Abstract
Rapamycin, an inhibitor of mechanistic target of rapamycin (mTOR), has the strongest experimental support to date as a potential anti-aging therapeutic in mammals. Unlike many other compounds that have been claimed to influence longevity, rapamycin has been repeatedly tested in long-lived, genetically heterogeneous mice, in which it extends both mean and maximum life spans. However, the mechanism that accounts for these effects is far from clear, and a growing list of side effects make it doubtful that rapamycin would ultimately be beneficial in humans. This Review discusses the prospects for developing newer, safer anti-aging therapies based on analogs of rapamycin (termed rapalogs) or other approaches targeting mTOR signaling.
A brief history of rapamycin and mechanistic target of rapamycin
Rapamycin was discovered in the soil of Easter Island as a compound produced by Streptomyces hygroscopicus that was capable of inhibiting the proliferation of the yeast Candida albicans but did not affect the growth of bacteria (1). In mammals, rapamycin was found to inhibit the immune response and was subsequently adopted as a standard therapy to prevent graft rejection in transplant recipients and to treat autoimmune disorders (2, 3). Rapamycin also broadly inhibits the growth and proliferation of mammalian cells, spurring more recent interest in its use as a cancer therapy (4).
Mechanistically, rapamycin binds FKBP12, an immunophilin with prolyl isomerase activity. Two additional proteins required for its effects in yeast were identified in a genetic screen in 1991 and termed target of rapamycin 1 (TOR1) and TOR2 (5). During 1994 and 1995, three separate groups isolated a 289-kDa kinase that is bound and inhibited by the rapamycin-FKBP12 complex in mammalian cells (6–8). This kinase is now known as the mechanistic target of rapamycin (mTOR) and is approximately 40% homologous to Saccharomyces cerevisiae TOR proteins and highly conserved among eukaryotes.
mTOR is found in two complexes that have distinct functions and different sensitivities to the action of rapamycin. mTOR complex 1 (mTORC1; consisting of mTOR, raptor, mLST8/GβL, PRAS40, DEPTOR) plays a key role in the regulation of translation and cell growth via phosphorylation of substrates that include S6 kinase (S6K) and eukaryotic initiation factor eIF4E binding protein (4E-BP), and is potently inhibited by rapamycin. In contrast, mTORC2 (consisting of mTOR, rictor, mLST8/GβL, mSIN1, protor, DEPTOR) regulates a diverse set of substrates, including AKT S473, serum/glucocorticoid regulated kinase, and PKC-α, and is acutely resistant to rapamycin, although it can become physically disrupted during chronic exposure. mTORCs receive inputs through a wide variety of signaling mechanisms and have roles in many aspects of physiology, which have been reviewed in depth (9). Briefly, mTORC1 responds to signals that include amino acids, glucose, WNT ligands, oxygen, cAMP, and insulin/IGF-1. The regulation of mTORC2 activity is less clear but may involve interaction with ribosomes (10). Insulin/IGF-1 signaling to mTORC1 is mediated in part by mTORC2 via AKT phosphorylation. In turn, mTORC1 activation feeds back to attenuate insulin/IGF-1 signaling via S6K1 and GRB10 (Figure 1 and ref. 11).
Figure 1. mTOR signaling.
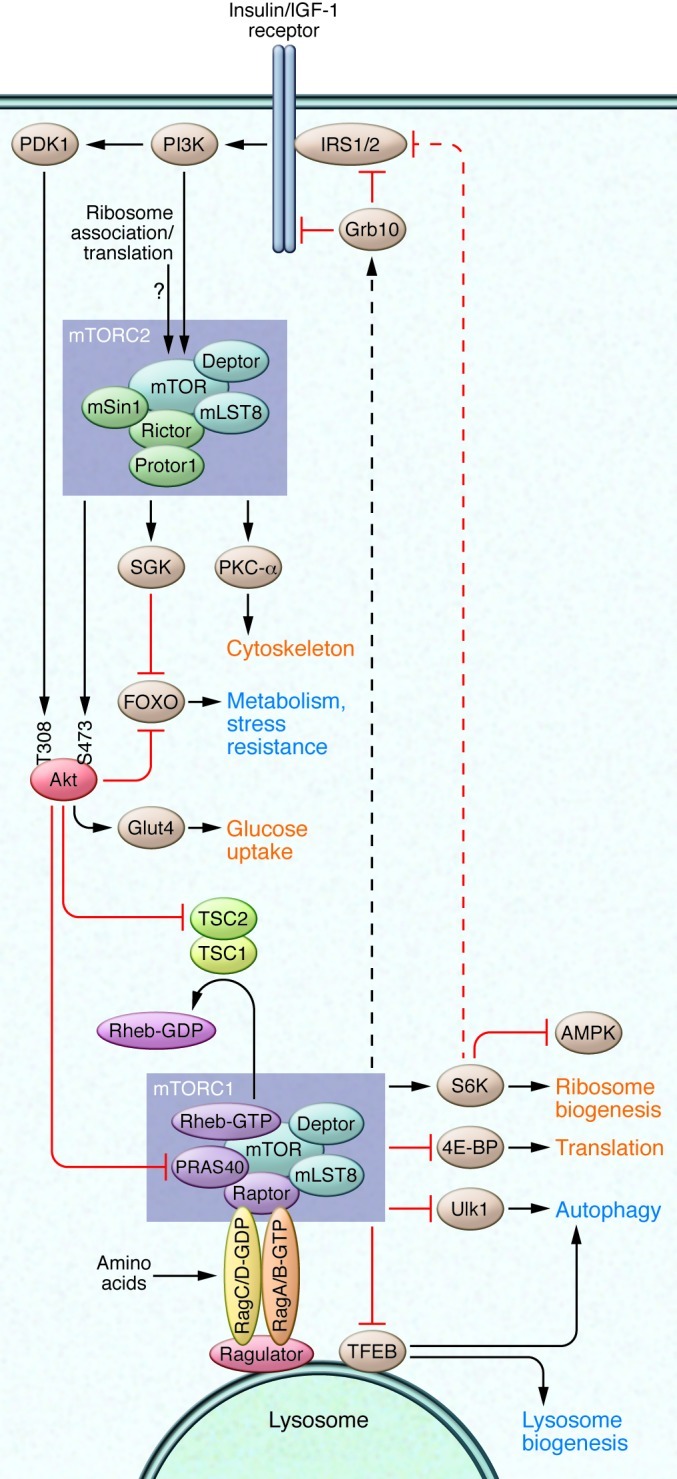
mTOR is found in two complexes, mTORC1 and mTORC2. mTORC1 is regulated in part via the TSC complex, which normally act as a GTPase-activating protein for Rheb to suppress mTORC1 signaling. mTORC1 is also regulated by amino acids via the Ras-related GTP binding (Rag) family of small GTPases. The Rag proteins activate mTORC1 by localizing mTORC1 to the lysosome via interaction with the ragulator complex (110). mTORC1 promotes growth by enhancing ribosomal biogenesis, translation, and other anabolic processes, while inhibiting autophagy. mTORC1 suppresses insulin/IGF-1 signaling via direct regulation of Grb10 and S6K, which subsequently reduces signaling to mTORC2. AKT, an inhibitor of TSC1/2, is one of several direct substrates of mTORC2. Processes that are upregulated by mTOR signaling are shown in red; those that are downregulated by mTOR signaling are shown in blue.
