Abstract
Human anti-glomerular basement membrane (GBM) disease strongly associates with HLA-DRB1*15:01. The target autoantigen in this disease is the noncollagenous domain of the α3 chain of type IV collagen, α3(IV)NC1, but critical early T cell epitopes presented by this human MHC class II molecule are unknown. Here, by immunizing HLA-DRB1*15:01 transgenic mice with whole recombinant α3(IV)NC1 and with overlapping α3(IV)NC1 peptides, we defined a HLA-DRB1*15:01–restricted α3(IV)NC1 T cell epitope (α3136–146) with four critical residues. This peptide was not immunogenic in HLA-DRB1*01:01 transgenic or C57BL/6 mice. The T cell epitope is naturally processed from α3(IV)NC1. CD4+ T cell clones, generated from HLA-DRB1*15:01 transgenic mice and specific for α3136–146, transferred disease into naive HLA-DRB1*15:01 transgenic mice, evidenced by the development of necrotizing crescentic GN, albuminuria, renal impairment, and accumulation of CD4+ T cells and macrophages in glomeruli. Because Fcγ receptors are implicated in disease susceptibility, we crossed HLA transgenic mice onto an FcγRIIb-deficient background. Immunization with either α3136–146 or α3(IV)NC1 induced GN in HLA-DRB1*15:01 transgenic FcγRIIb-deficient mice, but HLA-DRB1*01:01 transgenic FcγRIIb-deficient mice were unaffected. Taken together, these results demonstrate that the HLA-DRB1*15:01–restricted T cell epitope α3136–146 can induce T cell responses and injury in anti-GBM GN.
Goodpasture’s disease, also known as anti-glomerular basement membrane (GBM) disease, is characterized by rapidly progressive GN with crescentic and necrotizing glomerular lesions, and in some patients, pulmonary hemorrhage. It is caused by autoreactivity to the noncollagenous domain of the α3 chain of type IV collagen, α3(IV)NC1, expressed in the GBM.1,2 The diagnosis of anti-GBM disease is made by detecting serum α3(IV)NC1-reactive IgG autoantibodies, with linear IgG deposition in glomeruli.3 Treatment includes immunosuppressive agents and plasmapheresis to remove pathogenic autoantibodies.3 Cellular effectors also seem to play a role, based on studies in human anti-GBM disease4 and in experimental autoimmune anti-GBM GN.5–9
The MHC class II allele HLA-DRB1*15:01 is a key susceptibility element in anti-GBM disease.10 It confers a markedly increased relative risk for anti-GBM GN (odds ratio, 8.5; 95% confidence interval, 5.5–13.1). Most other HLA-DR alleles do not predispose people to developing anti-GBM disease and may even be protective (e.g., HLA-DRB1*01:01 [odds ratio, 0.6; 95% confidence interval, 0.3–1.0]). The strong HLA-DRB1*15:01 association and the accuracy of diagnostic criteria allow meaningful studies on how the MHC II molecule confers risk in mechanistic terms.
Key human B cell epitopes in anti-GBM disease have been defined. Autoantibodies bind to the conformational α3(IV)NC1 epitopes “EA” α317–31 and “EB” α3127–141 (all amino acid numbering, including other cited epitopes, follows Netzer et al.).11 Studies examining T cell epitopes in human anti-GBM disease are less common, although an important study showed reactivity to two peptides, α368–89 and α3129–148 (close to the EB autoantibody epitope), in all six patients studied.12 However, in the Wistar Kyoto (WKY) rat, T cell reactivity occurs in several different areas.13–15 T cell–mediated disease can be induced by α314–26 and α311–2515–17 and there is evidence of epitope spreading to involve B cell epitopes.16,18
Our hypothesis was based on studies in humans with anti-GBM GN showing strong MHC II association and suggesting key epitopes. We hypothesized that either the regions in or around α368–89 or α3129–148 (or both) would be immunogenic in the context of HLA-DRB1*15:01 but not other MHC II molecules, and could be defined as nephritogenic CD4+ T cell epitopes. Therefore, HLA-DRB1*15:01 would permit binding of one or both of these peptides at an appropriate avidity, allowing naïve autoreactive CD4+ cells to escape negative selection in the thymus, thus explaining why people who express HLA-DRB1*15:01 are more susceptible to anti-GBM GN. We studied mice that were entirely deficient in mouse MHC II,19,20 but that coexpressed the essentially nonpolymorphic HLA-DRA1*01:01, with either HLA-DRB1*15:01 or DRB1*01:01. HLA transgenic mice, especially those lacking all murine MHC II elements,19 have been powerful tools in studying the pathogenesis of autoimmune disease,20,21 because their CD4+ T cell repertoire is shaped by the presence of human (but not mouse) MHC II molecules. We found that in anti-GBM GN, α3136–146 is an immunodominant and nephritogenic CD4+ T epitope in DRB1*15:01 Tg mice, but not in DRB1*01:01 Tg or C57BL/6 mice. Importantly, CD4+ responses to this epitope induce severe anti-GBM GN.
