Abstract
Mitochondrial dysfunction is associated with a broad range of pathologies including diabetes, ethanol toxicity, metabolic syndrome, and cardiac failure. It is now becoming clear that maintaining mitochondrial quality through a balance between biogenesis, reserve capacity, and mitophagy is critical in determining the response to metabolic or xenobiotic stress. In diseases associated with metabolic stress, such as type II diabetes, non-alcoholic and alcoholic steatosis, the mitochondria are subjected to multiple “hits” such as hypoxia and oxidative and nitrative stress, which can overwhelm mitochondrial quality control pathways. In addition, the underlying mitochondrial genetics which evolved to accommodate high energy demand, low calorie supply environments may now be maladapted to modern lifestyles (low energy demand, high calorie environments). The pro-oxidant and pro-inflammatory environment of a sedentary western lifestyle has been associated with modified redox cell signaling pathways such as steatosis, hypoxic signaling, inflammation, and fibrosis. These data suggest that loss of mitochondrial quality control is intimately associated with the aberrant activation of redox cell signaling pathways under pathological conditions. In this short review, we will discuss evidence from alcoholic liver disease supporting this concept, the insights obtained from experimental models, and the application of bioenergetic based therapeutics in the context of maintaining mitochondrial quality.
Keywords: reserve respiratory capacity, mitochondria, cellular bioenergetics, steatosis, alcoholic liver disease, diabetes, metabolic syndrome, MitoQ
Introduction
Metabolic diseases including metabolic syndrome, diabetes, and alcoholic liver disease are prevalent causes of illness and death in developed nations. In all of these pathologies, bioenergetic dysfunction is a prominent feature which contributes significantly to the pathophysiology. Recent advances in mitochondrial biology now allow a concerted effort to address the mechanisms involved and the development of bioenergetic-specific therapies. A key feature of metabolic diseases is that a large proportion of the populations affected, possess multiple risk factors for developing severe life threatening symptoms but only a relatively small proportion progress to more severe pathologies. This observation has raised the possibility that secondary stressors or “hits” are factors that differentiate the severity of metabolic diseases among individuals. Examples of secondary hits vary and can include those associated with the cardiometabolic syndrome such as obesity, hyperlipidemia and insulin resistance, and environmental factors such as diet, pathogens and toxicants. In this review, we will use the example of alcohol induced hepatotoxicity to highlight these concepts.
Chronic alcohol consumption can cause severe liver diseases including steatohepatitis, fibrosis/cirrhosis, and hepatocellular carcinoma [1, 2]. Classically, it has been thought that development of alcoholic liver disease depends on the total amount and duration of alcohol consumption [3]. However, this simple hypothesis has been challenged as many heavy drinkers do not develop end-stage liver disease following decades of drinking suggesting more than one “hit” contributes to disease progression [4]. In the context of the second hit paradigm, data has emerged which show that genetic, epigenetic, metabolic, and environmental factors influence and drive the progression from simple steatosis to cirrhosis and cancer [5, 6]. This concept serves as the basis of the “multi-hit” mechanism of alcoholic liver disease in which secondary stressors or “hits” contribute to disease pathogenesis. Importantly, alcohol, obesity, and cigarette smoke may accelerate fibrosis and have been shown to be key synergistic risk factors for hepatocellular carcinoma [7, 8]. While correlative results from epidemiologic studies are important, the molecular mechanisms responsible for the interactive additive or synergistic effects of alcohol with other risk factors remain poorly understood. Furthermore, how these confounding factors work together to impact mitochondrial function as a key contributing factor to chronic alcohol-induced diseases is unclear.
In this review, we will address the emerging hypothesis that underlying differences in bioenergetics between individual patients is a key mechanism influencing the susceptibility and severity of the disease. At the molecular level, we will discuss how the balance between mitochondrial biogenesis, cellular bioenergetics, and mitophagy are integrated into a program to regulate mitochondrial quality control and how this may be altered by alcohol intake, diabetes, smoking, and in chronic alcohol disease pathogenesis. Furthermore, we will discuss the mitochondrial-targeted antioxidant, Mitoquinone (MitoQ) in experimental therapies against metabolic pathologies.
