Abstract
Reconstituted high density lipoprotein particles (rHDL) are powerful platforms used as a model phospholipid bilayer system to study membrane proteins. They consist of a discoidal-shaped planar bilayer of phospholipids that is surrounded by a dimer of apolipoproteinA-I (apoA-I). The amphipathic nature of apoA-1 shields the hydrophobic acyl chains of the lipids from solvent and keeps the particles soluble in aqueous environments. These monodispersed, nanoscale discoidal HDL particles are approximately 10-11 nm in diameter with a thickness that is dependent on the length of the phospholipid acyl chain. Reconstituted HDL particles can be assembled in vitro using purified apoA-1 and purified lipids. Investigators have utilized this model bilayer system to co-reconstitute membrane proteins, and take advantage of the small size and its monodispersion. Our laboratory and others have utilized the rHDL approach to study the behavior of G protein-coupled receptors. In this chapter we describe strategies for the preparation of rHDL particles containing GPCRs in their monomeric form and discuss various methodologies used to analyze the reconstituted receptors function.
Keywords: Apolipoprotein A-I, HDL particles, receptor, POPC, POPG, monomer, oligomers
1. Introduction
G protein-coupled receptors (GPCR) are the largest family of integral membrane proteins. Their vast diversity and their importance in cellular signaling make them prime therapeutic targets constituting nearly 50 percent of available drugs. Although their significance in biomedical research is undeniable, their study has been limited by the lack experimental systems that resemble their natural environment. Nevertheless, over the past decades membrane protein research has escalated due to a diverse array of membrane modeling systems such as detergent micelles, bicelles and liposomes (1-10). These have allowed the functional and structural characterization of a great number of membrane proteins, in particular GPCRs. However, in some instances it is unclear how these systems mimic the natural milieu. In most cases, the orientation and oligomerization state of the reconstituted proteins cannot be determined.
Recently, a new class of model membranes has been developed to study the function of isolated membrane proteins especially GPCRs (4). Now several integral membrane proteins a have been reconstituted into rHDL particles such as cytochrome 450 (3), GPCRs bacteriorhodopsin (2), chemoreceptor (5), voltage dependent anion channel (VDAC-1) (6) and EGF receptor (7) to name a few. In this approach membrane proteins in detergent micelles such as GPCRs may be reconstituted into the phospholipids bilayers of a discoidal high density lipoprotein (HDL) particle (Fig 1). The reconstituted HDL (rHDL) particles are monodispersed, homogenous and preferentially incorporate monomeric receptors in the case of GPCRs (1). Several forms of rHDL particles have been described and have successfully been used to reconstitute membrane proteins, e.g. nanodiscs and NABBs (nanoscale apolipoprotein-bound bilayers) (8). For the remainder of this chapter we will simply refer to these apolipoprotein particles as rHDL particles. A variety of GPCRs have now been reconstituted into rHDL particles: rhodopsin (8-10), β2-adrenergic receptor (1) and the μ-opioid receptor (11) each fully capable of activating its G protein when reconstituted as monomers in rHDL particles. Furthermore, reconstituted receptors display strong allosteric modulation by G proteins as well as arrestin (9).
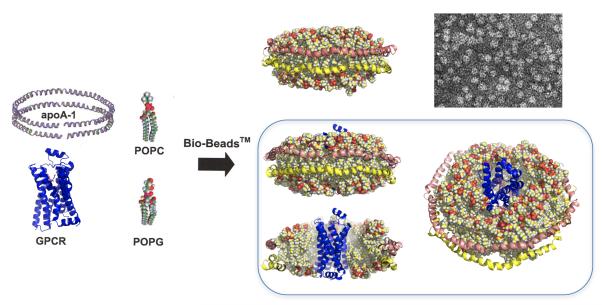
Figure 1. Reconstitution of a prototypical GPCR into rHDL particle.
Illustration of the procedure for the reconstitution of the b2AR (PDB: 2RH1) into rHDL particles. Detergent-solubilized purified lipids and purified β2AR are incubated with purified apolipoprotein A1 (apo-A1) as described in the text. Bilayer formation and self-assembly of the rHDL particle accompanies the detergent removal step through the addition of Biobeads™. Both empty and β2AR-containg rHDL particles are illustrated (the latter are illustrated from multiple perspectives). Also illustrated is the electron micrograph of a typical rHDL preparation (Whorton and Sunahara, unpublished). The coordinates for the rHDL particle were based on the model reported by Segrest et al (58) and used with the permission of Dr. Stephan Harvey (Georgia Institute of Technology).
In this chapter we discuss the methodology behind the formation and incorporation of GPCRs in rHDL particles. We will discuss in detail the isolation, purification of apolipoprotein A-I as well as the incorporation of GPCRs into rHDL particles.
2. Materials
2.1. Purification of Wild Type ApolipoproteinA-I (WT apoA-I)
Human Serum stored at −20°C in 10mM CaCl2.
