Abstract
Milk fat globule-EGF 8 (MFGE8) plays important, nonredundant roles in several biological processes, including apoptotic cell clearance, angiogenesis, and adaptive immunity. Several recent studies have reported a potential role for MFGE8 in regulation of the innate immune response; however, the precise mechanisms underlying this role are poorly understood. Here, we show that MFGE8 is an endogenous inhibitor of inflammasome-induced IL-1β production. MFGE8 inhibited necrotic cell–induced and ATP-dependent IL-1β production by macrophages through mediation of integrin β3 and P2X7 receptor interactions in primed cells. Itgb3 deficiency in macrophages abrogated the inhibitory effect of MFGE8 on ATP-induced IL-1β production. In a setting of postischemic cerebral injury in mice, MFGE8 deficiency was associated with enhanced IL-1β production and larger infarct size; the latter was abolished after treatment with IL-1 receptor antagonist. MFGE8 supplementation significantly dampened caspase-1 activation and IL-1β production and reduced infarct size in wild-type mice, but did not limit cerebral necrosis in Il1b-, Itgb3-, or P2rx7-deficient animals. In conclusion, we demonstrated that MFGE8 regulates innate immunity through inhibition of inflammasome-induced IL-1β production.
Introduction
Milk fat globule-EGF 8 (MFGE8), or lactadherin, is a secretory glycoprotein containing C domains that bind to anionic phospholipids (1) and extracellular matrices (2), and 1 or 2 EGF-like domains with an RGD motif that binds integrins αvβ3 and αvβ5 (3). As such, MFGE8 appears to be instrumental in cell-cell interactions and has been involved in diverse physiological functions, including fertilization (4), branching morphogenesis (5), angiogenesis (6), or preservation of epithelial integrity (7, 8). One of the prominent functions of MFGE8 is also to link phosphatidylserine of apoptotic cells to integrins αvβ3 and αvβ5 of phagocytic cells, thereby providing a nonredundant pathway for the clearance of apoptotic cells (3, 9–11). More recent studies have shown that MFGE8 regulates adaptive immune responses in part through the promotion of tolerogenic antigen presenting cells, which facilitate the generation and activity of regulatory T cells, thereby restraining pathogenic T helper responses (12–14). MFGE8 also controls the intracellular processing of apoptotic self antigens into MHC-antigen complexes and limits pathogenic antigen crosspresentation (15).
The role of MFGE8 in the regulation of the innate immune response is less clear. MFGE8 expression has been associated with antiinflammatory effects in settings such as postischemic injury (16–18), experimental colitis (19), or sepsis (20). However, no particular antiinflammatory mechanism was identified, and the effects were indirectly related to the known role of MFGE8 in the clearance of apoptotic cells. Recent data suggested that MFGE8 might interfere with osteopontin binding to integrin αvβ3 and limits NF-κB activation in response to LPS in vitro (19, 21). However, it is still unknown whether this is a major pathway by which MFGE8 exerts its antiinflammatory effects, and its relevance to inflammatory settings other than sepsis is uncertain.
In the present work, we addressed the role of MFGE8 in postischemic injury. We show that MFGE8 controls postischemic cerebral injury through a previously unsuspected mechanism involving integrin β3-dependent inhibition of the inflammasome.
Results and Discussion
MFGE8 reduces postischemic cerebral tissue damage and inflammatory response.
We first compared Mfge8–/– mice and control WT littermates in a model of focal cerebral ischemia. We found that infarct size was significantly larger in Mfge8–/– mice compared with their controls (Figure 1A), a noteworthy augmentation of 38% which was abrogated by supplementation of Mfge8–/– with recombinant murine MFGE8 (rMFGE8) (Figure 1B). These results clearly indicate that endogenous MFGE8 is required for protection against excessive postischemic cerebral damage. We also found that supplementation of WT mice (Figure 1C) with rMFGE8 induced a significant reduction of infarct volume, in agreement with the recently reported beneficial effect of recombinant human MFGE8 in a model of cerebral injury in rats (16).
