Abstract
Fourteen substances from the class of drugs sometimes known as “legal highs” were screened against a battery of human receptors in binding assays, and their potencies as inhibitors of monoamine uptake determined in functional in vitro assays. Thirteen of the test substances acted as inhibitors of monoamine uptake at submicromolar concentrations, including 9 potent inhibitors of the dopamine transporter (DAT), 12 potent inhibitors of the norepinephrine transporter (NET) and 4 potent inhibitors of the serotonin transporter (SERT). Seven compounds acted as submicromolar inhibitors of both DAT and NET, and three substances 1-(benzofuran-5-yl)propan-2-amine (5-APB),1-naphthalen-2-yl-2-pyrrolidin-1-ylpentan-1-one hydrochloride, (“naphyrone”) and 1-naphthalen-1-yl-2-pyrrolidin-1-ylpentan-1-one hydrochloride, (“1-naphyrone”) were submicromolar inhibitors of all three monoamine transporters. There was a lack of correlation between results of functional uptake experiments and in vitro binding assays for the monoamine transporters. There was also no correlation between the human behavioural effects of the substances and the results of bindings assays for a range of receptor targets, although 1-(benzofuran-5-yl)propan-2-amine(5-APB), 1-(benzofuran-6-yl)propan-2-amine hydrochloride(6-APB) and 5-iodo-2,3-dihydro-1H-inden-2-amine hydrochloride,(5-iodo-aminoindane) exhibited <100nM affinities for 5HT2B and α2C receptors. Functional assays revealed that 5-APB and 6-APB were potent full agonists at 5HT2B receptors.
Keywords: monoamine transporters, 5-HT2B receptor, cathinones, benzofurans, aminotetralins, aminoindanes, mephedrone, novel psychoactive substances
1. Introduction
The recent emergence of novel synthetic psychoactive drugs and their sale through internet sites has raised concerns about the potential harms associated with compounds for which formal toxicology profiles are lacking (Corazzo et al, 2012; Winstock et al, 2011; ACMD, 2011). Among the novel psychoactive substances that have emerged in recent years are a range of phenethylamine analogues designed to mimic the psychostimulant properties of amphetamine and/or ecstasy (3,4-methylenedioxymethamphetamine, “MDMA”). These include cathinone derivatives (e.g. mephedrone), aminoindanes, aminotetralins and benzofurans (e.g. 1-(benzofuran-5-yl)propan-2-amine (5-APB) and 1-(benzofuran-6-yl)propan-2-amine hydrochloride,(6-APB) –“benzo-fury”). The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) monitors the emergence of these psychoactive substances, reporting 42 new compounds during 2011 (EMCDDA Annual Reports, 2010, 2011). These substances are sold without any safety data and have been described as “plant food”, “bath salts”, “fish food” or simply as “research chemicals”. The use of untested novel chemical substances presents clear potential hazards.
Pharmacologically, the psychostimulant actions of amphetamine and ecstasy are thought to be related to their ability to promote the release of dopamine, norepinephrine and/or serotonin from nerve terminals in the brain (Iversen, 2008). These drugs act not only as competitive inhibitors of the respective monoamine transporters (DAT for dopamine), (NET for norepinephrine) and (SERT for serotonin), but also as substrates for these uptake systems acting to displace monoamines from their vesicular storage sites in the synaptoplasm (Rothman and Baumann, 2003).
