Abstract
Specifically, FK506-binding proteins 12 (FKBP12) and 12.6 (FKBP12.6) are cis–trans peptidyl prolyl isomerases that are expressed in the heart. Both FKBP12 and FKBP12.6 were previously known to interact with ryanodine receptors in striated muscles. Although FKBP12 is abundantly present in the heart, its function in the heart is largely uncertain. Recently, by generating FKBP12 transgenic overexpression and cardiac-restricted knockout mice, we showed that FKBP12 is critically important in regulating trans-sarcolemmal ionic currents, predominately the voltage-gated Na+ current, INa, but it appears to be less important for regulating cardiac ryanodine receptor function. Similar genetic approaches also confirm the role of FKBP12.6 in regulating cardiac ryanodine receptors. The current study demonstrated that FKBP12 and FKBP12.6 have very different physiologic functions in the heart.
Keywords: FK506-binding protein, Cardiac function, Arrhythmia, Voltage-gated sodium channel, Ryanodine receptor, Calcium release
The FK506-binding proteins (FKBP) are immunophilins that interact with immunosuppressants FK506 and rapamycin [23]. At least 10 mammalian FKBPs have been described to date [23]. They commonly contain conserved cis–trans peptidyl prolyl isomerase domains [23]. Both FKBP12 and FKBP12.6, encoded by Fkbp1a and Fkbp1b, respectively, are small (~12 kDa) cytoplasmic proteins that share 85 % amino acid homology and a near identical tertiary structure [12, 13, 23]. By binding to the immunosuppressive drugs FK506 and rapamycin, FKBP12–FK506 and FKBP12–rapamycin complexes inhibit calcineurin and mTOR (mammalian target of rapamycin), respectively, and subsequently suppress T-cell activation [3, 32].
Both FKBP12 and FKBP12.6 are ubiquitously expressed, including the cardiac and skeletal muscles [9]. A recent report has estimated that the protein concentrations of FKBP12 and FKBP12.6 in cardiomyocytes are, respectively, 1 μmol/l and about 150 nmol/l [6].
Both FKBP12 and FKBP12.6 were found to be associated with calcium release channel ryanodine receptors (RyR) [9]. The stoichiometry of binding is 4 FKBP molecules for every RyR tetramer (i.e., 1 FKBP to 1 RyR monomer). Tightly binding to the skeletal muscle ryanodine receptor (RYR1), FKBP12 can be co-purified through sucrose density gradient centrifugation [7].
Detailed biochemical and physiologic analyses further supported the role of FKBP12 as a channel modulator for RyR1 [9]. The FKBP12 binding site was mapped to a region between amino acids 2,458 and 2,468 of rabbit RyR1, and Val2461–Pro2462 residues were shown to be critical for FKBP12 binding. Each mutation of Val2461-Glu–RyR1, Val2461-Gly–RyR1, and Val2461-Ile–RyR1 abolished RyR1–FKBP12 interaction, and the mutant channels had altered gating frequencies [2, 5]. Additionally, FKBP12.6 can bind to normal RyR1, but with lower affinity [25]. Intriguingly, the normal channel function of Val2461-Ile–RyR1 can be restored by FKBP12.6 [5], suggesting a different binding property and specificity between FKBP12 and FKBP12.6.
Given the extensive studies and a series of pharmacophysiologic analyses, it is widely believed that FKBP12 is important for regulating skeletal muscle excitation–contraction coupling (E–C coupling) [9]. However, the role of FKBP12 in regulating cardiac ryanodine receptors (RyR2) has been highly debatable. Apparently, RyR2 has a significantly higher binding affinity to FKBP12.6 [25]. A recent work using a fluorescence-labeling approach to directly measure in situ binding of FKBP12 and FKBP12.6 in permeabilized cardiomyocytes confirmed a high FKBP12.6–RyR2 affinity (Kd = 0.7 ± 0.1 nmol/l) and a lower FKBP12–RyR2 affinity (Kd = 206 ± 70 nmol/l) despite the fact that both FKBP12.6 and FKBP12 were localized at Z-lines [6].
