Abstract
The purpose of this research was to determine whether motor cortex excitability assessed using transcranial magnetic stimulation (TMS) is less variable when subjects maintain a visually controlled low-level contraction of the muscle of interest. We also examined the dependence of single motor evoked potential (MEP) amplitude on stimulation intensity and pre-stimulus muscle activation level using linear and non-linear multiple regression analysis. Eight healthy adult subjects received single pulse TMS over the left motor cortex at a point where minimal stimulation intensity was required to produce MEPs in extensor digitorum communis (EDC). Voluntary activation of the muscle was controlled by visual display of a target force (indicated by a stable line on an oscilloscope) and the isometric force produced as the subject attempted to extend the fingers (indicated by a line on the oscilloscope representing the finger extension force) while subjects were instructed to: exert zero extension force (0%) and produce forces equal to 5 and 10% of maximum voluntary finger extension under separate conditions. Relative variability (coefficient of variation) of single MEPs at a constant stimulus intensity and of pre-stimulus muscle EMG was lower during maintained 5 and 10% contractions than at 0% contraction levels. Therefore, maintaining a stable low intensity contraction helps stabilize cortical and spinal excitability. Multiple regression analyses showed that a linear dependence of single MEPs on stimulation intensity and pre-stimulus muscle activation level produced similar fits to those for a non-linear dependence on stimulus intensity and a linear dependence on pre-stimulus EMG. Thus, a simple linear method can be used to assess dependence of single MEP amplitudes on both stimulus intensity (to characterize slope of the recruitment curve) and low intensity background muscle activation level.
Introduction
Transcranial magnetic stimulation (TMS) has gained considerable acceptance as a method to non-invasively study adaptive changes in motor cortex as a consequence of repeated practice or learning in able-bodied individuals (e.g., Tinazzi and Zanette 1998) and as a consequence of injury in individuals who have suffered strokes, limb amputation and spinal cord injury (e.g., Turton et al. 1996; Liepert et al. 1998; Capaday et al. 2000; Park et al. 2004). For example, effects of some rehabilitation techniques following brain injury have been evaluated by estimating the motor cortical area that is responsive to suprathreshold stimulation of a muscle of interest (e.g., Liepert et al. 1998). However, motor evoked potentials (MEPs) recorded by EMG in response to the same stimulation intensity at a particular motor cortex site can be highly variable because of independent fluctuations in excitability of motor cortex neurons and of spinal interneurons and motor neurons at the time of stimulation (Kiers et al. 1993; Thickbroom et al. 1999). Excitability of motor cortex and spinal neurons can fluctuate due to time-dependent variations in excitatory (subthreshold and suprathreshold) and inhibitory inputs from within the CNS and from peripheral sensory receptors (Magistris et al. 1998). Study of MEP variability under different experimental conditions is needed to improve understanding of the underlying mechanisms and to test whether such variability can be reduced to improve sensitivity of tests for adaptive changes in motor cortex excitability.
Many studies using TMS to assess motor cortex excitability have subjects attempt to maintain a relaxed state of minimal activation in the muscle of interest, although a few investigations have studied MEPs under varied levels of muscle activation (e.g., Kiers et al. 1993; Devanne et al. 1997). However, subliminal activations of motor cortex neurons due to assorted brain activities involving attention (Kiers et al. 1993), sleepiness/wakefulness and variations in sensory inputs (proprioceptive, visual, auditory, tactile, etc.) or mental imagery (Abbruzzese et al. 1996; Ikai et al. 1996) may influence excitability of these neurons and their response to a TMS stimulus. One method that may stabilize variations in excitability of neurons at cortical and segmental levels is to have subjects maintain a stable, low-level voluntary contraction controlled by visual input with a target and the limb of interest in a fixed position. This technique should minimize variations of attention and somatosensory inputs.
Most previous work has shown that the coefficient of variation of MEPs for a range of TMS stimulation intensities and levels of muscle activation is inversely related to the mean MEP amplitude (Kiers et al. 1993; Devanne et al. 1997; Carroll et al. 2001). In contrast, (Kamen 2004) reported lower coefficients of variation (and higher reliability) of TMS evoked MEPs in biceps brachii under resting conditions than when the muscle was actively contracted at 25, 50, 75 and 100% of maximum voluntary effort. However, none of those investigations assessed the dependence of individual MEP amplitudes on both stimulus intensity and muscle activation. Activation level of the muscle of interest under both relaxed and submaximal contraction conditions can vary considerably, especially in individuals who have suffered strokes or other brain injuries. A greater understanding of the dependence of individual MEP amplitudes on both stimulus intensity and muscle activation might be used to improve methodology of studies of cortical excitability by permitting estimates of recruitment curve slope based on data from all MEPs rather than average MEP amplitude at each stimulus intensity. We hypothesized: (1) that maintaining a low-level contraction equal to 10% of maximum would result in lower MEP variability (and better fits of the motor cortex recruitment curve) than under relaxed conditions and when producing 5% of maximum contraction and (2) that individual MEP amplitudes depend on both stimulus intensity and muscle activation level over a short interval preceding the TMS stimulus. The first hypothesis follows from previous findings of lower MEP variability with stronger pre-stimulus muscle contractions while the second is based on previous work showing that stronger stimuli and greater neuronal excitability (associated with voluntary activation of the muscle) should evoke greater amplitude MEPs.