Buffer A: 50 mM Tris-HCl, pH 8.0, 1mM CaCl2, 3M NaCl, and 5 mM EDTA.
Buffer B: 50 mM Tris-HCl, pH 8.0, 1mM CaCl2, and 5 mM EDTA.
Dilution Buffer: 25 mM Tris-HCl, pH 8.0, 1 mM CaCl2, 5 mM EDTA, 0.2% Triton X-100.
Buffer C: 20 mM Tris-HCl, pH 8.0, 1 mM CaCl2, 5 mM EDTA and 0.1% Triton X-100.
Exchange buffer: 100 mM K-acetate, pH 5.0, 1 mM EDTA, 0.1% Triton X-100.
Buffer D: 25 mM K-acetate, pH 5.0, 1 mM EDTA, 0.1% Triton X-100.
Buffer E: 20 mM Hepes, pH 8.0, 100 mM NaCl, 1 mM EDTA.
2.2. Purification of Recombinant ApolipoproteinA-I (apoA-I)
Luria Broth (LB) medium containing 50 μg/ml of carbenicillin
Buffer A: 10 mM Tris-HCl, pH 8.0, 100 mM NaH2PO4, 6 M Guanidine hydrochloride and 1% Triton X-100.
Buffer B: 10 mM Tris-HCl, pH 7.0, 100 mM NaH2PO4, 6 M Guanidine hydrochloride and 1% Triton X-100
Buffer C: 50 mM NaH2PO4, pH 8.0, 300 mM NaCl and 1% Triton X-100
Buffer D: 50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 250 mM Imidazole and 1% Triton X-100.
Buffer E: 20 mM Hepes, pH 8, 100 mM NaCl, 1 mM EDTA, 20 mM sodium cholate.
2.3. β2Adrenergic Receptor Purification
1. Sf9 and Hi5™ cells.
Sf900 (Gibco) supplemented with 1% FBS or Insect Xpress (Lonza) depending on the cell line being used.
Transfer vector: pFastBac (Invitrogen, Carlsbad, CA).
Human β2-Adrenergic Receptor DNA was generously provided by Dr. Brian Kobilka.
n-dodecyl-β-D-maltoside (Dojindo Molecular Technologies, Gaithersburg, MD).
50 mM Tris-HCl, pH 7.4 and 150 mM NaCl (TBS).
Protease inhibitors (PI): 3.2 μg/ml leupeptin, 3.2μg/ml ovomucoid trypsin inhibitor, 17.5μg/ml phenylmethanesulfonyl fluoride, 16 μg/ml tosyl-L-lysine-chloromethylketone (TLCK), 16 μg/ml tosyl-L-phenylalanine chloromethyl ketone (TPCK).
Buffer A: 50 mM Tris-HCl, pH 8.0, 50 mM NaCl and PIs.
Buffer B: 50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 0.1% DDM and PIs.
Buffer C: 50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 0.1% DDM, 2.5 mM Imidazole and PIs.
Buffer D: 50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 0.1% DDM, 100 mM Imidazole and PIs.
Buffer E: 20 mM Hepes pH 8.0, 1 mM EDTA, 0.1% DDM and PIs.
Buffer F: 20 mM Hepes pH 7.5, 100 mM NaCl, 1 mM CaCl2, 0.1% DDM.
Buffer G: 20 mM Hepes pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.1% DDM and 200 ug/mL Flag peptide (Invitrogen).
2.4. In vitro reconstitution of rHDL
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (Avanti Polar Lipids, Albaster, AL). Light sensitive, store at −20°C.
1-palmitoyl-2-oleoyl-sn-glycero-3-[phosphor-rac-(1-glycerol)] (POPG) (Avanti Polar Lipids, Albaster, AL). Light sensitive, store at −20°C.
Sodium Cholate (Sigma, St Louis, MO)
HNE: 20 mM Hepes, pH 8.0, 100 mM NaCl, 1 mM EDTA.
BioBeads™ (Bio Rad, Hercules, CA) at 0.5 mg/mL
3. Methods
3.1 High Density Lipoprotein (HDL) – Apolipoprotein A-I
Apolipoprotein A-I (apoA-I) is the major component of high density lipoprotein particles (HDL). It contains a globular domain in the N-terminus and a lipid binding domain in the C-terminal (residues 44-243). Two models have been proposed to explain its geometry; “double belt” (12-14) and the “picket fence” model (15). The “picket fence” model proposes that apoA-I forms a series of amphipathic short, 22 residue, α-helices that span the lipid bilayer (perpendicular to the bilayer) surrounding the particle and keeping the acyl moieties of the lipids protected from solvent (15). However, recent evidence from mutagenesis studies (16), cross-linking (17, 18) FRET (19), fluorescence spectroscopy (20) and infrared spectroscopy (21) studies suggest that the two molecules of apoA-I form continuous but antiparallel amphipatic α-helices that are aligned with the plane of the bilayer. ApoA-I therefore serves as a molecular belt surrounding an island of phospholipids (Fig 1).