This study also demonstrated that FKBP12.6, but not FKBP12, inhibits resting RyR2 activity [6]. Studies also suggest that the interaction site of FKBP12.6–RyR2 is very similar to that of FKBP12–RyR1 [21, 26, 27], and Ile2427–Pro2428 residues are essential for the FKBP12.6–RyR2 interaction [15]. However, another group demonstrated that a region between amino acids 1,815 and 1,855 near divergent region 3 is critical for FKBP12.6 binding [16, 31]. In addition, C-terminal region of RyR2 also was suggested as interacting with FKBP12.6 [33]. Apparently, despite a similar expression pattern and protein structure, FKBP12 and FKBP12.6 have distinctly different physiologic functions.
Mice deficient in FKBP12 die in utero due to severe ventricular defects including hypertrabeculation, ventricular noncompaction, and ventricular septal defects (VSD) [22], suggesting its essential role in cardiac development. In contrast, FKBP12.6-deficient mice display normal cardiac development and are viable. However, once mature, adult FKBP12.6-deficient mice display abnormal cardiac physiology including either enlarged hearts [30] or exercise-induced cardiac arrhythmias and sudden death [28]. It has been a highly debated question whether FKBP12, FKBP12.6, or both are key players in regulating cardiac function and various cardiac pathogenetic pathways. More recently, a series using mouse transgenic approaches to determine the biologic function of FKBP12 and FKBP12.6 led to several unexpected results. This brief review aims to summarize and update our recent findings.
Cardiomyocyte-Restricted FKBP12 Knockout Mice
Germline FKBP12 knockout mice (FKBP12-/-) demonstrated severe cardiac developmental defects, notably including ventricular hypertrabeculation, noncompaction, and VSD [22]. The majority of FKBP12-/- mice died between E14.5 and birth. A few FKBP12-/- survivors had severe compromised cardiac function and died before reaching to 2 months of age [22].
Our first thought was that the cardiac developmental defects seen in FKBP12-/- mice were due to its essential role in regulating calcium release channel RyR2. To test the functional properties of RyR1 and RyR2 in the absence of FKBP12, skeletal muscle and cardiac muscle membranes from wild-type and FKBP12-deficient mice were prepared and reconstituted into planar lipid bilayers. Both RyR1 and RyR2 from FKBP12-deficient mice demonstrated an increased probability of opening and stayed mostly in subconductance states compared with RyR1 and RyR2 from wild-type mice [22]. Thus, we concluded that FKBP12 was a key modulator for RyR2 [22]. However, the question of how altered RyR2 function led to these specific cardiac developmental defects in FKBP12-/- mice was very puzzling.
Given that FKBP12 is ubiquitously expressed, we generated FKBP12 conditional knockout mice to test a cardiomyocyte autonomous contribution to the cardiac developmental defects. A first report from Tang et al. [24] had demonstrated that ablation of FKBP12 in skeletal muscle using MCK-cre mice led to altered skeletal muscle E–C coupling, confirming the role of FKBP12 in regulating skeletal muscle function and RyR1. Surprisingly, this work also noted that the cardiac function was largely normal in the mutant mice, although FKBP12 expression also was ablated in cardiomyocytes [24], suggesting that FKBP12 was not critical for RyR2 function. These findings were later confirmed by using a cardiomyocyte-specific cre line, MHC-cre mice, further suggesting that the cardiac defects in FKBP12-deficient mice were not primarily derived from FKBP12 deficiency in cardiomyocytes [14]. Thus, the altered RyR2 function seen in FKBP12-deficient mice was not the mechanism underlying the cardiac developmental defects. We currently are in the process of determining whether RyR2 function is altered in the FKBP12 conditional knockout hearts.