Studies of the pharmacological profiles of novel psychoactive compounds have revealed their interactions with monoamine transporters. Nagai et al (2007) reported that a variety of synthetic amphetamine and tryptamine analogues inhibited monoamine uptake and release in rat brain synaptosomes. Simmler et al (2012) recently reported detailed studies on a range of cathinone derivatives, showing many of them to be potent inhibitors of monoamine transporters, using human targets, with a correlation between these properties and their behavioural stimulant effects in vivo. Martinez-Clemente et al (2011) reported that mephedrone inhibited dopamine and serotonin uptake in rat brain synaptosomes at submicromolar concentrations, and had appreciable affinity for displacing selective DAT and SERT radioligands in vitro. Mephedrone also had appreciable affinity for 5HT2 and dopamine D2 receptors. Lopez-Arnau et al (2012) compared the neuropharmacological profile of mephedrone with the related cathinones butylone and methylone, finding that all three compounds inhibited monoamine uptake via DAT, NET and SERT. In addition mephedrone had appreciable affinity for the vesicular monoamine transporter VMAT2 (Lopez-.Arnazu et al, 2012). In vivo the compounds also displayed amphetamine- and MDMA-like behavioural stimulant activity in rats. Baumann et al (2012) and Kehr et al, (2010) found that mephedrone and methylone increased the release of dopamine and serotonin in rat brain using in vivo microdialysis techniques.
In the present study the resources of the National Institute of Mental Health Psychoactive Drug Screening Program were used to obtain neurochemical profiles for 14 novel synthetic psychoactive substances and to compare these profiles with those of amphetamine, MDMA (‘ecstasy’), and other reference compounds. Unlike some previous studies, the present results were largely obtained using human receptor and transporter targets.
2. Materials and Methods
2.1 Chemicals
A sample of 2-methylamino-1-(4-methylphenyl)propan-1-one hydrochloride) “mephedrone” was acquired from an internet supplier. The full spectral data and elemental analysis were published (Gibbons and Zloh 2010) and were in close agreement with previously published data (Camilleri et al., 2010).
The remaining thirteen chemicals were supplied as reference materials by LGC Standards, as listed below:
1-(benzofuran-5-yl)propan-2-amine hydrochloride“5-APB”, “benzofury”
1-(benzofuran-6-yl)propan-2-amine hydrochloride, “6-APB”, ”benzofury”
2-(benzylamino)-1-(4-methylphenyl)propan-1-one hydrochloride,(“benzedrone”)
2-benzhydrylpiperidine hydrochloride, (“desoxypipradrol”)
6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine,(“methylenedioxyaminoindane, MDAI”)
2-ethylamino-1-(4-methylphenyl)propan-1-one hydrochloride,(“4-methylethcathinone”),
5-iodo-2,3-dihydro-1H-inden-2-amine hydrochloride(“5-iodo-2-aminoindane”),
4-methylhexan-2-amine hydrochloride, (“dimethylamylamine”)
methyl[(1-(2-thienyl)]propan-2-amine hydrochloride(“methiopropamine”)
2-(benzylamino)-1-(3,4-methylenedioxyphenyl)propan-1-one,(“methylenedioxybenzedrone, BMDP”)
1-naphthalen-1-yl-2-pyrrolidin-1-ylpentan-1-one hydrochloride, (“1-naphyrone”)
1-naphthalen-2-yl-2-pyrrolidin-1-ylpentan-1-one hydrochloride(“naphyrone”)
5,6,7,8-tetrahydrobenzo[f][1,3]benzodioxol-6-amine hydrochloride, (“methylenedioxyaminotetralin, MDAT”)
Certified purities ranged from 95.2 to 99.9% (full Certificates of Analysis for each material are available from the LGC Standards website, www.logical-standards.com).
Production of six of these reference materials (5-APB, 6-APB, benzedrone, methylenedioxybenzedrone., 5-iodo-2-aminoindane and methiopropamine) was supported by the UK’s Forensic Early Warning System (FEWS) and we thank the UK Home Office’s Centre for Applied Science and Technology (CAST) for permission to use these materials in this study.