Several native and recombinant forms of apoA-I have been used to support the bilayer such as apoA-I from human and zebrafish (zap1) as well as membrane scaffolding proteins (MSPs). For the incorporation of membrane proteins such as GPCRs into HDL particles it is possible to use both human and recombinant apoA-I interchangeably. Both can be purified to >90% homogeneity and have been shown to form stable and homogenous particles (1, 11). For recombinant proteins we can take advantage of bacterial expression as well as engineered affinity tags (6×His) and metal chelate affinity chromatography. ApoA-I, however, is highly abundant in serum (>1 g per liter of serum) making it a rich and inexpensive source of protein. The following paragraphs summarize the purification of native and recombinant forms of apoA-I.
3.1.1. Purification of apolipoproteinA-1
3.1.2. Wild Type (WT) Apoliprotein AI
WT human apoA-I is purified from serum by a protocol adapted from Gan et al (22) by Whorton et al (1). Frozen serum (200 to 1000mL at −20°C in 10mM CaCl2) is thawed at 37°C, filtered through cheesecloth, and centrifuged to pellet any debris (5, 000 × g for 10min).
The clarified serum is diluted in order to have a final concentration of (volume depends on the original serum volume); 50 mM Tris-HCl, pH 8.0, 1 mM CaCl2, 3 M NaCl and 5 mM EDTA (Buffer A). The solution is mixed with equal serum volume of blue agarose (Cibacron blue F3GA-agarose, Sigma) resin equilibrated in Buffer A, and stirred for 30 minutes at room temperature (RT).
The resin is filtered through a Whatman #1 filter in a Büchner funnel. The resin cake is resuspended in 3× resin volume of Buffer A and then re-filtered as before. The resin is washed until absorbance at 280 nm of the filtrate is less than 0.025, then it washed two more times with Buffer B. The cake is resuspended in an equal volume of Buffer B and loaded onto an empty column. The remaining apoA-I protein is eluted with Buffer B plus 5 mM Cholate. After this wash, apoA-I is ~80-90% pure.
ApoA-I containing fractions are pooled and concentrated using an Amicon stirred ultrafiltration cell affixex with a 10, 000 MWCO filter (Millipore) and then diluted (1:1) in dilution buffer.
The solubilized material is loaded into a 70 ml Q Sepharose (Amersham Pharmacia) column equilibrated in Buffer C.
The protein is eluted with a shallow linear gradient with Buffer C with 1 M NaCl where apoA-I usually elutes around 100-150 mM NaCl. Peak fractions were exchanged into 100 mM K-acetate, pH 5.0, 1 mM EDTA, 0.1% Triton X-100 and applied to a SP Sepharose (Amersham Pharmacia) column equilibrated in Buffer D and eluted with a linear gradient of Buffer D with 1 M NaCl.
Triton X-100 is exchanged for cholate by applying SP Sepharose fractions to a Superdex 200 size exclusion column (Amersham Pharmacia) in Buffer E with 20 mM cholate at 4°C.
ApoA-I fractions are pooled and concentrated to at least 10 mg/ml, dialyzed overnight against Buffer E with 5 mM cholate and stored in −80°C until further use.
3.1.3. Recombinant apoA-I
Secondary structure predictions, biochemical and crystallographic evidence suggest that the carboxy-terminal of apoA-I (residues 44-243) carries the predominant role to maintain the discoidal HDL structure (23). Deletion of the globular N-terminal region (aa 1-43) does not alter the HDL structure but does impair the capacity of apoA-I to interact with the ABC transporter (ABCA1). ABCA1 serves as the putative cholesterol transporter to load cellular cholesterol on to HDL in vivo (24).
The C-terminal half of apoA-I (44-243) can be expressed as a recombinant protein in E. coli as a hexahistidine tagged (6×His) version (11). Analysis of the secondary structure of apoA-I suggests that the C-terminal region is composed of repeating spans of 22 residues separated by proline residues. Positioning of the proline residues, reputable α-helix disrupters, fueled the original “picket fence” model but are now thought to serve as kinks within the “helical belt” that surrounds an island of phospholipids. Sligar and colleagues have taken advantage of these discrete repeating spans to engineer larger rHDL particles by inserting additional 22-residue cassettes within apoA-I (25). The modified forms of the apolipoprotein, termed membrane scaffolding proteins (MSPs) can produce particles with a Stokes radius of 10-13 nm, depending on the MSP subtype and the lipid:MSP ratio used during the reconstitution. The discoidal HDL formed by these MSPs are called nanodiscs. Apolipoproteins with 3 cassettes (MSP1E3) were successfully used to produce particles to accommodate the insertion of two rhodopsin particles (9, 26). Wild type apoA-I will comfortably generate rHDL particles of approximately 10 nm in diameter. However, increasing the lipid to protein ratios during the reconstitution can produce larger particles up to ~17 nm in diameter, albeit in a heterogeneous mixture with smaller particles (8, 23). While most reconstitution studies have been performed using sequences derived from human apoA-I, apolipoproteins from other species (zap1, from zebrafish, Danio rerio) (8) have been successfully used to support a phospholipid bilayer.