Connecting mTOR signaling to aging
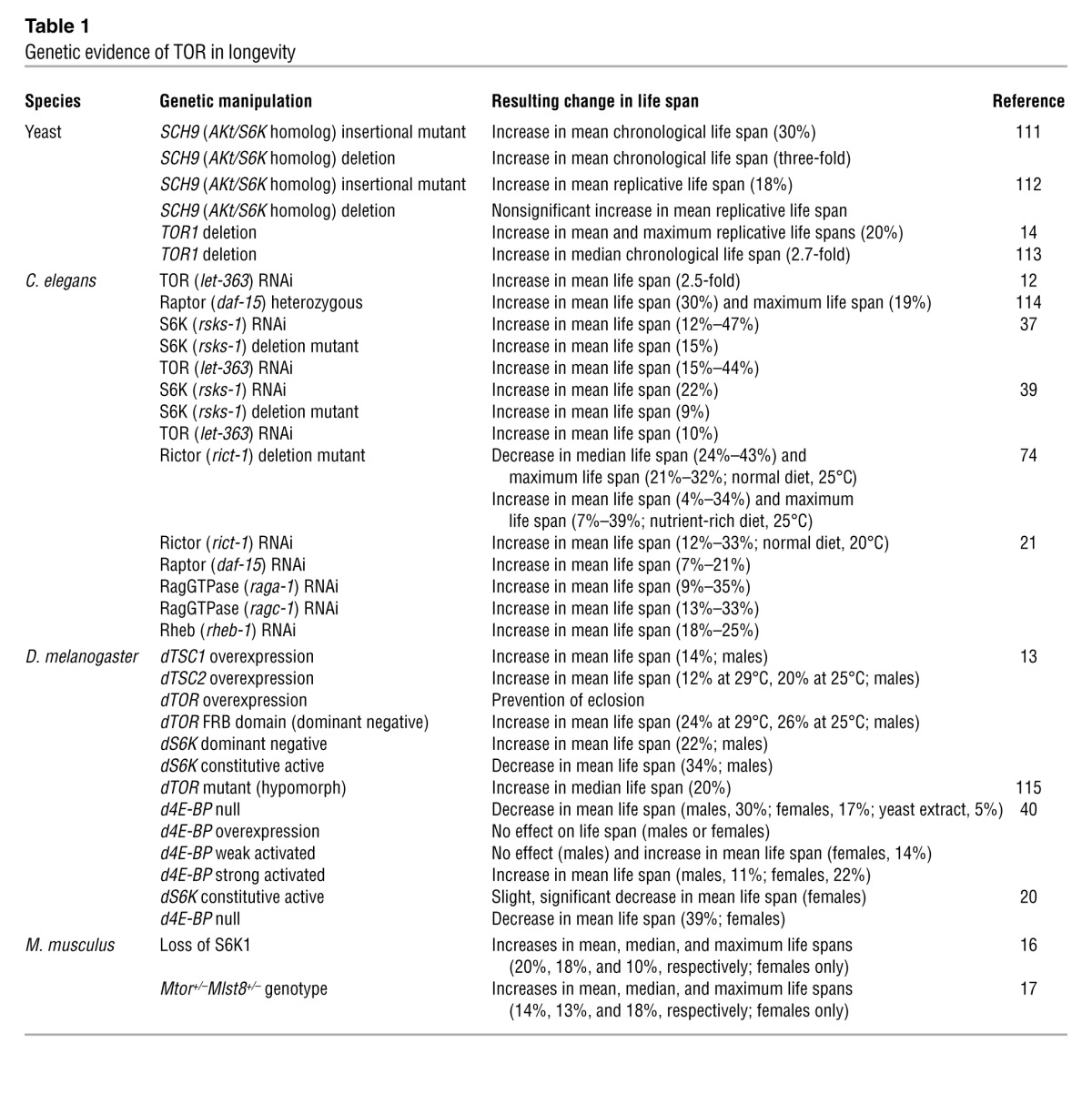
A role for TOR signaling in aging was first revealed in 2003, when Vellai and colleagues showed that RNAi against let-363/CeTor significantly extended the life span of Caenorhabditis elegans and functioned independently from daf-16, a FOXO homolog that had previously been shown to influence life span (12). This was rapidly followed by the demonstration that genetic inhibition of TOR signaling extends life span in Drosophila melanogaster and the budding yeast S. cerevisiae (13, 14). Genetic inhibition of mTOR signaling in mammals is a delicate matter, as the mTOR protein kinase, raptor, rictor, and mLST8 are all essential for development (15). Recently, we demonstrated that female Mtor+/–Mlst8+/– mice have reduced mTORC1 activity and increased longevity, similar to the phenotype reported by Selman and colleagues for mice that lack S6K1, one of the principal substrates of mTORC1 (16, 17). Therefore, the link between mTOR signaling and longevity appears to be conserved from yeast to mammals (Table 1).
Table 1.
