimental Medicine. 1992 Feb;175(2):331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011 Jul;25(11):1385–94. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 113.Kamat A, Ancuta P, Blumberg RS, Gabuzda D. Serological markers for inflammatory bowel disease in AIDS patients with evidence of microbial translocation. PLoS One. 2010;5(11):e15533. doi: 10.1371/journal.pone.0015533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006 Dec;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 115.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annual Review of Pathology. 2011;6:223–48. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 116.Chihara T, Hashimoto M, Osman A, Hiyoshi-Yoshidomi Y, Suzu I, Chutiwitoonchai N, et al. HIV-1 proteins preferentially activate anti-inflammatory M2-type macrophages. Journal of Immunology. 2012 Apr;188(8):3620–7. doi: 10.4049/jimmunol.1101593. [DOI] [PubMed] [Google Scholar]
- 117.Wherry EJ. T cell exhaustion. Nature Immunology. 2011 Jun;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 118.Rueda CM, Velilla PA, Chougnet CA, Montoya CJ, Rugeles MT. HIV-induced T-cell activation/exhaustion in rectal mucosa is controlled only partially by antiretroviral treatment. PLoS One. 2012;7(1):e30307. doi: 10.1371/journal.pone.0030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Reports. 2011 Apr;44(4):217–31. doi: 10.5483/BMBRep.2011.44.4.217. [DOI] [PubMed] [Google Scholar]
- 120.Jagannathan P, Osborne CM, Royce C, Manion MM, Tilton JC, Li L, et al. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. Journal of Virology. 2009 Mar;83(6):2728–42. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Medicine. 2008 May;5(5):e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. Journal of Immunology. 2009 Jun;182(11):6697–708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. Journal of Experimental Medicine. 2007 Oct;204(11):2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crawley AM, Angel JB. The influence of HIV on CD127 expression and its potential implications for IL-7 therapy. Seminars in Immunology. 2012 Jun;24(3):231–40. doi: 10.1016/j.smim.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 125.Porichis F, Kaufmann DE. Role of PD-1 in HIV pathogenesis and as target for therapy. Current HIV/AIDS Reports. 2012 Mar;9(1):81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. Journal of Immunology. 2008 Jun;180(11):7757–63. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006 Sep;443(7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 128.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Medicine. 2006 Oct;12(10):1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 129.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. Journal of Experimental Medicine. 2000 Oct;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunology. 2009 Jan;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpes-virus entry mediator. Nature Immunology. 2008 Feb;9(2):176–85. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 132.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. Journal of Immunology. 1999 Jun;162(12):6981–5. [PubMed] [Google Scholar]
- 133.Aldy KN, Horton NC, Mathew PA, Mathew SO. 2B4+ CD8+ T cells play an inhibitory role against constrained HIV epitopes. Biochemical and Biophysical Research Communications. 2011 Feb;405(3):503–7. doi: 10.1016/j.bbrc.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) Journal of Immunology. 2008 Jun;180(12):8159–67. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011 May;117(18):4805–15. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011 Jul;118(4):965–74. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Calarota SA, Weiner DB. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunological Reviews. 2004 Jun;199:84–99. doi: 10.1111/j.0105-2896.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 138.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. Journal of Immunology. 2005 Jul;175(1):112–23. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 139.Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007 Jun;25(26):4967–82. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 140.Berzofsky JA. A push–pull vaccine strategy using Toll-like receptor ligands, IL-15, and blockade of negative regulation to improve the quality and quantity of T cell immune responses. Vaccine. 2012 Jun;30(29):4323–7. doi: 10.1016/j.vaccine.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim JJ, Yang JS, Montaner L, Lee DJ, Chalian AA, Weiner DB. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. Journal of Interferon and Cytokine Research. 2000 Mar;20(3):311–9. doi: 10.1089/107999000312450. [DOI] [PubMed] [Google Scholar]
- 142.Marchetti G, Meroni L, Molteni C, Taskaris G, Gazzola L, Galli M, et al. IL-7/IL-7 receptor system regulation following IL-2 immunotherapy in HIV-infected patients. Antiviral Therapy. 2004 Jun;9(3):447–52. [PubMed] [Google Scholar]
- 143.Dhawan S, Heredia A, Wahl LM, Epstein JS, Meltzer MS, Hewlett IK. Interferon-gamma-induced downregulation of CD4 inhibits the entry of human immunodeficiency virus type-1 in primary monocytes. Pathobiology. 1995;63(2):93–9. doi: 10.1159/000163939. [DOI] [PubMed] [Google Scholar]
- 144.Vassena L, Proschan M, Fauci AS, Lusso P. Interleukin 7 reduces the levels of spontaneous apoptosis in CD4+ and CD8+ T cells from HIV-1-infected individuals. Proceedings of the National Academy of Sciences of the United States of America; 2007; Feb, pp. 2355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin YL, Portales P, Segondy M, Baillat V, de Boever CM, Le Moing V, et al. CXCR4 overexpression during the course of HIV-1 infection correlates with the emergence of X4 strains. Journal of Acquired Immune Deficiency Syndromes. 2005 Aug;39(5):530–6. [PubMed] [Google Scholar]
