Summary
The prevalence of Gastro Esophageal Reflux Disease (GERD) has been increasing worldwide. This increase is likely associated with the increased prevalence of obesity, the aging of the population and the decreased prevalence of Helicobacter pylori (Hp) infection. These different environmental factors interact with GERD pathogenesis in a potentially negative way. Esophago-gastric junction competence, esophageal clearance mechanisms and reflux causticity are involved in GERD pathophysiology. Obesity alters GERD pathogenesis by disrupting the EGJ and increasing intragastric pressure. Additionally, the number of transient lower esophageal sphincter relaxations is potentially increased in obese patients. The potential effect of obesity on esophageal peristalsis and the implication of impaired esophageal clearance in GERD pathogenesis are still to establish. Aging also plays an important role in GERD pathogenesis by decreasing lower esophageal sphincter pressure and impairing esophageal clearance. However a link between these abnormalities and an increased acid esophageal exposure has not yet been demonstrated in the elderly. The role of Helicobacter pylori and its eradication remain controversial. The type of Hp gastritis may explain the controversial effect. Hp with antral predominant gastritis is responsible for an increase gastric acid secretion and thus promotes GERD. On the opposite spectrum, Hp with diffuse gastritis induces a gastric atrophy and in this particular case, the Hp eradication may restore acid secretion and lead to a more caustic refluxate in patients with predisposing conditions for GERD. The association of GERD and the type of Hp gastritis remains to be confirmed.
Keywords: GERD, physiopathology, obesity, age, helicobacter pylori
Gastro-esophageal reflux is defined by the movement of gastric content into the esophagus. It could be associated with evocative symptoms such as heartburn or regurgitation or with extra-esophageal symptoms (cough, sore throat…). This phenomenon is physiologic, however, it becomes pathological when symptoms associated with reflux events impair a patient’s quality of life or when they are responsible for esophageal (esophagitis, Barrett’s mucosa…) or extra-esophageal lesions. Nevertheless, even if GERD impairs quality of life, it does not increase the long term mortality [1-2].
Commonly twice weekly reflux over several months is used to define Gastro-Esophageal Reflux Disease (GERD) as it is associated with impairment of quality of life [3]. Based on these criteria 10-20% of individuals in Western countries have pathological GERD [4-5]. This is higher than what is observed in Asia (5-7%) [4, 6-8]. Different factors may explain the variable prevalence among the countries [9]. The understanding of heartburn is not the same in the different languages. For example there is no translation for the word heartburn in most Asian languages [10]. Cultural differences in diagnostic practices and in physician recognition modify GERD reporting among the population. Finally the lifestyle, the environmental factors and the genetic backgrounds may influence GERD variations among the various geographic areas.
GERD physiopathology is complex and involves an interplay between many different aggressive and defensive forces promoting or retarding reflux: esophago-gastric junction (EGJ) competence, esophageal clearance and acid secretion are involved in determining the amount of gastric content refluxed into the esophagus. The GERD epidemiological risk factors interplay with these mechanisms at many different levels.
Despite the geographical variations, the prevalence of GERD is also increasing all over the world. This increase is associated with an increased prevalence of obesity, an aging population and a decreased prevalence of Helicobacter pylori (Hp) infection. This suggests that these factors could play a role in GERD occurrence. Moreover they interact with the different GERD mechanisms.
The aims of this review are to explore the different mechanisms involved in GERD pathogenesis and to determine how environmental and lifestyle risk factors interact with these mechanisms.
How may environmental factors and lifestyle influence GERD occurrence?
GERD Pathogenesis
The pathophysiology of GERD focuses on an interaction between an incompetent antireflux barrier, a default of esophageal clearance and/or the causticity of the gastric reflux (Figure 1).
Figure 1.
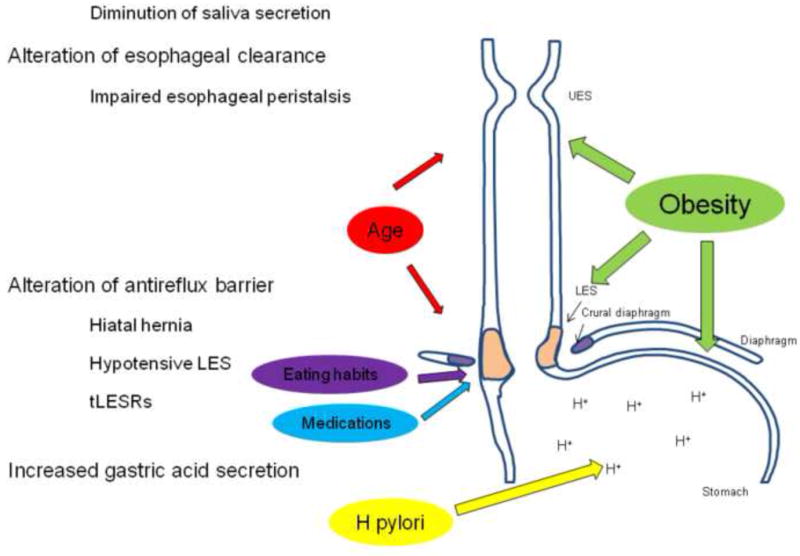
The environmental factors interact with the different mechanisms involved in GERD pathogenesis. Obesity alters esophago-gastric junction (EGJ) integrity, esophageal peristalsis and increases intra-gastric pressure. Age modifies EGJ integrity and impairs esophageal clearance by altering esophageal peristalsis and decreasing saliva secretion. Eating habits and medications decrease lower esophageal sphincter pressure (LES), increase the number of transient LES relaxations and delay gastric emptying. Helicobacter pylori modifies acid gastric secretion.
The esophago-gastric junction (EGJ)
The EGJ is synonymous with the antireflux barrier and is composed of the lower esophageal sphincter (LES), the crural diaphragm and the flap valve. An alteration of this anti-reflux barrier will promote opening of the EGJ; which is a prerequisite for reflux.
Transient LES relaxations (tLESRs) represents the main mechanism of GERD and these long duration LES relaxations are triggered by gastric distension, some food components (fat) or medications. Although the overall number of tLESRs is not increased in GERD, the number of acid reflux associated tLESRs is increased in GERD patients [11].
A hypotensive LES is also a favorable factor in promoting GERD symptoms and complications, such as esophagitis and stricture. Multiple studies have shown a relationship between severe erosive esophagitis and lower basal LES pressure [11-13].
Finally an anatomic disruption of the EGJ inclusive of, but not limited to hiatus hernia are also a major contributor to GERD occurrence [5, 14]. The flap valve formed by a musculomucosal fold created by the entry of the esophagus into the stomach along the lesser curvature could play a role in the EGJ competence [15-17].
