Abstract
Elucidating the neural basis of joint attention in infancy promises to yield important insights into the development of language and social cognition, and directly informs developmental models of autism. We describe a new method for evaluating responding to joint attention performance in infancy that highlights the 9 to 10 month period as a time interval of maximal individual differences. We then demonstrate that fractional anisotropy in the right uncinate fasciculus, a white matter fiber bundle connecting the amygdala to the ventral-medial prefrontal cortex and anterior temporal pole, measured in 6 month-olds predicts individual differences in responding to joint attention at 9 months of age. The white matter microstructure of the right uncinate was not related to receptive language ability at 9 months. These findings suggest that the development of core nonverbal social communication skills in infancy is largely supported by preceding developments within right lateralized frontotemporal brain systems.
Keywords: joint attention, DTI, amygdala, uncinate fasciculus, infancy, development
Triadic joint attention or the capacity to coordinate attention and engagement on objects in the distal visual field with another person marks a fundamental milestone during the latter half of the first year of life. These behaviors are critically important for providing a basis for the development of more sophisticated social-communicative and social-cognitive behaviors observed during childhood, including language skills and theory of mind (Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979; Brooks & Meltzoff, 2005; Kristen, Sodian, Thoermer, & Perst, 2011; Nelson, Adamson, & Bakeman, 2008). Joint attention, therefore, lays the foundation for perhaps the most complex and unique human attributes (Tomasello, Carpenter, Call, Behne, & Moll, 2005). One type of joint attention, first described by Scaife and Bruner (1975), is commonly referred to as responding to joint attention (RJA). RJA is defined as an infant response to a bid from a social partner intended to redirect the infant's attention to a location or object in the peripheral visual field. RJA consistently emerges around 9 months of age and is uniformly present in typically developing infants by 15 months (Carpenter, Nagell, & Tomasello, 1998). Over time, infants are increasingly able to respond to more subtle and less redundant bids for joint visual attention (Deák, Walden, Kaiser, & Lewis, 2008; Flom, Deák, Phill, & Pick, 2004). Developmental abnormalities in joint attention are thought to represent a core feature of autism, a neurodevelopmental disorder characterized in part by impaired social communication (Charman, 2003; Mundy, Sigman, Ungerer, & Sherman, 1986).
The methodological approaches available to social neuroscientists studying adults and nonhuman primates have fostered a rich literature characterizing the neural substrates of evaluating social information, gaze following, social attention, and sophisticated social-cognitive behaviors related to joint attention including mentalizing (for reviews see, Adolphs, 2003; Amodio & Frith, 2006; Frith & Frith, 2003; Klein, Shepherd, & Platt, 2009; Shepherd, 2010). Recently, behavioral assays designed specifically to elicit joint attention behaviors in neuroimaging contexts have yielded intriguing results. These data highlight a number of brain regions, namely the ventral-medial prefrontal cortex, right inferior frontal gyrus or fronto-insular cortex, right or bilateral posterior superior temporal sulcus, right or bilateral temporal-parietal junction, bilateral caudate, and the cuneus/precuneus (Bristow, Rees, & Frith, 2007; Ethofer, Gschwind, & Vuilleumier, 2011; Materna, Dicke, & Thier, 2008; Redcay, Dodell-Feder, Pearrow, Mavros, Kleiner, Gabrieli, &Saxe, 2010; Saito, Tanabe, Izuma, Hayashi, Morito, Komeda, Uchiyama, Kosaka, Okazawa, Fujibayashi, & Sadato, 2010; Schilbach, Wilms, Eickhoff, Romanzetti, Tepest, Bente, Shah, Fink, & Vogeley, 2009; Williams, Waiter, Perra, Perrett, & Whiten, 2005) that support a variety of social communication behaviors including gaze following. Additional studies using functional neuroimaging suggest that gaze following and monitoring information from the eye-region commonly elicits activation in the amygdala (Kawashima, Sugiura, Kato, Nakamura, Hatano, Ito, Fukuda, Kojima, & Nakamura, 1999; Okada, Sato, Kubota, Usui, Inoue, Murai, Hayashi, & Toichi, 2008; Sato, Kochiyama, Uono, & Yoshikawa, 2009). While informative, it remains unclear whether the subcortical and cortical neural circuitry underlying RJA behaviors in adults is the same as those functional circuits operating during infancy (Elman, Bates, Johnson, Karmiloff-Smith, Parisi, & Plunkett, 1996; Johnson, 2001).
Attempts to characterize the electrophysiological and hemodynamic correlates of RJA during infancy have also been instructive, yet inconsistent and implicate a broad number of brain regions. Some research has examined primary components of RJA such as gaze following and demonstrated that 1) early electrophysiological signals over frontal areas support the processing of gaze-to-object congruency in 9-month-olds (Senju, Johnson, & Csibra, 2006), and 2) hemodynamic activity in the left dorsal-medial prefrontal cortex, measured with near-infrared spectroscopy (NIRS), supports gaze-to-object congruency in 5-month-olds (Grossmann & Johnson, 2010). Additional research has revealed that the latency and amplitude of specific event-related potentials, especially over fronto-central electrodes, distinguishes object processing in an RJA context versus a non-joint attention context in 9-month-olds (Striano, Reid, & Hoehl, 2006). Another study explored the concurrent and predictive association of EEG coherence measured in 14 month-old infants, and joint attention behaviors measured at 14 months and 18 months (Mundy, Card, & Fox, 2000). Mundy and colleagues (2000) found that deactivation in left and right electrodes over the parietal cortex was significantly associated with RJA, measured concurrently at 14 months, and significantly predicted RJA at 18 months. Taken together, these studies represent the diversity of potential approaches for studying the neural correlates of RJA in infancy and suggest that a number of diverse cortical regions support RJA during infancy.
