Abstract
Background
Methadone plasma concentrations are decreased by nelfinavir. Methadone clearance and the drug interactions have been attributed to CYP3A4, but actual mechanisms of methadone clearance and the nelfinavir interaction are unknown. We assessed nelfinavir effects on methadone pharmacokinetics and pharmacodynamics, intestinal and hepatic CYP3A4/5 activity, and intestinal P-glycoprotein transport activity. CYP3A4/5 and transporters were assessed using alfentanil and fexofenadine, respectively.
Methods
Twelve healthy HIV-negative volunteers underwent a sequential crossover. On three consecutive days they received oral alfentanil plus fexofenadine, intravenous alfentanil, and intravenous plus oral methadone. This was repeated after nelfinavir. Plasma and urine analytes were measured by mass spectrometry. Opioid effects were measured by pupil diameter change (miosis).
Results
Nelfinavir decreased intravenous and oral methadone plasma concentrations 40-50%. Systemic clearance, hepatic clearance, and hepatic extraction all increased 1.6- and 2-fold, respectively, for R- and S-methadone; apparent oral clearance increased 1.7- and 1.9-fold. Nelfinavir stereoselectively increased (S>R) methadone metabolism and metabolite formation clearance, and methadone renal clearance. Methadone bioavailability and P-glycoprotein activity were minimally affected. Nelfinavir decreased alfentanil systemic and apparent oral clearances 50% and 76%, respectively. Nelfinavir appeared to shift the methadone plasma concentration-effect (miosis) curve leftward and upward.
Conclusions
Nelfinavir induced methadone clearance by increasing renal clearance, and more so by stereoselectively increasing hepatic metabolism, extraction and clearance. Induction occurred despite 50% inhibition of hepatic CYP3A4/5 activity and more than 75% inhibition of first-pass CYP3A4/5 activity, suggesting little or no role for CYP3A in clinical methadone disposition. Nelfinavir may alter methadone pharmacodynamics, increasing clinical effects.
Keywords: methadone, nelfinavir, CYP3A, CYP2B6, HIV, drug interactions
1. Introduction
Methadone is a cornerstone in the therapy of opiate and opioid addiction. It prevents withdrawal and illicit drug use, and is a vital public health strategy for HIV/AIDS risk reduction (Connock et al., 2007). Approximately 160,000 Americans are currently in methadone maintenance (Vlahov et al., 2007). Methadone is also widely used in pain treatment. Methadone use is confounded, however, by considerable and (currently) unpredictable inter- and intra-individual variability in methadone pharmacokinetics and pharmacodynamics, with the consequent risk of drug accumulation causing untoward side effects, or subtherapeutic plasma concentrations causing withdrawal (Kreek, 1996; Mitchell et al., 2006). One-third of well-maintained methadone patients regularly experience withdrawal symptoms for reasons not fully known (Dyer et al., 1999), and toxicity may occur at seemingly therapeutic plasma concentrations (Chugh et al., 2008).
Variability in methadone clearance, susceptibility to drug interactions, and a long elimination half-life can be major impediments to optimal methadone use (Ferrari et al., 2004; Foster et al., 2000). Despite considerable research, the causes of variability and the mechanism of drug interactions remain poorly understood. Methadone is mainly cleared via hepatic metabolism by cytochrome P450 (CYP) to the inactive metabolite 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), with some urinary excretion of unchanged drug. For over a decade, and based on extrapolation of in vitro drug metabolism studies, clinical methadone metabolism and clearance have been attributed to CYP3A4, and numerous dosing guidelines and the methadone label* warn about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly (Bruce et al., 2006; Coller et al., 2006; Crettol et al., 2006; Eap et al., 2002; Ferrari et al., 2004; Foster et al., 2000; McCance-Katz, 2005; Shinderman et al., 2003; Wang and DeVane, 2003). Methadone is also a substrate for the efflux transporter P-glycoprotein (P-gp) in vitro and in the intestine and brain in animals in vivo, where P-gp influences methadone absorption, brain access, pharmacodynamics, and analgesia (Bauer et al., 2006; Thompson et al., 2000). In humans, the role of P-gp in determining methadone intestinal absorption and bioavailability, and brain penetration, pharmacodynamics, and clinical effects, is poorly understood.
Due to the intertwined problems of opioid dependence and HIV/AIDS, concomitant treatment with methadone and antiretrovirals is increasingly frequent. Methadone-antiretroviral drug interactions are common, and are typically attributed to CYP3A4, although the actual mechanism(s) remain largely unknown (Bruce et al., 2006; Maas et al., 2006; Robertson et al., 2007). The HIV protease inhibitor nelfinavir decreases plasma concentrations of both S- and R-methadone (the active enantiomer) in half, yet there are generally no withdrawal symptoms or need for dose adjustment in patients on daily methadone (Hsyu et al., 2006; McCance-Katz et al., 2004), with some exception (Brown et al., 2006). Nelfinavir is a well-known inhibitor of CYP3A activity, yet the apparent paradox of nelfinavir induction of methadone elimination, despite CYP3A inhibition, has never been addressed. The mechanism of alteration in methadone plasma concentrations is unknown. Moreover, whether such methadone interactions occur secondary to modulation of hepatic or intestinal CYP, or influx/efflux transporters activity, is also unknown.
Methadone drug interaction studies provide therapeutically applicable information relevant to the specific drug combination, but may also yield broadly informative mechanistic insights. Therefore, the purpose of this clinical investigation was to determine: 1) the mechanism(s) of nelfinavir alterations in methadone disposition and clinical effect; 2) the role of CYP3A and/or P-gp-mediated methadone bioavailability, first-pass metabolism, and systemic clearance in methadone clearance and its alteration by nelfinavir; 3) the influence of nelfinavir on methadone pharmacodynamics, 4) the ability of a clinical CYP3A probe to rapidly and noninvasively detect drug interactions and predict methadone disposition, and 5) the effects of nelfinavir on hepatic CYP3A, first-pass CYP3A, and intestinal P-gp activities. A comprehensive crossover investigation was conducted in healthy volunteers. Hepatic and first-pass CYP3A activities were evaluated using intravenous and oral alfentanil (ALF) clearance (Kharasch et al., 2004b; Kharasch et al., 2007). ALF is metabolized similarly by CYP3A4 and CYP3A5, while CYP3A7 has significantly less activity (Klees et al., 2005), and CYP3A5 polymorphisms have no effect on intravenous or oral ALF clearances (Kharasch et al., 2007), hence we consider ALF to be a nonselective CYP3A4/5 (henceforth referred to as CYP3A) probe. Pupil diameter change (miosis) was used as a surrogate for ALF plasma concentrations to noninvasively estimate ALF clearance, and hence CYP3A activity. The P-gp substrate fexofenadine was used as the in vivo P-gp probe (Cvetkovic et al., 1999). Intravenous and oral (deuterium-labeled) methadone were simultaneously administered to concurrently assess IV and oral drug kinetics, hepatic extraction and bioavailability, and, by avoiding a crossover design (for different routes of administration on different days) thereby diminish interday variability and double the protocol efficiency (Kharasch et al., 2004a). Miosis was used to assess methadone clinical effects and pharmacodynamics.
2. Methods
2.1 Clinical Protocol
The investigation was approved by the University of Washington Institutional Review Board and subjects provided written informed consent. Eligibility criteria were 1) normal healthy volunteers 18-40 yr, 2) within 25% of ideal body weight (body mass index <30). Exclusion criteria were 1) major medical problems, 2) history of hepatic or renal disease, 3) family history of type 2 diabetes, 4) use of medications or nonprescription preparations known to alter CYP3A activity, 5) a known history of addiction to drugs or alcohol, or 6) access to and routine handling of addicting drugs in the regular course of employment. Females taking hormonal contraceptives were excluded. Both smokers and nonsmokers were enrolled. Subjects underwent a screening visit at which fasting blood glucose concentration and HIV serologic status were determined. The protocol was conducted in 2003 and HIV antibody testing was required for this study by the Institutional Review Board. Subjects were excluded if their glucose exceeded 110 mg/dl (because protease inhibitors can cause glucose intolerance) or they were HIV seropositive (because monotherapy can cause HIV resistance). The final study population was twelve healthy subjects (six men, six women; 23 ± 5 yr, range 19-34; 70 ± 12 kg, range 57-93).
The protocol was a 2-period sequential outpatient crossover (control period first, for logistical considerations) with each subject as their own control. Subjects were instructed to consume no food or beverages that contain grapefruit, apples or oranges for 7d before any study day, no alcohol or caffeine for 1d before each study session and on the study day, and no food or water after midnight before each study day. On each study day, a catheter was placed in an arm vein for blood sampling and (if needed) a second catheter placed for drug administration. Subjects (supine) were monitored with a pulse oximeter and automated blood pressure cuff, and received supplemental oxygen for saturations less than 94%.