Genetic evidence of TOR in longevity
Effects of rapamycin on longevity
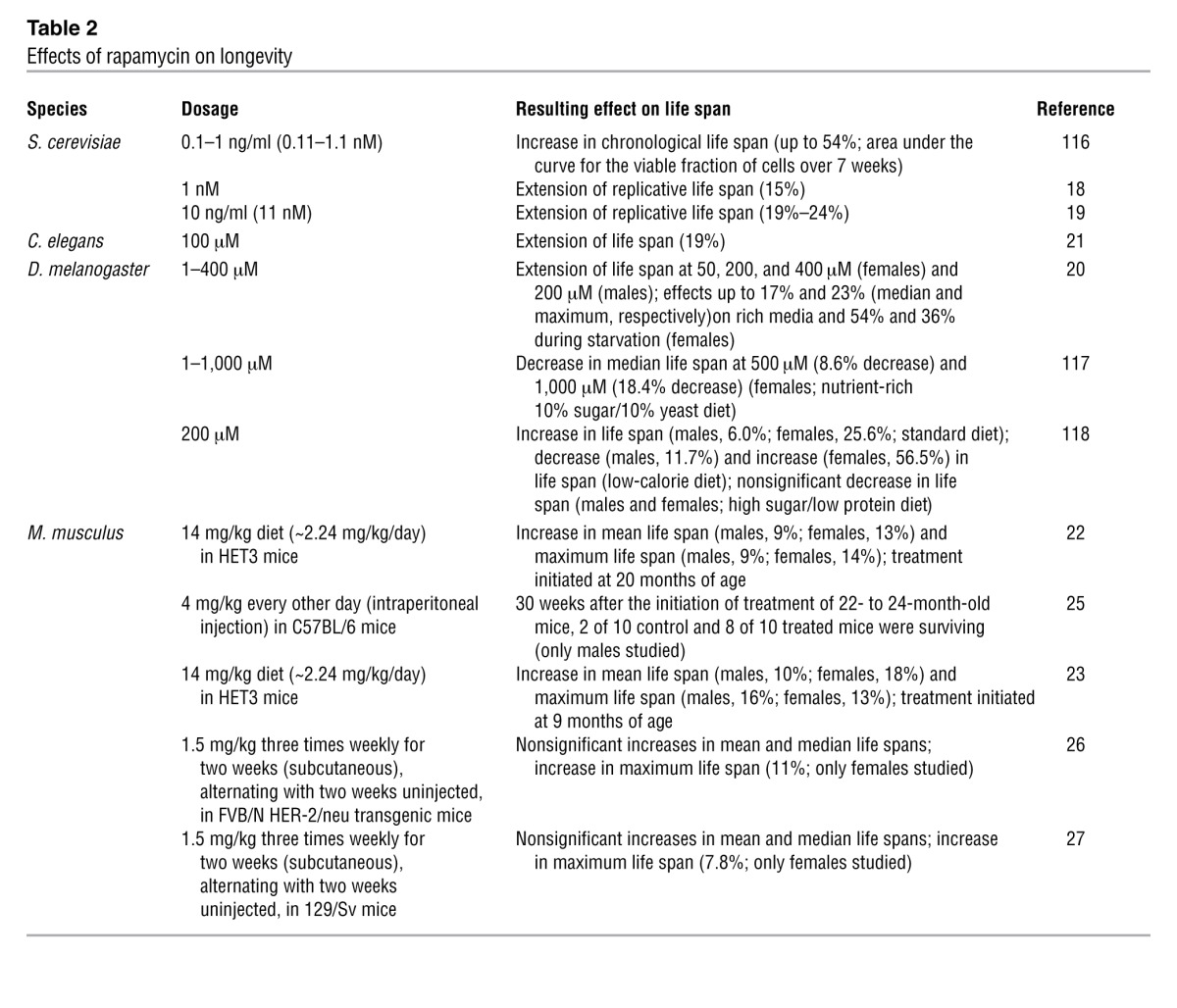
Rapamycin extends life span in yeast, worms, and flies (Table 2 and refs. 18–21). In 2009, rapamycin was shown to extend both mean and maximum life spans of male and female genetically heterogeneous mice (offspring of a four-way cross between long-lived, inbred strains) (22). Remarkably, the treatment was not initiated until the mice had reached an advanced age (20 months), roughly equivalent to a human age of 60 years. In a follow-up study beginning at 9 months of age, rapamycin extended median life span in males and females by 10% and 18%, respectively, and maximum life span by 16% and 13% (23). Rapamycin was microencapsulated in an enteric coating that enabled delivery in the food during these studies, and the blood level achieved was approximately three-fold higher than the typical therapeutic range for immunosuppression in humans (24).
Table 2.
Effects of rapamycin on longevity
Other studies have also found a positive effect of rapamycin on life span. Chen et al. found that rapamycin decreased mortality rate in aged male C57BL/6 mice (25). Anisimov et al. showed that rapamycin extends the maximum life span (mean life span of the last 10% surviving) in a short-lived, tumor-prone strain of mice (FVB/N HER-2/neu transgenic) (26). While this study provides strong evidence that rapamycin can be beneficial in the setting of cancer, the choice of strain makes it hard to separate anticancer effects from aging per se. However, rapamycin also extends life span in 129/Sv mice, an inbred strain with a more typical life span and tumor incidence (27). Impressively, 22.9% of the treated mice remained alive at the death of the last control animal.
Taken together, these observations make rapamycin the best-supported candidate for a mammalian longevity drug. Understanding its mechanism of action has the potential to offer insight into the nature of the underlying aging process and may lead to new therapeutic approaches to alleviate the burden of age-related diseases. However, the mechanism accounting for the anti-aging effects of rapamycin is not yet clear (Table 2).
Potential mechanisms of life span extension by rapamycin
Anticancer effects.
Cancer is the most common cause of death for laboratory mice, and rapamycin is an anticancer drug. Therefore, it remains possible that life span extension by rapamycin is secondary to tumor suppression and unrelated to the underlying aging process. There are several reasons why we do not favor this model. First, the initial experiments linking rapamycin and mTOR inhibition to longevity were performed in organisms that are mainly postmitotic (worms and flies) or single celled (yeast) and therefore do not experience cancer. Second, rapamycin increases maximum longevity, providing support for the idea that it slows multiple age-related pathologies. Targeting a single disease should not substantially increase the life spans of the longest-lived individuals in a group, as the oldest individuals will be at very high risk for most or all causes of death unless the underlying aging process has been postponed. Third, rapamycin has been shown to delay multiple age-related changes in mice, including loss of stem cell function (25), cognitive decline (28), retinopathy (29), accumulation of subcellular alterations in the myocardium, liver degeneration, endometrial hyperplasia, tendon stiffening, and decline in physical activity (30). Moreover, rapamycin is therapeutic in rodent models of cardiac hypertrophy (31, 32) and neurodegenerative diseases (33–35), conditions that affect aging humans. While cancer prevention clearly plays a major role in the survival benefit conferred by rapamycin, it is important to understand that cancer is an age-related disease, and its prevention is an expected consequence of any therapy that slows aging.
Translation.
mTORC1, via S6K and 4E-BP, plays a central role in the regulation of translation, and it is worth considering whether reduced protein synthesis per se might mediate the effects of rapamycin on longevity. For example, decreasing the overall rate of translation might allow better fidelity during synthesis and/or relieve stress on the mechanisms that degrade erroneous, misfolded, or damaged proteins (36). Indeed, experiments in S. cerevisiae, C. elegans, and D. melanogaster have demonstrated that deletion or siRNA-mediated knockdown of ribosomal subunits, S6K, or translation initiation factors results in increased life span and S6K1 deletion extends life span in female mice, whereas 4E-BP deletion blocks the life-extending effects of caloric restriction (CR) in flies (13, 37–40).