A modified version of apoA-I with an N-terminal 43 amino acid deletion and a 6×His tag (Δ1-43-His6-TEV-apoA-I) is expressed using a pET15b vector to transform Eschericia coli cells (BL21) (11).
A starter culture is prepared by inoculating 20 mls of Luria Broth (LB) medium containing 50 μg/ml of carbenicillin with a single colony overnight for no more than 10-12 hours at 37°C.
The starter culture is diluted 1:200 into the final culture. It is important that the cells are spun down and resuspended in fresh media before inoculating the final culture. Grow cells until OD600 reaches 0.6 (2-3 hours) and induce with 1 mM IPTG for 3-4 hours.
The cells are harvested by centrifugation at 4, 200 × g for 10 min. The cell pellets may be flash frozen in liquid nitrogen and stored at −80°C.
The cells are resuspended and lysed by gently vortexing in Buffer A. The lysate is fractionated by centrifugation at 10,000 × g for 20 min at room temperature (RT).
The supernatant is loaded onto a nickel-nitrilotriacetic acid column (Ni-NTA, Qiagen) by gravity flow. The column is washed with 10 column volumes of Buffer B and then with Buffer C.
Bound Δ1-43-His6-TEV-apoA-I is eluted with Buffer D. 7. The fractions containing the protein of interested are further purified on a Superdex 75 (Amersham Pharmacia) equilibrated with Buffer E.
Pooled Δ1-43-His6-apoA-I is dialyzed against Buffer E containing 5 mM cholate. Purified protein is concentrated in an Amicon Centricon 10,000 MWCO to ~10 mg/ml, flash frozen in liquid nitrogen and stored at −80°C until use.
This tagged version of Δ1-43-His6-TEV-apoA-I can be used for reconstitution experiments. HDL particles formed with the hexahistidine tag proteins are homogenous in size based on size exclusion chromatography and the incorporation efficiency is not affected. However, removal of the tag allows the separation of empty particles from those that contain hexahistidine-tagged proteins. To remove the tag the purified protein can be incubated with TEV protease.
TEV is the common name for the catalytic domain of the Nuclear Inclusion a (NIa) protein encoded by the tobacco etch virus (TEV) (27). This enzyme is commercially available; it contains a polyhistidine tag on the N-terminus and a polyarginine tag on the C-terminus. For digestion, we incubate TEV protease and Δ1-43-His6-TEV-apoA-I overnight at a ratio of 1:7.5. After cleavage, the proteins are separated using Ni-NTA (IMAC), the TEV protease will bind to the column and the un-tagged apoA-1 will be present in the flow through.
3.2. β2-Adrenergic Receptor (β2-AR) Purification
The reconstitution system has been adapted to incorporate various membrane proteins into the phospholipid bilayer, including cytochrome 450 (3), bacteriorhodopsin (2), chemoreceptor (5), β2Adrenergic Receptor (β2-AR) (1, 4), etc. In this report, we discuss the use of the HDL system to incorporate purified and fully functional monomeric β2-AR.
The β2-AR has several roles in the body, including the regulation of smooth muscle relaxation as well as other processes such as glycogenolysis and lipolysis. The β2-AR is primarily activated by endogenous epinephrine in the body. Receptor activation promotes the activation of the stimulatory G protein, Gs. GTP-bound Gs binds to and directly activates adenylyl cyclase causing an increase in levels of cAMP and activation of protein kinase A (PKA). PKA mediates various downstream effects. In recent years, work has shown that the β2-AR can signal through G protein-independent pathways, specifically arrestins (28).
To study the function of β2-AR we express either WT of CFP-fused receptor in Sf9 cells and solubilize using methods previously described (29, 30). The modified receptor is expressed by using recombinant baculoviruses that were created using transfer vectors (pFastBac™) that encoded a fusion protein of an N-terminal cleavable hemagglutinin signal sequence (MKTIIALSYIFCLVF), a FLAG epitope (DYKDDDD), a decahistidine tag, the monomeric and enhanced cyan fluorescent protein (Clontech), and the human β2-AR .
3.2.1. Preparation and solubilization of membranes
High titer viruses (107–108 plaque-forming units/ml) are used to infect Sf9 or High-Five™ (2-3 × 106 cells/mL) suspension cultures at a multiplicity of infection of 0.5-1.
FLAG-His10-mECFP-B2AR (CBAR) is expressed for 48–60 h.
After 60hrs of infection, spin the cultures for 10 min at 500 × g to pellet cells.
Resuspend the cells in 1/10 the original culture volume of TBS + PIs (see Note 1).