RJA behaviors are likely supported by computations performed by a circumscribed number of brain structures/regions. One approach capable of characterizing the structural organization of neural circuits underlying interregional processing is diffusion tensor imaging (DTI). While there are advantages and disadvantages to many of the imaging modalities discussed above (e.g., fMRI, EEG/ERP, and NIRS), two salient advantages of DTI bear mention: First, DTI can be employed from infancy to adulthood (Davis, Dennis, Buchler, White, Madden, & Cabeza, 2009; Dubois, Hertz-Pannier, Dehaene-Lambertz, Cointepas, & Le Bihan, 2006; Lebel & Beaulieu, 2011). Second, while NIRS and EEG/ERP are limited to cortical activation patterns, DTI is capable of characterizing the structural organization of subcortical white matter fiber pathways. Conceptual models of brain development along with empirical data suggest that subcortical structures and pathways contribute directly to cortical specialization across ontogeny, especially during the first year of life (Innocenti, 2007; Johnson, 2001; 2005).
There is accumulating evidence explicating the association between specific behavioral capacities and cognitive operations and individual differences in the microstructural organization of white matter fiber bundles (e.g., Klingberg, Hedehus, Temple, Salz, Gabrieli, Moseley, & Poldrack, 2000; Thiebaut de Schotten, Dell'Acqua, Forkel, Simmons, Vergani, Murphy, & Catani, 2011; Vestergaard, Madsen, Baare, Skimminge, Ejersbo, Ramsoy, Gerlach, Akeson, Paulson, & Jernigan, 2011). To our knowledge, no study has examined the association between white matter fiber pathways and joint attention, in either adults or infants. Furthermore, we are not aware of any research that has used DTI in infancy to longitudinally predict complex behavioral patterns or cognitive operations in typically developing infants.
We sought to characterize the predictive association between the structural organization of a priori hypothesized white matter fiber bundles measured at 6 months and individual differences in RJA at 9 months in order to understand the nature of neural `priors' necessary for the emergence of joint attention behaviors during infancy. The behavioral, cognitive, and neural antecedents of joint attention are largely unknown, despite conceptually rigorous theoretical accounts (Tomasello et al., 2005). The current longitudinal design rests on the assumption that structural variability in neural circuitry could function as one source of variance in later emerging complex behavioral patterns. Diffusion tensor imaging (DTI) was used to characterize the structural organization of white matter fiber tracts, as indexed by fractional anisotropy (FA), and measured at an age prior to the emergence of RJA. FA is a scalar index of directional water diffusion and is thought to represent the relative cumulative effects of myelin, axonal diameter or caliber, the density of axons, and/or the extent of membrane permeability on water diffusion in a given fiber bundle. We reasoned that making eye contact in response to a verbal elicitation, inferring intention from another individual, responding to biologically relevant directional cues (e.g., gaze shift, head turn and point), and shifting gaze and attention to an object in the peripheral visual field would be mediated by limbic/paralimbic (via the uncinate fasciculus) networks as well as separable but related ventral (via the inferior longitudinal fasciculus) and dorsal (via the fronto-occipital fasciculus) attention networks. As a control we measured the optic nerve as we hypothesized that RJA would engage representational operations above and beyond those involved in basic visual processing. In order to account for general brain development during a period of dramatic structural change (Knickmeyer, Gouttard, Kang, Evans, Wilber, Smith, Hamer, Lin, Gerig, & Gilmore, 2008), we controlled for total brain volume (TBV) in each analysis. Finally, to examine the specificity of an observed association, we examined whether FA in the putative tracts predicted receptive language at 9 months (Brooks & Meltzoff, 2005; Carpenter et al., 1998; Mundy, Block, Delgado, Pomares, Van Hecke, & Parlade, 2007).
RJA was measured at an age period hypothesized to maximize individual differences in competence and performance levels (Bates et al., 1979; Carpenter et al., 1998; Flom et al., 2004; Kristen et al., 2011; Scaife & Bruner, 1975) during naturalistic play-based social interaction. Individual differences in RJA performance were determined by varying the redundancy of the redirecting cue (e.g., gaze shift, head turn, redirecting vocalization, and pointing gesture). Evidence suggests that reliably responding to less redundant cues (e.g., gaze shift or gaze shift and head turn) indexes developmental advances in social communication (Butterworth & Jarrett, 1991; Deák, et al., 2008). This approach complements and extends previous attempts to characterize individual differences in joint attention (Mundy et al., 2007). We tested three primary hypotheses: 1) does our joint attention procedure elicit substantive individual differences in performance, such that RJA performance increases with age and intra-individual variability decreases with age? 2) Can we identify a time interval of maximal individual differences in RJA performance? 3) Do individual differences in white matter microstructure, assessed prior to the emergence of RJA, significantly predict individual differences in RJA performance in 9 month-olds?
Materials and Methods
Participants
Fifty-one typically developing infants participated in the joint attention assessment. Due to an administration error, data from one male infant (age = 55 weeks) were deemed invalid, yielding a final sample of 50 infants (26 males: age in weeks M(SD) = 46.2 (7.4), age range = 38 to 67 weeks). For one of the analyses, the sample was further classified into three groups based on age: 9 month-olds (n=23, age in weeks = 40.7 (1.6), age range = 38 to 43 weeks), 10 month-olds (n=12, age in weeks = 44.9 (1.0), age range = 44 to 47 weeks), and greater than 11 month-olds (n=15, age in weeks = 55.8 (5.9), age range = 48 to 67 weeks). Fourteen infants were recruited as part of a separate longitudinal brain imaging study, 12 (6 males) of whom contributed valid brain imaging data at 6 months (age in weeks = 28.2 (1.5), age range = 26 to 31 weeks) as well as language data at 9 months (age in weeks = 40.5 (1.2), age range = 39 to 42 weeks). All infants were recruited from the Chapel Hill, Raleigh, Durham area, born full-term (gestational age > 37 weeks; birth weight > 2500 grams), reared in homes where English was the primary language, and had no first or second degree relatives diagnosed with autism spectrum disorders. Parents provided written informed consent in accordance with the Internal Review Board at the University of North Carolina.
Behavioral Procedure
The joint attention procedure was adapted from previous research (Deák, Flom, & Pick, 2000; Deák et al., 2008; Flom et al., 2004; Presmanes, Walden, Stone, & Yoder, 2007). The primary goal of the procedure was to characterize a dimensional rating of responding to joint attention (RJA) that reflects individual differences in RJA performance. We reasoned that characterizing individual differences in RJA performance would be more suitable for brain-behavioral investigations than an ordinal rating of competence or an interval rating of proportion of correct responses.