Results
Mice with a Human HLA II Susceptibility Allele Exhibit T Responses to α3(IV)NC1133–152
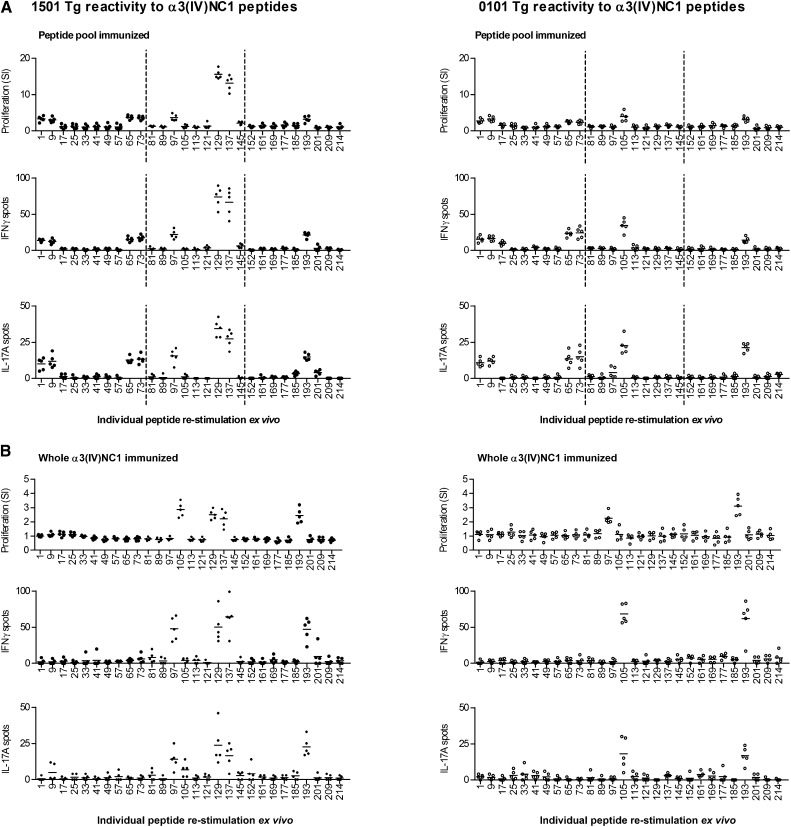
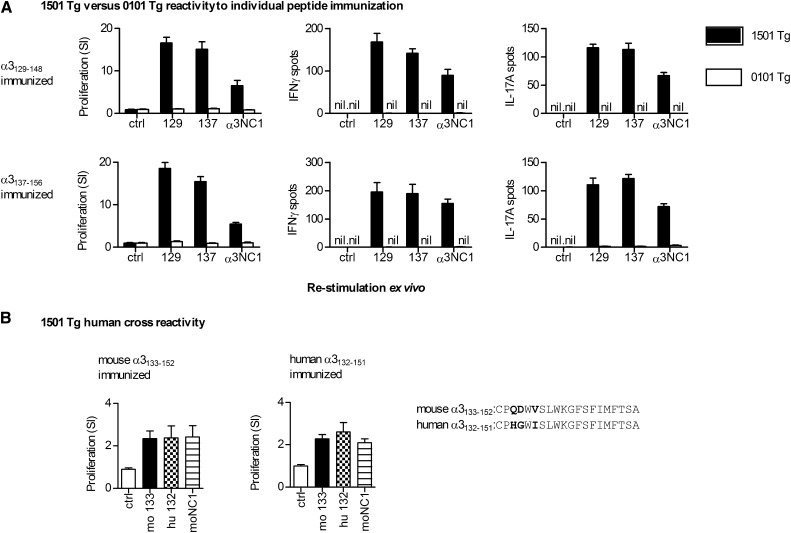
To determine immunogenic regions within the α3(IV)NC1 molecule, we immunized mouse MHC II–deficient mice transgenic for the Goodpasture disease susceptibility gene HLA-DRB1*15:01 or the protective HLA-DR1*01:01. Three pools of murine α3(IV)NC1 20-mers were used (each peptide overlapping by 12 aa; Supplemental Table 1), with each pool spanning one-third of the α3(IV)NC1 molecule. After 10 days, draining lymph node cells were re-stimulated with individual peptides within the pool and recall responses measured by [3H]-thymidine proliferation assays and IFN-γ and IL-17A enzyme-linked immunosorbent spots (ELISPOTs). Several regions within α3(IV)NC1 were immunogenic in both strains, but the striking difference was the strong reactivity to α3129–148 and α3137–156 in DRB1*15:01 Tg mice, but not in DR1*01:01 Tg mice (Figure 1A). No spontaneous reactivity to any peptide could be detected in unimmunized DRB1*15:01 Tg or DRB1*01:01 Tg mice (proliferation assay, IFN-γ and IL-17A ELISPOTs; Supplemental Figure 1). We immunized mice with whole recombinant murine α3(IV)NC122 and re-stimulated cells with each peptide individually. We found a similar, but more restricted pattern of responses, with cells from DRB1*15:01 Tg mice, but not from DRB1*01:01 Tg mice responding when stimulated with α3129–148 and α3137–156 (Figure 1B). Therefore, we immunized groups of mice with either α3129–148 or α3137–156 alone. Although there was no reactivity to these peptides in DR1*01:01 Tg mice, in DRB1*15:01Tg mice both α3129–148 and α3137–156 induced similar responses to each other, and to recombinant murine α3(IV)NC1 (rmα3(IV)NC1) (Figure 2A). The murine and human α3(IV)NC1 sequences are very similar, with only three differences at α3135, α3136, and α3138 (murine numbering). We immunized DRB1*15:01 Tg mice with either murine α3133–152 or the equivalent sequence in humans (hα3132–151). Responses to each could be induced by the other species’ peptide and human α3132–151–immunized mice responded to rmα3(IV)NC1 (Figure 2B).