Lyse the cells by nitrogen cavitation (see Note 2).
Spin down debris at 500 × g for 10 mins. Keep the supernatant and discard the pellet.
Spin the supernatant from the previous step for 35 min at 100, 000 × g to pellet the membranes.
Resuspend the membrane containing fractions in 1/20 the culture volume with Buffer A and either stored at −80°C or further processed to purify receptor (see Note 3).
Membrane preparations are diluted to 5mg/ml in Buffer A plus 1% DDM (w/v) final concentration and stir for 30-45 mins in ice
Spin down solubilized material for 35 mins at 100, 000 × g
3.2.2. Soluble receptor purification
3.2.2.1. CFP-β2AR
For CFP-β2AR: DDM-solublized extract is applied to a metal-chelate affinity column (Talon, Clontech) equilibrated by running 10× column volume of Buffer A with 0.1% DDM.
Wash the column with 10× column volume of Buffer B
Wash the column with 5× column volume of Buffer C
Elute 8 half column volume fractions with Buffer D
Peak fractions are applied to a 1mL Source Q anion exchange column (GE Healthcare) in Buffer E
CFP-β2AR is eluted with a 15mL 0-40% linear gradient of Buffer E with 1M NaCl.
Peak fractions are pooled and resolved on a Superdex 200 size exclusion column in Buffer E with 50 mM NaCl to resolve the CFP-β2AR from the clipped CFP.
The resultant CFP-β2AR is greater than 95% pure and stored with 10% glycerol until use.
3.2.2.2. WT-β2AR
For WT-β2AR: The solubilized extract can be purified with a metal-chelate affinity column following the procedure described above.
CaCl2 is added to the peak fractions from the previous step to a final concentration of 1mM
The β2AR is purified by M1-Flag affinity chromatography (Sigma) equilibrated with Buffer F
Wash the column with 10× column volume of Buffer F
Elute 8 half column volume fractions with Buffer G (see Note 4).
Determine the concentration of functional, purified receptor using a saturating concentration of [3H]dihydroalprenolol as previously described (29) (see Note 5).
3.3. In vitro reconstitution of GPCRs in rHDL
3.3.1. Empty rHDL
To date a number of membrane proteins have been functionally incorporated into rHDL particles, nanodiscs or NABBs: the list includes GPCRs (1, 2, 10, 11, 26), cytochrome P450 (3), the protein pump bacteriorhodopsin (2), SecYEG heterotrimer (31), bacterial chemoreceptors (32), and others. Reconstituted HDL particles have been used in a great number of biochemical and biophysical assays demonstrating their utility in membrane protein research.
Briefly, HDL particles are formed by adding apoA-I to detergent-solubilized phospholipids in a specific ratio and removal of the detergent. Detergent removal is achieved by addition of hydrophobic adsorbent Bio-Beads™ (Bio Rad, 0.05 mg/ml reconstitution volume). For the formation of empty HDL particles, lipids are solubilized in sodium cholate in a 3-fold molar excess to the lipid. The selection of lipids is critical for particle formation and efficient protein incorporation; it must be optimized for each protein of interest. A typical reconstitution has ratios of 1:50 to 1:100 apo:lipid ratio and a final concentration of 24 mM cholate.
POPC and POPG are combined at a 3:2 molar ratio, a mixture that mimics the zwitterionic environment of the cell membrane.
Dry lipids under argon (or nitrogen) from a chloroform solution and place in a vacuum dessicator for 30-60 min to residual traces of chloroform.
Solubilized lipids in HNE + 50 mM cholate, again flush with argon and reseal. For some lipids (i.e. POPC and POPG), it is hard to solubilized them with the working concentration of cholate. It helps to first solubilized in a higher concentration of cholate, then dilute this down to the working concentration before adding the receptor and apoA-I.
Allow the detergent buffer to solubilized the lipids about 10-15 min; protect from light. The solution should become clear or translucent, but with no particles. Make sure to solubilize all lipids, especially on the sides of the tube.
Further dilute with HNE to achieve the desired concentration of cholate.
Finally, a concentrated stock of apoA-I is added such that the final concentrations of the components are 24 mM detergent, 8 mM lipids, and 100 mM apoA-I.
Incubate the solution for 1-2 hours at 4°C. (TmPOPC/POPG = −2°C).
The mixture is added to Biobeads and incubated for a minimum of 3 hours to remove the detergent.
Samples can be store at 4°C until further use (see Note 6).
3.3.2. β2Adrenergic Receptor -rHDL
For the case of the β2-Adrenergic Receptor, zwitteronic environment was achieved by mixing POPC and POPG at a molar ratio of 3:2. The lipids are prepared the same way as the ones for empty rHDL (see Note 7).
Add purified β2AR (DDM solubilized and affinity purified as described (33)), and 100 μM apoA-I (see Note 8).