The assessment was designed to elicit naturalistic play-based social interaction between the infant and the examiner. Approximately 10 novel objects/toys (including 2 posters hung on the walls) were strategically placed around a standard clinical assessment room. Each assessment began with a warm-up period (approximately 3– 5 minutes) in which the infant and examiner played with a novel object. The parent(s) was asked to sit quietly and respond naturally to elicitations from the infant. Less mobile infants sat in front of their parent on the floor. After the warm-up period, the experimenter proceeded through 4 series of joint attention presses. Each series consisted of 4 presses or prompt types that varied in cue redundancy and were hierarchically ordered from most sophisticated (least redundant) to least sophisticated (most redundant).
At the beginning of each press, the experimenter positioned themselves directly in front of the infant, separated by approximately 60–90 cm, and ensured that an object/poster was positioned in a location that would require a shift in gaze of approximately 90° from the infant's midline. The experimenter then proceeded with an eliciting verbalization of the infant's name. After making eye contact with the infant, the hierarchical series of presses then followed: 1) gaze shift and head turn, 2) gaze shift, head turn, and verbal cue (i.e., “Look at that!”), 3) gaze shift, head turn, and point, and 4) gaze shift, head turn, point, and verbal cue. If the infant responded to a given press by shifting gaze and head position in the direction of the object in the distal visual field (i.e., between 1 and 2 meters from the infant and approximately 90° from the infant's midline), the hierarchy stopped and the infant was allowed to briefly play with the item prior to the onset of the next series of presses. If the infant did not respond to a given press, each subsequent press in the hierarchy was attempted. The examiner attempted each subsequent press after a brief delay between 20 and 60 seconds. If the infant did not respond to any of the 4 prompt types, the next series was initiated after a short delay.
The response scores were scaled to reflect the sophistication by which an infant responded to bids/cues to share joint attention on an object in the distal visual field. In each of the 4 series, a child was given a score of 0–4 (e.g., no response to any of the 4 prompt types = 0; response to gaze shift, head turn, point, and verbal cue = 1; response to gaze shift, head turn, and point = 2; response to gaze shift, head turn, and verbal cue = 3; response to gaze shift and head turn = 4). If in response to the pointing cue on press number 3 (i.e., gaze shift, head turn, and point) the infant looked at the hand of the experimenter, a provisional score of .5 was given (see Amano, Kezuka, & Yamamoto, 2004) and the final press was attempted. If there was no response on the final press, .5 became the final score for that series. However, if the child responded in the direction of the object on the 4th press, a score of 1 replaced the .5. A score of .5 could also be given on the final press if the infant looked to the hand of the experimenter. The total raw scores could range between 0 and 16. Analyses were conducted on the average score (total raw score/total presses successfully administered), as some individuals contributed less than 4 complete trials.
The digital recordings of thirty-seven assessments were coded by two raters. We employed a random effects model (Proc Mixed in SAS 9.2) to derive an intraclass correlation coefficient (ICC) to determine the amount of variance attributable to 1) differences in performance between individuals or 2) differences in rater scores. The intercept parameter (τ00 or variance between individuals) = 19.3790 and the residual parameter (σ2 or variance between raters) = 1.9696. The ICC was derived in the following manner, τ00 / (τ + σ2), yielding an ICC of 0.9077. 91% of the variance in RJA scores can be attributed to variation between participants and approximately 9% of the variance is attributable to variance between raters. ICC's greater than 0.9 are considered excellent.
Language Assessment
Language subscales from the Mullen Scales of Early Learning (MSEL; Mullen, 1995) were administered to assess current language functioning. The Mullen Scales are standardized measures of cognitive and motor development for infants and preschool aged children from birth to 68 months. The receptive and expressive language subscales were administered at 9 months to all participants who had brain imaging data at 6 months (n=12). As an index of verbal developmental level, we derived the verbal developmental quotient [(average age equivalent scores for the receptive and expressive language domains / chronological age) × 100] for the final analysis.
Diffusion Weighted Image (DWI) Acquisition
Diffusion weighted images were acquired during natural sleep on a Siemens 3T TIM Trio equipped with a 12-channel headcoil using the following parameters: FOV = 190 mm, 75 transversal slices, slice thickness = 2 mm isotropic, 2×2×2mm3 voxel resolution, TR = 12800 ms, TE = 102 ms, b-values of 0 to 1000 sec/mm2, 25 gradient directions, and 4–5 minute scan time.
DWI Data Quality Control and Preprocessing
Scans were screened for clinical abnormalities by a neuroradiologist and excluded if they had significant abnormalities. Image data were then screened for motion and common artifacts using DTIprep software (www.nitrc.org/projects/dtiprep), and expert raters manually removed images with clear, residual artifacts. Datasets with less than 70% gradient images remaining after this procedure were excluded. Twelve out of fourteen individuals in the current study passed this QC procedure.
Computational Anatomy Mapping
Unbiased atlas building (Joshi, Davis, Jomier, & Gerig, 2004) was used to provide one-to-one mapping between template and individual image data, where the reference atlas is constructed from the population of imaging data as a centered image corrected for minimum deformation distances. The population template was derived from ~200 six-month-old infants (see Wolff, Gu, Gerig, Elison, Styner, Gouttard, Botteron, Dager, Dawson, Estes, Evans, Hazlett, Kostopoulos, McKinstry, Paterson, Schultz, Zwaigenbaum, & Piven, 2012). Registration proceeded in two steps (Goodlett, Fletcher, Gilmore, & Gerig, 2009). First, linear affine registration of DWI baseline images was applied to a structurally weighted T2 atlas using B-spline registration and normalized mutual information (Rueckert, Sonoda, Hayes, Hill, Leach, & Hawkes, 1999; Schnabel, Tanner, Castellano-Smith, Degenhard, Leach, Hose, Hill, & Hawkes, 2003). Second, a large deformation diffeomorphic metric mapping transformation was applied for unbiased, deformable atlas building (Joshi et al., 2004). The procedure related each individual dataset to the study-specific atlas template via a nonlinear, invertible transformation. Tensor maps were calculated from each dataset using weighted least squares estimation (Salvador, Pena, Menon, Carpenter, Pickard, & Bullmore, 2005), and transformed into the atlas space with tensor re-orientation by a finite strain approach (Alexander, Pierpaoli, Basser, & Gee, 2001). Scalar diffusion measures were generated from tensor image data for each subject. Diffusion tensor images were transformed and averaged using the Riemannian and log-Euclidean frameworks (Arsigney, Fillard, Pennec, & Ayache, 2006; Fletcher & Joshi, 2007), resulting in the final 3D average tensor atlas.