First-pass CYP3A activity and intestinal P-gp (and other transporters) activity were evaluated on day 1 using oral ALF and fexofenadine as in vivo probes (Kharasch et al., 2004b, 2005; Kharasch et al., 2007). Subjects received ondansetron (4 mg IV) for antinausea prophylaxis followed 30 min later by 43 μg/kg oral ALF with 100 cc water. Fexofenadine (60 mg) was administered with 100 cc water 1 hr after ALF. Subjects received a standard breakfast and lunch 3 and 6 hr, respectively, after ALF. Venous blood was sampled for 48 hr after ALF dosing, and plasma stored at -20°C for later analysis. Coincident with blood sampling, dark-adapted pupil diameter was measured using a Pupilscan Model 12A infrared pupillometer with 0.1 mm resolution (Keeler Instruments, Broomall, PA) (Kharasch et al., 2004b; Kharasch et al., 2007). Each recorded value was the mean of triplicate measurements, which typically agreed to within 0.1-0.3 mm. Ondansetron has no effect on alfentanil or fexofenadine disposition or pupil diameter (Kharasch et al., 2005),
Hepatic CYP3A activity was evaluated on day 2 using intravenous ALF an in vivo probe (Kharasch et al., 2004b; Kharasch et al., 2007). Subjects received ondansetron, followed 30 min later by 15 μg/kg ALF bolus. Subject received a standard breakfast 4 hr after ALF, and free access to food and water thereafter. Venous blood was sampled for 24 hr after ALF dosing, and dark-adapted pupil diameter was measured coincident with blood sampling.
Methadone metabolism and clearance were assessed on day 3 (with follow-up on days 4-7) by simultaneously administering IV and oral methadone (Kharasch et al., 2004a). Subjects received IV ondansetron followed 30 min later by simultaneously administered oral deuterated racemic (d5)-methadone HCl (11.0 mg, equivalent to 9.86 mg free base) with 100 ml water, and IV racemic unlabelled (d0)-methadone HCl (6.0 mg, equivalent to 5.4 mg free base, Roxane Laboratories, Columbus, OH). Deuterated methadone hydrochloride was synthesized and used under Investigational New Drug approval (Kharasch et al., 2004a). Venous blood samples were obtained for 96 hr after methadone, plasma separated and stored at -20°C for later analysis, and dark-adapted pupil diameter was measured coincident with blood sampling. Subjects were fed a standard breakfast 4 hr after methadone and had free access to food and water thereafter. Continuous urine samples were collected at 24, 48, 72, and 96 hr. Nausea and/or vomiting were treated with ondansetron (4 mg IV or 8 mg orally) as needed.
Hepatic and first-pass CYP3A, intestinal transporters activity and methadone disposition were assessed as above at baseline and, after a several week washout, again as described above on the 15th-21st days of nelfinavir 1250 mg twice daily (approximately 7am and 7 pm). Nelfinavir dosing was adjusted on study days (morning dose at lunch, and evening dose at 11 pm) to preclude a potential acute effect of the morning nelfinavir dose which might mask the effects of chronic nelfinavir on CYP activity.
Sample size was determined using a simplified analysis (paired t-test) for comparing the outcome variable methadone systemic clearance. A previous study found 22 and 33% interday/intrasubject variability in IV and oral methadone clearances, respectively (Kharasch et al., 2004a). To detect a 30% change in clearance, using a paired t-test, with 33% variability, 1-ß=0.8, α=0.05, would require 12 subjects.
2.2 Analytical Methods
Plasma alfentanil and fexofenadine concentrations were simultaneously quantified using solid-phase extraction and electrospray liquid chromatography-mass spectrometry (Kharasch et al., 2005). Interday coefficients of variation for were 6, 4 and 6% for alfentanil (2, 10, 100 ng/ml) and 4% at all fexofenadine quality control concentrations (2, 10, 150, 600 ng/ml). Plasma and urine methadone and EDDP enantiomer concentrations were quantified using automated on-line extraction, d9-methadone and d3-EDDP as internal standards, stereoselective liquid chromatography, and electrospray mass spectrometry as described previously, except that standard curves (plasma and urine) for both d0- and d5-methadone were used (Whittington et al., 2004). Calibration curves were linear over the calibration range 0.08- 25 ng/ml EDDP enantiomer and 0.1- 100 ng/ml methadone enantiomer in plasma, and 0.5-750 ng/ml methadone or EDDP enantiomer in urine. Interday coefficients of variation for methadone enantiomers (1, 12.5, 25 ng/ml) were 8, 8, and 4%, and those for EDDP enantiomers (1,5, 12.5 ng/ml) were 6, 4, and 4%.
Methadone plasma protein binding was determined by ultrafiltration. Racemic methadone (10 ng/ml) was incubated (37°C) with outdated pooled human plasma with and without 1 μg/ml nelfinavir (n=4 each) for 45 min, and samples were processed (room temperature) through Amicon Ultracel YM-3 (Millipore Corp, Bedford, MA) centrifugal filters. Filtrate and retentate methadone enantiomer concentrations were quantified as above.
2.3 Data Analysis
Plasma methadone and EDDP data were analyzed using noncompartmental methods, assuming complete absorption (WinNonlin 5.2, Pharsight Corp, Mountain View, CA) (Kharasch et al., 2004b). Systemic clearance of intravenous ALF and d0-methadone was (CLIV)=doseIV/AUCIV, apparent oral clearance of ALF and d5-methadone was (CL/F)=doseoral/AUCoral, bioavailability was (Foral)= (AUCoral/doseoral) × (doseIV/AUCIV), volume of distribution based on the terminal phase was (Vz)=Dose/(AUC × λ) where λ is the terminal elimination rate constant, and steady-state volume of distribution was (Vss)=CL × mean residence time. Methadone renal clearance (Clr and CLr/F) was determined as: amount excreted in urine/AUC0-∞. EDDP formation clearance was determined from urine data as: Clf = fraction of dose recovered in urine × CLIV and Clf/F = fraction of dose recovered in urine × CL/F1, for IV and oral dosing, respectively. Hepatic clearance (CLH) was CLiv-CLr. Methadone hepatic extraction (EH) was CLH/Qp, where hepatic plasma flow (Qp) was estimated as 15.2 ml/kg/min. ALF plasma data were similarly analyzed (Kharasch et al., 2004b; Kharasch et al., 2007). Gastrointestinal extraction was EG = 1-FG where FG was Foral/(Fabs(1-EH)); the oral dose was assumed to be entirely absorbed and thus Fabs was considered to be unity. ALF and methadone effect (miosis) vs time curves were treated analogously to conventional plasma concentration curves, with similar noncompartmental analysis, to yield effect parameters similar to conventional pharmacokinetic parameters (Kharasch et al., 2004b; Kharasch et al., 2007). Hence, the area under the effect curve (AUEC) was obtained. ALF miosis was treated similarly to plasma concentration to obtain an effect clearance (CLmiosis, dose/AUEC), analogous to plasma clearance (dose/AUC). Methadone pharmacodynamics were assessed using R-methadone concentration (total of IV and oral)-effect relationships, evaluated graphically from hysteresis plots and AUC analysis, using the AUEC0-∞/ AUC0-∞ ratio (methadone miosis AUEC and the total IV plus oral plasma R-methadone concentration AUC).
2.4 Statistical Analysis
Differences between treatment groups for pharmacokinetic and effect parameters were analyzed using paired-t-tests (SigmaStat 3.5, Systat Corp, Point Richmond, CA). Non-normal data were log transformed for analysis, but reported as the non-transformed results. Statistical significance was assigned at p< 0.05. Results are reported as the arithmetic mean ± standard deviation (SD). Plasma AUC and urine data were also assessed as ratios (nelfinavir/control) and the geometric mean and 90% confidence interval of the geometric mean. Confidence intervals excluding 1.0 were considered statistically significant. Relationships between methadone and ALF clearances were evaluated by linear regression analysis.