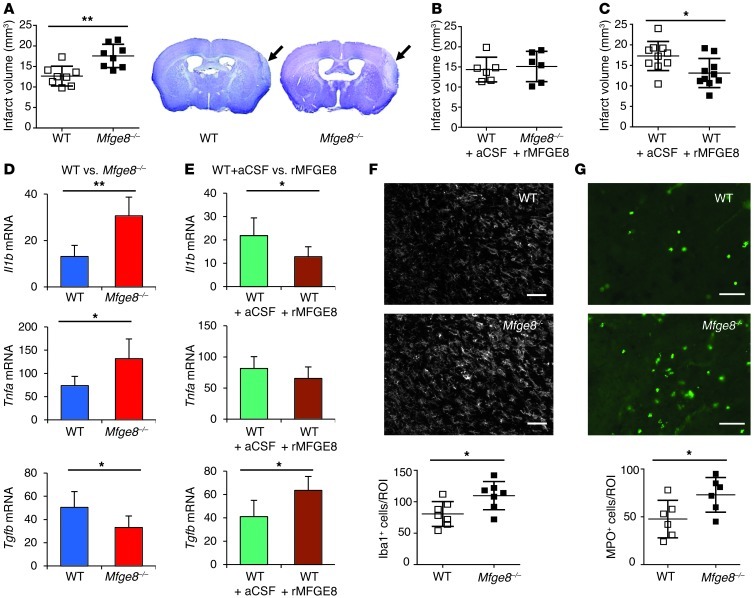
Figure 1. Effect of MFGE8 on cerebral infarct volume and brain inflammation.
(A–C) Representative photomicrographs of cresyl violet staining and infarct volume quantification in WT and Mfge8–/– mice, with or without supplementation with recombinant rMFGE8 (administered in artificial cerebrospinal fluid [aCSF], used as vehicle). (D and E) Cytokine expression (day 3 after artery occlusion) in brains of WT vs. Mfge8–/– mice (D) and brains of WT mice treated with artificial cerebrospinal fluid or rMFGE8 (E). (F and G) Representative photomicrographs and quantification of microglia/macrophages (Iba1 staining in F) and granulocyte accumulation (myeloperoxidase [MPO] staining in G) in ischemic brains of WT and Mfge8–/– mice at day 7 after artery occlusion. *P < 0.05; **P < 0.01; n = 7 to 8 mice per group. Scale bar: 50 μm.
We then investigated the potential mechanisms responsible for MFGE8 protective effect. MFGE8 has been shown to alter postischemic neovascularization (6) and fibrotic tissue response to injury (2). However, quantification of CD31-positive vascular area or collagen accumulation did not reveal any relevant difference between Mfge8–/– and WT mice (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI65167DS1).
MFGE8 is necessary for efficient clearance of apoptotic cells. To test this hypothesis in postischemic injury, we studied the association between macrophages/microglia and apoptotic cells. We observed reduced internalization of apoptotic material in Mfge8–/– mice with a concomitant increase in the percentage of noningested apoptotic cells (Supplemental Figure 2A). The results are very consistent with the higher density of dead cells, including dead neurons, that accumulate in the brains of Mfge8–/– mice at day 7 after cerebral ischemia (Supplemental Figure 2B). Conversely, treatment with rMFGE8 reduced the accumulation of apoptotic cells compared with vehicle-treated mice (Supplemental Figure 2C). These results highlight the important role of MFGE8 in apoptotic cell removal during postischemic cerebral injury.