Segmentation and Parameterization of Fiber Tracts
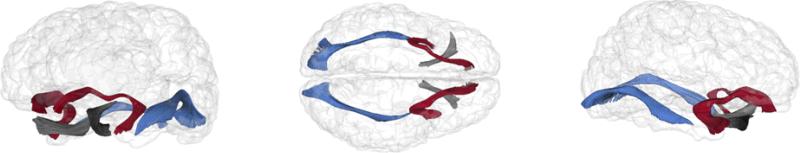
Seed label maps for the inferior longitudinal fasciculus (ILF), uncinate fasciculus (UF), and optic nerve were created based on methods described in existing white matter tractography atlases (Catani & Thiebaut de Schotten, 2008; Mori, Wakana, Nagae-Poetscher, & van Zilj, 2005) using 3D Slicer (www.slicer.org). Fiber tracts generated in 3D Slicer were processed for spurious or incomplete streamlines using software developed in-house (FiberViewer; www.ia.unc.edu/dev/). Fiber tracts were converted to ROIs and mapped to each coregistered individual dataset to generate FA values along each fiber bundle, from which mean FA values were calculated. Due to low signal related to maturational and imaging parameters, we were unable to extract the fronto-occipital fasciculus in this sample. See Figure 1 for representations of fiber tracts examined in the current study.
Figure 1.
Three-dimensional reconstructions of the white matter fiber tracts examined in the current study: red = uncinate fasciculi (UF), blue = inferior longitudinal fasciculi (ILF), grey = optic tract.
Analytic Strategy
First, we described the distribution of RJA scores. Intra-individual variability was characterized by the coefficient of variation (cv = σ/μ). We then employed two separate hierarchical multiple regression analyses (with RJA average score and cv as dependent variables) with the whole sample to test the null hypotheses that 1) RJA performance does not increase with age and that 2) intra-individual variability does not decrease with age, accounting for potential mediators such as sex of the child and the presence of siblings in the home. Next, we tested the hypothesis that 9–10 months is the time-interval of maximal individual differences in RJA performance and maximal intra-individual variability using two separate univariate ANOVAs (with RJA average score and cv as depedendent variables) and by creating 3 distinct age groups; 9 month-olds, 10 month-olds, and greater than 11 month-olds. Planned post-hoc comparisons were used to examine group differences in RJA performance and intra-individual variability. Finally, we examined a series of hierarchical multiple regression analyses in order to investigate the association between RJA performance and the six fiber tracts of interest (6 separate regressions), while controlling for age during the behavioral assessment and total brain volume. As an additional control, we examined the association between receptive language and the four primary fiber tracts (i.e., bilateral UF and bilateral ILF). The sample size and therefore the degrees of freedom constrained our ability to test these associations in a single model (e.g., multivariate multiple regression approach). Therefore, we employed an unweighted Bonferroni corrected alpha level (α/10 = p < 0.005) to control for multiple comparisons.
Results
Descriptive variables and RJA performance is summarized in Table 1. We successfully administered 4 series of RJA presses to 84% of the sample (42/50), 3 series of RJA presses to 12% of the sample (6/50), and 2 series of presses to 4% of the sample (2/50). Subsequent results did not change when the latter 2 participants were excluded from the analyses. With regard to competence, 10% of the sample (5/50) did not shift gaze in response to any cue or shifted gaze only to the hand during a pointing gesture. The age of these infants ranged from 38 to 43 weeks. 74% of the sample (37/50) responded to at least one of the least sophisticated or most redundant cue types (i.e., gaze shift, head turn, and pointing gesture that did or did not include a vocalization). 56% (28/50) responded at least once to one of the most sophisticated or least redundant cue types (i.e., gaze shift and head turn, may or may not include vocalization).
Table 1.
Participant characteristics and RJA performance
| Characteristic | Total n = 50 | 9 m n=23 | 10 m n=12 | > 11 m n=15 |
|---|---|---|---|---|
| RJA Chron. Age1 | 46.2 (7.4) | 40.7 (1.6) | 44.9 (1.0) | 55.8 (5.9) |
| Sex | ||||
| Male | 26 (52%) | 14 (61%) | 6 (50%) | 6 (40%) |
| Female | 24 (48%) | 9 (39%) | 6 (50%) | 9 (60%) |
| Siblings | ||||
| 0 | 21 (42%) | 5 (22%) | 8 (67%) | 8 (53%) |
| 1 or more | 29 (58%) | 18 (78%) | 4 (33%) | 7 (47%) |
| RJA Performance 2 | ||||
| RJA_raw total score | 7.1 (4.08) | 5.5 (3.41) | 5.5 (2.76) | 10.9 (3.51) |
| RJA_average score | 1.9 (1.11) | 1.4 (0.86) | 1.4 (0.73) | 3.0 (0.93) |
| RJA_CoV3 | 0.48 (0.34) | 0.53 (0.30) | 0.68 (0.36) | 0.23 (0.25) |
Chronological age during the joint attention assessment in weeks (SD).
Values represent mean and standard deviation.
CoV represents the coefficient of variation and represents intra-individual variability.