The esophageal clearance
The efficacy of esophageal clearance depends on the vigor of esophageal peristalsis and the acid buffering property of saliva. An alteration of these mechanisms leads to an increased contact time between noxious acid content and esophageal mucosa.
Ineffective esophageal motility is frequently encountered in GERD patients [18] and it is a risk factor for developing esophagitis [19]. In healthy volunteers and GERD patients, severe ineffective esophageal motility (>80% ineffective contractions) is also associated with prolonged clearance and acid exposure, especially in supine position [20-21].
Saliva contains bicarbonate that helps buffer the acidity of gastric juice during reflux events and increasing the volume of saliva secretion shortens esophageal acid clearance [22]. Epidermal growth factor is also present in saliva and may promote the healing mechanisms of esophageal mucosa [23-24].
The gastric content
The different components of the gastric refluxate are toxic for the esophageal mucosa. Acid is the main component and induces not only symptoms when it is in contact with esophageal mucosa but also esophageal ulcerations. The incidence of GERD is particularly high (around 80%) in patients with acid hypersecretion (with or without Zollinger-Ellison syndrome) [25].
The other components of gastric juice, pepsin but also bile acids and pancreatic enzymes are also harmful to esophageal epithelium [26-28]. Their toxicity is dependant on the background pH: pepsin is activated in an acidic environment; bile acids can penetrate through the cell membrane in a weakly acidic medium (pH 3-5) [29].
Lifestyle and Environmental risks for GERD
Obesity
The incidence of obesity in Western countries has dramatically increased [30] and this has occurred in concordance with an increase in the number of patients suffering from GERD [31] (Figure 2). Multiple epidemiological studies clearly demonstrate an association between obesity and GERD and physiologic investigations support a biologically plausible relationship between obesity and GERD.
Figure 2.
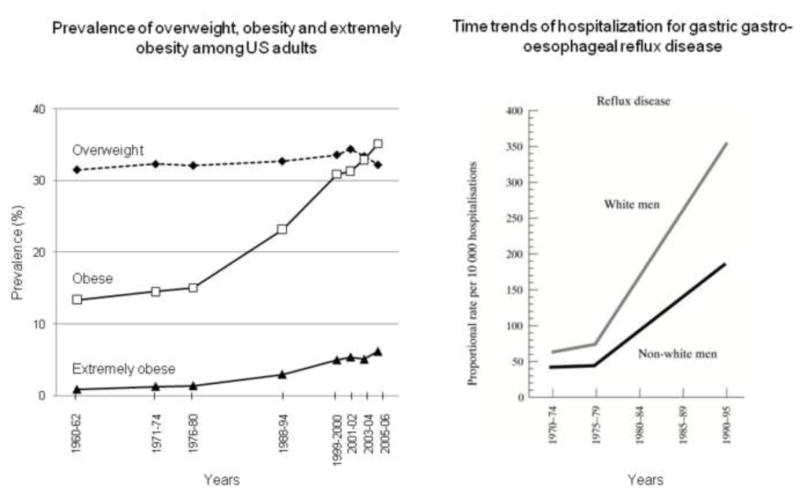
Increased prevalence of Obesity (left panel) and GERD (right panel) among US adults. The age-adjusted prevalence of overweight (BMI 25-30 kg/m2, black diamond, dashed line), obesity (BMI > 30 kg/m2, white square, black line) and extremely obesity (BMI > 40 kg/m2, black triangle, black line) increased from 1960 to 2006. This survey includes subjects aged from 20 to 74 years (source: Department of Health and Human Services. Centers for Disease Control and Prevention.http://www.cdc.gov/nchs/data/hestat/overweight/overweight_adult.htm [30]). The time trends of hospitalization for gastro-esophageal reflux disease also increased in the US Department of Vetrans Affairs from 1970 to 1995 [31].
Epidemiological data
Epidemiological studies point to obesity as a major risk factor for GERD in adults [32-33] and in children [34]. This association exists not only for the symptoms occurrence but also for the complications of GERD.
Different studies have shown an association between higher body mass index (BMI) and GERD [7, 35-38] and both obesity (BMI > 30 kg/m2) and overweight (BMI 25-30 kg/m2) are associated with GERD [1, 39-40]. Studies from Asia utilize a different cut off to define obesity (BMI > 25 kg/m2) due to different fat composition according to the race. In these studies, BMI is also a risk factor for GERD [7, 41-42]. Interestingly, in a prospective study in Korea a BMI > 25 was associated with erosive esophagitis whereas a BMI < 23 was associated with non erosive reflux disease [43]. A meta-analysis underlines the association between Barrett’s esophagus and being overweight (odds ratio (OR) 1.33, 95% confidence intervals (CI) 1.07 to 1.64, p=0.01) and obese (OR 1.70, 95% CI 1.36 to 2.12, p<0.01) [44].
The effects of BMI on GERD occurrence seems to be independent of total caloric intake, dietary intake of fiber, fruits and vegetables, or other macro or micronutrients [33]. Weight gain facilitates also GERD occurrence [42] and GERD symptoms may improve after weight loss [45] and bariatric surgery [46-47].
Controversies exist concerning the association between hiatus hernia (HH), GERD and obesity. Some studies reveal a positive association [42, 48]. El Serag noted that obesity and HH were independent risk factors for severe esophagitis [49]. Lee et al confirmed that BMI and HH were independent risk factors for erosive esophagitis [50]. On the contrary, Wu failed to find an association between BMI and HH [51].
Inter-relation between obesity and GERD mechanisms
Obesity may modify EGJ morphology and function. First, obesity generates a mechanical disruption of EGJ by promoting an axial separation between the LES and the extrinsic crural diaphragm [52]. LES incompetence has also been observed in obese patients [46, 53-54] and among morbidly obese patients a higher esophageal acid exposure was significantly associated with a lower LES pressure [55].
The distribution of fat may also play a role in GERD pathogenesis as GERD is also associated with an increased waist circumference [14, 33, 56]. Moreover a higher mean visceral fat area evaluated by computed tomography was found in patients with erosive esophagitis compared to controls [50]. It is possible that adipose tissue surrounding the stomach creates an extrinsic gastric compression which in turn leads to increasing intra gastric pressures and therefore a permissive gastro-esophageal pressure gradient. Finally an increasing number of tLESRs in obese patients has been observed in 2 studies [57-58]. Wu et al showed that, obese and overweight patients had an increase of tLESR number compared to patients with normal weight during the post-prandial period [57]. The proportion of tLESRs with acid reflux was also higher in overweight and obese patients as well as the gastro-esophageal pressure gradient. Schneider et al also noted a significantly increased frequency of tLESRs in obese patients compared to healthy volunteers, but not compared to GERD patients [58]. Theoretically, abdominal obesity could lead to more intense stimulation of both stretch and tension mechanoreceptors in the proximal stomach which will in turn provoke more post-prandial tLESRs.