The role of apoptotic cell removal in the induction of an antiinflammatory milieu has been well explored (22). In agreement with this concept, Mfge8–/– mice showed a marked increase in the expression of proinflammatory mediators IL-1β and TNF-α in the ischemic brain (respectively, +134%, P < 0.001, and +78%, P < 0.01), but a decrease of antiinflammatory TGF-β (–34%, P < 0.05) (Figure 1D) at day 3 after artery occlusion compared with controls. This was followed by enhanced accumulation of macrophages/microglia (Figure 1F) and neutrophils at day 7 after ischemic injury in Mfge8–/– mice (Figure 1G). Conversely, treatment of WT mice with rMFGE8 was associated with an antiinflammatory cytokine profile, revealed by an increase of TGF-β (+54%, P < 0.05) and a decrease of IL-1β (–41%, P < 0.05), although TNF-α expression was not altered (Figure 1E). Thus, the role of MFGE8 in the removal of apoptotic cells seems to be associated with the promotion of an antiinflammatory state in postischemic injury. However, the mechanistic pathways that directly mediate the protective effects of MFGE8 in this setting are still unknown.
Mfge8–/– antiinflammatory effect is mediated through the inflammasome/IL-1β pathway.
Focal cerebral ischemia leads to ischemic cell death both by necrosis (especially in the core of the infarct) and apoptosis (in the penumbra). Defective removal of apoptotic cells in Mfge8–/– mice would also lead to secondary necrosis. Given the major effect of MFGE8 modulation on IL-1β expression, we hypothesized that MFGE8 might directly alter inflammasome-mediated IL-1β production. The rationale for this hypothesis is also based on the following observations. Necrosis leads to accumulation of extracellular ATP, a potent activator of the inflammasome through the P2X7 receptor pathway (23), and induces the production of mature IL-1β. The inflammasome complex is activated after focal brain ischemia, and inhibition of inflammasome decreases caspase-1 activation and IL-1β processing (24). The IL-1 pathway has been implicated in the pathogenesis of ischemic brain damage (25, 26), and our results indicate a significant increase of IL-1β production in ischemic brains of Mfge8–/– mice compared with controls (Figure 1D and Supplemental Figure 3).
To test our hypothesis, we first used an in vitro model of BM-derived macrophages (BMDM). Incubation of LPS-primed BMDM with necrotic cells significantly induced IL-1β production, which was inhibited by incubation with apyrase, indicating an ATP-dependent process (Supplemental Figure 4). In addition, we checked using Nlrp3–/– macrophages, that ATP-induced IL-1β production was entirely dependent on NLRP3 (Xuan Li, unpublished observations). Interestingly, we found that preincubation of LPS-primed BMDM with rMFGE8 significantly reduced IL-1β production in response to necrotic cells (Supplemental Figure 4) or ATP stimulation (Figure 2A). Importantly, rMFGE8 suppressed caspase-1 activation in response to ATP (Figure 2B). Thus, MFGE8 directly alters inflammasome-mediated IL-1β production by macrophages in vitro. We then addressed the effect of rMFGE8 supplementation on this process in vivo. We found that cerebral ischemia significantly increased brain IL-1β production (Figure 2C) and caspase-1 activity (Figure 2D). Supplementation with rMFGE8 blunted both IL-1β production and the increase of caspase-1 activity after ischemia compared with vehicle-treated mice (Figure 2, C and D).