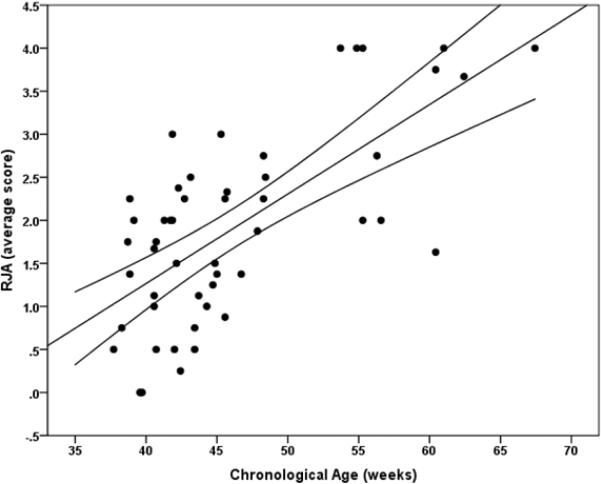
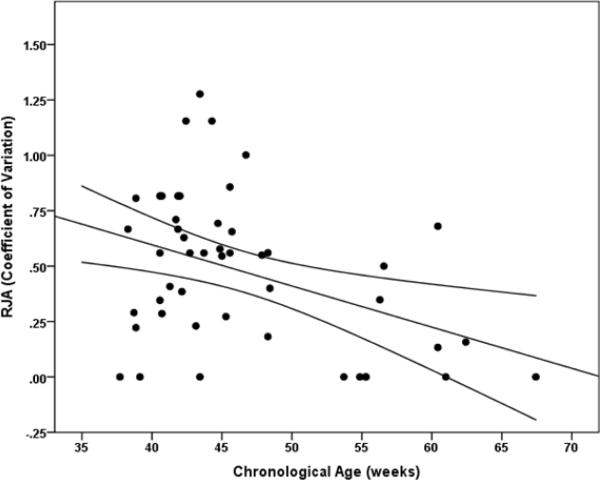
Scores representing average performance level (mean (SD) = 1.91 (1.11); range, 0–4) were not normally distributed according to a Shapiro-Wilk test of normality, p = 0.05, but represent a continuous range of individual differences. A hierarchical multiple regression analysis revealed that age significantly predicted RJA performance β = 0.68, t(46) = 6.17, p < 0.001. Age accounted for a unique portion of variance in RJA above and beyond the effect of sex and siblings in the home, ΔR2 = 0.43, F(1,46) = 38.1, p < 0.001 (see Figure 2a). Neither sex (p = .80) nor the number of siblings (p = .67) in the home significantly predicted RJA. Age also significantly predicted the coefficient of variation related to RJA performance β = −0.41, t(44) = −2.94, p = 0.005, and accounted for a unique portion of variance in the coefficient of variation above and beyond the effect of sex and siblings in the home, ΔR2 = 0.16, F(1,44) = 8.64, p = 0.005 (see Figure 2b). Neither sex (p = 0.33) nor the number of siblings in the home (p = 0.23) significantly predicted the coefficient of variation.
Figure 2a.
Scatterplot representing the association between RJA performance and age in weeks with 95% confidence intervals.
Figure 2b.
Scatterplot representing the association between intra-individual variability in RJA performance, indexed by the coefficient of variation, and age in weeks with 95% confidence intervals.
Next we examined the specificity of the age effects observed above by deriving three groups of infants based on their chronological age; 9 month-olds (n=23, age range from 38 to 43 weeks), 10 month-olds (n=12, age range from 43 to 47), and a group that included infants older than 11 months (n=15, age range from 48 to 67 weeks). See Table 1 for more details. The analyses revealed a main effect of group on RJA performance, F(2,45) = 15.89, p < 0.001, partial η2 = 0.41, and intra-individual variability in RJA performance, F(2,43) = 7.27, p = 0.002, partial η2 = 0.25. Planned post-hoc comparisons revealed that the 9 and 10 month-olds yielded statistically equivalent values in RJA performance (p = 0.76) and intra-individual variability (p = 0.29). RJA performance in the 11 to 14 month-olds was significantly more advanced than the 9 month-olds (p < 0.001) and the 10 month-olds (p < 0.001). Additionally, intra-individual variation decreased significantly in the 11 to 14 month-olds when compared with the 9 month-olds (p = 0.007) and the 10 month-olds (p = 0.001).
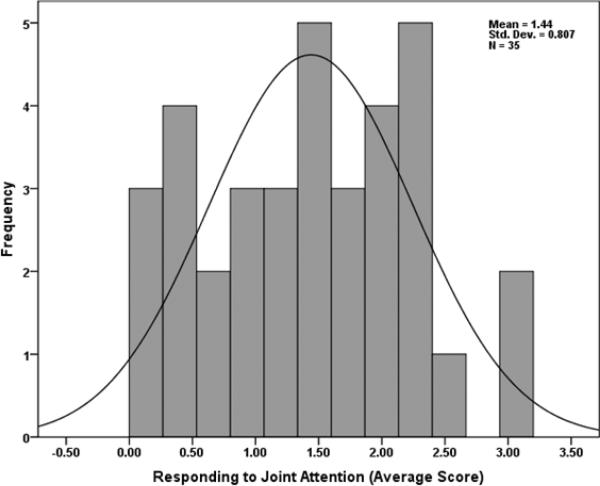
Considering only the 9 and 10 month-olds (n=35, average age 42 (2.4) weeks), age was no longer significantly associated with RJA performance, β = 0.26, t(31) = 1.32, p = 0.196, or intra-individual variability in performance, β = 0.29, t(29) = 1.50, p = 0.143. The distribution of scores across this age range was normal according to a Shapiro-Wilk test of normality (p = 0.55) and according to visual inspection of the data (see Figure 3). Characterizing normally distributed, individual differences in RJA during this time interval allowed us to test the final question: do individual differences in white matter microstructure significantly predict individual differences in RJA performance? Of note, there were no statistically significant difference in RJA performance among infants who did or did not receive a brain scan at 6 months, t(33) = 1.23, p = 0.23.
Figure 3.
Histogram with normal curve representing individual differences in RJA performance among 9 and 10 month-olds (n=35).