- 146.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 induces HIV replication in primary naive T cells through a nuclear factor of activated T cell (NFAT)-dependent pathway. Virology. 2006 Jul;350(2):443–52. doi: 10.1016/j.virol.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 147.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006 Jun;20(9):1247–52. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 148.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nature Reviews Immunology. 2011 Mar;11(3):176–86. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carini C, McLane MF, Mayer KH, Essex M. Dysregulation of interleukin-7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. European Journal of Immunology. 1994 Dec;24(12):2927–34. doi: 10.1002/eji.1830241202. [DOI] [PubMed] [Google Scholar]
- 150.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001 Aug;98(4):906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 151.Garcia F, Climent N, Assoumou L, Gil C, Gonzalez N, Alcami J, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. Journal of Infectious Diseases. 2011 Feb;203(4):473–8. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guillot B, Portales P, Thanh AD, Merlet S, Dereure O, Clot J, et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. British Journal of Dermatology. 2005 Apr;152(4):690–6. doi: 10.1111/j.1365-2133.2005.06512.x. [DOI] [PubMed] [Google Scholar]
- 153.Han X, Becker K, Degen HJ, Jablonowski H, Strohmeyer G. Synergistic stimulatory effects of tumour necrosis factor alpha and interferon gamma on replication of human immunodeficiency virus type 1 and on apoptosis of HIV-1-infected host cells. European Journal of Clinical Investigation. 1996 Apr;26(4):286–92. doi: 10.1046/j.1365-2362.1996.116271.x. [DOI] [PubMed] [Google Scholar]
- 154.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. Journal of Experimental Medicine. 2002 Jun;195(12):F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annual Review of Immunology. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 156.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. Journal of Medical Primatology. 2005 Oct;34(5/6):262–70. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 157.Boyer JD, Cohen AD, Ugen KE, Edgeworth RL, Bennett M, Shah A, et al. Therapeutic immunization of HIV-infected chimpanzees using HIV-1 plasmid antigens and interleukin-12 expressing plasmids. AIDS. 2000 Jul;14(11):1515–22. doi: 10.1097/00002030-200007280-00007. [DOI] [PubMed] [Google Scholar]
- 158.Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7(1):e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim JJ, Nottingham LK, Tsai A, Lee DJ, Maguire HC, Oh J, et al. Antigen-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12,orIL-18 gene adjuvants. Journal of Medical Primatology. 1999 Aug-Oct;28(4/5):214–23. doi: 10.1111/j.1600-0684.1999.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 160.Rodriguez AR, Arulanandam BP, Hodara VL, McClure HM, Cobb EK, Salas MT, et al. Influence of interleukin-15 on CD8+ natural killer cells in human immunodeficiency virus type 1-infected chimpanzees. Journal of General Virology. 2007 Feb;88(Pt 2):641–51. doi: 10.1099/vir.0.82154-0. [DOI] [PubMed] [Google Scholar]
- 161.Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, et al. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003 Feb;101(3):1024–9. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 162.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+Tcell expansion and function. Journal of Experimental Medicine. 2005 Jan;201(1):139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, Kumar S, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proceedings of the National Academy of Sciences of the United States of America; 2007; Nov, pp. 18648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chattergoon MA, Saulino V, Shames JP, Stein J, Montaner LJ, Weiner DB. Coimmunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine. 2004 Apr;22(13/14):1744–50. doi: 10.1016/j.vaccine.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 165.Dubie RA, Maksaereekul S, Shacklett BL, Lemongello D, Cole KS, Villinger F, et al. Co-immunization with IL-15 enhances cellular immune responses induced by a vif-deleted simian immunodeficiency virus proviral DNA vaccine and confers partial protection against vaginal challenge with SIV-mac251. Virology. 2009 Mar;386(1):109–21. doi: 10.1016/j.virol.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xin KQ, Hamajima K, Sasaki S, Tsuji T, Watabe S, Okada E, et al. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine. 1999 Feb;17(7/8):858–66. doi: 10.1016/s0264-410x(98)00271-0. [DOI] [PubMed] [Google Scholar]
- 167.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007 May;109(9):3873–80. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. Journal of Virology. 2011 Jan;85(2):733–41. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, et al. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. Journal of Immunology. 2006 Jul;177(1):177–91. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, et al. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011 Nov;29(49):9229–38. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parmigiani A, Pallin MF, Schmidtmayerova H, Lichtenheld MG, Pahwa S. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Human Immunology. 2011 Feb;72(2):115–23. doi: 10.1016/j.humimm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]