Methods
Subjects
Eight able-bodied volunteers (five males) with no history of neuromuscular pathology participated in this experiment. Ages ranged from 20–60 years. All subjects gave informed consent in accord with the local institutional review board.
Apparatus
Magnetic stimulation of the left motor cortex was accomplished using a Magstim 200 (Magstim Company Ltd., Whitland, Dyfed, UK) with a 9 cm diameter figure-8 coil at 0.2 Hz. The coil was oriented with the handle facing backward so the induced current in the brain was in the posterior–anterior direction during the rising phase of the monophasic pulse.
Bipolar electromyographic signals (EMGs) were recorded from the extensor digitorum communis (EDC) of the right forearm using disposable surface electrodes (Medtronic, Inc., Minneapolis, MN) placed 2 cm apart with a recording surface of 4 × 7 mm. The EMG signals were amplified (× 1,000) using a James Long Isolated Bioelectric Amplifier (SA Instrumentation Company, Encinitas, CA) and band-pass filtered (10–1,000 Hz) before digitizing at 1,000 Hz for 100 ms prior to and following each stimulus (i.e., 200 ms total recording time). We examined whether sampling at 1,000 Hz was sufficient by recording MEPs in one subject at 5,000 Hz. Regression analysis of peak-to-peak amplitudes (PPamp) of MEPs in the 5,000 Hz sampled data on PPamp from the same data down-sampled to 1,000 Hz (i.e., every 5th point) was used. Correlation coefficients exceeded 0.99 and regression slopes were near 1.0, showing that the 1,000 Hz sampling rate was sufficient to obtain PPamp of EDC MEPs.
Isometric force exerted while attempting to extend the fingers of the right hand was recorded using a load cell (Kistler® Model 9312A) rigidly connected to a platform and to a padded metal cuff into which subjects placed their fingers. The forearm was comfortably supported in full pronation with the wrist and hand (held in place by Velcro straps) in a neutral position on a wooden platform above the load cell. The finger extension force was displayed on an oscilloscope along with a target line such that subjects could visually match the exerted force to the target during magnetic stimulation of the left motor cortex.
Procedures
Subjects were comfortably seated in an adjustable chair and wearing a firm-fitting cap (Elector-cap International Inc. Eaton, OH) with imprinted 1 cm2 grids to serve as a reference for reproducible coil placement and orientation. The skin overlying the EDC muscle was located and prepared for EMG recording by abrading the skin using LemonPrep® (Mavidon Medical Products, Lake Worth, FL) until an erythemic response appeared. Skin impedance was maintained below 2 kΩ between the active electrodes and below 20 kΩ between each active electrode and the reference electrode. With the subject relaxed (audio EMG feedback from EDC was provided), the TMS hotspot for EDC was determined by moving the stimulating coil to various positions to find the grid location where the motor threshold was lowest. The hotspot was defined as the grid location where the motor threshold was the lowest while evoking the largest response (Wassermann 2002). Resting motor threshold (RMT) was defined as the stimulation intensity which elicited MEPs ≥ 50 μV in 5 of 10 consecutive trials (Butler et al. 2005).
After finding the EDC hotspot and RMT stimulation intensity, the subject’s fingers were inserted into the cuff attached to the load cell, and maximum finger extension force was recorded while the subject viewed the force signal on the oscilloscope and was verbally encouraged to maximize force output. The maximum finger extension force was the mean of the maximum forces from three trials as volunteers extended their fingers at the MCP joint as strongly as possible against the cuff. Recruitment curves were then generated by stimulation over the hotspot with five trials each at stimulation intensities of 0.9, 1, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6 × RMT (and greater stimulation intensities within the output range of the Magstim 200 in subjects with low resting motor threshold stimulus intensities) with preactivation of the muscle at 0% (i.e., relaxed), 5 and 10% of maximum voluntary contraction force. Subjects could hear an auditory signal of the EDC EMG and viewed the force output and target line on the oscilloscope during elicitation of each recruitment curve. Thus, even under relaxed conditions subjects had a visual display of force, which actually represented a small downward force due to gravitational effects on the fingers in the cuff, as well as an auditory signal representing the level of EDC muscle contraction. Subjects maintained the contraction for approximately 20 s while five TMS stimuli were applied at the same stimulation level and then had a short rest (about 10 s) before another set of five TMS stimuli was delivered while the subject again maintained the contraction.