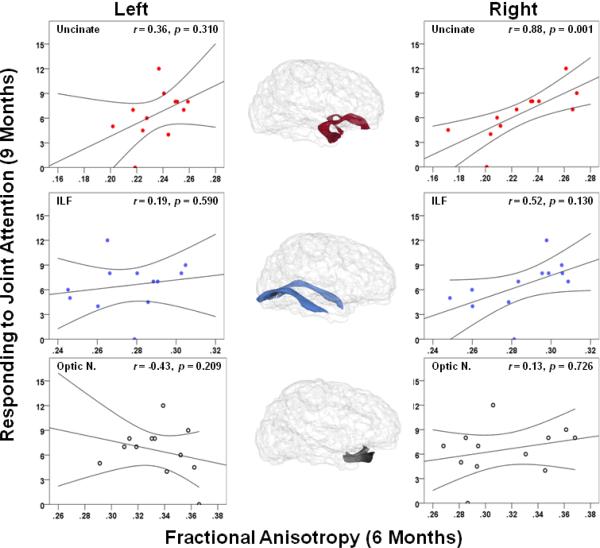
Of the 9–10 month-olds (n=35), a subset of 12 infants contributed brain imaging data at 6 months (average age in weeks = 28.2 (1.5), range = 26 to 31 weeks). Statistically significant associations were determined by employing an unweighted Bonferroni corrected α level (pB = 0.005) to control for multiple comparisons. Fractional anisotropy (FA) in the right UF at 6 months of age significantly predicted RJA at 9 months of age, β = 0.83, t(8) = 5.14, p = 0.001, and accounted for a unique portion of variance above and beyond the effects of total brain volume and age at the behavioral assessment, ΔR2 = 0.65, F(1,8) = 26.46, p = 0.001. Neither the left UF (p = 0.31) nor the left ILF (p = 0.59), right ILF (p = 0.13), left optic nerve (p = 0.21), or the right optic nerve (p = 0.73) significantly predicted RJA (see Figure 4). RJA showed a moderate but non-significant association with concurrent receptive language ability r(12) = 0.47, p = 0.121. However, the right UF did not significantly predict receptive language at 9 months of age (p = 0.40), nor did the left UF (p = 0.10), the left ILF (p = .99), or the right ILF (p = 0.79). Considering the putative association between the right UF and RJA, we conducted one final hierarchical regression that included total brain volume and verbal developmental quotient as predictors at step 1, and FA in the right UF as a predictor at step 2. The results revealed that FA in the right UF significantly predicts RJA at 9 months, β = 0.77, t(8) = 3.89, p = 0.005, and accounts for a significant portion of unique variance in RJA above and beyond the effects of verbal developmental level and totol brain volume, ΔR2 = 0.56, F(1,8) = 15.12, p = 0.005.
Figure 4.
Scatterplots representing the association between fractional anisotropy (along the abscissa) in the bilateral uncinate fasciculi, inferior longitudinal fasciculi, and optic nerves at 6 months and responding to joint attention (RJA) at 9 months (along the ordinate) with 95% confidence intervals (r = partial Pearson correlation coefficient, controlling for total brain volume at 6 months and chronological age during the behavioral assessment).
Discussion
The results suggest that the current behavioral procedure elicited substantive individual differences in RJA performance, the 9 to 10 month period may be a time interval of maximal individual differences in RJA, and that fractional anisotropy (FA) in the right uncinate fasciculus (UF) at 6 months strongly predicts RJA at 9 months of age. Individuals with increased microstrutural organization in the right UF at 6 months performed at higher levels during the joint attention procedure 3 months later (i.e., responded to more bids and to more subtle cues). The right ILF was not significantly associated with RJA, yet the moderate correlation coefficient raises the possibility that a larger sample size may reveal a significant association between this tract and RJA. In support of the specificity of the association between the right UF and RJA, the right UF was not associated with receptive language, a skill domain related to joint attention likely mediated by additional neural circuits. The results also suggest lateralization in social information processing early in development (see also Highley, Walker, Esiri, Crow, & Harrison, 2002; Iturria-Medina, Fernandez, Morris, Canles-Rodriguez, Haroon, Penton, Augath, Garcia, Logothetis, Parker, & Melie-Garcia, 2011), such that the microstructural organization of right lateralized neural circuitry anticipates the emergence of sophisticated social information processing capacities (Tomasello et al., 2005).
Fibers in the UF connect the inferior medial temporal lobe (including the amygdala), the rostral temporal pole, the fronto-insular cortex, and the orbital and ventral-medial prefrontal cortex. This tract likely transmits signals related to evaluating the biological relevance or the intentional nature of salient social information (Amodio & Frith, 2006; Schmahmann, Pandya, Wang, Dai, D'Arceuil, de Crespigny, & Wedeen, 2007; Uddin, Supekar, Ryali, & Menon, 2011). The uncinate fasciculus has been implicated in a number of psychiatric and neurodevelopmental disorders including autism (Wolff et al., 2012), major depression (Zhang, Leow, Ajilore, Lamar, Yang, Joseph, Medina, Zhan, & Kumar, 2012), social anxiety disorder (Baur, Bruhl, Herwig, Eberle, Rufer, Delsignore, Jancke, & Hanggi, in press), and psychopathy (Craig, Catani, Deeley, Latham, Daly, Kanaan, Picchioni, McGuire, Fahy, & Murphy, 2009; Motzkin, Newman, Kiehl, & Koenigs, 2011). We know that responding to joint attention is but one behavior that likely contributes directly to individual differences in social communication and social information processing. Across development, information flow through the uncinate, between the ventral-medial prefrontal cortex, the fronto-insular cortex, the amygdala, and the rostral pole of the temporal lobe, likely supports sophisticated but otherwise general social information processing. Individual differences in joint attention performance and/or the microstructural development of the right uncinate fasciculus, when considered in a broader context of ontogeny, may be patho-plastic mechanisms that support typical developmental processes under certain conditions but precipitate a variety of atypical outcomes under alternative conditions (Richters, 1997; von Bertalanffy, 1968). Longitudinal studies that track intra-individual change over time are required to refute the hypothesis that individual differences in the microstructural organization of white matter fiber pathways or foundational social-cognitive behaviors observed in infancy predict abnormal patterns of behavior later in development.
This is precisely the strategy adopted by many working on neurodegenerative disorders. There is emerging evidence that biomarkers, including brain imaging markers, represent prodromal characteristics that anticipate Alzheimer's disease, Huntington's disease, and Parkinson's disease (Aylward, Liu, Nopoulos, Ross, Pierson, Mills, Long, & Paulsen, 2012; Lo, Hubbard, Shaw, Trojanowski, Petersen, Aisen, Weiner, & Jagust, 2011; Rodriguez-Oroz, Jahanshahi, Krack, Litvan, Macias, Bezard, & Obeso, 2009; Tabrizi, Scahill, Durr, Roos, Leavitt, Jones, Landwehrmeyer, Fox, Johnson, Hicks, Kennard, Craufurd, Frost, Langbehn, Reilmann, & Stout, 2011). Prevention science and/or interventions designed to attenuate the progression of symptoms holds much promise for these conditions (Rosenberg, 2011; Schapira & Tolosa et al., 2010). In the current study, we present preliminary evidence that a brain imaging marker collected at 6 months of age predicts the performance level of an important social-cognitive behavior at 9 months of age.