2.2 Assays
Ki determinations, receptor binding profiles and functional assays were provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program essentially as previously described (Roth et al, 2002; Setola et al, 2003; Zolkowska et al, 2009; Jensen et al, 2008). A total of 14 compounds (mephedrone, methiopropamine, methylenedioxy-N-benzylcathinone, 5-APB, 6-APB, 5-Iodo-aminoindane, benzedrone, desoxypipradrol, dimethylamylamine, methylenedioxyaminotetralin, methylenedioxyaminoindane, 1-naphyrone, naphyrone and methylethcathinone) were screened against a total of 49 molecular targets listed in Table 1 initially in quadruplicate at 10 μM concentration (N=686 screening assays). Compounds which yielded inhibition of binding > 50% were subjected to Ki determinations via 12-point concentration-response studies in triplicate as described previously (Jensen et al, 2008). Functional assays of potencies of compounds as inhibitors of monoamine uptake at human cloned transporters were determined using the Molecular Devices Neurotransmitter Assay Kit (NET = norepinephrine transporter; DAT = dopamine transporter; SERT = serotonin transporter). h5-HT 2B serotonin receptor functional assays were performed as described using a FLIPRTETRA in the 384-well format (Jensen et al, 2008).
Table 1.
Primary and Secondary Radioligand Binding Assays
| Serotonin Receptors | 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT5A, 5-HT6, 5-HT7 |
| Dopamine Receptors | D1, D2, D3, D4, D5 |
| Glutamate Receptors | rNMDA Receptor (MK-801 binding site), mGluR5 |
| GABA Receptors | rGABA-A, rGABA-B, central and peripheral benzodiazepine sites |
| Biogenic Amine Transporters | SERT, NET, DAT |
| Adrenoceptors | α2A, α2B, α2C. β1, β2, β3, α1A, α1B, α1Δ |
| Cannibinoid | CB1, CB2 |
| Muscarinic Receptors | M1, M2, M3, M4, M5 |
| Opioid Receptors | MOR, KOR, DOR |
| Sigma Receptors | rSigma1, rSigma2 |
| Histamine Receptors | H1, H2, H3, H4 |
Shown are the human cloned neurotransmitter receptors, ion channels and transporters assayed in this study except where noted; r=Rat
3. Results
In keeping with the amphetamine/MDMA-like profiles of these compounds as subjectively reported by human users thirteen of the fourteen compounds tested were active at submicromolar concentrations as inhibitors of substrate uptake by at least one monoamine transporter (the exception being benzedrone – NET Ki = 1222 nM; DAT Ki = 1411 nM) (Table 2). Overall the compounds were more potent inhibitors of NET and DAT than of SERT. Seven compounds were active at submicromolar concentrations on DAT and NET; nine were active at submicromolar concentrations on DAT, and twelve were active at submicromolar concentrations on NET. Only three compounds (5-APB, 5-iodo-aminoindane and naphyrone) acted as submicromolar inhibitors of SERT. Some of the most potent inhibitors had Ki values <100nM for DAT inhibition (desoxypipradrol, naphyrone and 1-naphyrone).
Table 2.
Effects of novel psychoactive compounds on serotonin (SERT), dopamine(DAT) and norepinephrine (NET) transporter activity.