3. Results
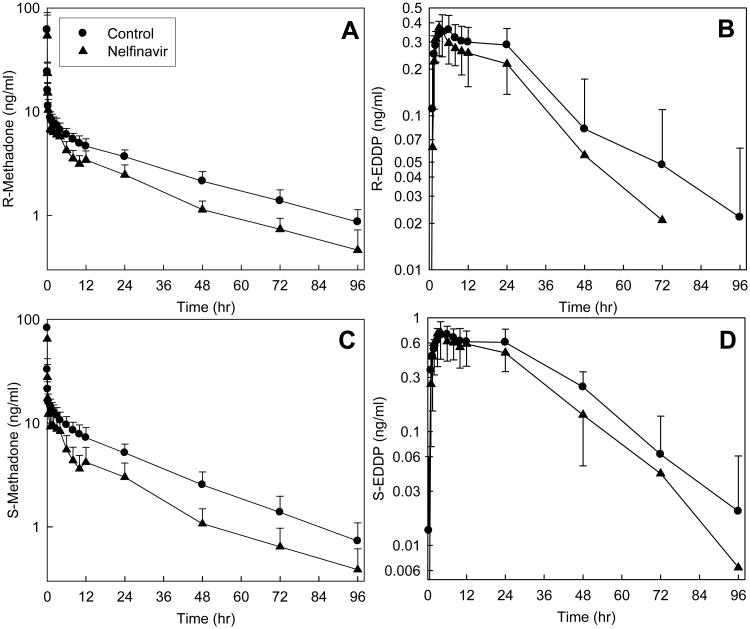
Plasma concentrations of both methadone enantiomers after IV administration were significantly diminished by nelfinavir (Fig 1, Tables 1 and 2). The plasma AUC0-∞ ratio (nelfinavir/control) for both enantiomers was reduced to 0.6. The systemic clearance, hepatic clearance, and hepatic extraction of IV R- and S-methadone were significantly increased 1.6- and 2-fold, respectively, by nelfinavir. Methadone N-demethylation was similarly affected by nelfinavir, which increased the plasma AUC∞ ratio (nelfinavir/control) for both R- and S-EDDP/methadone, with a greater effect on the latter enantiomer. Formation clearances of R- and S-EDDP were increased 11 and 38%, respectively, by nelfinavir. Renal clearance, which accounted for approximately 25% of both methadone enantiomers systemic clearance, was increased by nelfinavir approximately 30 and 50%, respectively, for R- and S-methadone. The fraction of total methadone clearance due to renal clearance was not significantly affected by nelfinavir.
Figure 1.
Effect of nelfinavir on intravenous methadone disposition. Shown are plasma (A) R-methadone, (B) R-EDDP, (C) S-methadone and (D) S-EDDP concentrations. Subjects received 6.0 mg IV methadone HCl (5.4 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity. R- and S-methadone concentrations were different from control (p<0.05) between 1–96 hr.
Table 1. Intravenous and oral methadone pharmacokinetic parameters.
| Control | Nelfinavir | Control | Nelfinavir | |
|---|---|---|---|---|
|
|
|
|||
| IV methadone | R- methadone | S-methadone | ||
|
|
|
|||
| Cmax (ng/ml) | 56 ± 29 | 54 ± 31 | 76 ± 37 | 65 ± 40 |
| AUC0-96 (ng •hr •ml-1) | 272 ± 46 | 179 ± 28a | 369 ± 86 | 209 ± 53a |
| AUC0-∞ (ng •hr •ml−1) | 318 ± 62 | 200 ± 32a | 398 ± 100 | 226 ± 55a |
| AUC0-∞ ratio | 0.63 (0.58,0.70) | 0.56 (0.51,0.63) | ||
| (nelfinavir/control) | ||||
| CLIV (ml•kg−1•min−1) | 2.13 ± 0.53 | 3.36 ± 0.78 a | 1.77 ± 0.60 | 3.10 ± 1.09a |
| CLH (ml•kg−1•min−1) | 1.54 ± 0.43 | 2.61 ± 0.76a | 1.39 ± 0.52 | 2.54 ± 1.04a |
| Elimination t1/2 (hr) | 35 ± 6 | 28 ± 6a | 25± 5 | 25± 10 |
| Vss (L/kg) | 5.9 ± 1.1 | 7.4 ± 1.2a | 3.3 ± 0.6 | 5.4± 1.5a |
| EH | 0.10 ± 0.03 | 0.17 ± 0.05a | 0.09 ± 0.03 | 0.17 ± 0.07a |
| R-EDDP | S-EDDP | |||
|
|
|
|||
| Cmax (ng/ml) | 0.40 ± 0.08 | 0.40 ± 0.11 | 0.78 ± 0.18 | 0.77 ± 0.33 |
| AUC0-96 (ng •hr •ml−1) | 14 ± 6 | 11 ± 3a | 29 ± 8 | 23 ± 7a |
| AUC∞ (ng •hr •ml−1) | 25 ± 15 | 17 ± 6a | 32 ± 9 | 27 ± 7a |
| Elimination t1/2 (hr) | 35 ± 11 | 30 ± 14 | 22 ± 6 | 22 ± 8 |
| AUC0-96 (EDDP/methadone) | 0.05 ± 0.02 | 0.06 ± 0.02a | 0.08 ± 0.03 | 0.12 ± 0.05a |
| AUC∞ (EDDP/methadone) | 0.08 ± 0.01 | 0.09 ± 0.04a | 0.09 ± 0.03 | 0.14 ± 0.07a |
| AUC∞ (EDDP/methadone) ratio (nelfinavir/control) | 1.20 (1.02, 1.42) | 1.45 (1.30,1.61) | ||
| Oral methadone | R-methadone | S-methadone | ||
|
|
|
|||
| Cmax (ng/ml) | 13 ± 3 | 11 ± 3a | 22 ± 6 | 16 ± 5a |
| Tmax (hr) | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| AUC0-96 (ng •hr •ml−1) | 441 ± 97 | 280 ± 56a | 591 ± 179 | 304 ± 92a |
| AUC0-∞(ng •hr •ml−1) | 540 ± 137 | 324± 80a | 652 ± 213 | 328 ± 98a |
| AUC0-∞ ratio (nelfinavir/control) | 0.60 (0.51, 0.70) | 0.51 (0.44, 0.59) | ||
| CL/F (ml•kg−1•min−1) | 2.39 ± 0.83 | 4.00 ± 1.33a | 2.09 ± 0.89 | 4.07 ± 1.74a |
| Elimination t1/2 (hr) | 39 ± 8 | 31 ± 9 | 27 ± 6 | 23 ± 7 |
| Vz/F | 7.6 ± 1.8 | 10.2 ± 3.1a | 4.5 ± 1.1 | 7.7 ± 2.3a |
| Foral | 0.92 ± 0.08 | 0.84 ± 0.18 | 0.88 ± 0.10 | 0.78 ± 0.10a |
| R-EDDP | S-EDDP | |||
|
|
|
|||
| Cmax (ng/ml) | 1.6 ± 0.3 | 1.7 ± 0.5 | 2.3 ± 0.4 | 2.7 ± 1.1 |
| AUC0-96 (ng •hr •ml−1) | 49 ± 12 | 44 ± 10 | 88 ± 18 | 74 ± 15 a |
| AUC∞ (ng •hr •ml−1) | 59 ± 14 | 54 ± 14 | 110 ± 27 | 88 ± 18a |
| Elimination t1/2 (hr) | 33 ± 16 | 32 ± 11 | 37 ± 13 | 31 ± 11 |
| AUC0-96 (EDDP/methadone) | 0.12 ± 0.04 | 0.17 ± 0.06a | 0.16 ± 0.05 | 0.27 ± 0.11a |
| AUC∞ (EDDP/methadone) | 0.12 ± 0.04 | 0.18 ± 0.09a | 0.18 ± 0.06 | 0.29 ± 0.11a |
| AUC∞ (EDDP/methadone) ratio (nelfinavir/control) | 1.51 (1.21,1.88) | 1.59 (1.36,1.86) | ||
Subjects received 6.0 mg IV and 11.0 mg oral methadone HCl at all sessions. Results are the arithmetic mean ± SD (n=12), except area under the concentration-time curve (AUC) ratios (nelfinavir/control), which are the geometric mean (90% CI).
AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CLH, hepatic clearance; CLIV, systemic clearance; CL/F, apparent oral clearance;; Cmax, peak plasma concentration; EDDP, 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine; EH, hepatic extraction; Foral, bioavailability; Vss, steady-state volume of distribution; Vz/F, apparent volume of distribution.
Significantly different from control (p<0.05)
Table 2. Methadone and metabolite renal excretion and clearance.
| Control | Nelfinavir | Control | Nelfinavir | |
|---|---|---|---|---|
|
|
|
|||
| R-methadone | S-methadone | |||
|
|
|
|||
| % dose recovered 0-96 hr | ||||
| IV d0-methadone | 28 ± 6 | 23 ± 6a | 22 ± 5 | 19 ± 6 |
| Oral d5-methadone | 24 ± 6 | 18 ± 5a | 18 ± 5 | 14 ± 5a |
| d0-EDDP | 19 ± 4 | 14 ± 2a | 33 ± 6 | 26 ± 5a |
| d5-EDDP | 20 ± 4 | 15 ± 2a | 33 ± 5 | 27 ± 4a |
| Renal or metabolite formation clearance (ml•kg−1•min−1) | ||||
| IV d0-methadone Clr | 0.59 ± 0.15 | 0.76 ± 0.17a | 0.38 ± 0.11 | 0.56 ± 0.16a |
| Oral d5-methadone Clr/F | 0.49 ± 0.11 | 0.59 ± 0.13a | 0.30 ± 0.07 | 0.41 ± 0.12a |
| d0-EDDP Clf | 0.40 ± 0.15 | 0.45 ± 0.12a | 0.58 ± 0.22 | 0.80 ± 0.26a |
| d5-EDDP Clf/F | 0.43 ± 0.14 | 0.51 ± 0.12a | 0.58 ± 0.19 | 0.84 ± 0.27a |
Results are the mean ± SD (n=12)
CLr, renal clearance; CLr/F, apparent renal clearance; CLf, formation clearance; CLf/F, apparent formation clearance; EDDP, 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine.