Cardiomyocyte-Restricted FKBP12 Overexpression Transgenic Mice
Overexpression of FKBP12 in cultured rabbit ventricular cardiomyocytes via adenovirus gene delivery apparently led to a slight increase in sarcoplasmic reticulum calcium load, which likely was due to the reduced calcium leak, as evinced by a reduced Ca2+ spark frequency at rest in the FKBP12 overexpressing cardiomyocytes [20]. To determine the biologic impact of FKBP12 upregulation on cardiac function, we generated cardiomyocyte-restricted FKBP12 transgenic mice using a-myosin heavy chain promoter (MHC-FKBP12). Three independent transgenic lines were generated. Western blot analyses demonstrated that all three transgenic lines exhibited about a ninefold increase in FKBP12 protein levels in the hearts compared with their nontransgenic control mice. All the transgenic mice showed normal development and growth. However, about 38 % of the MHC-FKBP12 transgenic mice died suddenly at 4–6 weeks of age [14].
Surface electrocardiograms (ECGs) recorded from adolescent MHC-FKBP12 transgenic mice showed significant prolongations of the PP interval, P-wave duration, PQ interval, and QRS duration compared with nontransgenic mice [14]. Almost all the MHC-FKBP12 mice presented with various degrees of higher-degree atrioventricular (AV) conduction block. Ambulatory ECG recordings also demonstrated intermittent complete AV block in MHC-FKBP12 mice [14], which was further confirmed by electrophysiologic studies using Langendorff-perfused hearts. The conduction velocity in left ventricular epicardium also was assessed using the optical mapping technique. The conduction velocity in MHC-FKBP12 transgenic hearts was significantly slower than in nontransgenic hearts [14]. Finally, microelectrode measurements of transmembrane action potentials (AP) from the left ventricular epicardium of isolated hearts showed deceleration of the maximal phase 0 upstroke velocity (dV/dt)max and a marked AP prolongation in transgenic hearts (Fig. 1a). These findings suggested that chronic FKBP12 overexpression was associated with abnormal cardiac conduction and repolarization [14].
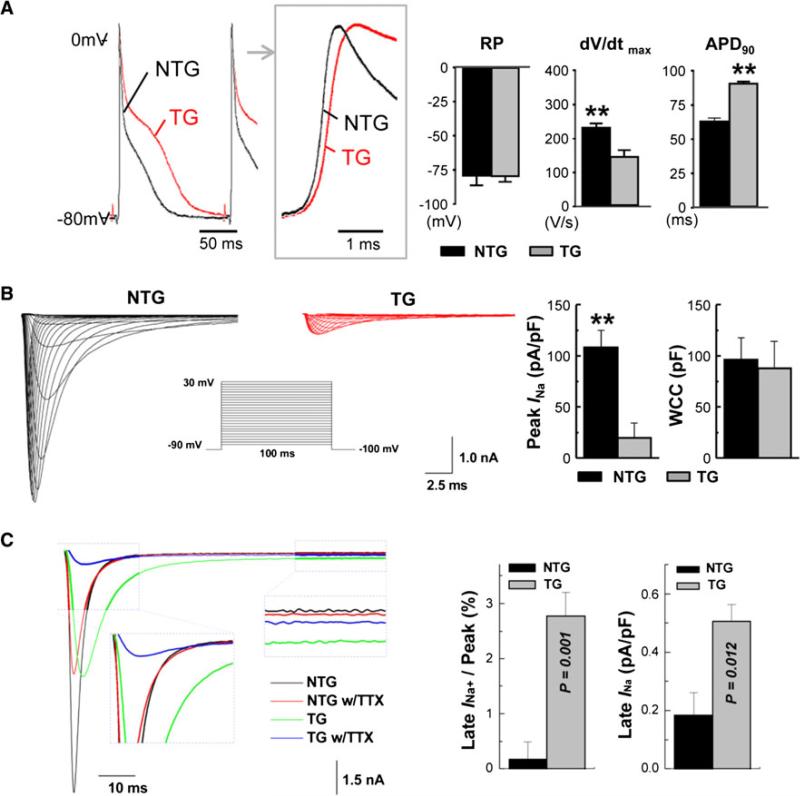
Fig. 1.