Data analysis
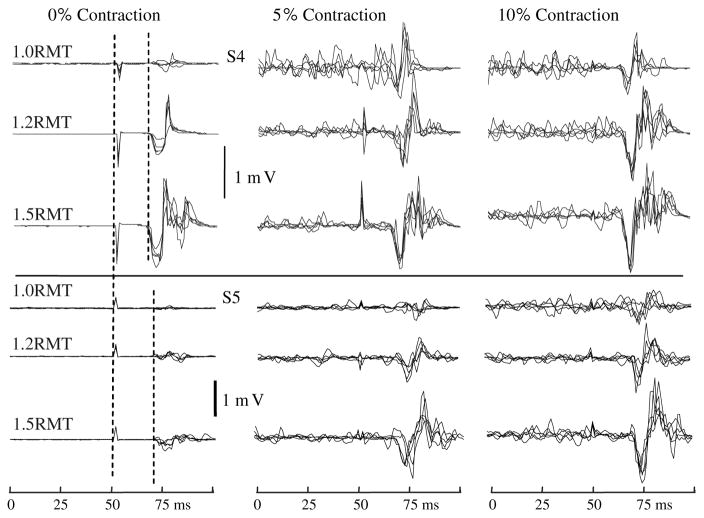
The peak-to-peak MEP amplitude was calculated following data collection by finding the maximum and minimum EMG potential occurring between the minimum latency of MEP onset after the stimulus and 50 ms after the stimulus (Fig. 1). The minimum latency of MEP onset after 100% intensity magnetic stimulus was determined in each subject under relaxed conditions. These latencies varied between 15–20 ms according to height of the subject. We also computed the area under the rectified MEP over the time interval from MEP onset to 50 ms after the stimulus, but report peak-to-peak MEP amplitude because the two methods produced very similar findings. Mean, standard deviation (SD) and coefficient of variation (CV = 100 × SD/mean) values of PPamp of MEPs were computed for the five trials at each stimulus intensity (from 0.9RMT to 1.6RMT) and contraction level. In addition, average pre-stimulus EMG level was measured over a 95 ms interval during the 100 ms prior to stimulation. All MEPs and pre-stimulus EMGs in each subject were normalized to that subject’s largest MEP. We chose this normalization method instead of maximum voluntary isometric contraction (MVIC) EMG level because previous work has shown that maximum MEPs with preactivation contractions of 10, 20 and 40% of maximum are similar (Devanne et al. 1997). Thus, the largest MEP observed at 5 and 10% contraction level probably represents maximal or near-maximal muscle activation. In contrast, MVICs can be quite variable, especially in sedentary subjects, because of the requirement for a maximal voluntary effort.
Fig 1.
TMS-induced MEPs recorded from EDC at resting motor threshold stimulus intensity (1.0RMT) and at 1.2RMT and 1.5RMT in two subjects (S4, S5). Each panel shows five superimposed MEPs at a single stimulus intensity at one activation level in the same subject. The stimulus was delivered at 50 ms in all records. Note stimulus artifact and onset of MEP response (dashed vertical lines at 0% contraction level)
We statistically compared the SDs and CVs of MEP amplitudes at different contraction levels and stimulus intensities using separate two-factor repeated measures analysis of variance (ANOVA) to assess whether variability of MEPs differed across stimulation intensities (0.9–1.6RMT) and contraction levels (0, 5, 10%). We also compared subjects’ abilities to maintain the three different contraction EDC EMG levels by comparing SDs and CVs of average pre-stimulus EMGs at different stimulus intensities and pre-contraction levels using a separate two-factor repeated measures ANOVA. Greenhouse-Geisser corrections were applied to ensure that the assumption of sphericity was met, resulting in adjusted P-values based on adjusted degrees of freedom. Adjusted P-values are given in the Results section.
We assessed the relationship between average MEP amplitude (dependent variable) and stimulus intensity (independent variable) at each contraction level using the Boltzmann equation (Devanne et al. 1997), except in a few cases where linear fits between these variables were better. We also assessed the dependence of single trial MEP amplitudes (dependent variable) on stimulus intensity and average muscle activity levels over the 95 ms prior to the stimulus (independent variables) using multiple linear regression and nonlinear regression. For multiple linear regression, we tested whether a linear combination of stimulus intensity and muscle activation level accurately predicted single trial MEP amplitudes (eq. 1) for the entire range of stimulus intensities and for the range from 0.9 to 1.4 × RMT, over which the relationship is nearly linear (Lewis et al. 2004). For non-linear regression, we examined whether single trial MEP amplitudes over the entire range of stimulus intensities could be accurately predicted using the Boltzmann equation to assess the effects of stimulus intensity on MEP amplitude and included a linear dependence on muscle activation level (eq. 2).
| (1) |
| (2) |
Dependent variable: MEPamp = normalized single trial peak-to-peak MEP amplitude
Independent variables: S = stimulus intensity, Pre- EMG = average rectified EMG amplitude over 95 ms prior to TMS stimulus
Variables estimated by fitting the model: MEPmax = maximum MEP amplitude, S50 = stimulus intensity that produces a half maximum MEP, K = slope parameter, a = coefficient for S in linear equation, b = coefficient for PreEMG in linear and nonlinear equations, c = constant
Results
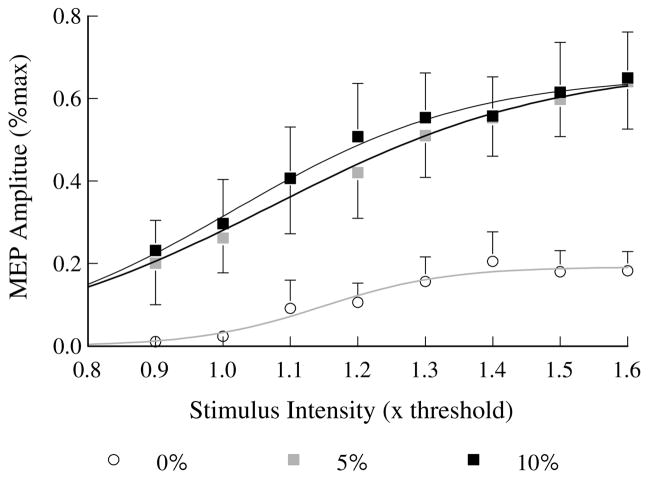
TMS evoked MEPs generally increased in amplitude with increases in both stimulation intensity (F6,42 = 22.9, P < 0.001) and contraction level (F2,14 = 41.3, P < 0.001) as expected (Figs. 1, 2). Large increases in MEP amplitude from 0 to 5% activation levels and from RMT to 1.2 RMT usually occurred, with only small further increases with higher stimulus intensities and activation level to 10% of maximum voluntary force (Fig. 1, S5; Fig. 2), although there was some intersubject variability (Fig. 1, compare MEPs of S5 and S4). Notably, there was often little difference in EDC muscle EMG level prior to the stimulus for 5% versus 10% of maximum contraction (e.g., Fig. 1). Analysis of the pre-stimulus average rectified EMG levels for all subjects showed that there was a significant increase (P = 0.004) from 5 to 10% contraction level, but only from 1.3 to 1.8% of maximum peak-to-peak MEP amplitude. Moreover, trial-to-trial variations in the pre-stimulus EDC EMG levels appeared high at both 5 and 10% of maximum MEP amplitude, indicating that production of these low forces is not associated with stable pre-stimulus muscle activation in EDC (Fig. 1). However, relative variability of pre-stimulus EMG clearly decreased with increasing contraction level from 0 to 5% (Fig. 3c, P < 0.001), but not from 5 to 10% contraction levels (Fig. 3c, P = 0.403).