Recent findings challenge the view that translation per se is the key to the benefits of TOR/mTOR inhibition. While female mice lacking S6K1 have extended life spans, there is no discernible effect on overall translation, at least in skeletal muscle (41). In addition, the long life spans of worms that lack a key translation initiation factor can still be further increased by TOR deletion, implying that distinct mechanisms are at play (38). Moreover, life span extension due to deletion of translation initiation factors is dependent on daf-16, whereas life span extension by depletion of TOR, S6K, or ribosomal subunits is not, again pointing to the involvement of multiple distinct mechanisms (12, 37). Interestingly, reducing TOR using RNAi fails to further extend the life spans of eat-2 mutant worms, a model for CR, despite suppressing the already low rate of protein synthesis by an additional 49% (37). Furthermore, inactivation of the worm homolog of AMPK is sufficient to suppress life span extension in animals lacking S6K, ostensibly without affecting translation (16). Clearly the relationship between translation and longevity is more complex than initially supposed.
Translation of specific mRNAs may influence life span. While complete loss of mTOR function has a major effect on general translation, rapamycin has a more subtle effect, most likely because a subset of the functions of 4E-BP is rapamycin resistant (42, 43). Both rapamycin and complete mTOR inhibition preferentially suppress translation of mRNAs with 5′ terminal oligopyrimidine motifs, suggesting a potential role for these genes in longevity (43, 44). Despite decreasing overall protein synthesis, CR in flies specifically enhances the translation of a subset of mRNAs that have short and less structured 5′ UTRs, including nuclear-encoded mitochondrial genes (40). The TOR substrate 4E-BP is required for this effect and for life span extension. In yeast lacking ribosomal subunits or TOR, full life span extension requires increased translation of a specific transcript, GCN4 (45). Expression of GCN4 is limited by multiple upstream ORFs that normally sequester ribosomes that bind to the mRNA. Under conditions of decreased TOR activity or large ribosomal subunit abundance, the upstream ORFs are more frequently bypassed to initiate translation of the GCN4 ORF. These examples highlight subtleties in the regulation of translation that we are only beginning to appreciate.
Autophagy.
Another effect of mTOR inhibition that has been linked to longevity is the induction of autophagy, a process by which cells recycle their proteins and organelles. Autophagy allows cells to survive nutrient-limited conditions and is a central mechanism by which damaged components are removed. Under conditions of nutrient sufficiency, mTOR phosphorylates and inhibits the autophagy-initiating kinase ULK1 (46). Inactivation of genes involved in autophagy decreases life span in yeast (chronological), C. elegans, and Drosophila, and promotion of autophagy in the fly nervous system extends life span (47–49). Furthermore, autophagy is required for the extension by rapamycin of yeast chronological life span (47) and for life span extension by CR or genetic inhibition of mTOR signaling in worms (50).
In mammals autophagy also appears to play a significant role in the aging process. Most dramatically, the induction of autophagy is sufficient to rejuvenate the liver histology and function of aged mice (51). Furthermore, autophagy seems to be upregulated in CR mice, and to mediate some of the beneficial effects of a CR diet on the heart, liver, and kidneys (52–54). Cells from long-lived Snell dwarf mice also show evidence of increased autophagy (55). Cardiomyocytes isolated from aged mice have lower autophagy and exhibit defects in calcium handling, both of which are corrected by exposure to rapamycin ex vivo (56). However, increased autophagy may not always be beneficial, and indeed may contribute to the pro-aging phenotype of progeroid mice (57).
Interestingly, rapamycin ameliorates nuclear blebbing and premature senescence in cells derived from patients with Hutchinson-Gilford progeria, a rare premature aging syndrome (58). The disease results from a misspliced variant of lamin A, termed progerin, that accumulates to a large degree in patients and is also detected in smaller amounts during normal cellular aging (59, 60). Rapamycin appears to stimulate clearance of progerin from diseased cells via autophagy, and thus may limit the normal age-related accumulation of progerin as well. Overall, the appropriate regulation of autophagy is likely to be a critical determinant of healthy aging.
Stem cell maintenance.
Rapamycin has a number of interesting effects on stem cell function. Hyperactive signaling upstream of mTORC1 due to deletion of Pten, deletion of tuberous sclerosis 1 (Tsc1), or constitutive activation of AKT reduces the number and functional capacity of HSCs (61–63). Rapamycin treatment can restore normal self-renewal capacity in a subpopulation of mouse HSCs that have spontaneously high oxidative stress and reduced functional capacity (64). More recently, Chen et al. noted that mTORC1 activity is elevated in HSCs derived from aged mice, which display functional deficits reminiscent of those caused by Tsc1 deletion (25). Rapamycin restored functional capacity in HSCs from aged mice and boosted the immune response to influenza virus. Rapamycin also increases intestinal stem cell self-renewal via inhibition of mTORC1 in the adjacent Paneth cells, similar to effects that have been in observed in CR animals (65). In addition, rapamycin enhances the reprogramming of somatic cells to generate induced pluripotent stem cells, suggesting a general promotion of stem cell function (66). On the other hand, rapamycin impairs pluripotency, reduces proliferation, and promotes differentiation in human embryonic stem cells (67, 68). In mouse embryonic stem cells, expression of pluripotency markers is more resistant to rapamycin treatment, yet cell size and proliferation are still reduced and differentiation is enhanced (67, 69). Intriguingly, rapamycin depletes leukemia-initiating cells and inhibits both the self-renewal and differentiation capacities of stem cells derived from infantile hemangioma, suggesting a protective effect against cancer stem cells (61, 70). Taken together, these results suggest that rapamycin modulates the behavior of stem cells and generally favors the retention of “stemness” and a more youthful phenotype in the adult stem cells types that have been studied.
Antiinflammatory mechanisms.
The original clinical use of rapamycin as an immunosuppressant should not be overlooked when it comes to longevity. Chronic, low-grade inflammation is a feature of aging, and almost every chronic disease has an inflammatory component (71). A complete discussion of the immunological effects of rapamycin is beyond the scope of this Review, and the topic has been covered elsewhere (72). Importantly, the drug has both positive and negative effects on innate and adaptive immunity, with a net outcome that is more complex than simple immunosuppression, as exemplified by its ability to enhance the immunization of aged mice against influenza virus (25).
mTORC2-dependent mechanisms.