Cellular electrophysiology of MHC-FKBP12 ventricular cardiomyocytes. a Representative ventricular transmembrane action potentials (APs) recorded from isolated transgenic (TG) and non-transgenic (NTG) hearts. The box shows the initial portions of the respective APs in an expanded time scale. On the average, the maximum upstroke velocity of phase 0 of the AP (dV/dt)max was decreased in TG hearts compared with NTG hearts despite similar mean resting membrane potentials (RP). Significantly longer action potential duration (APD) at 90 % repolarization (APD90) was noted in TG hearts. b Voltage-clamp analysis of macroscopic INa in ventricular cardiomyocytes isolated from MHC-FKBP12 and wild-type hearts. Overexpression of FKBP12 decreases the peak Na+ current. Representative INa traces are elicited by 120 ms depolarizing pulses to potentials of –90 to +30 mV from a holding potential of –100 mV in 5 mV increments (interpulse interval, 1 s). The insert shows a schematic of the voltage-clamp protocol. The bar graphs in (b) show Means ± Standard Deviations of maximal peak INa densities measured at –5 and –25 mV in MHC-FKBP12 and wild-type myocytes, respectively, and whole-cell capacitance (WCC), **p < 0.01. c FKBP12 overexpression enhances a persistent Na+ current. Original traces of INa elicited –100 to +30 mV for 400 ms before and after exposure to 3 μmol/l tetrodotoxin (TTX) in the external solution. TTX-sensitive components were averaged over the last 100 ms of the depolarizing pulse and normalized to the cell capacitance. Overexpression of FKBP12 increased mean late INa density (p < 0.05)
A major determinant of electrical conduction is the magnitude of Na+ influx through voltage-gated Na+ channels during the initial fast membrane depolarization [8]. Accordingly, using the whole-cell voltage-clamp technique, we compared the density and properties of the macroscopic voltage-gated Na+ current (INa) in MHC-FKPB12 and wild-type cardiomyocytes. The mean peak INa density was dramatically reduced (~80 %) in MHC-FKPB12 cardiomyocytes compared with their nontransgenic counterparts, whereas mean whole-cell capacitance was not significantly altered (Fig. 1b). Overexpression of FKBP12 shifted the peak INa–V curve to more positive potentials, indicating that the number of Na+ channels activated at a given membrane voltage was reduced.
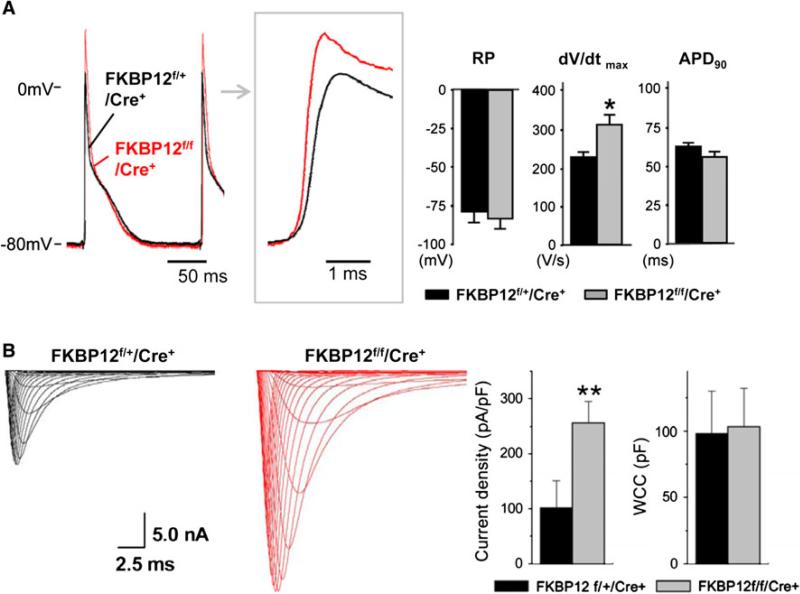
Furthermore, FKBP12 overexpression significantly slowed both the early and late component of INa inactivation compared with those of wild-type cardiomyocytes and enhanced a persistent and tetrodotoxin-resistant (3 μmol/l) late INa component (Fig. 1c) [14]. Interestingly, left ventricular AP recordings from Langendorff-perfused myocardial-specific FKBP12 conditional knockout hearts (FKBP12f/f/MHC-Cre) demonstrated a significant acceleration of the maximal phase 0 upstroke velocity compared with the control condition (Fig. 2a), suggesting an increase in INa density. Whole-cell voltage-clamp experiments confirmed that the peak INa density in FKBP12-deficient ventricular cardiomyocytes was more than twice as large as that in control cells (Fig. 2b). Thus, FKBP12 ablation profoundly affected peak INa density in ventricular cardiomyocytes and altered INa inactivation gating [14].