| Compound | NET | DAT | SERT |
|---|---|---|---|
| Mephedrone | 5.88±0.07 (511) | 6.08±0.07 (543) | 4.99±0.06 (4943) |
| Methiopropamine | 6.09±0.07 (313) | 6.05±0.07 (577) | 4.14±0.07 (>10000) |
| Methylenedioxy-N-benzylcathinone | 5.37±0.07 (1629) | 6.01±0.07 (637) | 4.53±0.06 (>10000) |
| 5-APB | 6.33±0.07 (180) | 6.30±0.07 (265) | 5.78±0.06 (811) |
| 6-APB | 6.52±0.07 (117) | 6.63±0.07 (150) | 5.26±0.06 (2698) |
| Desoxypipradrol | 6.26±0.07 (213) | 7.30±0.06 (32) | 4.27±0.07 (>10000) |
| 5-iod-2-aminoindane | 6.09±0.07 (311) | 5.61±0.06 (1590) | 5.75±0.06 (879) |
| Benzedrone | 5.50±0.07 (1222) | 5.64±0.06 (1491) | 4.75±0.06 (>10000) |
| Dimethylamylamine | 5.77±0.07 (649) | 4.74±0.06 (>10000) | 3.75±0.13 (>10000) |
| Methylenedioxy-aminoindane | 5.78±0.07 (641) | 5.12±0.06 (4924) | 4.93±0.06 (>10000) |
| Methylenedioxy-aminotetralin | 5.64±0.07 (894) | 5.20±0.06 (4067) | 5.65±0.06 (1104) |
| Naphyrone | 6.70±0.07 (200) | 7.28±0.06 (53) | 6.63±0.06 (235) |
| 1-Naphyrone | 6.27±0.07 (536) | 7.32±0.06 (48) | 6.45±0.06 (352) |
| Methylethcathinone | 5.36±0.07 (1668) | 6.08±0.06 (565) | 5.43±0.06 (1798) |
| Amitriptyline* | 6.14±0.07 (178) | 5.01±0.08 (4638) | 6.80±0.06 (123) |
| Nomifensine* | 7.04±0.07 (26) | 6.90±0.06 (66) | 5.56±0.06 (4731) |
| Cocaine* | 5.20±0.10 (1275) | 6.19±0.06 (249) | 5.62±0.08 (818) |
| (−)-MDMA* | 5.50±0.10 (704) | 5.21±0.06 (2425) | 5.17±0.,08 (2306) |
| (+)-MDMA* | 5.70±0.10 (398) | 5.62±0.06 (897) | 5.56±0.08 (948) |
| (+)-Amphetamine* | 6.20±0.10 (101) | 6.54±0.05 (109) | 4.78±0.09 (5728) |
| Fluoxetine* | 5.20±0.10 (1394) | 5.04±0.06 (3764) | 6.55±0.08 (98) |
Data represent mean pKi +/− S.E.M (N =3) and Ki in nM for inhibitory potencies of various novel psychoactive compounds with comparator data for psychostimulants and antidepressants. Values for comparator compounds (marked *) were obtained from assays performed at the same time as test compounds. Samples of the comparator compounds were obtained from the NIMH-PDSP. Ki values highlighted in bold type indicate the most potent interactions (Ki < 100 nM).
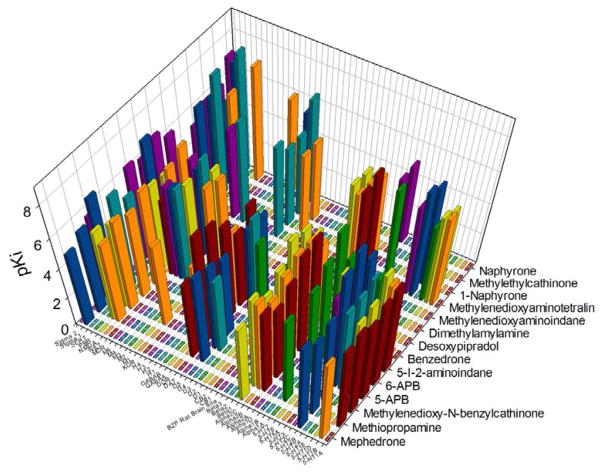
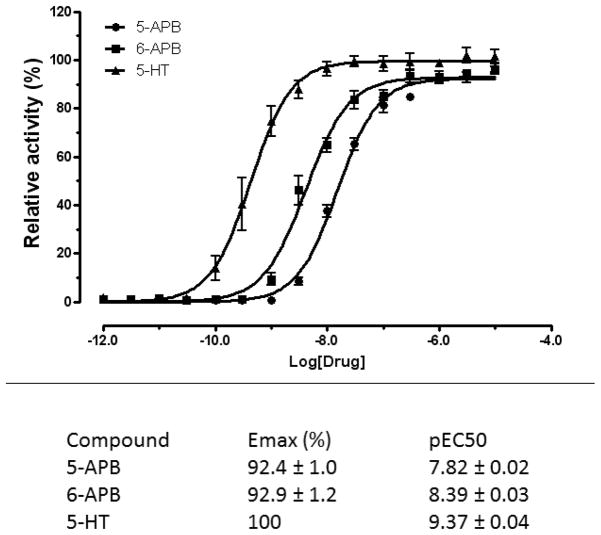
Screening of the test compounds on a range of neurotransmitter receptors revealed various submicromolar affinity interactions, but unlike the data for monoamine uptake, the pattern of activity did not correlate with the psychostimulant actions of these substances (Fig 1 and Supplementary Tables 1–6). Some notably high affinity interactions were observed at 5-HT2B serotonin receptors for 5-APB (Ki = 14nM) and 6-APB (Ki = 3.7nM), and 5-iodo-aminoindane (Ki = 70nM) (Supplemental Table 2). Functional assays confirmed that 5-APB and 6-APB were potent full agonists at human 5-HT2B serotonin receptors (Figure 2). Furthermore, 6-APB and 5-iodo-aminoindane exhibited high affinities (Ki values of 45nM and 89nM respectively) for α2C adrenoceptors (Supplementary Table 3). Submicromolar affinities were seen for some compounds at various histaminergic and muscarinic receptors as well as at σ1 and σ2 binding sites. No assayed compound had appreciable affinities for any of the following receptors: GABA-A or GABA-B orthosteric binding sites, central or peripheral benzodiazepine receptors, CB1 or CB2 cannabinoid receptors, NMDA receptors (MK801 site) or any of the other targets in the screen(Supplementary Tables 4-6).