An alteration of esophageal clearance is also suspected in obese patients. Esophageal motility disorders are encountered in 25 to 60% of obese patients [54, 59-61] and impaired bolus transit is more frequent in morbidly obese GERD patients than in GERD patients with normal weight [62]. However the prevalence of defective LES and abnormal peristalsis among obese patients with GERD is low in comparison with non obese patients with GERD [47, 54, 63-64]. This could reflect a physiologic compensatory response to increased gastric pressure.
Hormonal status could interplay with GERD and obesity [33]. Indeed upper GI symptoms and increased weight were found more frequently in perimenopausal and menopausal women than in premenopausal women [65]. In a large cohort of menopausal women receiving estrogen alone, estrogen plus progestin or placebo, Zheng et al. observed a trend toward a higher incidence of symptomatic GERD after one year of estrogen therapy whereas the combined hormonal therapy did not affect GERD occurrence [45]. In this study no association between BMI and hormonal treatment was observed.
Age
Studies support that the incidence of GERD increases with age. In a systematic review of the UK General Practice Research Database, El Serag noted that the incidence of GERD was highest in the 60-69 year range and decreased slightly thereafter [1]. This association with age was also found in studies from Asia [6]. Older patients, however, often complain of less severe or frequent heartburn than their younger cohorts [66] but the elderly have more complications including esophagitis [67], peptic strictures and Barrett’s esophagus [68]. The existence of prolonged acid exposure over many years could explain the prevalence of complicated GERD and an alteration of the sensorimotor response is also suspected to play a role in this blunted perception in older patients [69].
Higher frequency of hiatal hernia [67], impaired esophageal motility [70-71], and decreased saliva volume and bicarbonate concentration are involved in GERD occurrence in the elderly. The high prevalence of hiatal hernia may be related to the presence of kyphosis in elderly people [72]. Additionally, the use of medications that may interfere with LES pressure and esophageal peristalsis may also explain the higher frequency of GERD in elderly.
Helicobacter pylori
Since the discovery of the role of Helicobacter pylori (Hp) in the pathogenesis in ulcer disease, multiple studies have focused on the role of Hp in different gastrointestinal diseases. While the incidence of Hp decreased, the percentage of patients suffering from GERD has increased. Conflicting results exist concerning the influence of Helicobacter pylori and its eradication on GERD symptoms and complications.
In a meta-analysis of 20 case controls studies, the average prevalence of Hp infection in patients with GERD was 38.2% (95% CI 20.0-82.0%) compared to 49.5% (95% CI 29.0-75.6%) in non GERD patients (OR 0.58) [73]. In a systematic review of studies and trials extending up to September 2003, the same author concluded that successful eradication of Hp in patients with duodenal ulcer disease did not increase the risk of provoking de novo esophagitis [74]. Moreover there was a trend towards diminished prevalence of heartburn following eradication, successful or failed. The absence of an association between Hp eradication and development of new cases of GERD in dyspeptic patients was also observed in a meta-analysis recently published [75]. However, in the cohort studies for the subgroup of patients with peptic ulcer disease the odds ratio for GERD in Hp eradicated group versus persistent Hp was 2.04 (1.08-3.85, p=0.03). These heterogeneous results could be explained by the choice of control group (patients addressed for endoscopy with potential link between dyspepsia and Hp), the tools to diagnose Hp infection (serology, biopsies, urease test…) and GERD (heartburn score, pH studies, endoscopy).
Another possible explanation for the heterogeneous results regarding the relationship between GERD and Hp may be related to the variable effect of Hp on acid gastric. Helicobacter pylori infection has a major effect on somatostatin secreting D-cells in the gastric antrum. Thus the feedback inhibition by luminal acid on gastrin release is interrupted [76-77]. The gastrin levels are higher in subjects infected by Hp and do not exhibit normal feedback [78]. This lack of inhibition may be responsible for the increased acid secretion found in patients with Hp-induced duodenal ulcer or predominant antral gastritis. In the case of Hp-induced corpus gastritis, the gastrin levels are also increased, however, the local inflammation associated with increased levels of cytokines alters the parietal cell function [79]. This leads to hypochlorhydria and gastric atrophy. Reversal of Hp-induced corpus gastritis has the potential to increase gastric acid secretion [80]. Based on these effects, eradicating Hp in duodenal ulcer predominant antral gastritis patients may improve GERD symptoms whereas eradication in patients with predominant corpus gastritis may worsen GERD. Thus absence of gastric atrophy (diagnosed by a pepsinogen I/II ratio greater than 6) was found to be associated with a higher incidence of newly-developed GERD as well as negative Hp antibodies in Japanese population [81].
Finally some differences could be explained by the Hp strains and the genetic backgrounds of the patients. In a study conducted in Malaysia, an increased prevalence of Hp and Cag A positive strains was associated with reduced severity of GERD in Indian patients but not in Malaysian patients or Chinese patients [82]. The Indian patients had a higher prevalence of corpus atrophy and were more likely HLAB27 positive suggesting a higher predisposition to reduced acid secretion.
Other related GERD factors
Eating and lifestyle
Eating habits and lifestyle issues may interact with key factors involved in GERD physiopathology and thus, they are commonly implicated as risk factors for GERD occurrence.
Eating habits
Different foods may influence the occurrence of reflux events. An Italian study revealed less esophageal reflux events detected by pH-impedance monitoring in healthy volunteers [83] than what it was previously observed in American and Europeans cohorts [84-85]. One explanation given by the authors was the consumption of a Mediterranean diet (pasta or rice, meat or fish, vegetables and fruits). Foods listed in table 1 are associated with GERD through their presumed effects on reducing LES pressure and slowing gastric emptying.
Table 1.
Foods and effect on LES and gastric emptying
| Effects | Foods |
|---|---|
|
| |
| Decrease lower esophageal sphincter pressure | Fat food |
| Alcohol | |
| Chocolate | |
| Peppermint | |
|
| |
| Slower gastric emptying | Fat food |
High dietary fat intake is associated with an increased risk of GERD and erosive esophagitis [86]. In GERD patients, the consumption of carbonated soft drink is a predictor of heartburn during sleep [38]. Coffee may also be associated with an increase in heartburn occurrence [87]. However in a Norwegian case control study a negative association was found between GERD and coffee (OR 0.5 (95% CI 0.4-0.6) among subjects who drank 4-7 cups per day compared to those who didn’t drink coffee) [88]. In the same study, consumption of dietary fibers was found to be a protective factor whereas extra table salt use was considered a risk factor.