Fig. 2.
Cellular electrophysiologic analyses of FKPB12f/f/MHC-Cre+ and FKBP12f/+/MHC-Cre+ hearts. a Representative ventricular transmembrane APs recorded from an FKBP12f/f/MHC-Cre+ and an FKBP12f/+/MHC-Cre+ heart. The maximum upstroke velocity of phase 0 of the AP (dV/dt)max and the peak action potential amplitude were increased in FKBP12f/f/MHC-Cre+ hearts compared with control hearts. The means of RP and APD90 were similar between FKBP12f/f/Cre+ and FKBP12f/+/Cre+ hearts, *p < 0.05. b INa traces recorded from isolated FKBP12f/f/MHC-Cre+ and control FKBP12f/+/MHC-Cre+ ventricular cardiomyocytes. The maximal peak INa in FKBP12f/f/MHC-Cre+ myocytes was increased more than 2.5-fold compared with FKBP12f/+/MHC-Cre+ cells (15 cells/6 hearts), **p < 0.01
These findings suggested that FKBP12 is an important regulator of INa. In contrast to the dramatic alteration of INa, the voltage dependence of the inwardly rectifying K+ current (IK1), the transient outward K+ current (Ito), the sustained K+ current (IKsus), the L-type Ca2+ current (ICa,L), and the (Ca2+)i transients was either unchanged or only slightly affected [14], suggesting that these channels were not likely to underlie the delay in ventricular repolarization in the transgenic cardiomyocytes. Our current focus was to determine the underlying biochemical mechanism by which FKBP12 regulates voltage-gated sodium channels.
FKBP12.6 Knockout Mice
Two independent mouse strains deficient in FKBP12.6 had been reported. However, intriguingly, they demonstrated two distinctively different cardiac phenotypes. The first strain, generated via the targeting of 129SvEv mouse embryonic stem (ES) cells, displayed gender-specific adult cardiac hypertrophy [10, 30]. The second strain via DBA/lacJ ES cells displayed stress/exercise-induced cardiac sudden death [28]. Interestingly, intracellular calcium release appeared to be altered in both FKBP12.6-deficient mouse models, suggesting that secondary genetic factors may contribute to the final pathogenetic outcome.
To determine whether the cardiac enlargement in FKBP12.6-deficient males was associated with abnormal cardiac gene expression, we analyzed the expression levels of several cardiac markers such as atrial natriuretic peptide (ANP), αMHC, βMHC, skeletal α-actin (acta1), sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), and phospholamban (pln) [1, 4, 19]. Acta1, ANF, and βMHC, present only in embryonic hearts, are reactivated to persistently higher expression levels in hypertrophic hearts. Typically, SERCA2a and pln, found to be downregulated in hypertrophic and failing hearts, are involved in regulating intracellular Ca2+ homeostasis and contractile function of cardiomyocytes [1, 19]. Intriguingly, although cardiac hypertrophy was seen in FKBP12.6-deficient adult males, the mRNA levels of these cardiac hypertrophy markers were not elevated [10]. This observation suggests that FKBP12.6-mediated signaling is likely more relevant to physiologic than to pathologic hypertrophy [11, 17], consistent with the fact that FKBP12.6-deficient mice never display heart failure, a common end stage for the pathologic hypertrophic heart.