Significantly different from control (p<0.05)
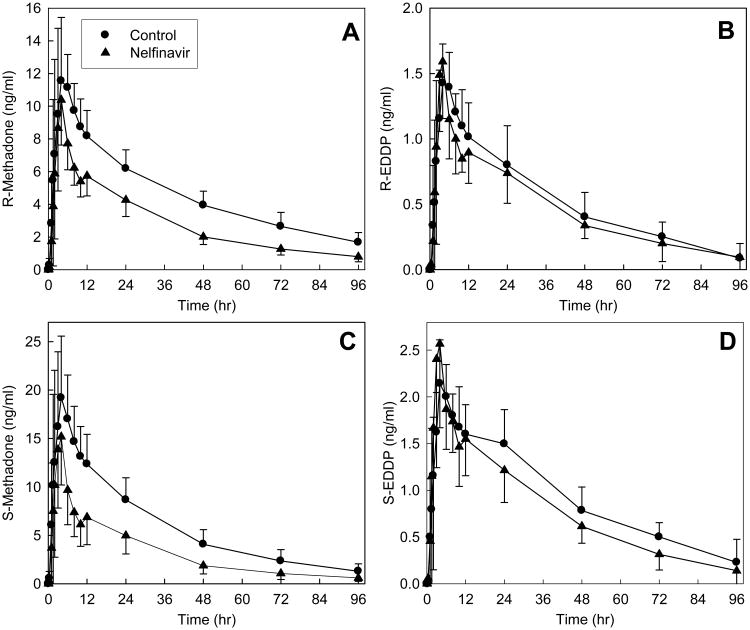
Nelfinavir also significantly diminished oral methadone plasma concentrations (Fig 2, Tables 1 and 2). The plasma AUC0-∞ ratio (nelfinavir/control) for the methadone enantiomers was reduced to 0.5-0.6. The apparent oral clearances of R- and S-methadone were significantly increased 1.7- and 1.9-fold, respectively. Nelfinavir had no effect on the oral bioavailability of R-methadone, but slightly reduced that of S-methadone. Nelfinavir induced oral methadone N-demethylation. The plasma AUC∞ ratios (nelfinavir/control) for R- and S- EDDP/methadone were significantly increased 1.5- and 1.7-fold, respectively, by nelfinavir. Nelfinavir increased R- and S-EDDP apparent formation clearances by 19% and 45%. Renal clearance accounted for approximately 20-30% methadone enantiomers systemic clearance. Nelfinavir increased R- and S-methadone renal clearance 20 and 37%, respectively, but did not significantly affect the fraction of apparent oral methadone clearance attributable to renal clearance.
Figure 2.
Effect of nelfinavir on oral methadone disposition. Shown are plasma (A) R-methadone, (B) R-EDDP, (C) S-methadone and (D) S-EDDP concentrations. Subjects received 11.0 mg oral methadone HCl (9.9 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity. Methadone concentrations were different from control (p<0.05) between 6-48 hr (R-methadone) and 4-24 hr (S-methadone).
Nefinavir effects on methadone plasma protein binding were determined at concentrations relevant to the present investigation. The free fraction of R- and S-methadone (13 ± 1 and 10 ± 2%, respectively) was not altered in the presence of 1 μg/ml nelfinavir (13 ± 1 and 12 ± 1%).
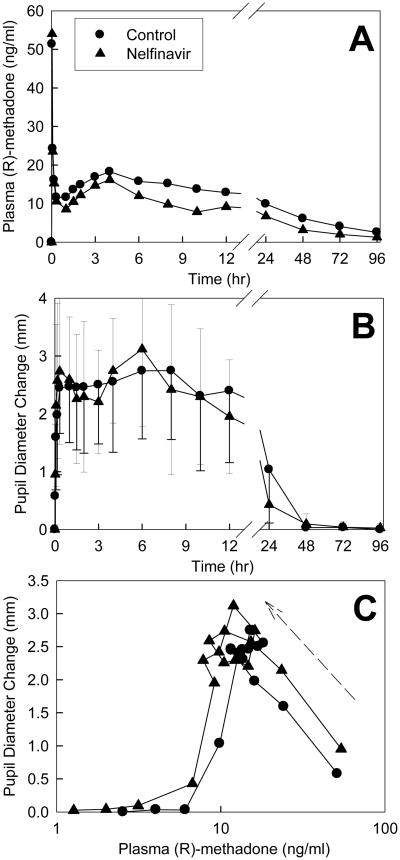
Methadone effects were quantified using changes in dark-adapted pupil diameter (miosis). Plasma concentrations of total (sum of IV d0- and oral d5) R-methadone (the pharmacologically active enantiomer) and pupil diameter changes are shown in Fig 3. Due to the slow absorption of oral methadone there was a second plasma concentration peak at 4 hr, after the initial IV peak (Fig 3A). Methadone caused immediate miosis, with effects detectable at the first time point (2 min), and peak effects occurring within 5-10 min (Fig 3B). Despite lower plasma total R-methadone concentrations and AUCs, miosis in the nelfinavir-treated subjects was not significantly different from controls (Fig 3B, Table 3). This may be better understood by examining R-methadone pharmacodynamics. The effect/concentration AUEC0-∞/AUC0-∞ ratio was significantly increased by nelfinavir (geometric mean 1.4, 90% CI 1,2-1.7), and there appeared to be a small shift upwards and to the left in the plasma concentration-effect curve by nelfinavir (Fig 3C).
Figure 3.
Effect of nelfinavir on methadone pharmacodynamics. Subjects simultaneously received 11.0 mg oral and 6.0 mg IV methadone HCl. Each data point is the mean ± SD (n=12). Some SD are omitted for clarity. (A) Total (IV and oral, d0 and d5) plasma R-methadone concentrations. Concentrations in the nelfinavir-treated subjects were significantly lower than in controls 6-96 hr after methadone administration (p<0.05). (B) Dark-adapted pupil diameter change from baseline (miosis). (C) Plasma concentration-effect relationships (miosis vs total R-methadone plasma concentration). Each data point is the mean concentration and mean effect at each time point. There was a counter-clockwise hysteresis (arrow) in the concentration effect curve showing that peak pupil effects are delayed from peak plasma concentrations.
Table 3. Methadone and alfentanil effect parameters.
| Control | Nelfinavir | |
|---|---|---|
| Methadone | ||
| Maximum miosis (mm) | 3.5 ± 0.9 | 3.5 ± 1.2 |
| AUEC0-∞ (mm•hr) | 78 ± 43 | 63± 34 |
| AUEC0-∞ ratio (nelfinavir/control) | 0.83 (0.68, 1.00) | |
| AUEC0-∞ / AUC0-∞ (mm•ml•ng−1) | 0.09 ± 0.04 | 0.13 ± 0.08 |
| AUEC0-∞ / AUC0-∞ ratio (nelfinavir/control) | 1.4 (1.2, 1.7) | |
| IV alfentanil | ||
| Maximum miosis (mm) | 4.9 ± 0.5 | 4.5 ± 0.8 |
| AUEC0-∞ (mm•hr) | 4.9 ± 2.9 | 8.6 ± 8.0 |
| AUEC0-∞ ratio (nelfinavir/control) | 1.5 (1.1, 2.0) | |
| CLIV miosis (μg•mm−1•hr−1•kg−1) | 4.2 ± 2.4 | 3.1 ± 1.9 |
| Effect t1/2 (hr) | 1.0 ± 0.5 | 1.9 ± 1.6 |
| Oral alfentanil | ||
| Maximum miosis (mm) | 2.5 ± 1.0 | 4.2± 0.8a |
| AUEC0-∞ (mm•hr) | 5.5 ± 2.2 | 15.7± 8.7a |
| AUEC0-∞ ratio (nelfinavir/control) | 2.7 (1.9, 3.8) | |
| CL/Fmiosis (μg•mm−1•hr−1•kg−1) | 9.8 ± 5.9 | 3.8 ± 2.3a |
| Effect t1/2 (hr) | 1.6 ± 0.5 | 2.0 ± 0.6 |
Subjects received 6.0 mg IV and 11.0 mg oral methadone HCl, 15 μg/kg IV ALF and 43 μg/kg oral ALF at all sessions. Results (n=12) are the arithmetic mean ± SD, except the AUC0-∞ ratio which is the geometric mean (90% CI).
AUEC, area under the effect (miosis)-time curve; CLIV miosis, effect clearance of IV alfentanil; CL/Fmiosis, effect clearance of oral alfentanil; AUEC0-∞ / AUC0-∞ ratio is the methadone miosis AUEC and the total (IV plus oral) plasma R-methadone concentration AUC.