Alcohol consumption
Alcohol consumption is a risk factor for GERD symptoms and complications [14, 35, 89-90]. Alcohol facilitates hydrogen ion penetration into the esophageal mucosa [91] and thus increase the risk of mucosal injuries. It can also decrease LES pressure and esophageal motility [92-93] resulting in an alteration of esophageal clearance [94]. In a study including 463 Japanese males, the OR for esophagitis was 1.88 in moderate drinkers (25 to 50 g alcohol per day) (p=0.04) and 1.99 in heavy drinkers (more than 50 g alcohol per day) (p<0.01). The risk to develop Barrett’s esophagus was also shown to be elevated in heavy drinkers (OR=1.91, p<0.01). However, in this study age and smoking could be confounding factors since heavy drinkers were older and more likely smokers than the other subjects included. In an Irish case-control study, only binge drinking and high liquor intake were associated with esophagitis (2 fold increase risk) while wine consumption tended to reduce the risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma.
Smoking
Smoking increases the risk of GERD occurrence [14, 35, 37, 50, 88, 90, 95]. In a Norwegian case control study, the odds ratio for GERD symptoms was 1.7 (95% CI 1.5 to 1.9) among people who had smoked daily for more than 20 years compared with non-smokers [88]. Smoking reduces the LES pressure and then promotes the reflux of gastric content into the esophagus [96]. Moreover it may diminish salivary base secretion and thus impair acid esophageal clearance [97].
Physical exercise
Physical exercise has been found to be a protective factor against reflux [88]. However esophageal acid exposure increases significantly in healthy volunteers and GERD patients during the exercise periods compared to the non exercise periods [17]. The intensity of exercise-induced reflux was correlated with the EGJ morphology in this study and thus, the integrity of the EGJ may be a key protective factor in preventing strain-induced reflux during exercise.
Medications
Table 2 summarizes different medications that may interfere with the EGJ function. These medications have been reported to promote GERD occurrence [81, 98]. Unfortunately, well done studies are lacking to truly assess the role on the different medications on GERD occurrence and controversy data exist. Some confounding factors limit the interpretation of the epidemiologic studies that underlies the relationship between medication and GERD occurrence. Thus, the association might be drug related or a result of the underlying condition. In case-control studies, asthma medication use was associated with Barrett’s esophagus [99] and esophageal adenocarcinoma [100]. The use of other medications known to have an effect on LES pressure (nitrates, calcium channel blockers , benzodiazepines) was not associated with Barrett’s mucosa or esophageal adenocarcinoma occurrence. Asthma has also been reported to be associated with GERD [1] and it is difficult to separate the role of the underlying condition from the role of the medication. Moreover, a randomized study focusing on the effect of theophylline failed to find any effect on GERD occurrence [101].
Table 2.
Medications and effect on esophago-gastric junction functions
| Effects | Medications |
|---|---|
|
| |
| Decrease lower esophageal sphincter pressure | Nitrates |
| Calcium channel blockers | |
| Anticholinergics | |
| β adrenergic agonists | |
| Theophylline | |
| Morphine | |
| Benzodiazepins | |
|
| |
| Increase transient lower esophageal sphincter | Sumatriptan |
A case-control study focusing on the potential role of tricyclic antidepressants on the occurrence of reflux esophagitis revealed that only clomipramine was associated with esophagitis [102]. NSAIDs are also associated with GERD [35-37, 90, 103] and it is postulated that they act by reducing the protective mechanisms against acid, however, they should also be considered to be an increased risk factor for pill esophagitis.
Underlying conditions
The association of GERD with other health conditions is frequent and typically is associated with some abnormality in esophageal or gastric motility [104].
Diabetes mellitus could be associated with GERD occurrence [105], however, different confounding factors could exist such as the BMI [106]. One Australian study noted that diabetes mellitus and high blood pressure were associated with GERD but were not independent risk factors while high cholesterol was independently associated with GERD [95]. A study reported a higher prevalence of GERD in diabetic patients with neuropathy than in those without [107]. And it is possible that the neuropathy associated with diabetes may facilitate reflux by delaying gastric emptying or impairing esophageal motility.
Cardiovascular diseases are also associated with GERD. In a case control study based on a large Norwegian health survey conducted in 1995-97, a positive association was noted between GERD and angina pectoris (OR 1.9, 95% CI 1.6-2.2) [108].
Finally, an interaction exists between pulmonary disease and GERD. For example chronic pulmonary diseases and asthma were associated with new GERD diagnosis in a study using the UK General Practice Research Database [1]. GERD may also be one of the contributing factors of interstitial lung disease in patients with scleroderma [109-111].
Genetic backgrounds
Data suggest that genetic background could be associated with GERD symptoms and complications occurrence. Some polymorphisms of interleukin-1gene (IL-1B) and IL-1RN (gene coding for an interleukin-1 antagonist receptor) are associated with GERD. Thus the IL-1B-511 CC genotype and C allele are associated with higher risk of GERD than the TT genotype (OR 2.0, 95% CI 1.12-3.57, p=0.01). In contrast, the IL-1B-511*T/IL-1RN-*1haplotype is associated with a lower risk of GERD particularly among patients with Hpinfection [112]. This haplotype is associated with higher gastric mucosal IL-1β levels and inflammatory response. This status may decrease the level of acid secretion and explain the protective effect on GERD occurrence.
The incidence of GERD complications could differ with race. Wang et al used the Clinical Outcomes Research Initiative database to evaluate ethnic trends in esophagitis and Barrett’s esophagus among various racial groups in USA [113]. This database included 280,075 patients with upper endoscopy from 2000 to 2005. Hispanics were found to be more likely to have esophagitis (prevalence 19.6% in Hispanics, 17.3% in Whites, 15.8% in Blacks and 9.5% in Asian/Pacific Islanders) whereas Whites were more likely to have Barrett’s esophagus (prevalence 5% in Whites, 2.9% in Hispanics, 1.8% in Asian/Pacific Islanders and 1.5% in Blacks). Although these differences are statistically significant, they are marginal and should not alter treatment or management strategies.
Conclusion
Gastro-esophageal reflux disease is frequently encountered in Western countries; however, its prevalence is increasing all over the world. Its complex physiopathology lays the foundation for numerous interactions with environmental and lifestyle risk factors. Obesity is one of the main risk factors and interacts not only with esophago-gastric junction integrity but also with the mechanisms of esophageal clearance and promoting a preferential pressure gradient. Aging also plays an important role by impairing LES function, esophageal peristalsis and saliva secretion. The role of Helicobacter pylori and its eradication remain controversial and clarifications are required before global management strategies can be supported.
Practice Points.
GERD prevalence is increasing worldwide
Obesity, age, absence of Helicobacter pylori infection are the main risk factor for GERD
The different risk factors alter esophago-gastric junction integrity, esophageal clearance and/or enhance acid gastric secretion
Research agenda.