To determine whether FKBP12.6-deficient mice in a 129SvEv/C57 background also can experience exercise-induced cardiac arrhythmia and sudden death in addition to hypertrophy, we compared conscious ECG parameters in these mutant mice and male littermates (age, 5–6 months) using a protocol similarly applied in characterizing another FKBP12.6 knockout strain [28]. Continuous recordings of ECG were collected from each mouse. No significant differences were observed for RR intervals, PR intervals, QRS duration, rate-corrected QT intervals, or resting heart rate. Neither FKBP12.6-deficient nor wild-type mice displayed arrhythmia or syncope during ECG probe implantation or under sedentary conscious conditions [10].
Furthermore, we subjected the mutant mice and the wild-type mice to a strenuous exercise protocol followed by intraperitoneal injection of epinephrine as previously described [18, 28]. Although both groups of mice had elevated heart rates after exercise, no syncope or polymorphic ventricular arrhythmia was observed in either group. After intraperitoneal injection of epinephrine, we did not observe differences between the two groups of mice. Again, both groups had elevations of heart rate, but neither FKBP12.6-deficient mice nor their wild-type littermates displayed syncope or polymorphic ventricular arrhythmia. These observations indicated that the 129SvEv/C57B6 strain of FKBP12.6-deficient mouse does not experience exercise-induced arrhythmia and sudden death.
Cardiomyocyte-Restricted FKBP12.6 Overexpression Transgenic Mice
Similarly, we generated MHC-FKBP12.6 transgenic mice [10]. These transgenic mice developed normally and had a normal life span. Histologic and functional characterization of these transgenic mice indicated that overexpression of FKBP12.6 did not significantly alter cardiac structure or function [10]. Expression of FKBP12.6 is ubiquitous. One important question concerned whether the abnormal cardiac hypertrophy seen with FKBP12.6-deficient mice in a 129SvEv/C57B6 background was directly caused by the loss of FKBP12.6 expression in cardiomyocytes. To test this, we generated MHC-FKBP12.6/FKBP12.6–/– (129SvEv/C57B6) compound mice in which FKBP12.6 overexpression was restricted to cardiomyocytes. Heart size and cardiac morphology were compared between littermate adult males (5-months-old) with different genotypes, namely, MHC-FKBP12.6/FKBP12.6–/–, FKBP12.6–/–, and FKBP12.6+/–, and wild type. Our data demonstrated that cardiomyocyte-specific expression of FKBP12.6 prevented cardiac enlargement in FKBP12.6-deficient adult males. This observation strongly suggested a cardiomyocyte autonomous mechanism underlying the development of cardiac hypertrophy in FKBP12.6-deficient males.
Previously, it had been shown that the properties of Ca2+ sparks and intracellular calcium transients were altered in FKBP12.6-deficient cardiomyocytes [30]. These findings were consistent with an increase in open probability of RyR2 in the absence of FKBP12.6 and pharmacologic dissociation of FKBP12.6 from RyR2-channel complex [28, 29].