Despite the high specificity of rapamycin for mTORC1 during acute treatment, chronic exposure can also inhibit mTORC2. This effect was first observed in certain cultured cell lines (73), and we have recently shown that it also occurs in vivo in multiple tissues including liver, muscle, and adipose (see Figure 2). It is currently unclear whether inhibition of mTORC2 plays a role in the pro-longevity effects of rapamycin. Female mice lacking S6K1 and female Mtor+/–Mlst8+/– mice are ostensibly long lived due to impairments in mTORC1-dependent signaling, but data from C. elegans suggest that inhibition of mTORC2 can also promote longevity (21, 74). Interestingly, life span extension by disruption of mTORC1 in worms requires skn-1 (the homolog of mammalian NRF1/2) and daf-16 (the homolog of mammalian FOXOs), both transcription factors that control genes involved in stress defenses. Life span extension by rapamycin or mTORC2 disruption, however, requires only SKN-1. Consistent with a role for general stress defenses in the benefits of rapamycin, both worms and flies with impaired TOR function are stress resistant, and induction of NRF1/2 and FOXO target genes has been detected in the livers of mice treated with rapamycin (2 mg/kg daily for two weeks) (20, 21).
Figure 2. Chronic rapamycin treatment disrupts mTORC2.
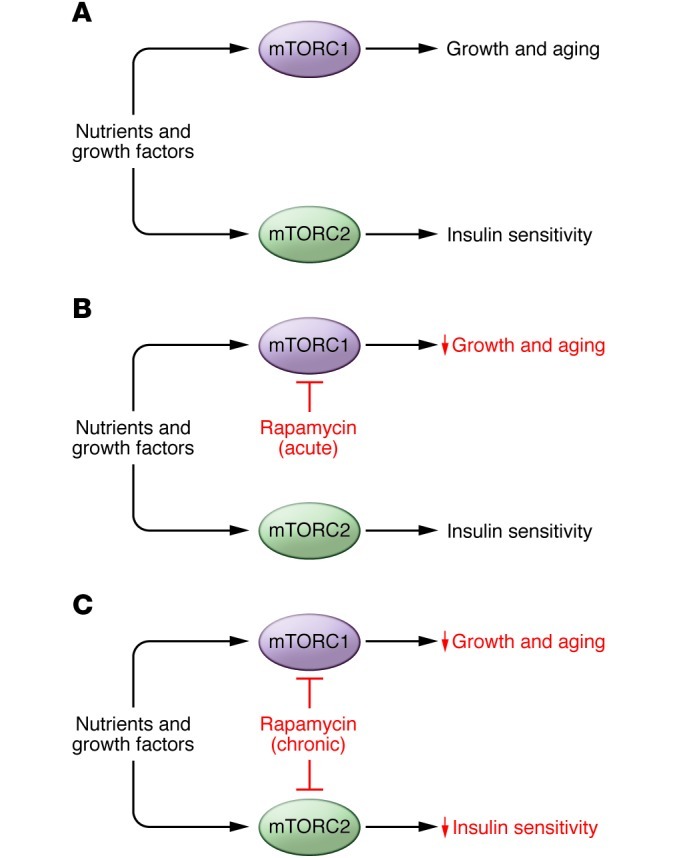
(A) In vivo, nutrients and growth factors drive the activity of mTORC1 and mTORC2, which promote growth, aging, and insulin sensitivity. (B) Acute treatment with rapamycin inhibits mTORC1 signaling, restricting growth and promoting longevity without reducing insulin sensitivity. (C) Chronic treatment with rapamycin inhibits both mTORC1 and mTORC2, restricting growth and impairing insulin signaling, but promoting longevity.
mTOR-independent mechanisms
Some in vivo effects of rapamycin may be independent of mTOR. FKBP12 proteins influence sodium and calcium currents in multiple excitable cell types, in part through binding to ryanodine receptors (75, 76). Moreover, rapamycin also binds to FKBP52, and analogs that favor interaction with FKBP52 over FKBP12 exhibit neuroprotective properties (77).
Endocannabinoid signaling.
Although rapamycin itself has not yet been tested, an intriguing connection between TOR and endocannabinoid signaling was recently described (78). Small molecules analogous to a mammalian endocannabinoid were identified in C. elegans, and depletion of these molecules was associated with life span extension by CR. One specific molecule, eicosapentaenoyl ethanolamide (EPEA), was also found to be lower in worms lacking S6K, and treatment with EPEA suppressed life span extension in both models while conferring increased susceptibility to heat stress. Clearly, there are many twists and turns remaining in the path to understanding life span extension by rapamycin, and the answers may offer insight into the 80-year-old mystery of how CR is able to delay mammalian aging.
Rapamycin side effects
Rapamycin is FDA approved for use as an immunosuppressant following transplant surgery and for the treatment of renal cell carcinoma, and has been used as a coating for coronary stents and in numerous clinical trials for conditions such as lymphangioleiomyomatosis (79) and autoimmune disorders. Although rapamycin has clinical utility in these settings, it is unlikely to be approved for use as a preventative measure in healthy individuals due to substantial side effects.
One of the greatest concerns with rapamycin is its ability to suppress the immune system. Rapamycin extends the life spans of mice, but these studies have been performed in pathogen-free facilities. Studies have found that rapamycin boosts the function of the immune system against certain pathogens (80), but human data are often complicated by the frequent use of rapamycin in conjunction with other immunosuppressants. A carefully controlled study of the use of rapamycin in renal transplant recipients found that 34% of patients experienced viral infection, while 16% suffered from fungal infection (24). Clearly, there are significant risks associated with long-term rapamycin treatment outside of a sanitized laboratory environment.
Rapamycin is also very frequently associated with dermatological adverse events. In renal transplant recipients, rapamycin was found to lead to edema in 60% of patients and aphthous ulcers in 55% of patients (24). Mucositis and rash have been observed in other patient populations (79). Rapamycin treatment has been associated with hair and nail disorders, with 90% of patients experiencing alopecia (24), and with loss of testicular function and reduced male fertility in both humans and mice (30, 81).
In addition, rapamycin treatment leads to metabolic changes, including hyperlipidemia, decreased insulin sensitivity, glucose intolerance, and an increased incidence of new-onset diabetes (79, 82). We recently found that rapamycin treatment promotes stem cell self-renewal in the intestinal crypt (65), but chronic rapamycin treatment of humans has also been associated with gastrointestinal events including diarrhea. Cancer and transplant patients are willing to tolerate these side effects as well as anemia, renal toxicity, impaired wound healing, and joint pain because the benefits outweigh the risks (83). However, the trade-offs are far less likely to be considered acceptable by healthy individuals considering preventative measures.
Prospects for safer mTOR inhibitors
Direct inhibition of mTOR.
Based on its ability to inhibit cell proliferation, there has been significant interest in treating cancers with rapamycin. Several derivatives of rapamycin (rapalogs) with improved pharmacokinetics have been developed, including temsirolimus, everolimus, ridaforolimus, 32-deoxo-rapamycin, and zotarolimus. Despite intense interest and promising results in animal models of cancer, rapalogs have generally disappointed in human trials and are currently approved only for the treatment of renal cell carcinoma (temsirolimus and everolimus) and for patients with specific types of pancreatic cancer or tuberous sclerosis (reviewed in ref. 84).