The effects of obesity on esophago-gastric junction and esophageal peristalsis have to be linked with increased acid esophageal exposure
The role of Helicobacter pylori and its eradication in GERD occurrence need to be clarified
Acknowledgments
declaration of all funding sources
This work was supported in part by R01 DK079902 from the Public Health Service
Abbreviations
- GERD
Gastro Esophageal Reflux Disease
- EGJ
Esophago-Gastric Junction
- Hp
Helicobacter pylori
- LES
Lower Esophageal Sphincter
- tLESR
transient Lower Esophageal Sphincter Relaxation
- BMI
Body Mass Index
- OR
Odds Ratio
- CI
Confidence Intervals
- HH
Hiatus Hernia
Footnotes
Conflicts of interest: none
References
- 1.El-Serag H, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther. 2009;29:470–80. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Locke GR, 3rd, McNally M, Schleck CD, Zinsmeister AR, Melton LJ., 3rd Impact of gastroesophageal reflux on survival in the community. Am J Gastroenterol. 2008;103:12–9. doi: 10.1111/j.1572-0241.2007.01546.x. [DOI] [PubMed] [Google Scholar]
- 3.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 43. [DOI] [PubMed] [Google Scholar]
- 4.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 6.Li YM, Du J, Zhang H, Yu CH. Epidemiological investigation in outpatients with symptomatic gastroesophageal reflux from the Department of Medicine in Zhejiang Province, east China. J Gastroenterol Hepatol. 2008;23:283–9. doi: 10.1111/j.1440-1746.2007.05045.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma XQ, Cao Y, Wang R, Yan X, Zhao Y, Zou D, et al. Prevalence of, and factors associated with, gastroesophageal reflux disease: a population-based study in Shanghai, China. Dis Esophagus. 2009;22:317–22. doi: 10.1111/j.1442-2050.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Yan X, Ma XQ, Cao Y, Wallander MA, Johansson S, et al. Burden of gastroesophageal reflux disease in Shanghai, China. Dig Liver Dis. 2009;41:110–5. doi: 10.1016/j.dld.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Wani S, Romero Y, Johnson D, Hamilton F. Racial and geographic issues in gastroesophageal reflux disease. Am J Gastroenterol. 2008;103:2669–80. doi: 10.1111/j.1572-0241.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheung TK, Wong BC, Lam SK. Gastro-oesophageal reflux disease in Asia : birth of a ‘new’ disease? Drugs. 2008;68:399–406. doi: 10.2165/00003495-200868040-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Iwakiri K, Kotoyori M, Sakamoto C. Mechanisms of acid gastroesophageal reflux in the Japanese population. Dig Dis Sci. 2008;53:1–6. doi: 10.1007/s10620-007-0038-4. [DOI] [PubMed] [Google Scholar]
- 12.Csendes A, Maluenda F, Burdiles P, Henriquez A, Quesada MS, Csendes P. Prospective study of esophageal motor abnormalities in patients with gastroesophageal disease reflux according to the severity of endoscopic esophagitis. Hepatogastroenterology. 1996;43:394–9. [PubMed] [Google Scholar]
- 13.Sugiura T, Iwakiri K, Kotoyori M, Kobayashi M. Relationship between severity of reflux esophagitis according to the Los Angeles classification and esophageal motility. J Gastroenterol. 2001;36:226–30. doi: 10.1007/s005350170107. [DOI] [PubMed] [Google Scholar]
- 14.Lee HL, Eun CS, Lee OY, Jeon YC, Sohn JH, Han DS, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–5. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 15.Hill LD, Kozarek RA, Kraemer SJ, Aye RW, Mercer CD, Low DE, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44:541–7. doi: 10.1016/s0016-5107(96)70006-8. [DOI] [PubMed] [Google Scholar]
- 16.Contractor QQ, Akhtar SS, Contractor TQ. Endoscopic esophagitis and gastroesophageal flap valve. J Clin Gastroenterol. 1999;28:233–7. doi: 10.1097/00004836-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfino JE, Bianchi LK, Lee TJ, Hirano I, Kahrilas PJ. Esophagogastric junction morphology predicts susceptibility to exercise-induced reflux. Am J Gastroenterol. 2004;99:1430–6. doi: 10.1111/j.1572-0241.2004.30515.x. [DOI] [PubMed] [Google Scholar]
- 18.Diener U, Patti MG, Molena D, Fisichella PM, Way LW. Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg. 2001;5:260–5. doi: 10.1016/s1091-255x(01)80046-9. [DOI] [PubMed] [Google Scholar]
- 19.Fornari F, Callegari-Jacques SM, Scussel PJ, Madalosso LF, Barros EF, Barros SG. Is ineffective oesophageal motility associated with reflux oesophagitis? Eur J Gastroenterol Hepatol. 2007;19:783–7. doi: 10.1097/MEG.0b013e3282748ecf. [DOI] [PubMed] [Google Scholar]
- 20.Fornari F, Blondeau K, Durand L, Rey E, Diaz-Rubio M, De Meyer A, et al. Relevance of mild ineffective oesophageal motility (IOM) and potential pharmacological reversibility of severe IOM in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:1345–54. doi: 10.1111/j.1365-2036.2007.03525.x. [DOI] [PubMed] [Google Scholar]
- 21.Simren M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784–90. doi: 10.1136/gut.52.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Schonfeld J, Hector M, Evans DF, Wingate DL. Oesophageal acid and salivary secretion: is chewing gum a treatment option for gastro-oesophageal reflux? Digestion. 1997;58:111–4. doi: 10.1159/000201432. [DOI] [PubMed] [Google Scholar]
- 23.Rourk RM, Namiot Z, Edmunds MC, Sarosiek J, Yu Z, McCallum RW. Diminished luminal release of esophageal epidermal growth factor in patients with reflux esophagitis. Am J Gastroenterol. 1994;89:1177–84. [PubMed] [Google Scholar]
- 24.Sarosiek J, Feng T, McCallum RW. The interrelationship between salivary epidermal growth factor and the functional integrity of the esophageal mucosal barrier in the rat. Am J Med Sci. 1991;302:359–63. doi: 10.1097/00000441-199112000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hirschowitz BI, Simmons JL, Johnson LF, Mohnen J. Risk factors for esophagitis in extreme acid hypersecretors with and without Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2004;2:220–9. doi: 10.1016/s1542-3565(04)00009-6. [DOI] [PubMed] [Google Scholar]
- 26.Oh DS, Hagen JA, Fein M, Bremner CG, Dunst CM, Demeester SR, et al. The impact of reflux composition on mucosal injury and esophageal function. J Gastrointest Surg. 2006;10:787–96. doi: 10.1016/j.gassur.2006.02.005. discussion 96-7. [DOI] [PubMed] [Google Scholar]
- 27.Salo J, Kivilaakso E. Role of luminal H+ in the pathogenesis of experimental esophagitis. Surgery. 1982;92:61–8. [PubMed] [Google Scholar]
- 28.Salo JA, Kivilaakso E. Role of bile salts and trypsin in the pathogenesis of experimental alkaline esophagitis. Surgery. 1983;93:525–32. [PubMed] [Google Scholar]
- 29.Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile acids as components of the duodenogastric refluxate: detection, relationship to bilirubin, mechanism of injury, and clinical relevance. Hepatogastroenterology. 1999;46:66–73. [PubMed] [Google Scholar]
- 30.Prevention. DoHaHSCfDCa Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1960-62 through 2005-06. [12 July 2010]; http://wwwcdcgov/nchs/data/hestat/overweight/overweight_adulthtm.