Significantly different from control (p<0.05)
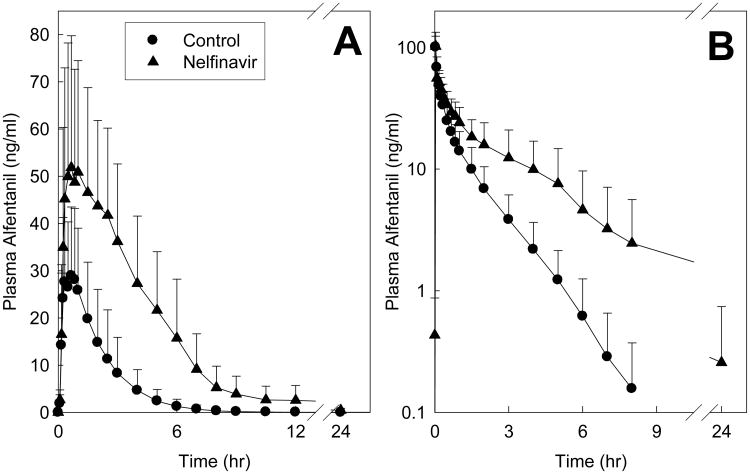
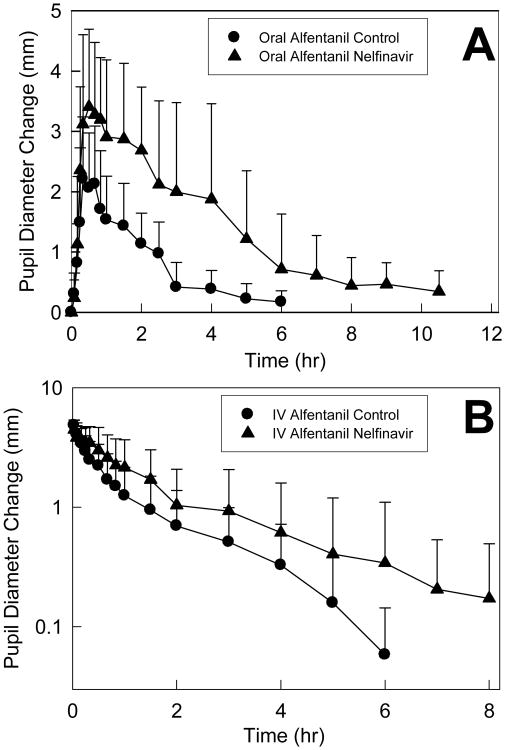
Nelfinavir significantly inhibited hepatic and first-pass CYP3A activity. Effects of nelfinavir on ALF plasma concentrations are shown in Fig 4, and pharmacokinetic parameters provided in Table 4. The AUC0-∞/dose ratio (nelfinavir/control) for IV and oral ALF was increased 2- and 4-fold, respectively, by nelfinavir. These results indicate 50% inhibition of hepatic ALF clearance and CYP3A activity, and 76% inhibition of first-pass ALF clearance and CYP3A activity. Nelfinavir significantly reduced ALF hepatic and intestinal extraction, and increased ALF bioavailability. Pupil data were available before plasma ALF concentrations, and used as an early surrogate for ALF clearance to assess nelfinavir effects on CYP3A (Fig 5 and Table 3). Nelfinavir increased and prolonged ALF miosis, and increased the AUEC0-∞ ratio for IV and oral ALF 1.5- and 2.7-fold.
Figure 4.
Nelfinavir effects on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Shown are ALF concentrations after (A) oral (43 μg/kg) and (B) intravenous (15 μg/kg) administration. Each data point is the mean ± SD (n=12).
Table 4. Intravenous and oral alfentanil pharmacokinetic parameters.
| Control | Nelfinavir | |
|---|---|---|
| IV alfentanil | ||
| Cmax (ng/ml) | 101 ± 23 | 100 ± 31 |
| AUC0-∞ (ng •hr •ml−1) | 54 ± 20 | 118 ± 62a |
| AUC0-∞ ratio (nelfinavir/control) | 2.1 (1.7, 2.5) | |
| CLIV (ml•kg−1•min−1) | 5.2 ± 1.8 | 2.6 ± 1.1a |
| Elimination t1/2 (hr) | 1.1 ± 0.2 | 1.8 ± 0.7a |
| EH | 0.34 ± 0.12 | 0.17 ± 0.07a |
| Oral alfentanil | ||
| Cmax (ng/ml) | 35 ± 16 | 66 ± 22a |
| AUC0-∞ (ng •hr •ml−1) | 68 ± 42 | 248 ± 95 |
| AUC0-∞ ratio (nelfinavir/control) | 4.0 (2.8, 5.8) | |
| CL/F (ml•kg−1•min−1) | 14.8 ± 9.63 | 3.5 ± 1.6a |
| Elimination t1/2 (hr) | 1.1 ± 0.3 | 2.0 ± 1.3a |
| Foral | 0.41 ± 0.14 | 0.71 ± 0.21a |
| EG | 0.38 ± 0.15 | 0.15 ± 0.20a |
Subjects received 15 μg/kg IV ALF and 43 μg/kg oral ALF. Results are the arithmetic mean ± SD (n=12), except the AUC0-∞ ratio (nelfinavir/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CLIV, systemic clearance of IV alfentanil; CL/F, apparent oral clearance of alfentanil; EH, hepatic extraction; EG, intestinal extraction; Foral, bioavailability.
Significantly different from control (p<0.05)
Figure 5.
Nelfinavir effects on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Pupil diameter change from baseline (miosis) was used as a surrogate for ALF plasma concentrations. Shown is miosis after (A) 43 μg/kg oral ALF and (B) 15 μg/kg intravenous ALF. Each data point is the mean ± SD (n=12).
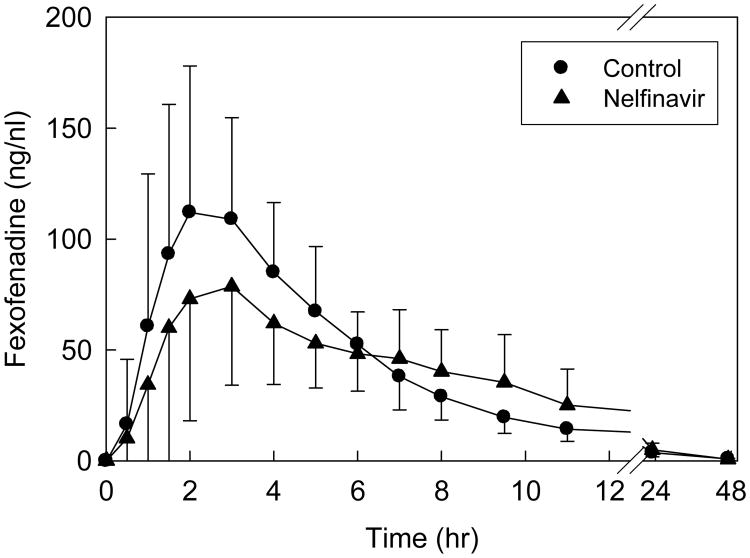
Disposition of oral fexofenadine was used to probe activity of the intestinal efflux pump P-gp, and other intestinal transporters. Nelfinavir decreased fexofenadine peak plasma concentration, but had no effect on overall AUC (Fig 6, Table 5).
Figure 6.
Nelfinavir effects on gastrointestinal transporter activity, assessed using fexofenadine as a transporter probe. Each subject received 60 mg oral fexofenadine on all occasions. Each data point is the mean ± SD (n=12).
Table 5. Fexofenadine pharmacokinetic parameters.
| Control | Nelfinavir | |
|---|---|---|
| Cmax (ng/ml) | 133 ± 67 | 100± 43a |
| AUC0-∞ (ng •hr •ml−1) | 744 ± 282 | 737 ± 192 |
| AUC0-∞ ratio (nelfinavir/control) | 1.02 (0.86,1.21) | |
| CL/F (ml•kg−1•min−1) | 22.8 ± 10.3 | 21.6 ± 9.0 |
| Elimination t1/2 (hr) | 11.1 ± 3.5 | 8.2 ± 2.7a |
Results are the arithmetic mean ± SD (n=12), except the AUC0-∞ ratio (nelfinavir/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CL/F, apparent oral clearance of fexofenadine.
Significantly different from control (p<0.05)
The relationship between methadone clearance and CYP3A activity, measured as ALF clearance, was evaluated by linear correlation analysis. For IV R- and S-methadone, there was no significant correlation between systemic methadone clearance and hepatic CYP3A activity (r2 = 0.01 for both, p>0.05). For oral R- and S-methadone, there was similarly no significant correlation between methadone oral clearance and first-pass CYP3A activity (r2 = 0.02 and 0.07, both p>0.05).