Figure 1. Radioligand binding profile of novel psychoactive compounds.
Shown is a three dimensional plot of mean pKi values of the 14 novel psychoactive compounds screened against 49 distinct molecular targets (see Supplementary Tables for mean +/− SEM data). Abbreviations are as in Table 1 except: BZP rat brain site=rat brain benzodiazepine receptor; PBR=peripheral benzodiazepine receptor. See Supplementary Table 6 for pIC50 values
Figure 2. Potent 5-HT2B agonist activity of 5- and 6-APB.
Shown are concentration-response curves for 5- and 6-APB as well as 5-HT (control) –induced mobilization of intracellular Ca++ release at human cloned 5-HT2B receptor expressed in HEK-T cells. Data represent mean +/− S.E.M of triplicate determinations; where no error bar is shown the error was smaller than the bar. The data in the table represent computer-derived estimates from N of at least 3 separate experiments +/− S.E.M.
4. Discussion
4.1
The present study confirms and extends previous reports regarding the relatively potent actions of novel psychostimulants on monoamine transporters. Previous studies have focused on cathinone derivatives (Setola et al, 2003; Simmler et al, 2012) including the most widely used drugs, mephedrone and methylone (Martinez-Clements et al 2012; López-Arnau et al, 2012; Baumann et al, 2012), the latter using rat tissue preparations as sources of molecular targets. As the synthetic drugs were designed to mimic the actions of amphetamine and/or MDMA these results are not particularly surprising. The potencies of several of the synthetic drugs tested were comparable to or greater than the reference compounds d-amphetamine, MDMA and cocaine (Supplementary Table 2). Some test compounds resembled MDMA in acting as submicromolar inhibitors of all three monoamine transporters (Supplementary Table 2). While there is no direct correlation between the subjective effects of any particular compound and inhibition of one or other monoamine transporter, this property is considered key. Twelve of the fourteen drugs tested acted as submicromolar inhibitors of norepinephrine uptake (NET). This can explain the common occurrence of sympathomimetic effects of these drugs on the cardiovascular system (Regan et al 2010; Wood et al, 2010). Inhibition of norepinephrine uptake in brain may also contribute importantly to the psychostimulant effects of these drugs. Administration of the selective NET inhibitor reboxetine to human subjects reduced the effects of MDMA in increasing blood pressure and heart rate and also reduced the subjective drug high, including stimulation and emotional excitation (Hysek et al., 2011).