Fig 2.
Peak-to-peak MEP amplitude versus stimulus intensity (relative to resting motor threshold) for three different levels of finger extensor force (0, 5, 10% of voluntary maximum). Each point is the average MEP amplitude for eight subjects at one stimulus intensity. The three plotted lines are best-fit non-linear (Boltzmann equation) regression lines for 0, 5 and 10% contraction levels. The error bars represent the mean of the standard deviations of MEP amplitude for eight subjects
Fig 3.
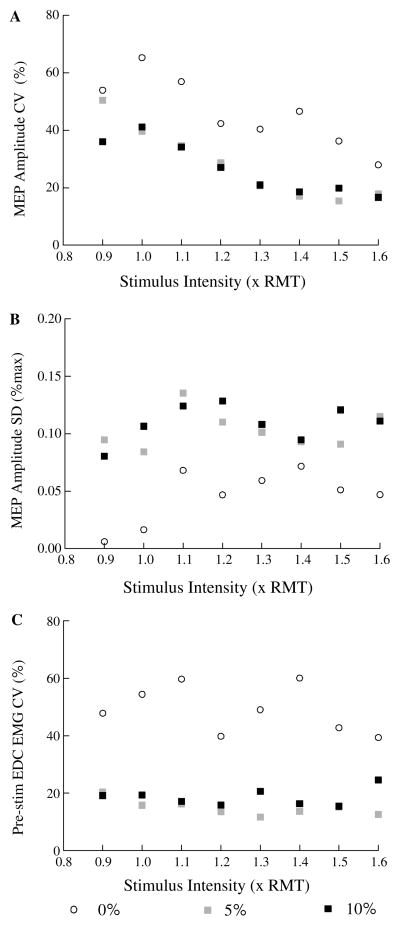
a Mean coefficient of variation (CV) of peak-to-peak MEP amplitude versus stimulation intensity at different muscle contraction levels. b Mean absolute variability (SD) of peak-to-peak MEP amplitudes (% of max MEP) versus stimulus intensity at different activation levels. c Mean coefficient of variation of pre-stimulus EMG levels versus stimulus intensity at three different finger extensor contraction levels (0, 5 and 10% of maximum voluntary force). Each plotted point is the mean of within-subject coefficients of variation or standard deviations from eight subjects at a single stimulus intensity and activation level
Relative variability of MEP amplitudes decreased with increases in muscle activation and stimulus intensity. Clear variations in minima and maxima of individual MEPs at all three stimulation intensities and contraction levels can be seen in the records from both subjects shown in Fig. 1. Also notable are the more complex and longer duration MEP waveforms at higher stimulus intensities and contraction levels. In general, the first minimum and maximum in the MEP waveforms were largest in absolute size, although there were some exceptions (e.g, S4 in Fig. 1 for 1.2 and 1.5RMT stimulus intensities at 10% contraction level). Analysis of group data showed that the coefficients of variation of peak-to-peak MEP amplitudes were lower at higher contraction levels (Fig. 3a, F2,14 = 10.32, P = 0.011). Post-hoc testing showed that the CVs were significantly lower at 5 and 10% activation than at 0% (P < 0.05), but there was no difference in CVs between 5 and 10% activation levels. CVs of MEP amplitudes were highest at RMT and generally decreased with higher stimulus intensities up to 1.4 × RMT, except at 0% contraction level (Fig. 3a, F7,49 = 8.86, P < 0.001). Taken together, these results suggest that the relative variability of MEP amplitudes decreases with increasing average MEP amplitudes. Examination of this relationship in individual subjects showed that each subject had significant (P < 0.05) negative correlation coefficients (ranging from −0.4 to −0.91) for the relationship between coefficient of variation and average MEP amplitude across all stimulus intensities and pre-stimulus EMG levels.
Absolute variability of MEPs increased with contraction level but did not show consistent changes with increasing stimulus intensity (Fig. 3b). Higher absolute variability was observed at 5 and 10% contraction levels than at 0% (Fig. 3b, F2,14 = 19.6, P < 0.001; P < 0.001 for post-hoc comparisons of 0% with 5 and 10% contraction levels) but did not differ between 5 and 10% contraction levels (P = 0.85). Although stimulus intensity significantly affected absolute variability of MEPs (Fig. 3b, F7,49 = 2.43, P = 0.032), post-hoc tests showed that variability differed only for 0.9, 1.0 and 1.1 × RMT stimulus intensities (P = 0.018) with no differences among all other stimulus intensities (P > 0.35).