To evaluate Ca2+ release in cardiomyocytes isolated from MHC-FKBP12.6 transgenic mice, we measured Ca2+ sparks in the cardiomyocytes derived from MHC-FKBP12.6 transgenic hearts. We observed normal Ca2+ release in MHC-FKBP12.6 cardiomyocytes compared with wild-type control mice. To determine whether the altered Ca2+ release in FKBP12.6-deficient cardiomyocytes was a direct consequence of FKBP12.6 ablation in cardiomyocytes or not, we compared the characteristics of calcium sparks in cardiomyocytes isolated from MHC-FKBP12.6/FKBP12.6–/– (male) with those of Ca2+ sparks in cardiomyocytes isolated from sex-matched littermate FKBP12.6–/–, MHC-FKBP12.6, and wild-type control mice [10]. These parameters included the frequency and amplitude of the Ca2+ spark, full width at half maximum (FWHM) spark size, spark rising time, and half decay time. For all the parameters measured, MHC-FKBP12.6/FKBP12.6–/– cardiomyocytes had normal calcium sparks [10]. These data indicated that cardiomyocyte-specific expression of FKBP12.6 was able to rescue abnormal calcium release in FKBP12.6-deficient cardiomyocytes, which further demonstrated a direct association of FKBP12.6 in regulating calcium release channel RyR2 in cardiomyocytes. Taken together, our findings provided a confirmation of the role played by FKBP12.6 in regulating cardiomyocyte calcium release and cardiac function as well as additional insight into that role.
Conclusion
The role of FKBP12 and FKBP12.6 in regulating cardiac function has been an intriguing question over the past decade. A number of FKBP12 and FKBP12.6 transgenic and knockout mice were generated and analyzed. The findings from these mice provided compelling new data supporting the concept that FKBP12 and FKBP12.6 have very different biologic functions in the heart. Whereas FKBP12 has an important physiologic role in regulating cardiac voltage-gated sodium channels, FKBP12.6 is more relevant to RyR2 function.
Contributor Information
Bai-Yan Li, Department of Pharmacology, Harbin Medical University, 157 Bao Jian Rd, Harbin 150081, People's Republic of China; Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Hanying Chen, Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Mitsunori Maruyama, Division of Cardiology, Department of Medicine, Krannert Institute of Cardiology, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Wenjun Zhang, Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Jin Zhang, Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Zhen-Wei Pan, Department of Pharmacology, Harbin Medical University, 157 Bao Jian Rd, Harbin 150081, People's Republic of China; Division of Cardiology, Department of Medicine, Krannert Institute of Cardiology, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Michael Rubart, Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Peng-Sheng Chen, Division of Cardiology, Department of Medicine, Krannert Institute of Cardiology, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
Weinian Shou, Department of Pediatrics, Riley Heart Research Center, Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA.
References
- 1.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994;74:555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 2.Avila G, Lee EH, Perez CF, Allen PD, Dirksen RT. FKBP12 binding to RyR1 modulates excitation-contraction coupling in mouse skeletal myotubes. J Biol Chem. 2003;278:22600–22608. doi: 10.1074/jbc.M205866200. [DOI] [PubMed] [Google Scholar]
- 3.Bram RJ, Hung DT, Martin PK, Schreiber SL, Crabtree GR. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993;13:4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn GW, II, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res. 2003;92:1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 5.Gaburjakova M, Gaburjakova J, Reiken S, Huang F, Marx SO, Rosemblit N, Marks AR. FKBP12 binding modulates ryanodine receptor channel gating. J Biol Chem. 2001;276:16931–16935. doi: 10.1074/jbc.M100856200. [DOI] [PubMed] [Google Scholar]