There was a weak correlation between the Ki values from functional assays (i.e., inhibition of substrate uptake) and from binding assays (i.e., inhibition of radioligand binding) for the transporter proteins, although six compounds had submicromolar Ki values for at least one or other transporter radioligand binding sites (Table 2). Notably high affinities (<100nM) were found for methylenedioxybenzedrone- and 5-APB at DAT, naphyrone and 1-naphyrone at SERT, and naphyrone at NET. The poor correlation probably reflects the fact that transporter inhibitors are likely to bind to different sites on the transporter than the radioligands used for displacement assays, and the fact that transporter substrate uptake assays were not performed at equilibrium. These results also suggest that functional measurement of drug effects on substrate reuptake may be the most reliable method of assessing drug effects on monoamine transporters. The present study included some cathinone derivatives already reported to be monoamine transport inhibitors (Martinez-Clemente et al, 2011; Lopez-Arnou et al, 2012; Simmler et al, 2012) – there was a good overall agreement between the current data and these findings.
In addition to their effects on monoamine transporters, screening of the drugs against a wide battery of human receptors and other CNS targets revealed a variety of other potentially significant interactions. Although among these interactions were several with submicromolar potency, there was no common target shared by all or most of the fourteen tested compounds.
Interactions with serotonin receptors could be particularly important, since modulation of serotoninergic transmission by drugs of abuse may underlie addiction vulnerability (Kirby et al, 2011). Some notable high affinity interactions were observed between 5-HT2B receptors for 5-APB (Ki =14nM) and 6-APB(Ki = 3.7nM), and 5-iodo-aminoindane (Ki = 70nM). Functional assays of 5-APB and 6-APB confirmed that these compounds acted as potent (i.e., nanomolar EC50 values) full agonists at 5-HT2B receptors (Figure 2). Four other compounds exhibited submicromolar affinities for the 5-HT2B receptor (mephedrone, naphyrone, 1-naphyrone and methylenedioxy-aminotetralin). This finding is potentially important because previous studies have shown that there was a correlation in a series of phenyl isopropylamines between hallucinogenic activity and affinity for the 5-HT2B receptor (Nelson et al, 1999), and activation of this receptor appears to play a key role in the behavioural stimulant and serotonin releasing effects of MDMA (Doly, 2008) and in the reinforcing effects of MDMA in mice (Doly et al 2009). The activation of 5-HT2B receptors by MDMA induces fenfluramine-like proliferation of cardiac valvular interstitial cells (Setola et al, 2003; Roth, 2007), and the drug was linked to increased prevalence of valvular heart disease in a group of Belgian MDMA users (Droogmans et al 2007). Indeed all drugs and drug metabolites that induce heart valvular disease in humans activate 5-HT2B receptors in vivo and in vitro and this activity is thought to be important for the development of fibrotic lesions upon chronic human administration (Huang et al 2009; Elengbam, 2010; Elengbam et al 2008; Roth, 2007). Therefore the finding that some of the compounds were as potent or more potent 5-HT2B receptor agonists than MDMA suggests the risk of potential cardiac toxicity with long term use) as has been reported for other 5-HT2B receptor agonists, including fenfluramine (Rothman et al, 2000), pergolide, cabergoline and MDMA (for review see Roth 2007 and Berger et al, 2009).
6-APB and 5-iodo-aminoindane also had high affinities (Ki’s 45nM and 89nM respectively) for α2C adrenoceptors although the significance of this is currently unknown.