Following incubation on ice (1-2 hrs), Biobeads™ are added to the mix for detergent removal and spontaneous formation of homogenous particles that contain monomeric receptor.
The reconstitution sample can be centrifuged briefly to promote the sedimentation of the Bio-Beads, recover the supernatant without disturbing the beads.
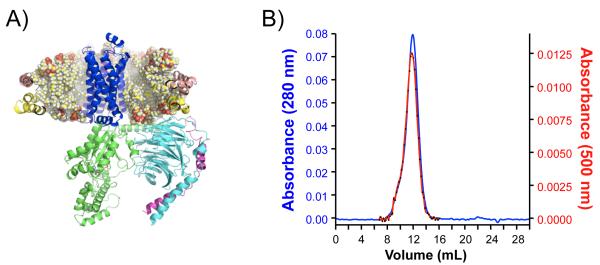
Subsequent separation of the reconstituted sample in a Superdex 200 column (Size Exclusion Chromatography, SEC) will allow determination of size and homogeneity of the rHDL particles; size changes in the peak fractions indicate improper particle formation. In addition, empty particles can be separated from those containing membrane proteins by affinity chromatography if a tag is present (See Fig 2B) (see Note 9).
Figure 2. Reconstitution of a prototypical GPCR with G proteins in rHDL particles.
A) Illustration of the β2AR (PDB: 2RH1) and heterotrimeric G protein (Giabg, PDB: 1GP2) reconstituted into rHDL particles. B) Size exclusion chromatography of rhodopsin-rHDL particles. Indicated are the UV absorption of protein (A280) and dark-state rhodopsin (A500). Adapted from Whorton et al (4).
3.4. GPCR Oligomers in rHDL
Clearly one of the major advantages of the rHDL technology is the isolation of homogeneous populations of reconstituted particles where the oligomeric state of the GPCRs may be controlled. The physical size limitations of the rHDL particle and the conditions under which the receptors are reconstituted can dictate the stoichiometry of receptors reconstituted within each particle. However, a technically challenging step is determining how many receptors are reconstituted within each particle. Equally important is the assessment of the GPCR:rHDL ratio based on biochemical properties that are not related to receptor function or activity. Since cooperatively factors, such as potential consequences of oligomerization, may influence receptor activity (e.g. radio-ligand binding or photoactivation), it is critical that the receptor:rHDL be quantified using other biochemical properties of the receptor rather than activity alone.
While it was clearly demonstrated that monomeric GPCRs are fully functional in apolipoprotein particles, the contributions of oligomeric forms to signaling are far more complicated. Reconstituted particles containing two or more rhodopsin molecules (9, 26) have been reported to display an activity (i.e. interaction with G protein or arrestin) that is lower than expected for two receptors. The assumption, of course, is that both reconstituted receptors share the same topology within the lipid bilayer, i.e. the N-termini are both on the same side of the membrane. However, as acknowledged by each group and as demonstrated by Banerjee et al (8), this assumption should be taken with caution. Single molecule studies by electron microscopy (EM) analysis indicate that insertion of two or more rhodopsins into rHDLs leads to a random orientation where equally distributed between parallel and anti-parallel forms. Thus only ~50% of the particles containing two receptors have inserted with the correct topology, i.e. with their N-termini on the same side of the phospholipid bilayer. With this caveat in mind the data suggest that dimeric forms couple to transducin half as efficiently as monomeric forms (8, 26). Similar observations are noted for arrestin-dimeric rhodopsin interactions (9)
The intricate binding characteristics of various ligands to hormone receptors have implicated receptor oligomerization to account for their complex behavior. Although it would seem likely that the rHDL system could be a logical preparation to address these properties, the role of oligomeric receptors reconstituted in rHDL particles have yet to be reported.
4. Notes
To help maintain functional and stable receptor 10 μM alprenolol can be added to the buffers used in the membrane solubilization. It should not be present in the buffers used in the consecutive steps as it can remain bound and interfere with subsequent functional assays.
The nitrogen cavitation chamber should be pre-chilled and filled with Nitrogen up to 600psi. Allow it to equilibrate in the cold room for 30-45 mins to allow for optimal cell rupture.
All receptor purification procedures are performed at 4°C unless noted.
Add half a column volume at a time; let incubate 3-5 mins on the column before adding the next elution.
WT-β2AR can be further purified to separate active vs. inactive receptor. Flag-purified receptor can be purified by alprenolol-Sepharose chromatography as previously described (29, 30). The peak fractions from this column can be loaded onto M1-Flag resin in order to remove free alprenolol. Two liters of Sf9 cells typically yield 500 μL of a 5 μM solution of β2AR.
Reconstituted HDL particles are composed of two molecules of apo A-I, for particles with a Stokes diameter of ~10.8 nm the final preparation consists of 1molecule of apo A-I per 80 lipid molecules.