These findings have direct implications for the field of autism, as there is accumulating evidence that the constellation of behaviors that defines this neurodevelopmental disorder emerge during the latter half of the first year of life (Ozonoff, Iosif, Baguio, Cook, Hill, Hutman, Rogers, Rozga, Sangha, Sigman, Steinfeld, & Young, 2010; Zwaigenbaum, Bryson, Rogers, Roberts, Brian, & Szatmari, 2005). Ongoing research is attempting to characterize prodromal features of autism in the first year of life that precede the consolidation of clinical/diagnostic profile. Though developmental abnormalities in joint attention and other social communication behaviors have long been considered central to developmental models of autism (Charman, 2003; Mosconi, Cody-Hazlett, Poe, Gerig, Gimpel-Smith, & Piven, 2009; Mundy et al., 1986), no work to date has quantified performance level in RJA at a time of maximal individual differences, namely the age interval of 9–10 months.
The current study also highlights a novel design strategy for determining the structural neural circuitry necessary for performance of a complex behavior during infancy. Multiple lines of evidence indicate that triadic RJA emerges around 9–10 months of age (Carpenter et al., 1998; Corkum & Moore, 1998; Morales, Mundy, Delgado, Yale, Messinger, Neal, & Schwartz, 2000; Kristen et al., 2011). Collecting brain imaging data prior to the emergence of a complex behavioral pattern allowed us to make inferences about the necessary role of the uncinate fasciculus in the emergence of RJA. The potential for a causal association is supported by the fact that the microstructure of the UF was not related to language levels at 9 months. However, this causal association would have been stronger had we demonstrated an association/dissociation pattern similar to those described in the neuropsychological lesion literature (Bechara, Tranel, Damasio, Adolphs, Rockland, & Damasio, 1995). The findings would also be strengthened by an increased sample size, a noted limitation of the current study. A larger sample should increase the power to determine whether the right ILF significantly predicts RJA performance, and perhaps whether microstructural organization of the left UF predicts receptive language ability at 9 months of age.
In conclusion, the amygdala, fronto-insular prefrontal cortex, ventral-medial prefrontal cortex, and temporal poles are consistently implicated in social information processing (Adolphs, 2003; Amodio & Frith, 2006) and this report suggests that these regions play a critical role in the development of sophisticated social communication behaviors during the first year of life. More specifically, the mircostructural organization of white matter connecting these regions appears to account for a large portion of variance in later emerging joint attention behaviors. These results have promising implications for future examinations of infants at risk for developing autism. Abnormal joint attention has been hypothesized to function as the behavioral pivot that initiates a cascade of events leading to the constellation of behaviors characteristic of this neurodevelopmental disorder (Charman, 2003). Neuroimaging data acquired in infancy that predict individual differences in later emerging behavioral patterns have the potential to alter the landscape of neurodevelopmental disorders and childhood disorders more broadly as targeted intervention to prevent symptom expression becomes a possibility (Schapira & Tolosa, 2010).
Acknowledgments
This research was supported by grants awarded to J.P. from NIH/NICHD (Autism Center of Excellence R01 #HD055741; IDDRC, P30, #HD03110), and the Foundation of Hope. J.J.W. and D.C.H. were supported by an NIH training grant (T32-HD40127) and J.T.E. was supported by an NRSA award (5-T32-HD007376) from NICHD. The authors declare no competing financial interests. We thank the families that participated in this work and Whitney Weigold for her contribution to data collection.
References
- Adolphs R. Cognitive neuroscience of human social behavior. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Alexander D, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Transactions on Medical Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Amano S, Kezuka E, Yamamoto A. Infant shifting attention from an adult's face to an adult's hand: a precursor of joint attention. Infant Behavior and Development. 2004;27:64–80. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arsigny V, Fillard P, Pennec X, Ayache N. Log-euclidean metrics for fast and simple calculus on diffusion tensors. Magnetic Resonance in Mediciney. 2006;56:411–421. doi: 10.1002/mrm.20965. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Liu D, Nopoulos PC, Ross CA, Pierson RK, Mills JA, Long JD, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group Striatal volume contributes to the prediction of onset of Huntington disease in incident cases. Biological Psychiatry. 2012;71:822–828. doi: 10.1016/j.biopsych.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The emergence of symbols: Cognition and communication in infancy. Academic Press; New York: 1979. [Google Scholar]
- Baur V, Bruhl AB, Herwig U, Eberle T, Rufer M, Delsignore A, Jancke L, Hanggi J. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Human Brain Mapping. doi: 10.1002/hbm.21447. in press. doi: 10.1002/hbm.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bristow D, Rees G, Frith CD. Social interaction modifies neural response to gaze shifts. Social Cognitive and Affective Neuroscience. 2007;2:52–61. doi: 10.1093/scan/nsl036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs for the Society for Research in Child Development. 1998;63:1–143. [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society, London, B. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum V, Moore C. The origins of joint visual attention in infants. Developmental Psychology. 1998;34:28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, McGuire PK, Fahy T, Murphy DG. Altered connections on the road to psychopathy. Molecular Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler N, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractogrphy. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák GO, Flom RA, Pick AD. Effects of gesture and target on 12- and 18-month-olds' joint visual attention to objects in front and behind them. Developmental Psychology. 2000;36:511–523. [PubMed] [Google Scholar]
- Deák GO, Walden TA, Kaiser MY, Lewis A. Driven from distraction: how infants respond to parents' attempts to elicit and re-direct their attention. Infant Behavior and Development. 2008;31:34–50. doi: 10.1016/j.infbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants' cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Elman JL, Bates EA, Johnson MH, Karmiloff-Smith A, Parisi D, Plunkett K. Rethinking innateness: A connectionist perspective on development. MIT Press; Cambridge, MA: 1996. [Google Scholar]
- Ethofer T, Gschwind M, Vuilleumier P. Processing social aspects of human gaze: a combined fMRI-DTI study. Neuroimage. 2011;55:411–419. doi: 10.1016/j.neuroimage.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Joshi S. Riemannian geometry for statistical analysis of diffusion tensor data. Signal Processing. 2007;87:250–262. [Google Scholar]