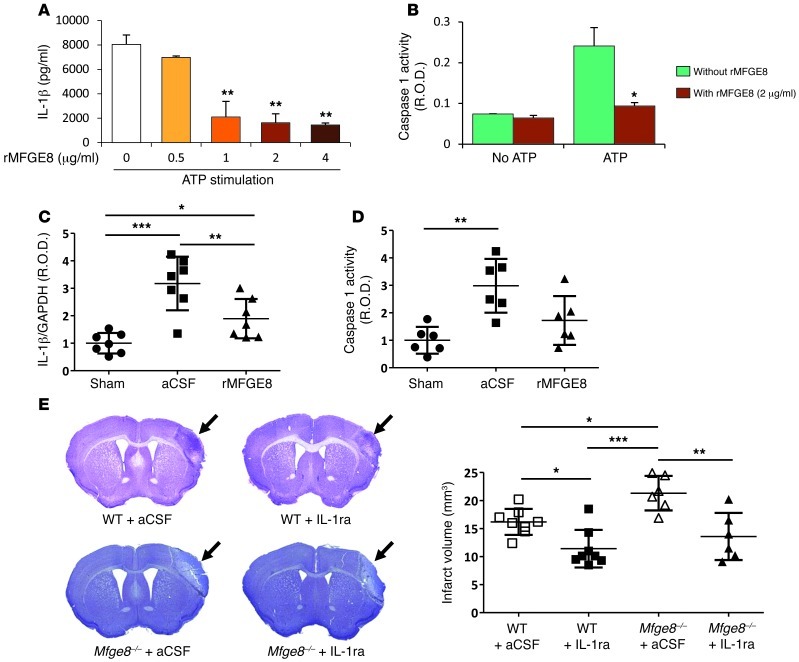
Figure 2. MFGE8 protects against postischemic cerebral injury through inhibition of inflammasome-mediated IL-1β production.
(A and B) rMFGE8 inhibits ATP-induced IL-1β production (A) and caspase-1 activity (B) (see Methods). Data are representative of 3 independent experiments for in vitro experiments. Results are given as relative optic density (R.O.D.). (C and D) Cerebral ischemia significantly increased brain IL-1β production (C) and caspase-1 activity (D) in mice treated with artificial cerebrospinal fluid vehicle. Supplementation with rMFGE8 blunted both IL-1β production and the increase of caspase-1 activity. (E) Representative photomicrographs of cresyl violet staining and infarct volume quantification in WT and Mfge8–/– mice, with or without treatment with IL-1 receptor antagonist (IL-1ra) (administered in artificial cerebrospinal fluid, used as vehicle). *P < 0.05; **P < 0.01; ***P < 0.001; n = 6 to 8 mice per group for in vivo experiments.
We then examined the direct contribution of the IL-1 pathway to the protective effect of MFGE8 in postischemic injury. We found that treatment with IL-1 receptor antagonist completely abrogated the increase of infarct size seen in Mfge8–/– mice compared with controls (Figure 2E). In addition, rMFGE8 supplementation did not reduce infarct size in Il-1β–/– mice (Supplemental Figure 5). Thus, MFGE8-dependent regulation of IL-1β production plays a nonredundant role in protection against postischemic injury.
Mfge8–/– inhibits the inflammasome pathway via β3 integrin.
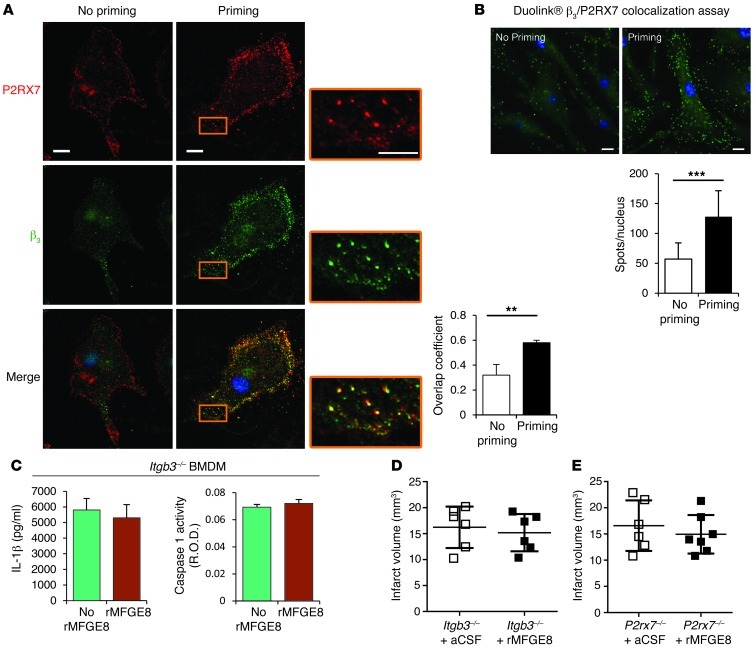
We then focused on the potential underlying mechanism through which MFGE8 modulates necrotic cell– or ATP-induced IL-1β production. αvβ3 integrin is one of the main MFGE8 receptors. P2X7 receptor is an ATP sensor and activates the NLRP3 pathway (23). As expected, ATP-induced IL-1β production was entirely dependent on P2X7 receptor as checked by use of P2rx7–/– macrophages (Xuan Li, unpublished observations). We therefore hypothesized that MFGE8 inhibits the inflammasome pathway through interaction between β3 integrin and P2X7 receptor. To test our hypothesis, we first studied the putative interaction between β3 integrin and P2X7 receptor in BMDM. Using Duolink in situ proximity ligation assay and confocal microscopy, we found a low level of colocalization between β3 integrin and P2X7 receptor in the absence of inflammatory stimuli. Interestingly, however, priming of BMDM with LPS increased β3 expression (Supplemental Figure 6) and induced tight spatial association between β3 integrin and P2X7 receptor (Figure 3, A and B), suggesting a potential functional role under inflammatory settings. This is supported