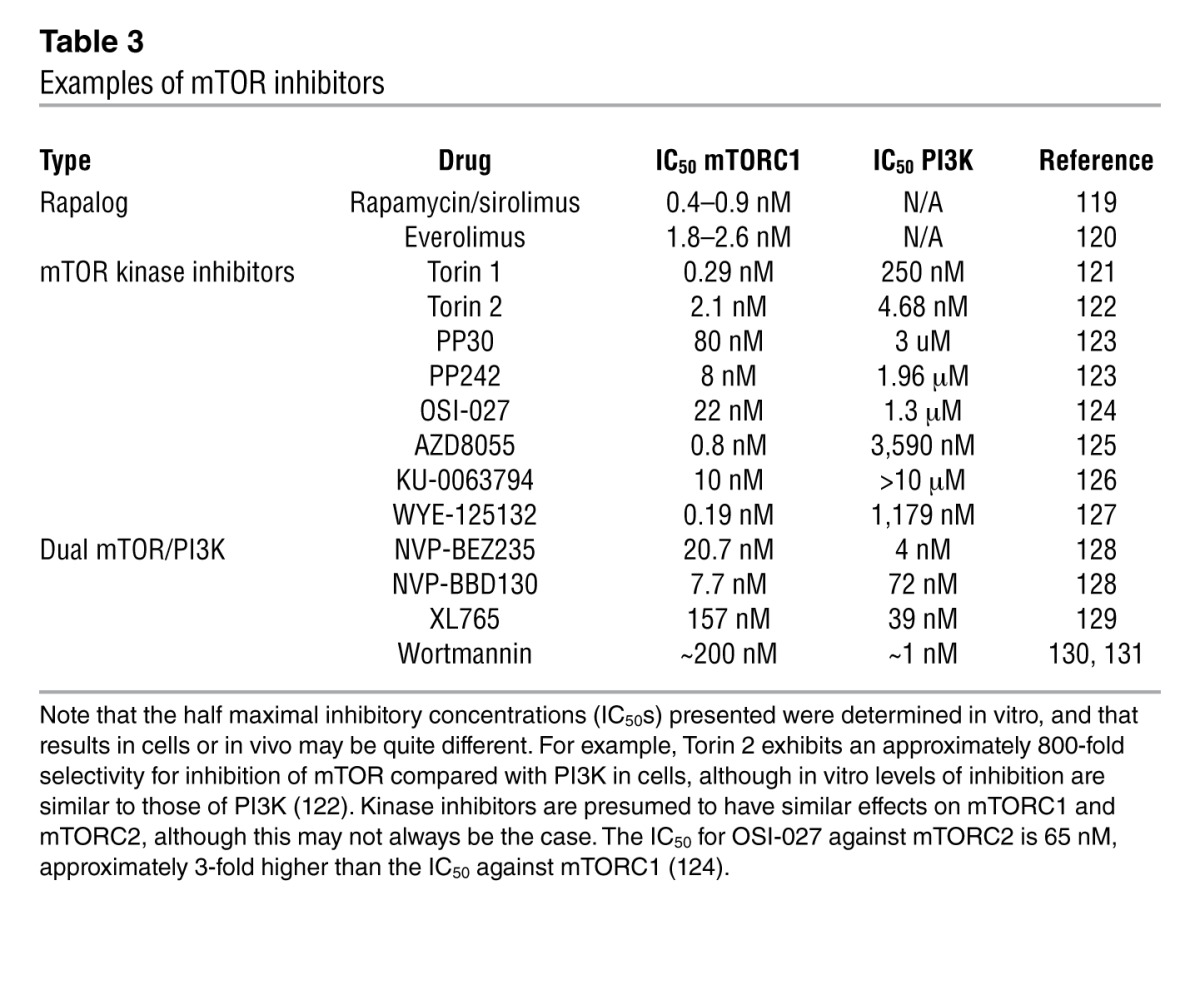
One possible explanation for the disappointing results to date is that in human cancer, rapalogs predominately inhibit mTORC1, leading to increased PI3K and AKT signaling by preventing negative feedback through S6K and GRB10 (Figure 1). AKT activity may be attenuated by subsequent mTORC2 disruption during chronic treatment but, if not sufficiently controlled, can promote cancer growth. Pharmaceutical interest has therefore focused on two new classes of compound: mTOR kinase inhibitors that inhibit both mTORC1 and mTORC2, and dual PI3K/mTOR kinase inhibitors (Table 3). mTOR kinase inhibitors such as torin 1 and WYE-125132 in particular have revealed important but previously unknown biological mechanisms, including rapamycin-resistant functions of mTORC1 (85, 86). However, as these compounds strongly inhibit both mTORC1 and mTORC2, it is unlikely that they will prove to have fewer undesirable side effects than rapamycin. One interesting possible exception is caffeine, which is a weak inhibitor of TOR. TOR inhibition mediates life span extension in yeast exposed to caffeine, and it is possible that the dose received from coffee might be sufficient to have a mild effect on mTOR in humans (87).
Table 3.
Examples of mTOR inhibitors
Indirect inhibitors.
Studies showing that S6K1–/– mice and Mtor+/–Mlst8+/– mice have extended longevity (16, 17) suggest that specific inhibition of mTORC1, or perhaps of S6K1, may provide many of the same benefits for age-related diseases as rapamycin. S6K1 inhibitors are now being developed (88), but even if sufficient selectivity is achieved, these compounds will require many years of development before FDA approval.
Fortunately, a number of FDA-approved compounds reduce mTORC1 activity. The most widely used by far is aspirin, which has been shown to decrease S6K phosphorylation in response to TNF-α signaling (89). Aspirin may act in part by inhibiting the phosphorylation of TSC1 by IKKβ (90), but it was recently demonstrated that aspirin can also activate AMPK (91). AMPK inhibits mTORC1 activity through two independent mechanisms, the activating phosphorylation of TSC2 and the inhibitory phosphorylation of raptor, an essential component of mTORC1 (92, 93). We might therefore expect other compounds that activate AMPK to specifically inhibit mTORC1 activity. In fact, this is the case: activation of AMPK by 5-aminoimidazole-4-carboxamide-1β-d-ribonucleoside (AICAR) results in decreased mTORC1 activity (94). Interestingly, aspirin influences longevity in rodent models, extending the average but not maximum life span of male mice (95), and has been found to decrease cancer-related and all-cause mortality in humans (96).