4. Discussion
One major finding of this investigation was that nelfinavir significantly inhibited CYP3A activity. ALF miosis AUEC0-∞ ratios and ALF plasma AUC ratios were both increased by nelfinavir. Nelfinavir inhibited 50%, 40% and 76% of hepatic, intestinal, and first-pass CYP3A activities, respectively, assessed using ALF clearance. Nelfinavir reduced ALF hepatic extraction from 0.34 to 0.17, intestinal extraction from 0.38 to 0.15, and increased bioavailability from 41 to 71%. Nelfinavir inhibition of CYP3A was, as expected, consistent with impaired clearance of numerous CYP3A substrates, whose oral AUC is increased 2- to 5-fold by nelfinavir (Perry et al., 2005). Nelfinavir has also been suggested to induce CYP3A4, based on autoinduction of nelfinavir metabolism and clearance in humans and rats, and induction of hepatic but not intestinal CYP3A protein expression in rats (Bardsley-Elliot and Plosker, 2000; Huang et al., 2001). Nevertheless, there are considered to be few well-characterized nelfinavir interactions with model CYP3A substrates (Ernest II et al., 2005). This is the first investigation to evaluate nelfinavir effects on both hepatic and first-pass CYP3A. Results show that this protease inhibitor diminished both intestinal and hepatic CYP3A activities, without evidence for CYP3A induction. Thus, both liver and intestine are the site(s) of nelfinavir-CYP3A drug interactions.
A second major finding was that nelfinavir increased apparent gastrointestinal P-gp activity. Nelfinavir significantly decreased maximum fexofenadine plasma concentrations, suggesting enhanced intestinal efflux, although AUC was not altered. Fexofenadine is a well-characterized human P-gp substrate and is frequently used as an in vivo probe to assess P-gp pharmacogenetics and drug interactions (Dresser et al., 2002; Hamman et al., 2001; Wang et al., 2002). For example, the P-gp inducers rifampin and St. John's wort diminished plasma fexofenadine concentrations (Hamman et al., 2001; Wang et al., 2002). The present results are consistent with nelfinavir induction of P-gp expression in cultured human intestinal cells and hepatocytes, and induction of intestinal P-gp in rats in vivo (Dixit et al., 2007; Huang et al., 2001; Perloff et al., 2000). P-gp is expressed on both enterocytes and hepatocytes, and the site of action (intestinal vs hepatic) of other P-gp modulators on first-pass fexofenadine extraction remains controversial (Tannergren et al., 2003), hence the present results do not discriminate the site of P-gp induction by nelfinavir. Fexofenadine is not, however, exclusively transported by P-gp, but is also a substrate for organic anion uptake transporters, and decreased fexofenadine bioavailability by citrus and apple juices was attributed to inhibition of intestinal organic anion uptake transporter-mediated uptake (Dresser et al., 2002). Nelfinavir might also inhibit fexofenadine uptake by organic anion uptake transporters (Cvetkovic et al., 1999). Exact mechanisms by which nelfinavir modulates drug transporters remain unknown.
The primary purpose of this investigation was to determine the mechanism of nelfinavir effects on methadone disposition. It is the first to evaluate nelfinavir effects on IV methadone disposition, oral and IV methadone concurrently, methadone metabolism, and renal excretion. Nelfinavir caused an average 1.8-fold induction of systemic and apparent oral methadone clearances. Induction was attributable to an increase in renal clearance of unchanged drug, and more so to induction of hepatic methadone N-demethylation, extraction, and clearance. Induction of IV and oral methadone metabolism was evidenced by increases in both plasma EDDP/methadone ratios and EDDP formation clearances. Induction of methadone N-demethylation was stereoselective, with a greater effects on S than R-EDDP formation clearances and plasma EDDP/methadone ratios, although changes in renal clearance were less stereoselective, as were changes in total systemic and apparent oral clearances. Intestinal contributions to nelfinavir alterations of first-pass methadone metabolism, if any, appear small, because nelfinavir effects on IV and oral methadone disposition were similar and bioavailability was negligibly changed. Together, these results suggest that nelfinavir decreases methadone plasma concentrations by induction of systemic clearance, in turn attributable to stereoselective induction of hepatic N-demethylation and clearance, and, to a lesser extent, relatively non-stereoselective induction of methadone renal clearance.
Nelfinavir effects on methadone disposition resembled those in previous, albeit more limited observational studies. In patients on daily methadone, nelfinavir 1250 mg twice daily reduced the AUC of oral RS-methadone by 38% (McCance-Katz et al., 2004), and the AUC of oral R- and S-methadone by 43 and 51%, respectively (Hsyu et al., 2006). This is essentially identical to the 40 and 49% reduction of single-dose oral methadone enantiomers in the present study. Nelfinavir increased the geometric mean apparent oral clearance of RS-, R- and S-methadone 1.6-, 1.8- and 2.0-fold, respectively (Hsyu et al., 2006; McCance-Katz et al., 2004), comparable to the present 1.7- and 2.0-fold increases for R- and S-methadone. Thus, nelfinavir effects on a single oral methadone dose in volunteers at concentrations much lower than typically used in opioid-dependent patients were highly representative of nelfinavir effects on chronic high-dose methadone in patients on methadone maintenance therapy.
Although methadone plasma concentrations are known to be reduced by nelfinavir, the mechanism has remained unexplained (Hsyu et al., 2006; McCance-Katz et al., 2004). Clinical methadone elimination in general has been attributed for years to CYP3A4. Initial in vitro studies reported methadone N-demethylation by human recombinant and hepatic and intestinal CYP3A4 (Foster et al., 1999; Gerber et al., 2004; Iribarne et al., 1996; Kharasch et al., 2004a; Wang and DeVane, 2003). Based on extrapolation of these in vitro studies, methadone metabolism and clearance in vivo have been widely attributed to CYP3A4 (Crettol et al., 2006; Crettol et al., 2005; Eap et al., 2002; Foster et al., 2000; Shinderman et al., 2003). Interindividual variability in CYP3A4 activity was considered the major factor responsible for large differences in methadone bioavailability (Ferrari et al., 2004). Protease inhibitor and other drug interactions with methadone have been attributed to CYP3A4-mediated alterations in methadone disposition (McCance-Katz, 2005; McCance-Katz et al., 2003). Specifically, nelfinavir reductions in methadone concentrations were attributed to induction of CYP3A4 (Eap et al., 2002). Based on these considerations, the working hypothesis was that hepatic and/or intestinal CYP3A would mediate nelfinavir effects on methadone disposition. Nevertheless, the results provide strong and unambiguous evidence against a role for CYP3A in nelfinavir induction of IV and oral methadone clearance. Specifically, nelfinavir caused 1.7- to 2-fold increases in IV and oral methadone enantiomers N-demethylation and clearance, despite 50% inhibition of hepatic CYP3A activity and more than 75% inhibition of first-pass CYP3A activity. There was no correlation between IV methadone clearance and hepatic CYP3A activity, nor between oral methadone apparent clearance and first-pass CYP3A activity. The hypothesis that CYP3A mediates nelfinavir effects on methadone disposition is rejected.
The present investigation does not identify the CYP isoform responsible for clinical methadone metabolism and clearance, and induction by nelfinavir. Compelled however by results of the current and previous clinical investigations which were contrary to accepted belief (Kharasch et al., 2008a; Kharasch et al., 2008b; Kharasch et al., 2004a), we and others reevaluated methadone metabolism by CYP. These recent investigations identified the prominent role of CYP2B6 in methadone metabolism in vitro, and the present observations may be consistent with a role for CYP2B6 in vivo. Recombinant CYP2B6 catalyzed methadone N-demethylation as effectively as CYP3A4, and moreover, unlike CYP3A4, metabolism by CYP2B6 was stereoselective (Gerber et al., 2004; Gerber et al., 2001; Kharasch et al., 2004a; Totah et al., 2007a; Totah et al., 2007b). In human liver microsomes, greater stereoselective metabolism (S>R) occurred in livers expressing high levels of CYP2B6 compared to CYP3A4, inhibition of CYPB6 caused the greatest reduction in methadone metabolism, and only CYP2B6 inhibition was stereoselective (Totah et al., 2007b). Clinically, the CYP2B6 inducer rifampin increased methadone clearance, while CYP3A inhibition had no effect (Kharasch et al., 2004a; Totah et al., 2007b). Other clinical evidence, based on the influence of CYP2B6 polymorphisms, supports a role for CYP2B6 in methadone metabolism and clearance (Crettol et al., 2006; Crettol et al., 2005). While nelfinavir is a well-known inhibitor of CYP3A isoforms in vitro (Ernest II et al., 2005; Lillibridge et al., 1998), nelfinavir was recently shown to induce CYP2B6 mRNA, protein expression and catalytic activity in human hepatocytes (Dixit et al., 2007). Nelfinavir characteristically impairs the clearance of numerous CYP3A substrates, but induces the clearance of some other drugs (Fellay et al., 2005; Perry et al., 2005). Nelfinavir induction of CYP2B6 might explain the apparently paradoxical induction of methadone clearance despite inhibition of CYP3A, although nelfinavir effects on clinical CYP2B6 activity are not presently known.