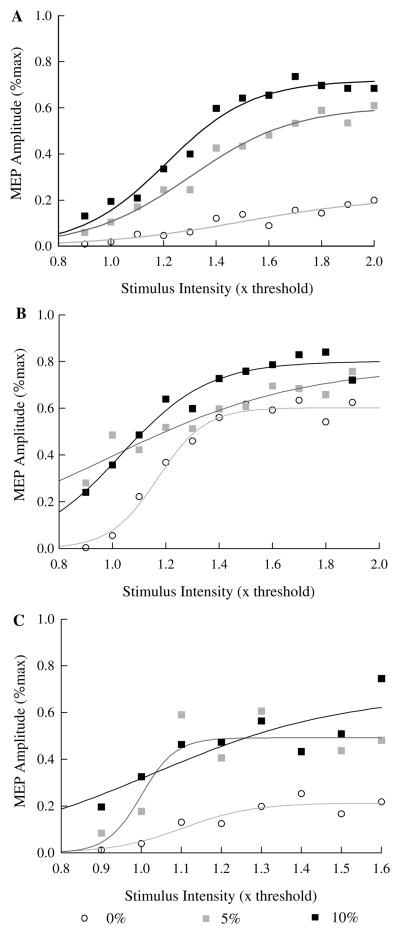
Recruitment curves varied in shape for different subjects (Fig. 4, compare a, b, c) and, in some cases, exhibited quite different shapes for different muscle activation levels within the same subject (e.g., Fig. 4, compare b, c). The Boltzmann equation produced excellent fits to the average MEP amplitude at each stimulus intensity in most cases as coefficients of determination were usually above 0.8. However, in a few cases the fit was nearly linear (e.g., Fig. 4b, 5% activation level, Fig. 4c, 10% activation level). Mean coefficients of determination were slightly higher for recruitment curves recorded under relaxed conditions (mean R2 = 0.82) than during 5 and 10% contraction levels (mean R2 = 0.80, 0.77 respectively), although there were no statistical differences (F2,14 = 0.226, P = .801). As reported previously, the threshold stimulus intensity always decreased with increasing muscle activation such that 0.9RMT stimulus intensities always produced MEPs at 5 and 10% contraction levels. The slope of the recruitment curve usually increased with increasing contraction level, but there were some exceptions (e.g., Fig. 4b).
Fig 4.
Motor cortex recruitment curves (peak-to-peak MEP amplitude versus stimulus intensity) at different pre-stimulus activation levels for three subjects (a, b, c). Each plotted point is the mean MEP amplitude for five consecutive stimuli at one stimulus intensity and contraction level (0, 5, 10% of maximum voluntary finger extensor force) in a single subject. The three plotted lines are best-fit non-linear (Boltzmann equation) regression lines for 0, 5 and 10% contraction levels
Despite the non-linear relationship between stimulus intensity and average MEP amplitude at a single activation level in most subjects, multiple regression analysis showed that single trial MEP amplitudes depended on stimulus intensity and muscle activation level in a linear manner. Peak-to-peak MEP amplitudes depended on a linear combination of stimulus intensity and pre-stimulus EMG level in individual subjects when data from all three muscle activation levels were considered (e.g., Fig. 5). Multiple linear correlation coefficients were significant (P < 0.05) in all eight subjects. Coefficients of determination ranged from 0.44 to 0.79 when including all stimulus intensities and when including only stimulus intensities from 0.9RMT to 1.4RMT (Table 1). Thus, including only stimulus intensities which produce a nearly linear recruitment curve (Lewis et al. 2004) did not improve the multiple linear regression fits. Furthermore, both stimulus intensity and activation level were significant (P < 0.05) predictors of single trial MEP amplitude in all but one subject (Table 1) whose data showed a strong dependence on pre-stimulus EMG and no dependence on stimulus intensity (Table 1, subject 1). It is also noteworthy that there was an inverse relationship between the regression coefficients for stimulus intensity and pre-stimulus muscle activation (r = −0.69, P < 0.05). Thus, for subjects in whom stimulus intensity strongly affected MEP amplitude, pre-stimulus muscle activation level tended to have weaker effects on MEP amplitude.
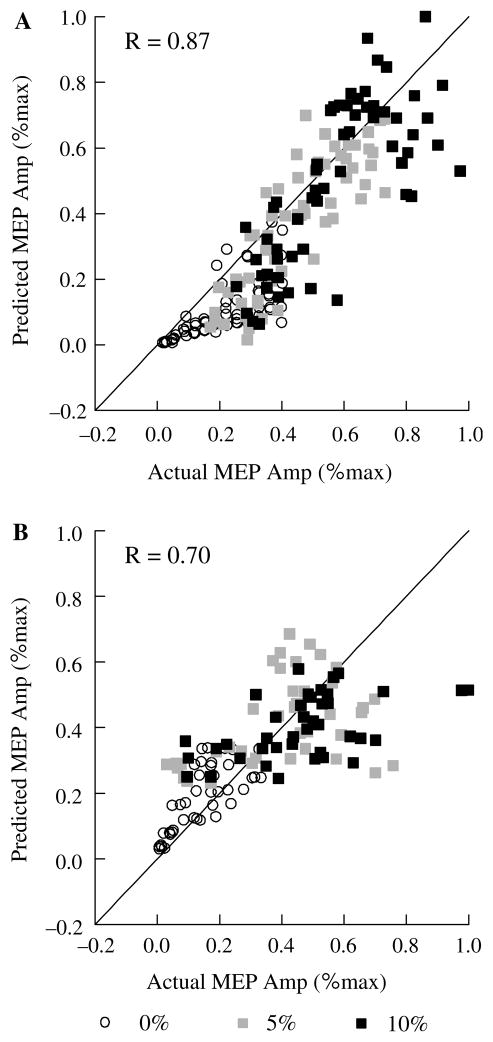
Fig 5.