Mitochondrial Quality Control and Dysfunction in Alcoholic Liver and Metabolic Disease
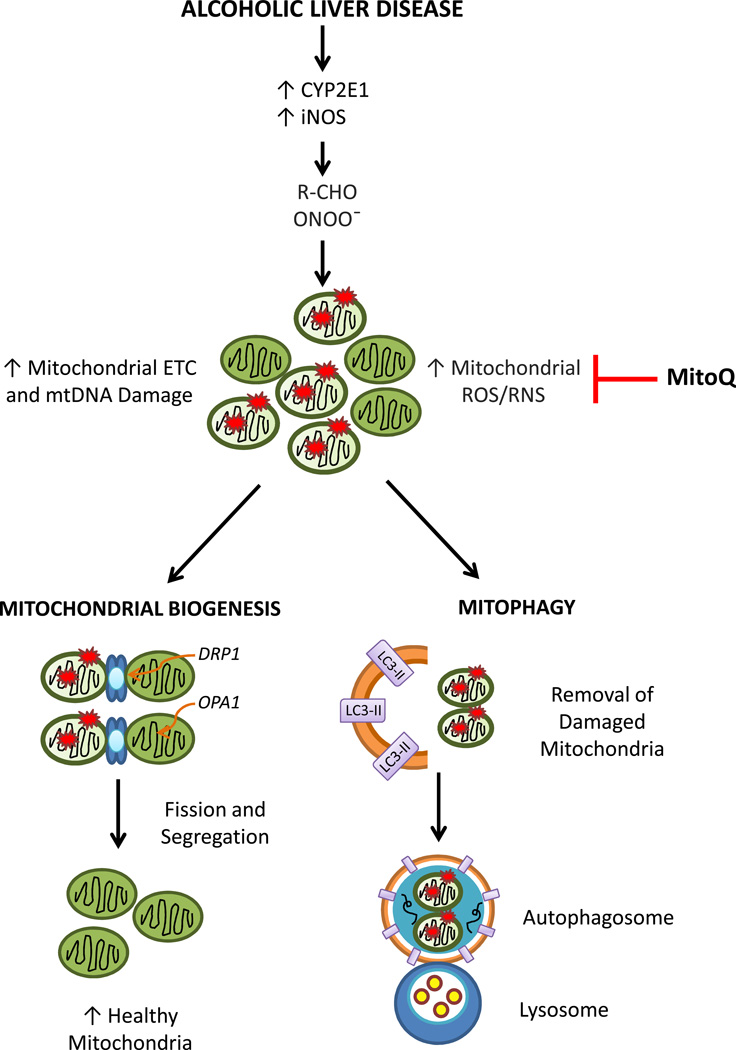
Mitochondria are important intracellular organelles that provide cellular energy, control reactive species, and retrograde signaling to the nucleus for gene expression [9–12]. Within the cell, the quality of the mitochondrial population varies due to protein expression, post-translational modifications, assembly of respiratory chain proteins, membrane potential, and specific and non-specific modifications by reactive species to lipids, proteins, DNA and RNA. Dysfunctional mitochondria may arise due to accumulation of mitochondrial DNA mutations and mis-folded and oxidatively modified proteins [13–15]. In alcoholic liver disease (ALD), it has been well established that mitochondria are targeted in cellular injury and that cytochrome P450 (CYP2E1) and inducible nitric oxide synthase (iNOS) expression are upregulated[16–18]. This results in aldehyde and peroxynitrite formation, which further induces mitochondrial reactive oxygen and nitrogen species formation, respiratory chain dysfunction, and mtDNA damage [19–21](Figure 1). These events are all thought to contribute to liver steatosis. This naturally raises the question whether bioenergetic dysfunction is an epiphenomenon or intimately related to the pathology of the disease.
Figure 1. Mitochondrial Quality Control is Regulated by Mitochondrial Biogenesis and Mitophagy.