In some cases (e.g. μ-opioid receptor) the lipid component may be modified with porcine polar brain lipid extract (Avanti Polar Lipids) in addition to POPC and POPG for a final concentration of 7 mM lipids and at a molar ratio of 1.07:1.5:1 brain lipid:POPC:POPG.
The final concentration of the GPCR varies from 100 nM-1 μM, bust must comprise no more than 20% of the total reconstitution volume, in order to minimize detergent concentration.
It is critical that the particles are stored at 4°C in the absence of divalent cations as we have observed (unpublished results) that the presence of <5 mM (MnCl2, CaCl2, and/or MgCl2) promotes rHDL aggregation. HDL particles have been shown to form “rouleau” or stacks of particles resembling stacks of coins (34). The interaction between HDL particles is thought to be facilitated through the bridging action of divalent cations between the phospholipid headgroups of each HDL particle. HDL aggregation leads to the loss of protein yield, homogeneity and unknown stoichiometries. In addition HDL particles are sensitive to detergents where exposure may lead to instantaneous re-solubilization of both membrane proteins and the lipids. To verify the homogeneity of the particles it is useful to analyze the samples through a size exclusion chromatography column, although dynamic light scattering (35) and ultracentrifugation may be used also (31).
5. Summary
These examples illustrate the usefulness of rHDL particles a powerful approach to study membrane proteins such as GPCRs. They provide the means to maintain membrane proteins embedded in a lipid environment as a soluble and monodispersed particle, mimicking that of the plasma membrane. This approach has allowed investigators to address a central question regarding the role of receptor oligomerization: what is the minimal functional unit required to activate a G protein? The physical properties of rHDL particles and their accessibility to G proteins on both sides of the bilayer are qualities that make this approach an adequate system to investigate this question. Further studies will undoubtedly reveal the functions of oligomerization and the contributions it makes toward the recruitment of signaling partners.
As with other model membrane preparations, rHDL particles have been limited to the use of purified-functional protein (especially GPCRs), thus limiting the number of targets that can be studied. This has recently been overcome with a plant cytochrome P450 where it was expressed, solubilized and directly incorporated in particles (36). In addition, reconstituted HDL technology has recently been commercialized by Invitrogen and sold as an in vitro membrane protein expression kit (MembraneMax™). Reconstituted HDL approaches have garnered subtle criticism as it is considered an ultra reductionist and simplistic method allowing direct characterization of GPCRs and its cognate partners in vivo, but removing the plethora of signaling partners that could normally have either direct or indirect effects on their activity in the cell. That being said, as new membrane protein-interactors are identified we predict that the utilization of rHDL methologies will continue to provide valuable information about membrane protein biophysics complementing other known membrane model systems.
Acknowledgements
This work is supported through funding of the National Institutes of Health (GM-068603 and GM-083118), the University of Michigan Biological Sciences Scholars Program and the Cellular and Molecular Biology Training Grant and the University of Michigan Rackham Merit Program.
References
- 1.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayburt TH, Sligar SG. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci. 2003;12:2476–2481. doi: 10.1110/ps.03267503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baas BJ, Denisov IG, Sligar SG. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch Biochem Biophys. 2004;430:218–228. doi: 10.1016/j.abb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Leitz AJ, Bayburt TH, Barnakov AN, Springer BA, Sligar SG. Functional reconstitution of Beta2-adrenergic receptors utilizing self-assembling Nanodisc technology. Biotechniques. 2006;40:601–602. doi: 10.2144/000112169. 604, 606, passim. [DOI] [PubMed] [Google Scholar]
- 5.Amin DN, Hazelbauer GL. The chemoreceptor dimer is the unit of conformational coupling and transmembrane signaling. J Bacteriol. 2010;192:1193–1200. doi: 10.1128/JB.01391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc. 2009;131:17777–17779. doi: 10.1021/ja907918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mi LZ, Grey MJ, Nishida N, Walz T, Lu C, Springer TA. Functional and structural stability of the epidermal growth factor receptor in detergent micelles and phospholipid nanodiscs. Biochemistry. 2008;47:10314–10323. doi: 10.1021/bi801006s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S, Huber T, Sakmar TP. Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J Mol Biol. 2008;377:1067–1081. doi: 10.1016/j.jmb.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto H, Sinha A, Dewitt M, Farrens DL. Monomeric rhodopsin is the minimal functional unit required for arrestin binding. J Mol Biol. doi: 10.1016/j.jmb.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK. Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem. 2009;284:26732–26741. doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers DP, Roberts LM, Lebowitz J, Datta G, Anantharamaiah GM, Engler JA, Brouillette CG. The lipid-free structure of apolipoprotein A-I: effects of amino-terminal deletions. Biochemistry. 1998;37:11714–11725. doi: 10.1021/bi973112k. [DOI] [PubMed] [Google Scholar]