Scattergraphs showing actual MEP amplitude versus MEP amplitude predicted from linear multiple regression with stimulus intensity and average pre-stimulus EMG amplitude as independent variables in two subjects. Each plotted point represents the peak-to-peak amplitude of a single MEP in one subject
Table 1.
Coefficients of determination and regression coefficients for each subject
| Subject | Coefficient of determination
|
Regression coefficients
|
||||
|---|---|---|---|---|---|---|
| Linear All SIa | Linear 0.9-1.4RMTb | Non-linear All SI | PreEMGc | 0,5,10%d | 0%e | |
| 1 | 0.8 | 0.79 | 0.78 | 39.39 | 0.12* | 0.09 |
| 2 | 0.56 | 0.67 | 0.61 | 7.05 | 0.83 | 0.56 |
| 3 | 0.64 | 0.72 | 0.60 | 18.85 | 0.53 | 0.26 |
| 4 | 0.64 | 0.71 | 0.68 | 8.62 | 0.95 | 1.11 |
| 5 | 0.76 | 0.74 | 0.76 | 15.18 | 0.60 | 0.26 |
| 6 | 0.44 | 0.44 | 0.44 | 27.31 | 0.52 | 0.15 |
| 7 | 0.49 | 0.53 | 0.44 | 24.37 | 0.62 | 0.48 |
| 8 | 0.64 | 0.66 | 0.67 | 32.25 | 0.84 | 0.24 |
Stimulus intensity
Resting motor threshold
Pre-stimulus activation level
0, 5, 10% contraction levels
0% contraction level only
P > 0.05 (ns not significant)
Prediction of MEP amplitudes using a non-linear model (Boltzmann equation) for the stimulus intensity effects on MEP amplitude combined with a linear effect of muscle activation level produced similar fits to the single trial MEP amplitude data. Coefficients of determination for this more complex regression model ranged 0.44–0.78 among the eight subjects and were not statistically different from those for the linear model (Table 1, t7 = 0.066, P > 0.2).
Discussion
We have shown that relative variability (coefficient of variation) of pre-stimulus EMG amplitude and MEPs evoked by TMS over motor cortex is lower when subjects maintain a low submaximal muscle contraction (5 and 10% of maximum) than when maintaining a relaxed state. However, contrary to our first hypothesis, lower variability of MEPs when producing submaximal contractions did not result in better fits between average MEP amplitude and stimulus intensity than under relaxed conditions. Consistent with our second hypothesis, individual MEP amplitudes depended on the combined influence of stimulus intensity and pre-stimulus EMG activation level. Despite the non-linear relationship between average MEP amplitude and stimulus intensity, there was a linear dependence of MEP amplitude on stimulus intensity and pre-stimulus muscular activation for a wide range of stimulus intensities and limited range of muscle activation levels. Thus, obtaining recruitment curves from subjects exhibiting variable levels of background muscle activation should be possible using this multiple regression analysis technique. The regression coefficient for stimulation intensity in the multiple regression equation may provide an appropriate measure of motor cortex (and spinal) excitability from these recruitment curves. Moreover, the regression coefficient for pre-stimulus muscle activation may indicate the effect of voluntary muscle activation level on motor cortex excitability, which could also change with learning or upper limb hemiparesis following a stroke.
Although previous research has reported that relative variability of MEPs decreases with increasing stimulus intensity and with increased background contraction level, this report is the first to examine variability of single trial MEP amplitudes in relation to both stimulation intensity and pre-stimulus muscle activation level. Clearly, variability of single MEP amplitudes for a constant TMS stimulus intensity cannot be explained only by variations in activation level of the muscle from which MEPs are recorded. TMS activates variable numbers of motor cortex neurons and spinal interneurons and many of these neurons may be associated with a number of muscles that are synergistic and antagonistic to the muscle of interest (Amassian et al. 1990; Cracco et al. 1990). Thus, excitability levels of motor cortex neurons and spinal interneurons, especially those associated with the propriospinal system in the cervical cord (Alstermark et al. 1991b; Alstermark et al. 1991a; Tantisira et al. 1996), likely contribute substantially to the variations in EDC MEP amplitude to constant TMS stimuli. Other factors including small trial-to-trial variations in location of the TMS coil and attention-level of the subject, location of the hotspot relative to synergist and antagonist muscles, and number of applied stimuli can also affect MEP variability. Indeed, previous work has shown that subjects imagining muscle contractions or movements (e.g., Gandevia and Rothwell 1987; Hashimoto and Rothwell 1999; Niyazov et al. 2005) and simply thinking about intrinsic muscles (Gandevia and Rothwell 1987) can influence MEPs. Thus, trial-to-trial variations in focus of attention on synergist or antagonist muscles could influence variability of MEPs.