- 31.El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–33. doi: 10.1136/gut.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–12. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281–4. doi: 10.1136/gut.2007.127878. [DOI] [PubMed] [Google Scholar]
- 34.Pashankar DS, Corbin Z, Shah SK, Caprio S. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. J Clin Gastroenterol. 2009;43:410–3. doi: 10.1097/MCG.0b013e3181705ce9. [DOI] [PubMed] [Google Scholar]
- 35.Hansen JM, Wildner-Christensen M, Schaffalitzky de Muckadell OB. Gastroesophageal reflux symptoms in a Danish population: a prospective follow-up analysis of symptoms, quality of life, and health-care use. Am J Gastroenterol. 2009;104:2394–403. doi: 10.1038/ajg.2009.391. [DOI] [PubMed] [Google Scholar]
- 36.Ruigomez A, Garcia Rodriguez LA, Wallander MA, Johansson S, Graffner H, Dent J. Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharmacol Ther. 2004;20:751–60. doi: 10.1111/j.1365-2036.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 37.Nasseri-Moghaddam S, Mofid A, Ghotbi MH, Razjouyan H, Nouraie M, Ramard AR, et al. Epidemiological study of gastro-oesophageal reflux disease: reflux in spouse as a risk factor. Aliment Pharmacol Ther. 2008;28:144–53. doi: 10.1111/j.1365-2036.2008.03708.x. [DOI] [PubMed] [Google Scholar]
- 38.Fass R, Quan SF, O’Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest. 2005;127:1658–66. doi: 10.1378/chest.127.5.1658. [DOI] [PubMed] [Google Scholar]
- 39.Dore MP, Maragkoudakis E, Fraley K, Pedroni A, Tadeu V, Realdi G, et al. Diet, lifestyle and gender in gastro-esophageal reflux disease. Dig Dis Sci. 2008;53:2027–32. doi: 10.1007/s10620-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 40.Nocon M, Labenz J, Jaspersen D, Meyer-Sabellek W, Stolte M, Lind T, et al. Association of body mass index with heartburn, regurgitation and esophagitis: results of the Progression of Gastroesophageal Reflux Disease study. J Gastroenterol Hepatol. 2007;22:1728–31. doi: 10.1111/j.1440-1746.2006.04549.x. [DOI] [PubMed] [Google Scholar]
- 41.Choi CW, Kim GH, Song CS, Wang SG, Lee BJ, I H, et al. Is obesity associated with gastropharyngeal reflux disease? World J Gastroenterol. 2008;14:265–71. doi: 10.3748/wjg.14.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi M, Oka H, Hashimoto T, Asakuma Y, Takao M, Gon G, et al. Obesity as a risk factor for GERD in Japan. J Gastroenterol. 2008;43:57–62. doi: 10.1007/s00535-007-2128-7. [DOI] [PubMed] [Google Scholar]
- 43.Kim N, Lee SW, Cho SI, Park CG, Yang CH, Kim HS, et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008;27:173–85. doi: 10.1111/j.1365-2036.2007.03561.x. [DOI] [PubMed] [Google Scholar]
- 44.Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:655–62. doi: 10.1016/j.athoracsur.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Z, Margolis KL, Liu S, Tinker LF, Ye W. Effects of estrogen with and without progestin and obesity on symptomatic gastroesophageal reflux. Gastroenterology. 2008;135:72–81. doi: 10.1053/j.gastro.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iovino P, Angrisani L, Tremolaterra F, Nirchio E, Ciannella M, Borrelli V, et al. Abnormal esophageal acid exposure is common in morbidly obese patients and improves after a successful Lap-band system implantation. Surg Endosc. 2002;16:1631–5. doi: 10.1007/s00464-001-9225-0. [DOI] [PubMed] [Google Scholar]
- 47.Weiss HG, Nehoda H, Labeck B, Peer-Kuhberger MD, Klingler P, Gadenstatter M, et al. Treatment of morbid obesity with laparoscopic adjustable gastric banding affects esophageal motility. Am J Surg. 2000;180:479–82. doi: 10.1016/s0002-9610(00)00511-0. [DOI] [PubMed] [Google Scholar]
- 48.Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94:2840–4. doi: 10.1111/j.1572-0241.1999.01426.x. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB, Johanson JF. Risk factors for the severity of erosive esophagitis in Helicobacter pylori-negative patients with gastroesophageal reflux disease. Scand J Gastroenterol. 2002;37:899–904. doi: 10.1080/003655202760230847. [DOI] [PubMed] [Google Scholar]
- 50.Lee HL, Eun CS, Lee OY, Jeon YC, Han DS, Yoon BC, et al. Association between erosive esophagitis and visceral fat accumulation quantified by abdominal CT scan. J Clin Gastroenterol. 2009;43:240–3. doi: 10.1097/MCG.0b013e318167b88a. [DOI] [PubMed] [Google Scholar]
- 51.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–8. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 52.Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Fisher BL, Pennathur A, Mutnick JL, Little AG. Obesity correlates with gastroesophageal reflux. Dig Dis Sci. 1999;44:2290–4. doi: 10.1023/a:1026617106755. [DOI] [PubMed] [Google Scholar]
- 54.Suter M, Dorta G, Giusti V, Calmes JM. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients. Obes Surg. 2004;14:959–66. doi: 10.1381/0960892041719581. [DOI] [PubMed] [Google Scholar]
- 55.Sabate JM, Jouet P, Merrouche M, Pouzoulet J, Maillard D, Harnois F, et al. Gastroesophageal reflux in patients with morbid obesity: a role of obstructive sleep apnea syndrome? Obes Surg. 2008;18:1479–84. doi: 10.1007/s11695-008-9508-9. [DOI] [PubMed] [Google Scholar]
- 56.Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56:756–62. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu JC, Mui LM, Cheung CM, Chan Y, Sung JJ. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology. 2007;132:883–9. doi: 10.1053/j.gastro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Schneider JH, Kuper M, Konigsrainer A, Brucher B. Transient lower esophageal sphincter relaxation in morbid obesity. Obes Surg. 2009;19:595–600. doi: 10.1007/s11695-009-9809-7. [DOI] [PubMed] [Google Scholar]
- 59.Hong D, Khajanchee YS, Pereira N, Lockhart B, Patterson EJ, Swanstrom LL. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg. 2004;14:744–9. doi: 10.1381/0960892041590854. [DOI] [PubMed] [Google Scholar]
- 60.Jaffin BW, Knoepflmacher P, Greenstein R. High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg. 1999;9:390–5. doi: 10.1381/096089299765552990. [DOI] [PubMed] [Google Scholar]