A screen of FDA-approved compounds for regulators of autophagy identified four compounds that reduce mTORC1 activity without affecting mTORC2: perhexiline, niclosamide, rottlerin, and amiodarone (97). Rottlerin regulates mTORC1 in a TSC-dependent fashion, but the mechanisms of action for perhexiline, niclosamide, and amiodarone are TSC independent (97). At least one natural product, phenethyl isothiocyanate, has also been shown to inhibit mTORC1 activity in a TSC-dependent manner (98). Given the wide variety of factors that can influence signaling through the mTOR complexes, many drugs are likely to have downstream effects on these pathways, particularly those that target insulin/IGF-1 signaling.
Is metformin a safer mTOR inhibitor?
A widely used FDA-approved AMPK activator is metformin, the first-line drug for the treatment of type 2 diabetes (99). Treatment with metformin lowers blood glucose levels, inhibits lipolysis, and decreases circulating free fatty acids, while producing few undesired side effects (100). The exact mechanism by which metformin acts is uncertain, but much attention has been focused on its ability to activate AMPK (101). Metformin inhibits phosphorylation of the mTORC1 substrates S6K1 and 4E-BP1 and decreases translation (102). While these effects were originally believed to result solely from the action of AMPK, it was recently demonstrated that metformin also regulates mTORC1 directly via inhibition of the Ras-related GTP binding (Rag) GTPases (see Figure 3 and ref. 103) and indirectly via upregulation of REDD1, which promotes TSC2 activity (104).
Figure 3. Metformin regulates mTORC1 signaling.
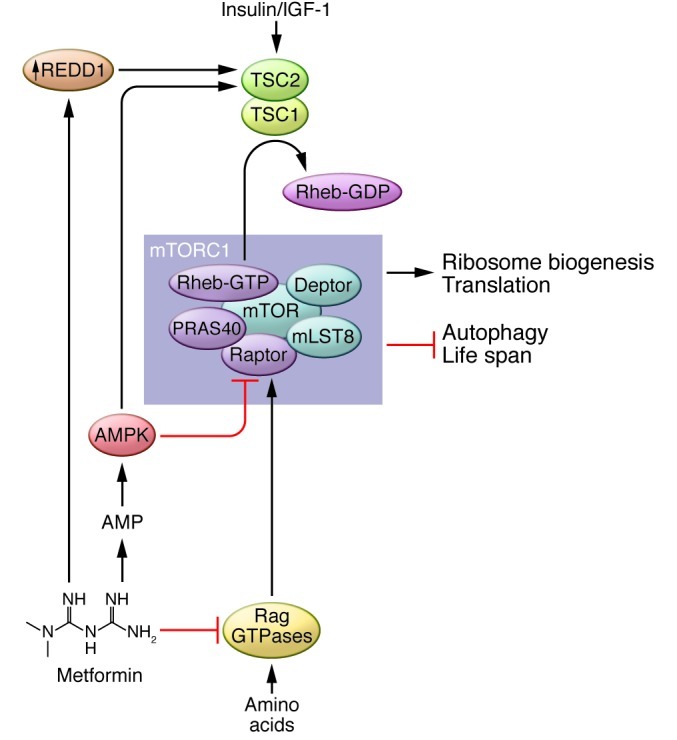
Metformin activates AMPK by inhibiting oxidative phosphorylation, which in turn negatively regulates mTORC1 signaling via activation of TSC2 and inhibitory phosphorylation of raptor. In parallel, metformin inhibits mTORC1 signaling by suppressing the activity of the Rag GTPases and upregulating REDD1.
Substantial evidence suggests that metformin functions to promote longevity in worms, rodents, and humans. Metformin extends both the life span and health span of the nematode C. elegans (105). These effects are independent of the insulin signaling pathway but are dependent on AMPK, as well as the oxidative stress transcription factor SKN-1/NRF2 (105). Metformin extends the life span of short-lived, tumor-prone HER2/neu mice and female SHR mice (106, 107). The National Institute on Aging Intervention Testing Program is currently treating genetically heterogeneous mice with metformin in order to definitively test its effect on life span. Interestingly, a long-term study in human patients found that treatment with metformin in patients with diabetes decreased mortality from all causes, including diabetes-related mortality, cancer, and myocardial infarction (108, 109). Importantly, an effect on maximum life span in humans or long-lived rodents has yet to be demonstrated.
Conclusion
Rapamycin shows significant promise in animal models as a pharmaceutical agent for the treatment of age-related disease. However, the significant side effects limit its long-term utility in humans. Similar problems are likely to emerge for rapalogs and mTOR kinase inhibitors. Moving forward, mTORC1-specific inhibitors that avoid disruption of mTORC2 signaling or that only reduce, rather than abolish, the activity of the mTORC1 pathway, may offer a safer method for the treatment of age-related diseases. The exploration of different dosing regimens for rapamycin and further testing of metformin have significant promise in this regard, but further research will be required to determine whether any of the available strategies for targeting mTOR will ultimately prove beneficial to human longevity and protect against age-related diseases.
Acknowledgments
We would like to thank all members of the Baur and Sabatini labs. The Baur lab is supported by a grant from the National Institute on Aging and a New Scholar Award from the Ellison Medical Foundation. The Sabatini lab is supported by grants from the NIH and awards from the American Federation for Aging Research, the Starr Foundation, the Koch Institute Frontier Research Program, and the Ellison Medical Foundation to D.M. Sabatini. D.W. Lamming is a Charles A. King Trust Postdoctoral Research Fellow. L. Ye is an American Heart Association Postdoctoral Fellow. D.M. Sabatini is an investigator of Howard Hughes Medical Institute.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(3):980–989. doi:10.1172/JCI64099.