by the finding that Itgb3–/– BMDM produce significantly more IL-1β than WT cells in response to ATP stimulation (Supplemental Figure 7). We then examined the direct role of β3 integrin in MFGE8-mediated IL-1β inhibition. We found that rMFGE8 did not alter ATP-induced IL-1β production or caspase-1 activity in Itgb3–/– BMDM (Figure 3C). Finally, we addressed the requirement for β3 integrin and P2X7 receptor pathways for MFGE8 protective effect in vivo. Interestingly, Itgb3–/– and P2rx7–/– mice were insensitive to the protective effects of rMFGE8 on infarct size (Figure 3, D and E).
Figure 3. MFGE8 inhibits the inflammasome pathway via β3 integrin.
(A) Representative photomicrographs of costaining for P2RX7 and β3 integrin in BMDM before and after LPS priming and analysis using confocal microscopy. A tight spatial association (quantified by the overlap coefficient) was observed between β3 integrin and P2RX7 after LPS priming. (B) Duolink in situ PLA colocalization assay proving the spatial association between β3 integrin and P2RX7 in BMDM after LPS priming. The spots in B indicate colocalized P2X7R and β3. Data are representative of 3 independent experiments. (C) MFGE8 did not inhibit ATP-induced IL-1β production or caspase-1 activity in BMDM recovered from Itgb3–/– mice. (D and E) Quantification of infarct volume in Itgb3–/– and P2rx7–/– mice treated or not with rMFGE8; rMFGE8 did not reduce infarct volume in Itgb3–/– and P2rx7–/– mice. **P < 0.01; ***P < 0.001; n = 6 mice per group for in vivo experiments. Scale bars: 10 μm.
MFGE8 expression is associated with immunomodulatory effects in various inflammatory settings, but the specific underlying mechanisms are still poorly described. Our work identifies a previously unsuspected antiinflammatory mechanism by which MFGE8 controls postischemic inflammatory response and injury. Necrotic cells generated after ischemic injury release extracellular ATP, which activates the inflammasome pathway and leads to IL-1β production. MFGE8 plays a nonredundant role in the control of this innate immune response through β3 integrin and limits P2X7 receptor–dependent IL-1β production. Our results describe a new immunomodulatory function of MFGE8 and might have broad pathophysiological and therapeutic implications, particularly in diseases associated with enhanced activation of the inflammasome.
Methods
Methods are described in detail in Supplemental Methods. All mice were fully backcrossed to a C57BL/6 background.
Permanent focal cerebral ischemia.
Under anesthesia and using a small craniotomy, the middle cerebral artery (MCA) was electrocoagulated. Assessment of infarct volume was performed at day 7 after ischemia on sections stained with Cresyl violet.