- 6.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument-Bromage H, Tempst P, Marks AR. FK506-binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- 8.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lehnart SE, Huang F, Marx SO, Marks AR. Immunophilins and coupled gating of ryanodine receptors. Curr Top Med Chem. 2003;3:1383–1391. doi: 10.2174/1568026033451907. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Chen H, Ji G, Li B, Mohler PJ, Zhu Z, Yong W, Chen Z, Xu X, Xin H, Shou W. Transgenic analysis of the role of FKBP12.6 in cardiac function and intracellular calcium release. Assay Drug Dev Technol. 2011;9:620–627. doi: 10.1089/adt.2011.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 13.Marks AR. Ryanodine receptors, FKBP12, and heart failure. Front Biosci. 2002;7:d970–d977. doi: 10.2741/A822. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama M, Li BY, Chen H, Xu X, Song LS, Guatimosim S, Zhu W, Yong W, Zhang W, Bu G, Lin SF, Fishbein MC, Lederer WJ, Schild JH, Field LJ, Rubart M, Chen PS, Shou W. FKBP12 is a critical regulator of the heart rhythm and the cardiac voltage-gated sodium current in mice. Circ Res. 2011;108:1042–1052. doi: 10.1161/CIRCRESAHA.110.237867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 16.Masumiya H, Wang R, Zhang J, Xiao B, Chen SR. Localization of the 12.6-kDa FK506-binding protein (FKBP12.6) binding site to the NH2-terminal domain of the cardiac Ca2 ? release channel (ryanodine receptor). J Biol Chem. 2003;278:3786–3792. doi: 10.1074/jbc.M210962200. [DOI] [PubMed] [Google Scholar]
- 17.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase (p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez P, Kranias EG. Phospholamban: a key determinant of cardiac function and dysfunction. Arch Mal Coeur Vaiss. 2005;98:1239–1243. [PubMed] [Google Scholar]
- 20.Seidler T, Loughrey CM, Zibrova D, Kettlewell S, Teucher N, Kogler H, Hasenfuss G, Smith GL. Overexpression of FK-506 binding protein 12.0 modulates excitation contraction coupling in adult rabbit ventricular cardiomyocytes. Circ Res. 2007;101:1020–1029. doi: 10.1161/CIRCRESAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 21.Sharma MR, Jeyakumar LH, Fleischer S, Wagenknecht T. Three-dimensional visualization of FKBP12.6 binding to an open conformation of cardiac ryanodine receptor. Biophys J. 2006;90:164–172. doi: 10.1529/biophysj.105.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, Mathews LM, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- 23.Snyder SH, Sabatini DM. Immunophilins and the nervous system. Nat Med. 1995;1:32–37. doi: 10.1038/nm0195-32. [DOI] [PubMed] [Google Scholar]
- 24.Tang W, Ingalls CP, Durham WJ, Snider J, Reid MB, Wu G, Matzuk MM, Hamilton SL. Altered excitation–contraction coupling with skeletal muscle-specific FKBP12 deficiency. Faseb J. 2004;18:1597–1599. doi: 10.1096/fj.04-1587fje. [DOI] [PubMed] [Google Scholar]
- 25.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 26.Wagenknecht T, Grassucci R, Berkowitz J, Wiederrecht GJ, Xin HB, Fleischer S. Cryoelectron microscopy resolves FK506-binding protein sites on the skeletal muscle ryanodine receptor. Biophys J. 1996;70:1709–1715. doi: 10.1016/S0006-3495(96)79733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagenknecht T, Radermacher M, Grassucci R, Berkowitz J, Xin HB, Fleischer S. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J Biol Chem. 1997;272:32463–32471. doi: 10.1074/jbc.272.51.32463. [DOI] [PubMed] [Google Scholar]
- 28.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 29.Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. The immunophilin FK506-binding protein modulates Ca2 ? release channel closure in rat heart. J Physiol. 1997;500(Pt. 2):343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Liu Z, Masumiya H, Wang R, Jiang D, Li F, Wagenknecht T, Chen SR. Three-dimensional localization of divergent region 3 of the ryanodine receptor to the clamp-shaped structures adjacent to the FKBP binding sites. J Biol Chem. 2003;278:14211–14218. doi: 10.1074/jbc.M213164200. [DOI] [PubMed] [Google Scholar]
- 32.Zheng XF, Florentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 33.Zissimopoulos S, Lai FA. Interaction of FKBP12.6 with the cardiac ryanodine receptor C-terminal domain. J Biol Chem. 2005;280:5475–5485. doi: 10.1074/jbc.M412954200. [DOI] [PubMed] [Google Scholar]