References
- 1.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 2.Martel RR, Klicius J, Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol. 1977;55(1):48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- 3.Ingle GR, Sievers TM, Holt CD. Sirolimus: continuing the evolution of transplant immunosuppression. Ann Pharmacother. 2000;34(9):1044–1055. doi: 10.1345/aph.19380. [DOI] [PubMed] [Google Scholar]
- 4.Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo). 1984;37(10):1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- 5.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 6.Brown EJ, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 7.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 8.Sabers CJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270(2):815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Hsu PP, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 13.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5(10):e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha CW, Huh WK. Rapamycin increases rDNA stability by enhancing association of Sir2 with rDNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2011;39(4):1336–1350. doi: 10.1093/nar/gkq895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjedov I, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robida-Stubbs S, et al. TOR Signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15(5):713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahe E, et al. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation. 2005;79(4):476–482. doi: 10.1097/01.TP.0000151630.25127.3A. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anisimov VN, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176(5):2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anisimov VN, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 28.Halloran J, et al. Chronic inhibition of mTOR by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, and Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol. 2012;181(2):472–477. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77(6):1040–1052. doi: 10.1161/01.RES.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 32.Shioi T, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107(12):1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 33.Spilman P, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner JP, et al. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 1997;3(4):421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- 36.Hipkiss AR. On why decreasing protein synthesis can increase lifespan. Mech Ageing Dev. 2007;128(5–6):412–414. doi: 10.1016/j.mad.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 38.Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445(7130):922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 39.Pan KZ, et al. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6(1):111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zid BM, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139(1):149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mieulet V, et al. S6 kinase inactivation impairs growth and translational target phosphorylation in muscle cells maintaining proper regulation of protein turnover. Am J Physiol Cell Physiol. 2007;293(2):C712–C722. doi: 10.1152/ajpcell.00499.2006. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen S, Celis JE, Nielsen J, Christiansen J, Nielsen FC. Distinct repression of translation by wortmannin and rapamycin. Eur J Biochem. 1997;247(1):449–456. doi: 10.1111/j.1432-1033.1997.00449.x. [DOI] [PubMed] [Google Scholar]
- 43.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16(12):3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffen KK, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133(2):292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvers AL, Wood MS, Hu D, Kaywell AC, Dunn WA, Jr, Aris JP. Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy. 2009;5(6):847–849. doi: 10.4161/auto.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hars ES, et al. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3(2):93–95. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 49.Lionaki E, Markaki M, Tavernarakis N. Autophagy and ageing: insights from invertebrate model organisms. Ageing Res Rev. 2013;12(1):413–428. doi: 10.1016/j.arr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4(2):e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14(9):959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 53.Han X, Turdi S, Hu N, Guo R, Zhang Y, Ren J. Influence of long-term caloric restriction on myocardial and cardiomyocyte contractile function and autophagy in mice. J Nutr Biochem. 2012;23(12):1592–1599. doi: 10.1016/j.jnutbio.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kume S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11(4):668–674. doi: 10.1111/j.1474-9726.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106(6):1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 57.Marino G, et al. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008;17(14):2196–2211. doi: 10.1093/hmg/ddn120. [DOI] [PubMed] [Google Scholar]
- 58.Cao K, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3(89):89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 59.Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci U S A. 2007;104(12):4949–4954. doi: 10.1073/pnas.0611640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McClintock D, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2(12):e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 62.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205(10):2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105(49):19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yilmaz OH, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486(7404):490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen T, et al. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10(5):908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106(19):7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee KW, et al. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010;19(4):557–568. doi: 10.1089/scd.2009.0147. [DOI] [PubMed] [Google Scholar]
- 69.Murakami M, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24(15):6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenberger S, et al. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131(12):2467–2476. doi: 10.1038/jid.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franceschi C, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23(6):707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 74.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23(4):496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li BY, et al. The role of FK506-binding proteins 12 and 12.6 in regulating cardiac function. Pediatr Cardiol. 2012;33(6):988–994. doi: 10.1007/s00246-012-0298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z, et al. FKBP12.6-knockout mice display hyperinsulinemia and resistance to high-fat diet-induced hyperglycemia. FASEB J. 2010;24(2):357–363. doi: 10.1096/fj.09-138446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruan B, et al. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U S A. 2008;105(1):33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucanic M, et al. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 2011;473(7346):226–229. doi: 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCormack FX, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuber J, et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant. 2008;8(7):1471–1479. doi: 10.1111/j.1600-6143.2008.02267.x. [DOI] [PubMed] [Google Scholar]
- 82.Gyurus E, Kaposztas Z, Kahan BD. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: a long-term analysis of various treatment regimens. Transplant Proc. 2011;43(5):1583–1592. doi: 10.1016/j.transproceed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87(8 suppl):S23–S26. doi: 10.1097/TP.0b013e3181a05b7a. [DOI] [PubMed] [Google Scholar]
- 84.Populo H, Lopes JM, and Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thoreen CC, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shor B, et al. Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J Biol Chem. 2010;285(20):15380–15392. doi: 10.1074/jbc.M109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wanke V, et al. Caffeine extends yeast lifespan by targeting TORC1. Mol Microbiol. 2008;69(1):277–285. doi: 10.1111/j.1365-2958.2008.06292.x. [DOI] [PubMed] [Google Scholar]
- 88.Bilanges B, Vanhaesebroeck B. A new tool to dissect the function of p70 S6 kinase. Biochem J. 2010;431(2):e1–e3. doi: 10.1042/BJ20101445. [DOI] [PubMed] [Google Scholar]
- 89.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278(27):24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 90.Lee DF, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130(3):440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 91.Din FV, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–1515. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18(13):1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 95.Strong R, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 97.Balgi AD, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS ONE. 2009;4(9):e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cavell BE, Syed Alwi SS, Donlevy AM, Proud CG, Packham G. Natural product-derived antitumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. J Nat Prod. 2012;75(6):1051–1057. doi: 10.1021/np300049b. [DOI] [PubMed] [Google Scholar]
- 99.Nathan DM, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29(8):1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 100.Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108(8):1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 103.Kalender A, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ben Sahra I, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 105.Onken B, aDriscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anisimov VN, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8–9):685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 107.Anisimov VN, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 108.Scarpello JH. Improving survival with metformin: the evidence base today. Diabetes Metab. 2003;29(4 pt 2):S36–S43. doi: 10.1016/S1262-3636(07)70005-8. [DOI] [PubMed] [Google Scholar]
- 109.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 112.Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557(1–3):136–142. doi: 10.1016/S0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- 113.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5(4):265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131(16):3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 115.Luong N, et al. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4(2):133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 116.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20(2):174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harrison B, Tran TT, Taylor D, Lee SD, Min KJ. Effect of rapamycin on lifespan in Drosophila. Geriatr Gerontol Int. 2010;10(1):110–112. doi: 10.1111/j.1447-0594.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 118.Sun X, et al. Nutrient-dependent requirement for SOD1 in lifespan extension by protein restriction in Drosophila melanogaster. Aging Cell. 2012;11(5):783–793. doi: 10.1111/j.1474-9726.2012.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hedge S, Schmidt M. In: Doherty AM, ed Annual Reports in Medicinal Chemistry. To market, to market — 2004. San Diego, California, USA: Elsevier; 2005. [Google Scholar]
- 120.Brown EJ, et al. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377(6548):441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 121.Liu Q, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53(19):7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Q, et al. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2( 1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem. 2011;54(5):1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7(2):e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhagwat SV, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10(8):1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 125.Chresta CM, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 126.Garcia-Martinez JM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J. 2009;421(1):29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu K, et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70(2):621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 128.Marone R, et al. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7(4):601–613. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- 129.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27(41):5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 130.Brunn GJ, et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15(19):5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 131.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296(pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]