One theoretical explanation for increased methadone clearance by nelfinavir is displacement of protein binding. Methadone is highly bound to α1-acid glycoprotein, and displacement interactions can potentially alter drug clearance, although this is controversial.(Benet and Hoener, 2002) Previous studies found that ritonavir/saquinavir and ritonavir/fosamprenavir increased methadone free fraction, to which was attributed an increase in methadone systemic clearance (Cao et al., 2008; Gerber et al., 2001). Nelfinavir also binds to α1-acid glycoprotein (Schön et al., 2003). In the present investigation, nelfinavir had no effect on methadone plasma protein binding, using pooled human plasma. This suggests that protein displacement may not be mechanism by which nelfinavir increases methadone hepatic or renal clearance, although protein binding was not determined on subject samples.
Induction of methadone renal clearance by nelfinavir was an unanticipated finding. This investigation is apparently the first to report such a renal contribution to the methadone-nelfinavir interaction, although the data do not identify the mechanism(s) for nelfinavir induction of methadone renal clearance. Induction of methadone renal clearance by ritonavir was also recently reported (Kharasch et al., 2008a). Little is known about the potential involvement of renal transporters in methadone renal clearance, or more generally about the mechanisms of renal methadone elimination. Nelfnavir, in addition to modulating P-gp activity, is an effective inhibitor of human organic cation transporter (indeed the most potent among all the HIV protease inhibitors) (Zhang et al., 2000), and breast cancer resistance protein (Gupta et al., 2004; Huls et al., 2008), and both transporters are expressed in human kidney. Mechanisms of alteration in renal methadone clearance by nelfinavir, indeed methadone renal clearance in general, require further evaluation.
The fourth major purpose of this investigation was to determine nelfinavir effects on methadone pharmacodynamics. Clinical studies in patients on stable methadone treatment found an absence of withdrawal symptoms after initiating nelfinavir therapy and no need to adjust methadone doses, despite 50% decreases in methadone plasma concentrations, a finding which has remained unexplained (Brown et al., 2006; Hsyu et al., 2006; McCance-Katz et al., 2004). Using pupil diameter changes to assess methadone pharmacodynamics, nelfinavir increased the effect/concentration AUC ratio, and appeared to cause a small leftward and upward shift of the total R-methadone concentration-response curve, consistent with a small increase in apparent potency and efficacy. This is the first clinical investigation to demonstrate an apparent effect of nelfinavir on methadone pharmacodynamics. This offset the increased methadone clearance and reduced plasma concentrations by nelfinavir, such that miosis was not different from controls. Thus altered methadone pharmacodynamics may explain the lack of clinical withdrawal or need for dose adjustment despite nelfinavir induction of methadone clearance and reduced plasma concentrations. The mechanism by which this occurs is unknown, but one plausible explanation is that methadone brain access is an active process, influenced by one or more blood brain barrier drug efflux and/or influx transporters, and that nelfinavir causes a transport-mediated drug interaction. For example, methadone is a substrate for brain P-gp in vitro and in animals in vivo, where it influences methadone absorption, brain access, pharmacodynamics, and analgesic effects, and diminished brain P-gp activity increases methadone brain penetration, analgesia, and shifts the methadone dose-response curve to the left (Bauer et al., 2006; Thompson et al., 2000). Nelfinavir was a potent inhibitor of P-gp in primary cultured bovine brain microvessel endothelial cells (Bachmeier et al., 2006). The role of P-gp in human methadone brain transport remains unknown and controversial (Coller et al., 2008; Crettol et al., 2008).
The present findings have significant clinical implications for practitioners. Methadone use is growing, evidenced by a 1300% increase in methadone prescriptions between 1997 and 2006 (Governale, 2007). Coincident with this increased clinical use, however, has been an exponential, decade-long increase in methadone toxicity, with methadone-related adverse events increasing ∼1800% between 1997-2004, and fatalities increasing 390% from 1999 to 2004 (the most recent data available) (2007). Methadone has a long elimination half-life, and extreme variability in clearance (pharmacogenetic and/or drug interaction-related) is considered the greatest impediment to predictable dosing and clinical effect (Eap et al., 2002). Concerted research efforts for over a decade have endeavored to identify the enzymes responsible for methadone elimination, pertinent drug interactions, and provide practitioner guidance. Based on well-meaning extrapolation of in vitro studies, methadone metabolism and clearance in vivo have been ubiquitously attributed to CYP3A4 (Crettol et al., 2006; Crettol et al., 2005; Eap et al., 2002; Foster et al., 2000; Shinderman et al., 2003). Methadone dosing guidelines warn about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly (Bruce et al., 2006; Coller et al., 2006; Crettol et al., 2006; Eap et al., 2002; Ferrari et al., 2004; Foster et al., 2000; Shinderman et al., 2003; Wang and DeVane, 2003). Even the most recent FDA-approved methadone label states “Since the metabolism of methadone is mediated primarily by CYP3A4, coadministration of drugs that inhibit CYP3A4 may cause decreased clearance of methadone. The expected clinical results would be increased or prolonged opioid effects.”† Nevertheless, neither the role of CYP3A4 in methadone clearance nor effects of CYP3A4 inhibition were ever clinically tested. We performed the first ever assessment of CYP3A activity and methadone clearance (Kharasch et al., 2004a). CYP3A inhibition (60%) had no effect on methadone plasma concentrations or clearances, and there was no correlation between methadone clearance and hepatic CYP3A activity. More recently, ritonavir (more than 90%) (Kharasch et al., 2008c) and in this investigation nelfinavir (more than 75%), were shown to inhibit hepatic and first-pass CYP3A profoundly, yet without reducing methadone clearance, and the lack of correlation between methadone clearance and CYP3A activity is a consistent finding across all studies (Crettol et al., 2006; Kharasch et al., 2004a; Kharasch et al., 2008c). These results provide substantive evidence against a role for CYP3A in methadone drug interactions, and clearance in general. Practitioner guidelines identifying methadone as a CYP3A substrate, and warning of CYP3A-mediated drug interactions, require thorough and thoughtful reevaluation. Such ongoing misunderstandings significantly limit the use of methadone in the most learned and rationale manner possible. Indeed, the important message is that guidelines used by clinicians to direct methadone therapy may be incorrect.
There are potential limitations with this investigation. First, a single methadone dose was evaluated, and at concentrations lower than typically used in opioid-dependent patients. It is neither possible nor ethical to study healthy volunteers at “therapeutic“ (80-100 mg in methadone maintenance therapy) doses, due to the need for a several day dose-escalation with the risk of causing addiction. Nonetheless, the kinetics of a single low methadone dose are similar to those of higher, steady-state methadone doses, and the present effects of nelfinavir on single low dose methadone plasma concentrations in volunteers were comparable to those on chronic high-dose methadone in patients. Second, nelfinavir effects were evaluated in healthy volunteers, not HIV-infected patients. This was deliberate, because antiretroviral therapy involves several drugs, thereby precluding a mechanistic evaluation and attribution of results to any one specific drug. Third, the pharmacodynamics of d0 and d5 methadone have not been directly compared, although the generally accepted premise is that unlabeled and labeled ligands interact similarly with receptors. Fourth, protein binding was not determined on the subject's plasma samples. Lastly, clinical nelfinavir use is decreasing. Nonetheless, the insights gained into methadone pharmacology are broadly applicable to drug abuse treatment.
In summary, systemic and apparent oral methadone clearances were induced 1.7- and 1.8-fold by nelfinavir, due to increased renal clearance and stereoselective induction of hepatic methadone metabolism, extraction, and clearance. Induction of methadone clearance occurred despite significant (50-76%) inhibition of hepatic and first-pass CYP3A activity. This suggests little or no role for CYP3A in clinical methadone metabolism and clearance. Nelfinavir may alter methadone pharmacodynamics, suggesting a brain transport-mediated drug interaction, and hence that human blood brain barrier transport proteins may influence methadone transport and clinical effects.