The relationship between MEP amplitude and stimulus intensity is of primary research interest because this relationship reflects the excitability of motor cortex and subcortical structures. We confirmed the results of previous studies showing that submaximal activation of the muscle of interest causes larger TMS-evoked MEPs (Kiers et al. 1993; Devanne et al. 1997). Increased excitability of cortical and spinal neurons likely contribute to larger MEPs because excitability of neurons participating in directly activating the muscle and of neighboring (subliminal fringe) neurons will be increased (Capaday and Stein 1987; Butler et al. 1993). Motor neurons that contribute directly to the muscle activation may be refractory at the time the stimulus is applied, but for low level contractions one would expect only a small percentage would be in this state because force is maintained by asynchronous activation of a number of motor units. By the same reasoning, one would expect absolute variability (SD) of the MEP amplitudes to increase with increasing muscle activation because the number of subliminal fringe neurons near threshold should increase. Increased absolute variability of MEPs with increasing muscle activation level was observed in the present work (Fig. 3).
An important question is whether a multiple linear regression technique can be used to measure slope of the recruitment curve relating MEP amplitude to TMS stimulus intensity accurately in subjects who have variable muscle activation levels. We further examined this issue by comparing the multiple linear regression coefficients for stimulus intensity in the condition when subjects were instructed to remain relaxed (0% contraction level) with the regression coefficients computed using the data from all three activation levels. However, these regression coefficients were 1.5–3.5 times larger when all data were combined than in the relaxed (0%) condition in five of eight subjects (Table 1). This observation is consistent with previous reports that the maximum slope of the Boltzmann equation fit to recruitment curve data increases with pre-stimulus muscle activation level (Devanne et al. 1997). Moreover, of the three subjects (2, 4, 5 in Table 1) who had the most difficulty maintaining a relaxed state of EDC (as indicated by coefficients of variation of pre-stimulus EDC EMG > 60%), only one had similar regression coefficients for stimulus intensity for all activation levels and for 0% contraction levels (subject 4, Table 1). Thus, even for subjects who have difficulty maintaining a relaxed state in the muscle of interest, uncontrolled activation of the muscle produces a substantially higher recruitment curve slope. Perhaps when attempting to maintain a relaxed state the fluctuations in neuronal excitability at cortical and segmental levels are relatively independent as suggested by Kiers and colleagues (Kiers et al. 1993). In contrast, during voluntary activation these fluctuations of neuronal excitability may be positively correlated. If so, the lower recruitment curve slope during the relaxation state, in spite of varied muscle activation, may be due to periods of high motor cortex excitability when segmental excitability is low and vice-versa. In contrast, higher slopes during voluntary activation may occur because excitability of both cortical and segmental levels is high due to the focus on activation of the muscle of interest. Furthermore, the extent to which voluntary activation would stabilize cortical and spinal excitability levels in people who have suffered brain damage is unclear because the greater effort required to voluntarily activate certain muscles in these individuals may cause greater variability in cortical and segmental excitability levels due to concomitant activation of neurons controlling other muscles, including antagonists (Canning et al. 2000). Alternatively, positioning of the limb in these patients by placing hyperactive flexor muscle in a lengthened position could induce reflex shortening or persistent muscle spindle input that could alter cortical and spinal excitability to TMS and hence affect variability. Moreover, recent work has shown that propriospinal neurons may be hyperexcitable following stroke (Mazevet et al. 2003; Stinear and Byblow 2004), which could also cause greater variability in TMS-evoked MEPs.
The inverse relationship between the regression coefficients for stimulus intensity and pre-stimulus muscle activation level suggests that the effects of increasing voluntary activation on MEP amplitude are lower for subjects who have a more excitable motor cortex. One might expect a positive relationship between the effects of stimulus intensity and voluntary activation on motor cortex because a more excitable cortex would presumably be more easily activated both voluntarily and by TMS. A more likely explanation, however, is that even low-level voluntary activation may lead to a ceiling effect in terms of the number of neurons available for activation by external stimulation. This notion is consistent with findings of similar maximum MEP amplitudes for background contractions of 10–40% of maximum voluntary effort (Devanne et al. 1997) and of lower maximum MEP amplitudes at 50–100% than at 25% of maximum effort (Kamen 2004).
In conclusion, results of the present work show that single MEP amplitudes in response to TMS applied over motor cortex depend in a linear manner on stimulus magnitude and pre-stimulus muscle activation level. Voluntary activation to produce a visually controlled force reduces variability of pre-stimulus muscle activation level and MEPs, but this does not improve traditional non-linear fits of recruitment curves relating average MEP amplitudes to stimulus intensity (c.f., Devanne et al. 1997). Other techniques such as visual feedback of smoothed EMG of the muscle of interest or triggering of stimulation based on EMG of the muscle of interest over a short time interval (Kaelin-Lang and Cohen 2000) could also be used to control pre-stimulus muscle activation and attention during the task. Whether such techniques would cause greater reductions of variability of motor cortex and spinal neurons is not clear given the noisy nature of the EMG signal and because the muscle EMG occurs some time after activation of cortical and spinal neurons. However, the multiple regression method to evaluate recruitment curve parameters suggested in this work could also be applied when using these EMG-based techniques. The major advantages of the multiple regression technique used here is that all single trial MEPs are included in the determination of slope of the recruitment curve and the dependence of MEP amplitude on pre-stimulus muscle activation is also assessed. In contrast, previous work has used average MEP amplitude at each stimulus intensity and has not considered variations in pre-stimulus activation level. When using average MEP amplitudes different pre-stimulus activation levels for different stimulus intensities would produce an inaccurate recruitment curve slope. However, the percentage of unexplained variability in single trial MEP amplitudes remained relatively high (range: 21–66%) even when accounting for variations in pre-stimulus activation levels. Although some of this unexplained variability may be related to uncontrolled experimental factors including small variations in location and orientation of the coil at the instant of stimulation, time-varying neuronal excitability at cortical and segmental levels is a more likely cause.