It is well known that during alcoholic liver disease cytochrome P450 (CYP2E1) and inducible nitric oxide synthase (iNOS) are upregulated and this leads to aldehyde (R-CHO) and peroxynitrite (ONOO−) generation. These highly oxidative/nitrative compounds further induce mitochondrial ROS/RNS generation, electron transport chain (ETC) dysfunction, and mtDNA damage. Collectively, these events are thought to contribute to liver steatosis. To maintain mitochondrial population and quality, the cell stimulates mitochondrial biogenesis and mitophagy pathways. Mitochondrial biogenesis is a complex process that is controlled by communication between the nucleus and the organelle to activate transcription factors that control fusion and fission processes. DRP1 and OPA1 are fission proteins located on the mitochondrial outer and inner membranes, respectively that divide and segregate damaged mitochondria to increase the population of healthy mitochondria. In contrast, mitophagy is initiated by the formation of cytoplasmic double membrane structures that recognize damaged mitochondria. Further, expansion of the autophagosomal structures is dependent on the conversion of LC3-I to LC3-II, and insertion of LC3-II into the autophagosomal membranes. Completion of autophagic flux requires fusion of autophagosomes with lysosomes, where damaged mitochondria are degraded and cleared from the cell. Mitoquinone (MitoQ), a mitochondrial targeted antioxidant has been shown to prevent alcoholic liver disease-mediated oxidative and nitrative stress and steatosis, supporting the pivotal role of mitochondrial oxidants in alcoholic liver disease pathogenesis.
How do cells maintain normal mitochondrial bioenergetics? Typically this is carried out by a mitochondrial quality control system to remove damaged proteins through mitochondrial specific repair pathways[22, 23]. If this process is overwhelmed, then the entire organelle is targeted for disposal and removed by the autophagic machinery in a process known as autophagy of the mitochondria, or mitophagy [23](Figure 1). Mitophagy is a complex program that the cell uses to recognize damaged mitochondria by mitochondrial network morphologies (e.g. fission) and bioenergetic parameters (e.g. decreased mitochondrial membrane potential). Because intracellular protein and organelle degradation can be a dangerous undertaking, autophagy is highly regulated by the participation of more than 30 proteins which are important for synthesis and maturation of double membrane vesicles encircling cellular content, including damaged mitochondria, and formation of autophagosomes[23]. The autophagosomes then fuse to lysosomes in order for the contents of the autophagosomes including mitochondria to be degraded. This may seem to be a drastic measure but low quality mitochondria can damage remaining healthy mitochondria through the release of ROS and Ca2+ from the organelle. Concomitantly, the cell also stimulates mitochondrial biogenesis to maintain the mitochondrial population (Figure 1). This is a complex process that is controlled by communication between the nucleus and the organelle to activate transcription factors that control fusion and fission processes to divide and segregate damaged mitochondria to increase the population of healthy mitochondria [24]. For example, damaged mitochondria undergo fission to segregate the dysfunctional components, and fusion to supplement the deficiencies of damaged mitochondria with segments of the respiratory chain[24–26]. Thus, maintaining mitochondrial dynamics has emerged as a critical element in the contribution of mitochondria to cell division and physiological maintenance. Preservation of mitochondrial quality in alcohol toxicity could represent a threshold for cell survival since damaged mitochondria which are not removed by mitophagy are more susceptible to uncoupling and contribute to cell death [27–30].
In alcohol-dependent hepatotoxicity, primary (alcohol) and secondary stressors conspire to promote an inflammatory response in the liver. Specifically, previous studies implicate mechanistic links between alcohol (primary hit) and cigarette smoke, insulin resistance, and hyperlipidemia (secondary hits), which include oxidative and/or nitrative damage, disrupted redox signaling, and hypoxic stress [6, 17, 31–33]. Notably, mitochondria are primary targets for alcohol and secondary stressors, which can also heighten liver inflammatory processes. Studies show that chronic alcohol consumption alone increases mitochondrial ROS production, causes mtDNA damage, impairs oxidative phosphorylation, and alters mitochondrial dynamics [34, 35]. Cigarette smoke and hyperlipidemia also disrupt mitochondrial function [36]. Thus, chronic alcohol consumption when combined with these secondary stressors such as cigarette smoke or hyperlipidemia amplifies liver injury with increased mtDNA damage, mitochondrial proteome alterations, and enhanced hypoxia via mitochondrial damage [37]. Nitric oxide (NO) is central to these mechanisms and is exacerbated by the fact that the responsive of the respiratory chain to nitric oxide (NO) is increased by chronic alcohol consumption [16, 17]. Indeed, hypoxia and disrupted NO signaling increase vulnerability of mitochondria in the alcohol consumer to additional damage when exposed to metabolic and/or environmental stressors. These conditions will lead to irreversible modification and inactivation of mitochondrial proteins, which will negatively impact mitochondrial bioenergetics. The inability to generate ATP needed for metabolism, detoxification, and repair renders hepatocytes vulnerable to secondary stressors leading to hepatocyte cell death; a factor critical in alcoholic liver disease progression and severity.