Footnotes
www.fda.gov/CDER/Drug/infopage/methadone/default.htm (Accessed on January 5, 2009)
www.fda.gov/CDER/Drug/infopage/methadone/default.htm (Accessed on January 5, 2009).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Methadone mortality - A reassessment Background Information Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration. DHHS; Rockville, MD: 2007. [Accessed on January 5, 2009]. http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%2007_Brief%20w%20attch.pdf. [Google Scholar]
- Bachmeier CJ, Trickler WJ, Miller DW. Comparison of drug efflux transport kinetics in various blood-brain barrier models. Drug Metab Dispos. 2006;34:998–1003. doi: 10.1124/dmd.105.006999. [DOI] [PubMed] [Google Scholar]
- Bardsley-Elliot A, Plosker GL. Nelfinavir: An update on its use in HIV infection. Drugs. 2000;59:581–620. doi: 10.2165/00003495-200059030-00014. [DOI] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71:115–121. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- Brown LS, Jr, Kritz S, Chu M, Madray C. Safety, efficacy, and tolerability of nelfinavir-containing antiretroviral therapy for patients coinfected with HIV and hepatitis C undergoing methadone maintenance. J Subst Abuse Treat. 2006;30:331–335. doi: 10.1016/j.jsat.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–572. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Smith PF, Wire MB, Lou Y, Lancaster CT, Causon RC, Bigelow GE, Martinez E, Fuchs EJ, Radebaugh C, McCabe S, Hendrix CW. Pharmacokinetics and pharmacodynamics of methadone enantiomers after coadministration with fosamprenavir-ritonavir in opioid-dependent subjects. Pharmacotherapy. 2008;28:863–874. doi: 10.1592/phco.28.7.863. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–690. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Coller JK, Barratt DT, Somogyi AA. Response to “No Influence of ABCB1 Haplotypes on Methadone Dosage Requirement”. Clin Pharmacol Ther. 2008;83:669–670. doi: 10.1038/sj.clpt.6100305. [DOI] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–190. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–681. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. No Influence of ABCB1 haplotypes on methadone dosage requirement. Clin Pharmacol Ther. 2008;83:668–669. doi: 10.1038/sj.clpt.6100305. [DOI] [PubMed] [Google Scholar]
- Crettol S, Deglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hammig R, Monnat M, Huttemann H, Baumann P, Eap CB. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35:1853–1859. doi: 10.1124/dmd.107.016089. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999;65:685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Ernest CS, II, Hall SD, Jones DR. Mechanism-based inactivation of cytochrome P450 3A (CYP3A) by HIV protease inhibitors. J Pharmacol Exp Ther. 2005;312:583–591. doi: 10.1124/jpet.104.075416. [DOI] [PubMed] [Google Scholar]
- Fellay J, Marzolini C, Decosterd L, Golay KP, Baumann P, Buclin T, Telenti A, Eap CB. Variations of CYP3A activity induced by antiretroviral treatment in HIV-1 infected patients. Eur J Clin Pharmacol. 2005;60:865–873. doi: 10.1007/s00228-004-0855-8. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–559. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–412. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–440. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- Gerber JG, Rosenkranz S, Segal Y, Aberg J, D'Amico R, Mildvan D, Gulick R, Hughes V, Flexner C, Aweeka F, Hsu A, Gal J. Effect of ritonavir/saquinavir on stereoselective pharmacokinetics of methadone: results of AIDS Clinical Trials Group (ACTG) 401. J Acquir Immune Defic Syndr. 2001;27:153–160. doi: 10.1097/00126334-200106010-00010. [DOI] [PubMed] [Google Scholar]
- Governale L. Methadone utilization in the US 2002 - 2006. [Accessed on January 5, 2009];FDA Center for Drug Evaluation and Research. 2007 ttp://www.dpt.samhsa.gov/ppt/FINAL%20Methadone%20Governale%20SAMHSA%207-20-07.ppt#270,1,MethadoneUtilizationintheU.S.,2002-2006.
- Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–121. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- Hsyu PH, Lillibridge J, Daniels E, Kerr BM. Pharmacokinetic interaction of nelfinavir and methadone in intravenous drug users. Biopharm Drug Dispos. 2006;27:61–68. doi: 10.1002/bdd.482. [DOI] [PubMed] [Google Scholar]
- Huang L, Wring SA, Woolley JL, Brouwer KR, Serabjit-Singh C, Polli JW. Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metab Dispos. 2001;29:754–760. [PubMed] [Google Scholar]
- Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- Iribarne C, Berthou F, Baird S, Dréano Y, Picart D, Bail JP, Beaune P, Ménez JF. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–373. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008a;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008b;84:506–512. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004a;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass CYP3A activity. Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004b;76:452–466. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Walker A, Hoffer C, Sheffels P. Evaluation of first-pass cytochrome P4503A (CYP3A) and P-glycoprotein activities using alfentanil and fexofenadine in combination. J Clin Pharmacol. 2005;45:79–88. doi: 10.1177/0091270004269705. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–426. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Walker A, Whittington D, Hoffer C, Sheffels P. Mechanism of ritonavir influence on methadone pharmacokinetics, pharmacodynamics, and clinical effect (abstract) Clin Pharmacol Ther. 2008c;83:S40. [Google Scholar]
- Klees TM, Sheffels P, Dale O, Kharasch ED. Metabolism of alfentanil by cytochrome P4503A (CYP3A) enzymes. Drug Metab Dispos. 2005;33:303–311. doi: 10.1124/dmd.104.002709. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Long-term pharmacotherapy for opiate (primarily heroin) addiction: Opioid agonists. Handbook Exp Pharmacol. 1996;118:487–562. [Google Scholar]
- Lillibridge JH, Liang BH, Kerr BM, Webber S, Quart B, Shetty BV, Lee CA. Characterization of the selectivity and mechanism of human cytochrome P450 inhibition by the human immunodeficiency virus-protease inhibitor nelfinavir mesylate. Drug Metab Dispos. 1998;26:609–616. [PubMed] [Google Scholar]
- Maas B, Kerr T, Fairbairn N, Montaner J, Wood E. Pharmacokinetic interactions between HIV antiretroviral therapy and drugs used to treat opioid dependence. Expert Opin Drug Metab Toxicol. 2006;2:533–543. doi: 10.1517/17425255.2.4.533. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: the importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005;41 Suppl 1:S89–95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Friedland G, Jatlow P. The protease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Smith P, Morse G, Friedland G, Gourevitch M, Jatlow P. Drug interactions between opioids and antiretroviral medications: interaction between methadone, LAAM, and nelfinavir. Am J Addict. 2004;13:163–180. doi: 10.1080/10550490490436037. [DOI] [PubMed] [Google Scholar]
- Mitchell TB, Dyer KR, Newcombe D, Somogyi AA, White JM. Fluctuations in (R,S)-methadone pharmacokinetics and response among long-term methadone maintenance patients. Addict Biol. 2006;11:170–174. doi: 10.1111/j.1369-1600.2006.00014.x. [DOI] [PubMed] [Google Scholar]
- Perloff MD, von Moltke LL, Fahey JM, Daily JP, Greenblatt DJ. Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. AIDS. 2000;14:1287–1289. doi: 10.1097/00002030-200006160-00034. [DOI] [PubMed] [Google Scholar]
- Perry CM, Frampton JE, McCormack PL, Siddiqui MA, Cvetkovic RS. Nelfinavir: a review of its use in the management of HIV infection. Drugs. 2005;65:2209–2244. doi: 10.2165/00003495-200565150-00015. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Penzak SR, Pau A. Drug interactions in the management of HIV infection: an update. Expert Opin Pharmacother. 2007;8:2947–2963. doi: 10.1517/14656566.8.17.2947. [DOI] [PubMed] [Google Scholar]
- Schön A, del Mar Ingaramo M, Freire E. The binding of HIV-1 protease inhibitors to human serum proteins. Biophys Chem. 2003;105:221–230. doi: 10.1016/s0301-4622(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Shinderman M, Maxwell S, Brawand-Amey M, Golay KP, Baumann P, Eap CB. Cytochrome P4503A4 metabolic activity, methadone blood concentrations, and methadone doses. Drug Alcohol Depend. 2003;69:205–211. doi: 10.1016/s0376-8716(02)00320-4. [DOI] [PubMed] [Google Scholar]
- Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernas H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin Pharmacol Ther. 2003;74:423–436. doi: 10.1016/S0009-9236(03)00238-8. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Koszdin KK, Bernards CM. Opiate-induced analgesia as increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–1399. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007a;321:389–399. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2007b;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- Vlahov D, O'Driscoll P, Mehta SH, Ompad DC, Gern R, Galai N, Kirk GD. Risk factors for methadone outside treatment programs: implications for HIV treatment among injection drug users. Addiction. 2007;102:771–777. doi: 10.1111/j.1360-0443.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31:742–747. doi: 10.1124/dmd.31.6.742. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hamman MA, Huang SM, Lesko LJ, Hall SD. Effect of St John's wort on the pharmacokinetics of fexofenadine. Clin Pharmacol Ther. 2002;71:414–420. doi: 10.1067/mcp.2002.124080. [DOI] [PubMed] [Google Scholar]
- Whittington D, Sheffels P, Kharasch ED. Stereoselective determination of methadone and the primary metabolite EDDP in human plasma by automated on-line extraction and liquid chromatography mass spectrometry. J Chrom B. 2004;809:313–321. doi: 10.1016/j.jchromb.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gorset W, Washington CB, Blaschke TF, Kroetz DL, Giacomini KM. Interactions of HIV protease inhibitors with a human organic cation transporter in a mammalian expression system. Drug Metab Dispos. 2000;28:329–334. [PubMed] [Google Scholar]