Figure 1.
Distribution of the α3(IV)NC1 T cell epitopes in HLA-DRB1*15:01 and HLA-DRB1*01:01 transgenic mice. Mice deficient in murine MHC II but possessing human DRB1*15:01 (1501 Tg) or DRB1*01:01 (0101 Tg) after immunization with either (A) peptide pools (with each pool encompassing a third of α3(IV)NC1, dotted lines) or (B) whole α3(IV)NC1. Reactive peptides are defined by re-stimulation of draining lymph node cells by ex vivo proliferation, IFN-γ ELISPOT, and IL-17A assays. Each dot represents the response from a mouse (n=5 per group). Numbers on the x axis represent the α3(IV)NC1 N-terminal number of a 20-mer. Several areas of reactivity are present, but one area of strong reactivity, α3129–148 and α3137–156 (corresponding to one of two epitopes found in humans with anti-GBM disease), is present only in 1501 Tg mice.
Figure 2.
DRB1*15:01 (1501 Tg) or DRB1*01:01 (0101 Tg) responses to individual peptide immunizations. (A) 1501 Tg or 0101 Tg mice (n=4 per group) are immunized with either α3129–148 or α3137–156, and then re-stimulated with either a control peptide, α3129–148 (129) or α3137–156 (137), or recombinant murine α3(IV)NC1 (α3NC1). Immunized 1501 Tg mice react to both peptides and to α3(IV)NC1. There is no reactivity in 0101 Tg mice. (B) Because there is consistent cross-reactivity between α3129–148 and α3137–156 in 1501 Tg mice, a composite peptide is synthesized (α3133–152). The 1501 Tg mice (n=4 per group) are immunized with murine α3133–152 or the equivalent human peptide α3132–151. Proliferative recall responses show cross-reactivity of mouse (mo 133) and human peptides (hu 132), and that mice immunized with the human sequence respond to recombinant murine α3(IV)NC1 (moNC1). Results are representative of two independent experiments.