Since alcohol consumption is a severe metabolic stress due to the production of ROS/RNS, and reactive aldehydes leading to protein and mtDNA modification then it compromises the mitochondrial population in the cell and this should induce the autophagic process. Indeed, acute exposure of the liver or hepatocytes to alcohol, modeling binge drinking, results in the activation of autophagy with the abundant formation of autophagosomes and the targeting of damaged mitochondria (mitophagy) and lipid droplets (lipophagy). Inhibition of the autophagic process resulted in increased mitochondrial-dependent apoptosis suggesting a protective role which appears to be overwhelmed as alcohol toxicity progresses [38, 39]. This inhibition of the autophagic process may be linked to increased steatosis and marked deterioration of mitochondrial quality possibly since damaged ROS-generating mitochondria can no longer be removed. Indeed, it appears that chronic alcohol consumption is associated with a dysfunctional engagement of the autophagic process[40]. The suppression of autophagy can also contribute to the development of steatosis and mitochondrial dysfunction due to damaged mitochondria promoting cell death [27–30]. The mechanisms through which alcohol suppresses autophagy are still under investigation but could include suppression of the AMP kinase pathway, disruption of protein trafficking or the formation of oxidative protein adducts [28, 41].
As with alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD) is a serious medical problem as the percentage of obese individuals is growing in many countries [42, 43]. Moreover, the etiology of NAFLD is also linked to multiple risk factors including obesity, visceral adiposity, inflammation, type 2 diabetes, insulin resistance, and hyperlipidemia. Mitochondrial dysfunction and proteomic changes are a feature of NAFLD, suggesting that overlapping mechanisms between obesity-dependent and alcoholic liver disease, including enhanced hypoxia and increased mitochondrial sensitivity to NO [44, 45]. These results are important as they reveal novel targets in mitochondria that should be amenable for future NAFLD therapeutic studies.
Mitochondrial Dynamics and Therapeutics
As oxidative stress is implicated as a key player in alcoholic liver disease, numerous studies have been undertaken using antioxidant therapies. Unfortunately, results from these studies show minimal effects of antioxidants to prevent and/or reverse alcohol hepatotoxicity in experimental animal models and clinical trials with alcoholic patients. Importantly, few studies have assessed whether antioxidants prevent alcohol-dependent mitochondrial dysfunction and oxidative damage. Recently, we have shown that S-adenosylmethionine (SAM) and betaine; key methionine metabolism intermediates, prevent alcohol-dependent steatosis, with preservation of the mitochondrial thiol proteome and inhibition of oxidative and nitrative stress [37, 46, 47]. These results are important as they support the concept that SAM serves a key role in maintaining mitochondrial quality.
Recently, a new category of antioxidant-based therapeutics have been developed which target mitochondria. The strategy employed attaches the antioxidant to a lipophilic cationic moiety triphenylphosphonium (TPP+), which results in a several hundred-fold accumulation of the antioxidant into the mitochondrial matrix compartment [48]. To date, the most successful mitochondrial-targeted antioxidant is Mitoquinone which is referred to as MitoQ [49–52]. Studies show that the protective effects of MitoQ stem from changes in mitochondrial ROS production, which affect redox and hypoxia sensitive signaling pathways. For example, MitoQ prevented alcohol-dependent oxidative damage, hypoxia, and steatosis [16, 20]. MitoQ has also been shown to decrease liver injury in hepatitis C patients[53]. Whether the mechanisms of action of MitoQ in hepatoprotection involve regulation of mitochondrial biogenesis, dynamics and mitophagy are currently being investigated. In other cell models, MitoQ has been shown to decrease mitochondrial fragmentation, and increase autophagy [54].
Mitochondrial Genetics and the Implications for personalized medicine
The mitochondrial genome codes for key proteins in oxidative phosphorylation and it is now clear that polymorphisms in this genome can influence disease susceptibility or progression [55, 56]. In terms of liver disease, recent studies have implicated certain mtDNA polymorphisms with increased risk for the development of hepatocellular carcinoma associated with either hepatitis virus infection or alcohol abuse [57, 58]. Similar associations have also been found linking mtDNA background and epigenetic modifications of the mtDNA with NAFLD. While these representative studies specifically investigated liver disease, relationships between metabolic diseases and mtDNA genetic background have frequently been reported.