In vitro stimulation, assessment of IL-1β production, and caspase-1 activity.
BMDM cells were primed for 6 hours with LPS unless otherwise specified. Cells were then incubated with the indicated amount of MFGE8 prior to stimulation with ATP or necrotic thymocytes. Cell media were collected for ELISA assay, caspase-1 activity (colorimetric assay), and/or Western blot.
Real-time PCR.
Ready-to-use primers for IL-1β, TNF-α, IL-10, TGF-β, and cyclophilin A were used (QIAGEN).
Immunohistochemistry.
The sources of primary antibodies are listed in Supplemental Methods. Apoptosis assay was performed using Roche In Situ Cell Death Detection Kit (Roche). Duolink in situ proximity ligation assay was performed according to the manufacturer’s instructions (Olink).
Efferocytosis assessment.
Association between CD68+ cells (macrophage/microglia) and TUNEL+ cells was determined on 3 magnification fields. Internalization was assessed on deconvoluted pictures using a Zeiss ApoTome (Imager Z1 with ApoTome, Carl Zeiss International).
Statistics.
Statistical analyses were performed with Prism 5 software (GraphPad). All data are expressed as mean ± SD. Comparisons of 2 different groups were analyzed by Mann-Whitney U test. For more than 2 groups, we used ANOVA test with Bonferroni’s post-test analysis. A P < 0.05 was considered statistically significant.
Study approval.
Experiments were performed under French Ministry of Agriculture permit no. 02934. The study was also approved by the Home Office, PPL 80/2426, United Kingdom.
Supplementary Material
Acknowledgments
This work was supported by grants from the British Heart Foundation, Fondation pour la Recherche Médicale, France, and by an ERC Starting Grant (to Z. Mallat). We are indebted to Feriel Azibani and Stéphane Potteaux for their help and advice.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(3):1176–1181. doi:10.1172/JCI65167.
References
- 1.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106(6):957–966. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atabai K, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119(12):3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 4.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114(4):405–417. doi: 10.1016/S0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 5.Ensslin MA, Shur BD. The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc Natl Acad Sci U S A. 2007;104(8):2715–2720. doi: 10.1073/pnas.0610296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvestre JS, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11(5):499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 7.Bu HF, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117(12):3673–3683. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond AS, Shur BD. A novel role for SED1 (MFG-E8) in maintaining the integrity of the epididymal epithelium. J Cell Sci. 2009;122(pt 6):849–858. doi: 10.1242/jcs.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 10.Kranich J, et al. Engulfment of cerebral apoptotic bodies controls the course of prion disease in a mouse strain-dependent manner. J Exp Med. 2010;207(10):2271–2281. doi: 10.1084/jem.20092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117(7):1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jinushi M, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206(6):1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ait-Oufella H, et al. Lactadherin-defciency induces apoptotic cell accumulation, alters the regulatory immune response, and accelerates atherosclerosis in mice. Circulation. 2007;115(16):2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 15.Peng Y, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest. 2011;121(6):2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheyuo C, et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology. 2012;62(2):890–900. doi: 10.1016/j.neuropharm.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui T, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181(3):238–246. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda A, et al. Protective effect of milk fat globule-epidermal growth factor-factor VIII after renal ischemia-reperfusion injury in mice. Crit Care Med. 2011;39(9):2039–2047. doi: 10.1097/CCM.0b013e3182227a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz MM, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182(11):7222–7232. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 20.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25(6):586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 21.Aziz M, et al. Pre-treatment of recombinant mouse MFG-E8 downregulates LPS-induced TNF-alpha production in macrophages via STAT3-mediated SOCS3 activation. PLoS One. 2011;6(11):e27685. doi: 10.1371/journal.pone.0027685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407(6805):784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 23.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29(3):534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 25.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21(15):5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18(4):269–276. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.