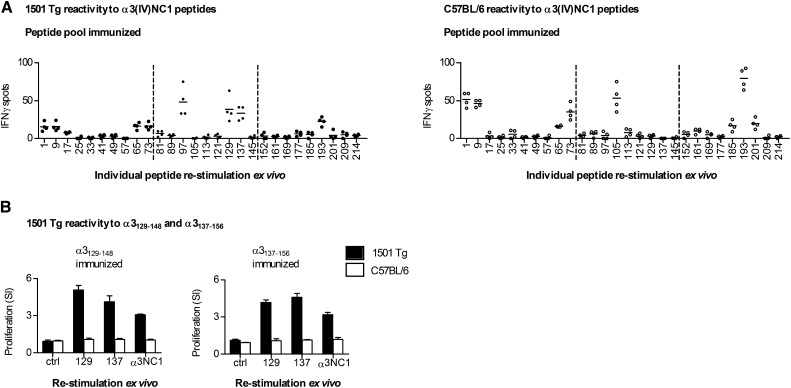
We then defined reactivity in DRB1*15:01 Tg mice compared with C57BL/6 mice (murine I-Ab). After peptide pool immunization, several peptides were immunogenic, similar to the comparison between DRB1*15:01 Tg and DRB1*01:01 Tg mice (Figure 3A). Although responses were seen in DRB1*15:01 Tg mice after re-stimulation with α3129–148 or α3137–156, there were, as with 0101 Tg mice, no responses in C57BL/6 mice. After α3129–148 or α3137–156 immunization, DRB1*15:01 Tg mice, but not C57BL/6 mice, responded to either peptide or to rmα3(IV)NC1 (Figure 3B). Because α3129–148 and α3137–156 both induce responses in DRB1*15:01 Tg mice, we then used a 20-mer derived from the common 12 aa of both peptides with 4 aa at each end (α3133–152).
Figure 3.
The α3129–156 epitope present in HLA-DRB1*15:01 Tg (1501 Tg) mice is not present in C57BL/6 mice possessing I-Ab. (A) Groups of mice immunized with peptide pools (dotted lines) react to several areas, including α3129–156. However, C57BL/6 mice immunized in an identical manner respond (by IFN-γ ELISPOT) to similar areas, with the exception of α3129–156. Each dot represents the response from a mouse (n=4 per group). (B) Groups of 1501 Tg and C57BL/6 mice (n=4 per group) are immunized with either α3129–148 or α3137–156 and are then re-stimulated with either a control peptide, α3129–148 (129) or α3137–156 (137), or recombinant murine α3(IV)NC1. Immunized 1501 Tg mice react to both peptides and to α3(IV)NC1. There is no reactivity in C57BL/6 mice. Results are representative of two independent experiments. Numbers on the x axis represent the α3(IV)NC1 N-terminal number of the re-stimulating 20-mer.
The DRB1*15:01 Restricted T Cell Epitope Is Immunogenic after Whole Antigen Immunizations
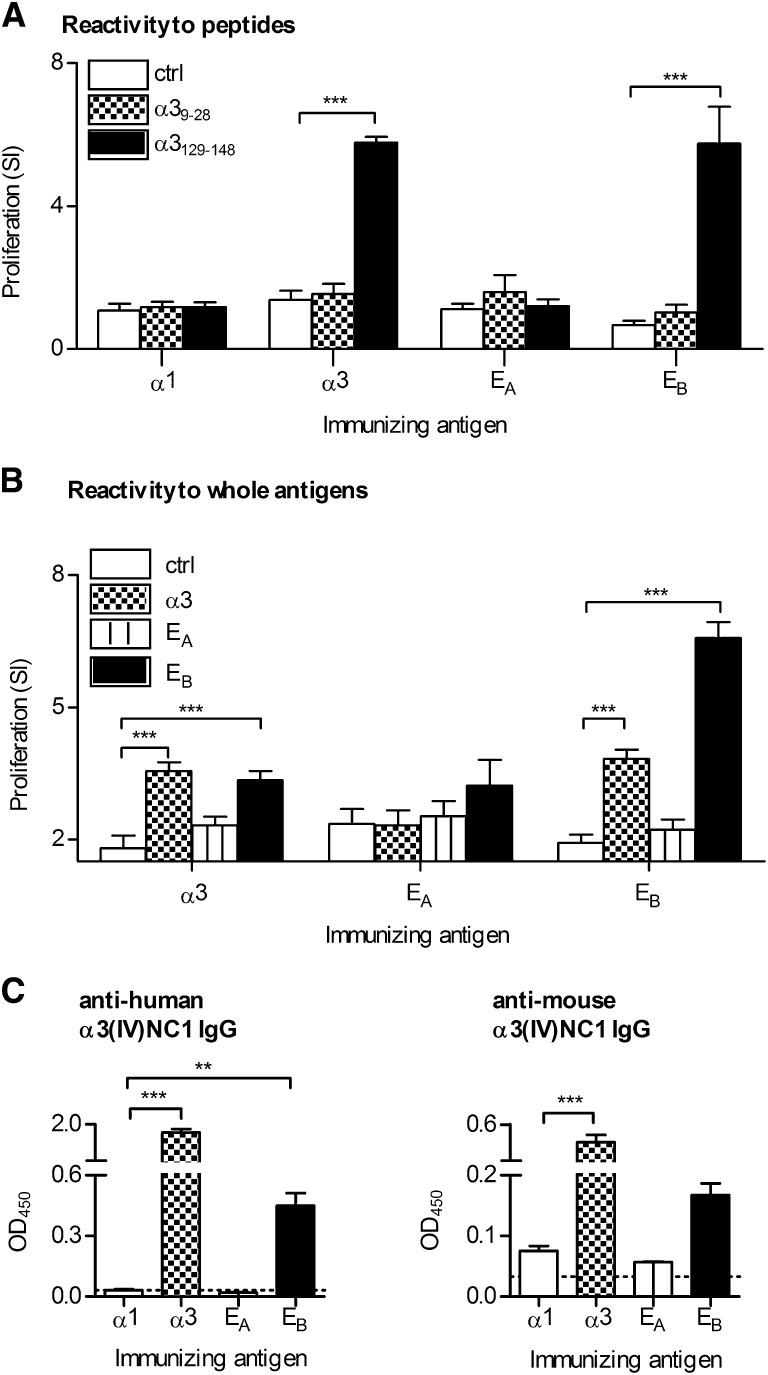
To define anti-α3(IV)NC1 responses using whole proteins, we used chimeric human α3/α1(IV)NC1 domains, with the conformational α3(IV)NC1 autoantibody epitopes EA or EB substituted into the α1(IV)NC1 domain, which is not a common or primary autoimmune target in humans.11 DRB1*15:01 Tg mice were immunized with α1(IV)NC1, α3(IV)NC1, or the EA chimera (containing