The rationale connecting disease susceptibility to mtDNA genetic background is based upon the hypothesis that certain mtDNA missense mutations were bioenergetically advantageous during human prehistory [59]. These changes in mitochondrial function were inter-related with caloric availability (diet) and environmental conditions (latitude) in humans which gave them a reproductive and survival advantage in prehistoric times. For example, it has been hypothesized that mtDNA haplotypes associated with sub-Saharan latitudes have mutations that made the mitochondrion more “economical”, enabling them to utilize fewer electrons for the generation of a transmembrane potential and thus, ATP synthesis. This would make them well adapted for a low calorie diet at warm latitudes (no need to generate heat). In contrast, mtDNAs evolving in northern latitudes have altered mitochondrial economies in that more electrons would be utilized for ATP generation due to decreased efficiency – translating into generation of greater thermal energy (heat); a metabolic advantage at colder latitudes. Although these mutations decreased mitochondrial efficiency for ATP generation, they were accommodated by a change in diet (increased caloric intake associated with animal fats). Today these changes (mutations) in the mtDNA can be maladapted for contemporary diets (excess fat) and sedentary lifestyle common in western society. Under conditions of high caloric intake and physical inactivity, individuals having mtDNA backgrounds originating from prehistoric clines characterized by low caloric availability and tropical latitudes have mitochondria that generate increased mitochondrial oxidants relative to those with geographic origins typified by animal fats and northern latitudes.
An additional factor impacting mitochondrial function in addition to mtDNA background is overall organelle integrity, or the level of epigenetic modification and damage present. It is well established that age and the environment can influence mitochondrial integrity. Numerous studies have shown that environmental oxidants cause significant mtDNA damage and that developmental exposure can significantly impact downstream organelle function and condition [60, 61]. Because these factors can significantly influence mitochondrial function and response to exogenous stimuli, it is plausible that future therapeutic strategies for disease treatment and/or prevention will utilize information about mitochondrial genetic background, function, and integrity to determine appropriate choice of pharmaceutical or treatment regimen. Interventional strategies could be personalized based upon mtDNA background, mtDNA damage, function, and oxidative stress measures from a surrogate tissue. For example, mtDNA genetic background may be linked to changes in mitochondrial function and cellular oxidant production in the presence and absence of a particular therapeutic (e.g. SAM or MitoQ) in a surrogate tissue, as a predictive measure for overall efficacy. Recent studies are revealing the “mitochondrial priming” may provide a means for increasing the efficiency of cytotoxins for the treatment of certain cancers [62].
Summary
As many chronic alcohol consumers are now more likely to be obese, studies are needed to investigate the impact of cardiometabolic risk factors such as type 2 diabetes and hyperlipidemia to increase alcoholic liver disease. Moreover, because exposure to environmental pollutants like second-hand cigarette smoke remains widespread, and concomitant exposure to alcohol and cigarette smoke frequently occurs, investigations of these confounding factors on liver disease are also essential. It is rapidly becoming clear that the central therapeutic strategy in the context of bioenergetics is to maintain mitochondrial quality (Figure 1). The aspect which has received most attention is the suppression of the dysregulation of mitochondrial hydrogen peroxide generation so correcting dysfunctional cell signaling and oxidative damage to proteins, lipids and DNA. This is the mechanism of action through which MitoQ appears to be working. However, if the mitophagy pathway is overwhelmed or inhibited and the removal of damaged mitochondria fails then the impact of compounds such as MitoQ will be limited. Similarly, if biogenesis cannot supply new functional mitochondria to replace those removed by mitophagy the remaining mitochondrial population will be stressed and turnover increased so amplifying the initial insult. This framework provides both a model to understand the interaction of multiple hits on the development of metabolic syndromes and the rationale for combination therapies to improve mitochondrial quality in disease.
Acknowledgements
This work was supported by NIH T32 DK007545 (Tanecia Mitchell); HL 94518 and HL 103859 (Scott Ballinger); DK073775, AA015172, and AA018841 (Shannon Bailey); NS064090 and a VA merit award (Jianhua Zhang), and AA13395 (Victor Darley-Usmar).