- 61.Koppman JS, Poggi L, Szomstein S, Ukleja A, Botoman A, Rosenthal R. Esophageal motility disorders in the morbidly obese population. Surg Endosc. 2007;21:761–4. doi: 10.1007/s00464-006-9102-y. [DOI] [PubMed] [Google Scholar]
- 62.Quiroga E, Cuenca-Abente F, Flum D, Dellinger EP, Oelschlager BK. Impaired esophageal function in morbidly obese patients with gastroesophageal reflux disease: evaluation with multichannel intraluminal impedance. Surg Endosc. 2006;20:739–43. doi: 10.1007/s00464-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 63.Herbella FA, Sweet MP, Tedesco P, Nipomnick I, Patti MG. Gastroesophageal reflux disease and obesity Pathophysiology and implications for treatment. J Gastrointest Surg. 2007;11:286–90. doi: 10.1007/s11605-007-0097-z. [DOI] [PubMed] [Google Scholar]
- 64.Di Francesco V, Baggio E, Mastromauro M, Zoico E, Stefenelli N, Zamboni M, et al. Obesity and gastro-esophageal acid reflux: physiopathological mechanisms and role of gastric bariatric surgery. Obes Surg. 2004;14:1095–102. doi: 10.1381/0960892041975622. [DOI] [PubMed] [Google Scholar]
- 65.Infantino M. The prevalence and pattern of gastroesophageal reflux symptoms in perimenopausal and menopausal women. J Am Acad Nurse Pract. 2008;20:266–72. doi: 10.1111/j.1745-7599.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- 66.Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92:2007–11. [PubMed] [Google Scholar]
- 67.Fujimoto K, Iwakiri R, Okamoto K, Oda K, Tanaka A, Tsunada S, et al. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol. 2003;38(Suppl 15):3–6. [PubMed] [Google Scholar]
- 68.Richter JE. Gastroesophageal reflux disease in the older patient: presentation, treatment, and complications. Am J Gastroenterol. 2000;95:368–73. doi: 10.1111/j.1572-0241.2000.t01-1-01791.x. [DOI] [PubMed] [Google Scholar]
- 69.Chen CL, Yi CH, Liu TT, Orr WC. Altered sensorimotor responses to esophageal acidification in older adults with GERD*. Scand J Gastroenterol. 2010 doi: 10.3109/00365521.2010.496493. [DOI] [PubMed] [Google Scholar]
- 70.Achem AC, Achem SR, Stark ME, DeVault KR. Failure of esophageal peristalsis in older patients: association with esophageal acid exposure. Am J Gastroenterol. 2003;98:35–9. doi: 10.1111/j.1572-0241.2003.07188.x. [DOI] [PubMed] [Google Scholar]
- 71.Lee J, Anggiansah A, Anggiansah R, Young A, Wong T, Fox M. Effects of age on the gastroesophageal junction, esophageal motility, and reflux disease. Clin Gastroenterol Hepatol. 2007;5:1392–8. doi: 10.1016/j.cgh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Kusano M, Hashizume K, Ehara Y, Shimoyama Y, Kawamura O, Mori M. Size of hiatus hernia correlates with severity of kyphosis, not with obesity, in elderly Japanese women. J Clin Gastroenterol. 2008;42:345–50. doi: 10.1097/MCG.0b013e318037556c. [DOI] [PubMed] [Google Scholar]
- 73.Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. doi: 10.1136/bmj.326.7392.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raghunath AS, Hungin AP, Wooff D, Childs S. Systematic review: the effect of Helicobacter pylori and its eradication on gastro-oesophageal reflux disease in patients with duodenal ulcers or reflux oesophagitis. Aliment Pharmacol Ther. 2004;20:733–44. doi: 10.1111/j.1365-2036.2004.02172.x. [DOI] [PubMed] [Google Scholar]
- 75.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol. 2010;105:1007–13. doi: 10.1038/ajg.2009.734. [DOI] [PubMed] [Google Scholar]
- 76.Calam J. Helicobacter pylori and somatostatin cells. Eur J Gastroenterol Hepatol. 1998;10:281–3. doi: 10.1097/00042737-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol. 2002;282:G175–83. doi: 10.1152/ajpgi.00287.2001. [DOI] [PubMed] [Google Scholar]
- 78.el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–91. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 79.Souza RC, Lima JH. Helicobacter pylori and gastroesophageal reflux disease: a review of this intriguing relationship. Dis Esophagus. 2009;22:256–63. doi: 10.1111/j.1442-2050.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 80.Feldman M, Cryer B, Sammer D, Lee E, Spechler SJ. Influence of H pylori infection on meal-stimulated gastric acid secretion and gastroesophageal acid reflux. Am J Physiol. 1999;277:G1159–64. doi: 10.1152/ajpgi.1999.277.6.G1159. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto M, Haruma K, Kuwabara M, Nagano M, Okamoto T, Tanaka M. High incidence of newly-developed gastroesophageal reflux disease in the Japanese community: a 6-year follow-up study. J Gastroenterol Hepatol. 2008;23:393–7. doi: 10.1111/j.1440-1746.2007.05043.x. [DOI] [PubMed] [Google Scholar]
- 82.Rajendra S, Ackroyd R, Robertson IK, Ho JJ, Karim N, Kutty KM. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter. 2007;12:177–83. doi: 10.1111/j.1523-5378.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 83.Zentilin P, Iiritano E, Dulbecco P, Bilardi C, Savarino E, De Conca S, et al. Normal values of 24-h ambulatory intraluminal impedance combined with pH-metry in subjects eating a Mediterranean diet. Dig Liver Dis. 2006;38:226–32. doi: 10.1016/j.dld.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 85.Zerbib F, des Varannes SB, Roman S, Pouderoux P, Artigue F, Chaput U, et al. Normal values and day-to-day variability of 24-h ambulatory oesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther. 2005;22:1011–21. doi: 10.1111/j.1365-2036.2005.02677.x. [DOI] [PubMed] [Google Scholar]
- 86.El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11–7. doi: 10.1136/gut.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DiBaise JK. A randomized, double-blind comparison of two different coffee-roasting processes on development of heartburn and dyspepsia in coffee-sensitive individuals. Dig Dis Sci. 2003;48:652–6. doi: 10.1023/a:1022860019852. [DOI] [PubMed] [Google Scholar]