- Flom R, Deák GO, Phill CG, Pick AD. Nine-month-olds' shared visual attention as a function of gesture and object location. Infant Behavior & Development. 2004;27:181–194. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London, B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CB, Fletcher PT, Gilmore JH, Gerig G. Group analysis of DTI fiber tract statistics with application to neurodevelopment. Neuroimage. 2009;45:S133–S142. doi: 10.1016/j.neuroimage.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. Selective prefrontal cortex responses to joint attention in early infancy. Biology Letters. 2010;6:540–543. doi: 10.1098/rsbl.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortum study of normal subjects and patients with schizophrenia. Cerebral Cortex. 2002;12:1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Subcortical regulation of cortical development: some effects of early, selective deprivations. Progress in Brain Research. 2007;164:23–37. doi: 10.1016/S0079-6123(07)64002-3. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Fernandez AP, Morris DM, Canales-Rodriguez EJ, Haroon HA, Penton LG, Augath M, Garcia LG, Logothetis N, Parker GJM, Melie-Garcia L. Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cerebral Cortex. 2011;21:56–67. doi: 10.1093/cercor/bhq058. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23:S151–S160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, Fukuda H, Kojima S, Nakamura K. The human amygdala plays an important role in gaze monitoring. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Current Biology. 2009;19:R958–R962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristen S, Sodian B, Thoermer C, Perst H. Infants' joint attention skills predict toddlers' emerging mental state language. Developmental Psychology. 2011;47:1207–1219. doi: 10.1037/a0024808. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, Weiner MW, Jagust WJ, the Alzheimer's Disease Neuroimaging Initiative Longitudinal change of biomarkers in cognitive decline. Archives of Neurology. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materna S, Dicke PW, Thier P. Dissociable roles for the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Delgado CEF, Yale M, Messinger D, Neal R, Schwartz HK. Responding to joint attention across the 6- through 24-month age period and early language acquisition. Journal of Applied Developmental Psychology. 2000;21:283–298. [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zilj PCM. MRI atlas of human white matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Archives of General Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. American Guidance Services, Inc.; Circle Pines: 1995. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contributions of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Adamson LB, Bakeman R. Toddlers' joint engagement experience facilitates preschoolers' acquisition of theory of mind. Developmental Science. 2008;11:847–852. doi: 10.1111/j.1467-7687.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sato W, Kubota Y, Usui K, Inoue Y, Murai T, Hayashi T, Toichi M. Involvement of medial temporal structures in reflexive attentional shift by gaze. Social Cognitive and Affection Neuroscience. 2008;3:80–88. doi: 10.1093/scan/nsm027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Redcay E, Dodell-Feder D, Pearrow MJ, Mavros PL, Kleiner M, Gabrieli JDE, Saxe R. Live face-to-face interaction during fMRI: A new tool for social cognitive neuroscience. Neuroimage. 2010;50:1639–1647. doi: 10.1016/j.neuroimage.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters JE. The Hubble hypothesis and the developmentalist's dilemma. Development and Psychopathology. 1997;9:193–229. doi: 10.1017/s0954579497002022. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurology. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg RN. Treat Alzheimer disease before it is symptomatic. Archives of Neurology. 2011;68:1237–1238. doi: 10.1001/archneurol.2011.135. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Saito DN, Tanabe HC, Izuma K, Hayashi MJ, Morito Y, Komeda H, Uchiyama H, Kosaka H, Okazawa H, Fujibayashi Y, Sadato N. “Stay tuned”: Inter-individual neural synchronization during mutual gaze and joint attention. Frontiers in Integrative Neuroscience. 2010;4 doi: 10.3389/fnint.2010.00127. doi: 10.3389/fnint.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Commonalities in the neural mechanisms underlying automatic attentional shifts by gaze, gestures, and symbols. Neuroimage. 2009;45:984–992. doi: 10.1016/j.neuroimage.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Salvador R, Pena A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human Brain Mapping. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife M, Bruner JS. The capacity for joint visual attention in the infant. Nature. 1975;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Tolosa E. Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nature Reviews Neurology. 2010;6:309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience. 2009;22:2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil H, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schnabel JA, Tanner C, Castellano-Smith AD, Degenhard A, Leach MO, Hose DR, Hill DL, Hawkes DJ. Validation of nonrigid image registration using finite-element methods: application to breast MR images. IEEE Transactions on Medical Imaging. 2003;22:238–247. doi: 10.1109/TMI.2002.808367. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH, Csibra G. The development and neural basis of referential gaze perception. Social Neuroscience. 2006;1:220–234. doi: 10.1080/17470910600989797. [DOI] [PubMed] [Google Scholar]
- Shepherd SV. Following gaze: gaze-following behavior as a window into social cognition. Frontiers in Integrative Neuroscience. 2010;4 doi: 10.3389/fnint.2010.00005. doi: 10.3389/fnint.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano T, Reid VM, Hoehl S. Neural mechanisms of joint attention in infancy. European Journal of Neuroscience. 2006;23:2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill R, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, Kennard C, Craufurd D, Frost C, Langbehn DR, Reilmann R, Stout JC, TRACK-HD Investigators Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12 month longitudinal analysis. Lancet Neurology. 2011;10:31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM, Catani M. A lateralized brain network for visuospatial attention. Nature Neuroscience. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behavioral and Brain Sciences. 2005;28:675–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M, Madsen KS, Baare WFC, Skimminge A, Ejersbo LR, Ramsoy TZ, Gerlach C, Akeson P, Paulson OB, Jernigan TL. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. Journal of Cognitive Neuroscience. 2011;23:2135–2146. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- von Bertalanffy L. General system theory: Foundations, development, applications. George Braziller, Inc.; New York: 1968. [Google Scholar]
- Williams JHG, Waiter GD, Perra O, Perrett DI, Whiten A. An fMRI study of joint attention experience. Neuroimage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans A, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, the IBIS Network Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J, Medina J, Zhan L, Kumar A. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]