Abbreviations
- ALD
Alcoholic Liver Disease
- NAFLD
Non-Alcoholic Fatty Liver Disease
- NO
Nitric Oxide
- RCR
Respiratory Control Ratio
- ROS/RNS
Reactive Oxygen Species/Reactive Nitrogen Species
- MitoQ
mitochondrial targeted ubiquinone (Mitoquinone)
- SAM
S-adenosylmethionine
- iNOS
inducible nitric oxide synthase
- TPP
Triphenylphosphonium
Literature Cited
- 1.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 2.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. Journal of gastroenterology and hepatology. 2011;26:1089–1105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 3.Corrao G, Ferrari P, Zambon A, Torchio P, Arico S, Decarli A. Trends of liver cirrhosis mortality in Europe, 1970–1989: age-period-cohort analysis and changing alcohol consumption. Int. J. Epidemiol. 1997;26:100–109. doi: 10.1093/ije/26.1.100. [DOI] [PubMed] [Google Scholar]
- 4.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CP. Genes or environment to determine alcoholic liver disease and nonalcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Shukla SD, Aroor AR. Epigenetic effects of ethanol on liver and gastrointestinal injury. World J. Gastroenterol. 2006;12:5265–5271. doi: 10.3748/wjg.v12.i33.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Cassetti T, La Rosa F, Rossi L, D'Alo D, Stracci F. Cancer incidence in men: a cluster analysis of spatial patterns. BMC Cancer. 2008;8:344. doi: 10.1186/1471-2407-8-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circulation research. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 11.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 13.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and nonalcoholic liver injury. J. Gastroenterol. Hepatol. 2008;23(Suppl 1):S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osna NA, Donohue TM., Jr Implication of altered proteasome function in alcoholic liver injury. World J. Gastroenterol. 2007;13:4931–4937. doi: 10.3748/wjg.v13.i37.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pezzotti A, Kraft P, Hankinson SE, Hunter DJ, Buring J, Cox DG. The mitochondrial A10398G polymorphism, interaction with alcohol consumption, and breast cancer risk. PLoS One. 2009;4:e5356. doi: 10.1371/journal.pone.0005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochimica et biophysica acta. 2011;1807:1573–1582. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, Bailey SM, Darley-Usmar VM. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 18.Bailey SM. A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic. Res. 2003;37:585–596. doi: 10.1080/1071576031000091711. [DOI] [PubMed] [Google Scholar]
- 19.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic. Biol. Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondriatargeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatraman A, Shiva S, Davis AJ, Bailey SM, Brookes PS, Darley- Usmar VM. Chronic alcohol consumption increases the sensitivity of rat liver mitochondrial respiration to inhibition by nitric oxide. Hepatology. 2003;38:141–147. doi: 10.1053/jhep.2003.50293. [DOI] [PubMed] [Google Scholar]
- 22.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch.Biochem.Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak I. Mitophagy: A Complex Mechanism of Mitochondrial Removal. Antioxidants & redox signaling. 2012;17:794–802. doi: 10.1089/ars.2011.4407. [DOI] [PubMed] [Google Scholar]
- 25.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxidants & redox signaling. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto K, Kondo-Okamoto N. Mitochondria and autophagy: Critical interplay between the two homeostats. Biochimica et biophysica acta. 2012;1820:595–600. doi: 10.1016/j.bbagen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Nitta T, Mohuczy D, O'Malley KA, Moldawer LL, Dunn WA, Jr, Behrns KE. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donohue TM., Jr Autophagy and ethanol-induced liver injury. World J. Gastroenterol. 2009;15:1178–1185. doi: 10.3748/wjg.15.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochimica et biophysica acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Aspects Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley- Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am. J. Physiol. Gastrointest Liver Physiol. 2004;286:G521–G527. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- 34.Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Hajnoczky N, Antony AN, Csordas G, Gaspers LD, Clemens DL, Hoek JB, Hajnoczky G. Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflugers Archiv : European journal of physiology. 2012;464:101–109. doi: 10.1007/s00424-012-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 37.Andringa KK, King AL, Eccleston HB, Mantena SK, Landar A, Jhala NC, Dickinson DA, Squadrito GL, Bailey SM. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol. 2010;298:G732–G745. doi: 10.1152/ajpgi.00332.