Acknowledgments
Support received from the Emory University School of Medicine and the Emory Department of Rehabilitation Medicine, and NIH Grants HSD 37606 and 40984. We would also like to thank Jim Hudson for his help in the fabrication of the force measurement device. Special thanks to Charles Gibson, Jean Ko, and Amir Ahmadian, Emory Department of Rehabilitation Medicine, for assisting with recruitment of participants, data collection and analysis.
Contributor Information
Warren G. Darling, Email: warren-darling@uiowa.edu, Motor Control Laboratories, Department of Exercise Science, University of Iowa, 526 Field House, Iowa City, IA 52242, USA
Steven L. Wolf, Department of Rehabilitation Medicine, Emory University School of Medicine, Atlanta, GA, USA, Department of Cell Biology, Emory University School of Medicine, Atlanta, GA, USA, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Andrew J. Butler, Department of Rehabilitation Medicine, Emory University School of Medicine, Atlanta, GA, USA
References
- Abbruzzese G, Trompetto C, Schieppati M. The excitability of the human motor cortex increases during execution and mental imagination of sequential but not repetitive finger movements. Exp Brain Res. 1996;111:465–472. doi: 10.1007/BF00228736. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 18. Morphology, axonal projection and termination of collaterals from C3-C4 propriospinal neurones in the segment of origin. Exp Brain Res. 1991a;84:561–568. [PubMed] [Google Scholar]
- Alstermark B, Isa T, Tantisira B. Pyramidal excitation in long propriospinal neurones in the cervical segments of the cat. Exp Brain Res. 1991b;84:569–582. doi: 10.1007/BF00230969. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex.[see comment] Electroencephalogr Clin Neurophysiol. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- Butler A, Kahn S, Wolf S, Weiss P. Finger extensor variability in TMS parameters among chronic stroke patients. J Neuroengineering Rehabil. 2005;2:10. doi: 10.1186/1743-0003-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AJ, Yue G, Darling WG. Variations in soleus H-reflexes as a function of plantarflexion torque in man. Brain Res. 1993;632:95–104. doi: 10.1016/0006-8993(93)91143-g. [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, O’Dwyer NJ. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J Neurol Sci. 2000;176:45–56. doi: 10.1016/s0022-510x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- Capaday C, Richardson MP, Rothwell JC, Brooks DJ. Long-term changes of GABAergic function in the sensorimotor cortex of amputees. A combined magnetic stimulation and 11C-flumazenil PET study. Exp Brain Res. 2000;133:552–556. doi: 10.1007/s002210000477. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods. 1987;21:91–104. doi: 10.1016/0165-0270(87)90107-5. [DOI] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, MacCabee PJ, Cracco JB. Excitatory and inhibitory effects of magnetic coil stimulation of human cortex. Electroencephalogr Clin Neurophysiol Suppl. 1990;41:134–139. doi: 10.1016/b978-0-444-81352-7.50016-9. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999;125:75–81. doi: 10.1007/s002210050660. [DOI] [PubMed] [Google Scholar]
- Ikai T, Findley TW, Izumi S, Hanayama K, Kim H, Daum MC, Andrews JF, Diamond BJ. Reciprocal inhibition in the forearm during voluntary contraction and thinking about movement. Electromyogr Clin Neurophysiol. 1996;36:295–304. [PubMed] [Google Scholar]
- Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. 2000;102:81–89. doi: 10.1016/s0165-0270(00)00284-3. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:415–423. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Polych MA, Byblow WD. Proposed cortical and sub-cortical contributions to the long-latency stretch reflex in the forearm. Exp Brain Res. 2004;156:72–79. doi: 10.1007/s00221-003-1767-z. [DOI] [PubMed] [Google Scholar]
- Liepert J, Miltner WHR, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials.[see comment] Brain. 1998;121:437–450. doi: 10.1093/brain/121.3.437. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Niyazov DM, Butler AJ, Kadah YM, Epstein CM, Hu XP. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin Neurophysiol. 2005;116:1601–1610. doi: 10.1016/j.clinph.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Park SW, Butler AJ, Cavalheiro V, Alberts JL, Wolf SL. Changes in serial optical topography and TMS during task performance after constraint-induced movement therapy in stroke: a case study. Neurorehabil Neural Repair. 2004;18:95–105. doi: 10.1177/0888439004265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–434. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Tantisira B, Alstermark B, Isa T, Kummel H, Pinter M. Motoneuronal projection pattern of single C3-C4 propriospinal neurones. Can J Physiol Pharmacol. 1996;74:518–530. [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Mastaglia FL. A model of the effect of MEP amplitude variation on the accuracy of TMS mapping. Clin Neurophysiol. 1999;110:941–943. doi: 10.1016/s1388-2457(98)00080-7. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci Lett. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]