- 13.Rogers DP, Roberts LM, Lebowitz J, Engler JA, Brouillette CG. Structural analysis of apolipoprotein A-I: effects of amino- and carboxy-terminal deletions on the lipid-free structure. Biochemistry. 1998;37:945–955. doi: 10.1021/bi9713512. [DOI] [PubMed] [Google Scholar]
- 14.Segrest JP. Amphipathic helixes and plasma lipoproteins: thermodynamic and geometric considerations. Chem Phys Lipids. 1977;18:7–22. doi: 10.1016/0009-3084(77)90023-8. [DOI] [PubMed] [Google Scholar]
- 15.Nolte RT, Atkinson D. Conformational analysis of apolipoprotein A-I and E-3 based on primary sequence and circular dichroism. Biophys J. 1992;63:1221–1239. doi: 10.1016/S0006-3495(92)81698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorshkova IN, Liu T, Kan HY, Chroni A, Zannis VI, Atkinson D. Structure and stability of apolipoprotein a-I in solution and in discoidal high-density lipoprotein probed by double charge ablation and deletion mutation. Biochemistry. 2006;45:1242–1254. doi: 10.1021/bi051669r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat S, Sorci-Thomas MG, Alexander ET, Samuel MP, Thomas MJ. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J Biol Chem. 2005;280:33015–33025. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MJ, Bhat S, Sorci-Thomas MG. The use of chemical cross-linking and mass spectrometry to elucidate the tertiary conformation of lipid-bound apolipoprotein A-I. Curr Opin Lipidol. 2006;17:214–220. doi: 10.1097/01.mol.0000226111.05060.f4. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Lyles DS, Thomas MJ, Pan W, Sorci-Thomas MG. Structural determination of lipid-bound ApoA-I using fluorescence resonance energy transfer. J Biol Chem. 2000;275:37048–37054. doi: 10.1074/jbc.M005336200. [DOI] [PubMed] [Google Scholar]
- 20.Panagotopulos SE, Horace EM, Maiorano JN, Davidson WS. Apolipoprotein A-I adopts a belt-like orientation in reconstituted high density lipoproteins. J Biol Chem. 2001;276:42965–42970. doi: 10.1074/jbc.M106462200. [DOI] [PubMed] [Google Scholar]
- 21.Koppaka V, Silvestro L, Engler JA, Brouillette CG, Axelsen PH. The structure of human lipoprotein A-I. Evidence for the “belt” model. J Biol Chem. 1999;274:14541–14544. doi: 10.1074/jbc.274.21.14541. [DOI] [PubMed] [Google Scholar]
- 22.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- 23.Rogers DP, Brouillette CG, Engler JA, Tendian SW, Roberts L, Mishra VK, Anantharamaiah GM, Lund-Katz S, Phillips MC, Ray MJ. Truncation of the amino terminus of human apolipoprotein A-I substantially alters only the lipid-free conformation. Biochemistry. 1997;36:288–300. doi: 10.1021/bi961876e. [DOI] [PubMed] [Google Scholar]
- 24.Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res. 2001;42:1717–1726. [PubMed] [Google Scholar]
- 25.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 26.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 27.Lucast LJ, Batey RT, Doudna JA. Large-scale purification of a stable form of recombinant tobacco etch virus protease. Biotechniques. 2001;30:544–546. doi: 10.2144/01303st06. 548, 550 passim. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 29.Kobilka BK. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem. 1995;231:269–271. doi: 10.1006/abio.1995.1533. [DOI] [PubMed] [Google Scholar]
- 30.Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 31.Alami M, Dalal K, Lelj-Garolla B, Sligar SG, Duong F. Nanodiscs unravel the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J. 2007;26:1995–2004. doi: 10.1038/sj.emboj.7601661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boldog T, Grimme S, Li M, Sligar SG, Hazelbauer GL. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc Natl Acad Sci U S A. 2006;103:11509–11514. doi: 10.1073/pnas.0604988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devanathan S, Yao Z, Salamon Z, Kobilka B, Tollin G. Plasmon-waveguide resonance studies of ligand binding to the human beta 2-adrenergic receptor. Biochemistry. 2004;43:3280–3288. doi: 10.1021/bi035825a. [DOI] [PubMed] [Google Scholar]
- 34.Forte T, Norum KR, Glomset JA, Nichols AV. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: structure of low and high density lipoproteins as revealed by elctron microscopy. J Clin Invest. 1971;50:1141–1148. doi: 10.1172/JCI106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima ES, Maranhao RC. Rapid, simple laser-light-scattering method for HDL particle sizing in whole plasma. Clin Chem. 2004;50:1086–1088. doi: 10.1373/clinchem.2004.032383. [DOI] [PubMed] [Google Scholar]
- 36.Civjan NR, Bayburt TH, Schuler MA, Sligar SG. Direct solubilization of heterologously expressed membrane proteins by incorporation into nanoscale lipid bilayers. Biotechniques. 2003;35:556–560. 562–553. doi: 10.2144/03353rr02. [DOI] [PubMed] [Google Scholar]