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poso AR, Hirsimaki P. Inhibition of proteolysis in the liver by chronic ethanol feeding. Biochem. J. 1991;273:149–152. doi: 10.1042/bj2730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donohue TM, Jr, Zetterman RK, Tuma DJ. Effect of chronic ethanol administration on protein catabolism in rat liver. Alcoholism, clinical and experimental research. 1989;13:49–57. doi: 10.1111/j.1530-0277.1989.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 40.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. Journal of hepatology. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanolinduced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochemical and biophysical research communications. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petta S, Muratore C, Craxi A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis. 2009;41:615–625. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Zafrani ES. Non-alcoholic fatty liver disease: an emerging pathological spectrum. Virchows Arch. 2004;444:3–12. doi: 10.1007/s00428-003-0943-7. [DOI] [PubMed] [Google Scholar]
- 44.Eccleston HB, Andringa KK, Betancourt AM, King AL, Mantena SK, Swain TM, Tinsley HN, Nolte RN, Nagy TR, Abrams GA, Bailey SM. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal. 2011;15:447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver diseases. World J Gastroenterol. 2007;13:4967–4973. doi: 10.3748/wjg.v13.i37.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G857–G867. doi: 10.1152/ajpgi.00044.2006. [DOI] [PubMed] [Google Scholar]
- 47.Kharbanda KK, Todero SL, King AL, Osna NA, McVicker BL, Tuma DJ, Wisecarver JL, Bailey SM. Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. International journal of hepatology. 2012;2012:962183. doi: 10.1155/2012/962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10. improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 50.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Lowes DA, Wallace C, Murphy MP, Webster NR, Galley HF. The mitochondria targeted antioxidant MitoQ protects against fluoroquinolone-induced oxidative stress and mitochondrial membrane damage in human Achilles tendon cells. Free Radic. Res. 2009;43:323–328. doi: 10.1080/10715760902736275. [DOI] [PubMed] [Google Scholar]
- 52.Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM, Ross MF, Logan A, Cocheme HM, Trnka J, Prime TA, Abakumova I, Jones BA, Filipovska A, Murphy MP. Mitochondria-targeted antioxidants in the treatment of disease. Annals of the New York Academy of Sciences. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- 53.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondriatargeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver international. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 54.Solesio ME, Prime TA, Logan A, Murphy MP, Del Mar Arroyo-Jimenez M, Jordan J, Galindo MF. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson's disease. Biochimica et biophysica acta. 2012 doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Kimura Y, Selmi C, Leung PS, Mao TK, Schauer J, Watnik M, Kuriyama S, Nishioka M, Ansari AA, Coppel RL, Invernizzi P, Podda M, Gershwin ME. Genetic polymorphisms influencing xenobiotic metabolism and transport in patients with primary biliary cirrhosis. Hepatology. 2005;41:55–63. doi: 10.1002/hep.20516. [DOI] [PubMed] [Google Scholar]
- 56.Lu MY, Huang JF, Liao YC, Bai RK, Trieu RB, Chuang WL, Yu ML, Juo SH, Wong LJ. Mitochondrial polymorphism 12361A>G is associated with nonalcoholic fatty liver disease. Transl. Res. 2012;159:58–59. doi: 10.1016/j.trsl.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Onishi M, Sokuza Y, Nishikawa T, Mori C, Uwataki K, Honoki K, Tsujiuchi T. Different mutation patterns of mitochondrial DNA displacement-loop in hepatocellular carcinomas induced by N-nitrosodiethylamine and a choline-deficient l-amino acid-defined diet in rats. Biochem. Biophys. Res. Commun. 2007;362:183–187. doi: 10.1016/j.bbrc.2007.07.175. [DOI] [PubMed] [Google Scholar]
- 58.Zhang R, Zhang F, Wang C, Wang S, Shiao YH, Guo Z. Identification of sequence polymorphism in the D-Loop region of mitochondrial DNA as a risk factor for hepatocellular carcinoma with distinct etiology. J. Exp. Clin. Cancer Res. 2010;29:130. doi: 10.1186/1756-9966-29-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Human molecular genetics. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 60.Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM, Ballinger SW. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. American journal of physiology. Lung cellular and molecular physiology. 2009;297:L209–L216. doi: 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reproductive biomedicine online. 2003;7:65–70. doi: 10.1016/s1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 62.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]