4.2
In summary, we evaluated the molecular pharmacology of 14 novel psychoactive compounds and discovered that many of them had relatively potent activities as biogenic amine transporter antagonists—similar to the widely abused drugs cocaine, amphetamine and MDMA. As might be expected from the diversity of chemical structures, a variety of ‘off-target’ actions at miscellaneous CNS targets was also discovered. Notably, several compounds were potent 5-HT2B serotonin receptor agonists, raising the possibility that long-term abuse of these compounds could be associated with valvular heart disease similar to that induced by fenfluramine, MDMA and various ergolines.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- ACMD. Advisory Council on the Misuse of Drugs. [accessed 25.11.2012];Consideration of the Novel Psychoactive Substances (‘Legal Highs’) 2011 Oct;2011 [ http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/acmdnps2011?view=Binary] [Google Scholar]
- Baumann MH, Ayestas MH, Jr, Partilla JS, Sink JR, Shulgin AT, Dazley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain. Neuropsychoparmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DGE. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, α-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197:59. doi: 10.1016/j.forsciint.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Corazzo O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27:145–149. doi: 10.1002/hup.1242. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacobill P, Rhuolo AE. Inhibition of plasma membrane monoamine transporters by betaketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Doly S, Valient E, Setola V, Callebert J, Launy JM, Maroteaux L. Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J Neurosci. 2008;28:2933–2940. doi: 10.1523/JNEUROSCI.5723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly S, Bertran-Gonzalez J, Cellebert J, Bruneau A, Banas SM, Belmer A, Boutourlinsky K, Hervé D, Launay JM, Maroteaux L. Role of serotonin via 5-HT2B receptors in the reinforcing effects of MDMA in mice. PLoS One. 2009;4:e7952. doi: 10.1371/journal.pone.0007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans S, Cosyns B, Dhaenen H, Creeten E, Weytjens C, Franken PR, Scott B, Schoors D, Kemdem A, Close L, Vandenbossche JL, Bechet S, Van Camp G. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Amer J Cardiol. 2007;100:14421445. doi: 10.1016/j.amjcard.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Elengbam CS. Drug-induced valvulopathy –an update. Toxicol Pathol. 2010;38:837–848. doi: 10.1177/0192623310378027. [DOI] [PubMed] [Google Scholar]
- Elengbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, Slocum N. 5-Hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol. 2008;60:253–262. doi: 10.1016/j.etp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) [accessed 25/11/2012];Annual Report 2010 & 2011. 2011 [ www.emcdda.europa.eu/publications/annualreport2010]
- Gibbons S, Zloh M. An analysis of the legal high mephedrone. Bioorg Med Chem Lett. 2010;20:4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Huang XP, Setola V, Allen JA, Rogan SC, Hanson BJ, Revankar C, Robers M, Doucette C, Roth BL. Parallel functional activity profiling reveals vavulopathogens are potent 5-hydroxtryptamine (2B) receptor agonists: implications for drug safety assessment. Mol Pharmacol. 2009;76:710–722. doi: 10.1124/mol.109.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, Huwyler J, Liechti ME. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clin Pharmacol Ther. 2011;90:246–250. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Iversen L. Speed, Ecstasy, Ritalin: the Science of Amphetamines. Oxford University Press; New York: 2008. [Google Scholar]
- Jensen NH, Rodriguez RM, Caron MG, Wetsel WC, Rothman RB. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology. 2008;33:2303–2312. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goimy M, Sievertsson T, Nyberg F, et al. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby IG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three stimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Neuropsychopharmacology. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Measham F, Moore K, Newcombe R, Welch Z. Tweaking, bombing, dabbing and stockpiling: the emergence of mephedrone and the perversity of prohibition. Drugs and Alcohol Today. 2010;10:14–21. [Google Scholar]
- Nagai F, Nonaka R, Kamimura KSH. The effects of non-medically used psychoactive drugs on monoamine transmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT(2B) and 5-HT2C receptors. NaunynSchmeidebergs Arch Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- Nichols D. Legal highs: the dark side of medicinal chemistry. Nature. 2011:469. doi: 10.1038/469007a. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice K, Steinberg B, Bernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci US. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac vulvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychotropic drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, DeLuca P, Corazza O, et al. Mephedrone (4-methylmethcathjinone); “Meow meow”): chemical, pharmacological and clinical issues. Psychopharmacology. 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen J, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. Methylenedioxymethamphtamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser CA, Donzelli M, Schram Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Leichti ME. Pharmacological characterization of designer cathinones. Br J Pharmacol. 2012 doi: 10.111/j.1476-5381. 2012.02145x. online August 17,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Wood D, Dargan P. ‘Mephedrone’ (4-methylmethcathinone): what is it, how commonly is it used and what are the risks associated with its use? Pharmacology Matters. 2010;3:11–14. [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.