- 88.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut. 2004;53:1730–5. doi: 10.1136/gut.2004.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akiyama T, Inamori M, Iida H, Mawatari H, Endo H, Hosono K, et al. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett’s epithelium in Japanese men. BMC Gastroenterol. 2008;8:58. doi: 10.1186/1471-230X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gunasekaran TS, Dahlberg M, Ramesh P, Namachivayam G. Prevalence and associated features of gastroesophageal reflux symptoms in a Caucasian-predominant adolescent school population. Dig Dis Sci. 2008;53:2373–9. doi: 10.1007/s10620-007-0150-5. [DOI] [PubMed] [Google Scholar]
- 91.Bor S, Bor-Caymaz C, Tobey NA, Abdulnour-Nakhoul S, Orlando RC. Esophageal exposure to ethanol increases risk of acid damage in rabbit esophagus. Dig Dis Sci. 1999;44:290–300. doi: 10.1023/a:1026646215879. [DOI] [PubMed] [Google Scholar]
- 92.Mayer EM, Grabowski CJ, Fisher RS. Effects of graded doses of alcohol upon esophageal motor function. Gastroenterology. 1978;75:1133–6. [PubMed] [Google Scholar]
- 93.Keshavarzian A, Polepalle C, Iber FL, Durkin M. Esophageal motor disorder in alcoholics: result of alcoholism or withdrawal? Alcohol Clin Exp Res. 1990;14:561–7. doi: 10.1111/j.1530-0277.1990.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 94.Pehl C, Frommherz M, Wendl B, Pfeiffer A. Gastroesophageal reflux induced by white wine: the role of acid clearance and “rereflux”. Am J Gastroenterol. 2002;97:561–7. doi: 10.1111/j.1572-0241.2002.05530.x. [DOI] [PubMed] [Google Scholar]
- 95.Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol. 2009;43:111–7. doi: 10.1097/MCG.0b013e31815ea27b. [DOI] [PubMed] [Google Scholar]
- 96.Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4–10. doi: 10.1136/gut.31.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kahrilas PJ, Gupta RR. The effect of cigarette smoking on salivation and esophageal acid clearance. J Lab Clin Med. 1989;114:431–8. [PubMed] [Google Scholar]
- 98.Lagergren J, Bergstrom R, Adami HO, Nyren O. Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med. 2000;133:165–75. doi: 10.7326/0003-4819-133-3-200008010-00007. [DOI] [PubMed] [Google Scholar]
- 99.Corley DA, Levin TR, Habel LA, Buffler PA. Barrett’s esophagus and medications that relax the lower esophageal sphincter. Am J Gastroenterol. 2006;101:937–44. doi: 10.1111/j.1572-0241.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 100.Vaughan TL, Farrow DC, Hansten PD, Chow WH, Gammon MD, Risch HA, et al. Risk of esophageal and gastric adenocarcinomas in relation to use of calcium channel blockers, asthma drugs, and other medications that promote gastroesophageal reflux. Cancer Epidemiol Biomarkers Prev. 1998;7:749–56. [PubMed] [Google Scholar]
- 101.Hubert D, Gaudric M, Guerre J, Lockhart A, Marsac J. Effect of theophylline on gastroesophageal reflux in patients with asthma. J Allergy Clin Immunol. 1988;81:1168–74. doi: 10.1016/0091-6749(88)90886-x. [DOI] [PubMed] [Google Scholar]
- 102.van Soest EM, Dieleman JP, Siersema PD, Schoof L, Sturkenboom MC, Kuipers EJ. Tricyclic antidepressants and the risk of reflux esophagitis. Am J Gastroenterol. 2007;102:1870–7. doi: 10.1111/j.1572-0241.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 103.Mostaghni A, Mehrabani D, Khademolhosseini F, Masoumi SJ, Moradi F, Zare N, et al. Prevalence and risk factors of gastroesophageal reflux disease in Qashqai migrating nomads, southern Iran. World J Gastroenterol. 2009;15:961–5. doi: 10.3748/wjg.15.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dean BB, Aguilar D, Johnson LF, McGuigan JE, Orr WC, Fass R, et al. Night-time and daytime atypical manifestations of gastro-oesophageal reflux disease: frequency, severity and impact on health-related quality of life. Aliment Pharmacol Ther. 2008;27:327–37. doi: 10.1111/j.1365-2036.2007.03574.x. [DOI] [PubMed] [Google Scholar]
- 105.Bonatti H, Achem SR, Hinder RA. Impact of changing epidemiology of gastroesophageal reflux disease on its diagnosis and treatment. J Gastrointest Surg. 2008;12:373–81. doi: 10.1007/s11605-007-0294-9. [DOI] [PubMed] [Google Scholar]
- 106.Horikawa A, Ishii-Nozawa R, Ohguro M, Takagi S, Ohtuji M, Yamada M, et al. Prevalence of GORD (gastro-oesophageal reflux disease) in Type 2 diabetes and a comparison of clinical profiles between diabetic patients with and without GORD. Diabet Med. 2009;26:228–33. doi: 10.1111/j.1464-5491.2009.02671.x. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–12. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jansson C, Nordenstedt H, Wallander MA, Johansson S, Johnsen R, Hveem K, et al. Severe symptoms of gastro-oesophageal reflux disease are associated with cardiovascular disease and other gastrointestinal symptoms, but not diabetes: a population-based study. Aliment Pharmacol Ther. 2008;27:58–65. doi: 10.1111/j.1365-2036.2007.03537.x. [DOI] [PubMed] [Google Scholar]
- 109.Marie I, Dominique S, Levesque H, Ducrotte P, Denis P, Hellot MF, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001;45:346–54. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 110.Marie I, Ducrotte P, Denis P, Hellot MF, Levesque H. Oesophageal mucosal involvement in patients with systemic sclerosis receiving proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1593–601. doi: 10.1111/j.1365-2036.2006.03180.x. [DOI] [PubMed] [Google Scholar]
- 111.Savarino E, Bazzica M, Zentilin P, Pohl D, Parodi A, Cittadini G, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179:408–13. doi: 10.1164/rccm.200808-1359OC. [DOI] [PubMed] [Google Scholar]
- 112.Chourasia D, Achyut BR, Tripathi S, Mittal B, Mittal RD, Ghoshal UC. Genotypic and functional roles of IL-1B and IL-1RN on the risk of gastroesophageal reflux disease: the presence of IL-1B-511*T/IL-1RN*1 (T1) haplotype may protect against the disease. Am J Gastroenterol. 2009;104:2704–13. doi: 10.1038/ajg.2009.382. [DOI] [PubMed] [Google Scholar]
- 113.Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci. 2009;54:964–71. doi: 10.1007/s10620-009-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