hα317–31) or the EB chimera (containing hα3127–141 overlapping the restricted T cell epitope), and lymphocytes were then re-stimulated with either murine α39–28 or α3129–148. Mice immunized with α1(IV)NC1 or the EA chimera responded to neither peptide (Figure 4A), whereas mice immunized with the EB chimera or full-length α3(IV)NC1 responded to α3129–148 but not α39–28. Further groups of mice were immunized with α3(IV)NC1, the EA chimera, or the EB chimera and re-stimulated with chimeric and whole proteins. Mice immunized with α3(IV)NC1 responded to α3(IV)NC1 and to the EB chimera, but not to the EA chimera (Figure 4B). Immunization with the EA chimera did not induce recall responses, whereas EB chimera immunization induced recall responses both to itself and to full-length human α3(IV)NC1. By 10 days, serum anti-human α3(IV)NC1 and anti-murine α3(IV)NC1 autoantibodies were detectable in mice immunized with either human α3(IV)NC1 or the EB chimera (Figure 4C).
Figure 4.
Responses of HLA-DRB1*15:01 Tg (1501 Tg) mice to human α3 and chimeric α3/α1 domains. (A) Groups of mice (n=4 per group) are immunized with control nonimmunogenic α1(IV)NC1, α3(IV)NC1, the EA chimera (with human α3 aa 17–31 from α3(IV)NC1 substituted into α1(IV)NC1), or the EB chimera (with human α3 aa 127–141 from α3(IV)NC1 substituted into α1(IV)NC1 and encompassing the HLA DRB1*15:01 restricted epitope). Lymph node cells are re-stimulated ex vivo 10 days later with either control peptide (ctrl, α3161–180) or α39–28 or α3129–148. (B) Groups of 1501 Tg mice (n=4 per group) are immunized with full-length α3, the EA chimera, or the EB chimera, and lymphocytes are then re-stimulated with either α2(IV)NC1 (ctrl), α3, EA chimera, or EB chimera. The SI of approximately 2 in the control group represents the response to the FLAG tag of the protein (cells re-stimulated with α2(IV)NC1 without FLAG had an SI of 1, the same as cells incubated with media alone). (C) Serum IgG, 10 days after immunization, specific for human or murine α3(IV)NC1 from mice immunized with α1(IV)NC1 (α1), α3(IV)NC1 (α3), EA, or EB measured by ELISA. **P<0.01; ***P<0.001 compared with control.
The Core Immunogenic HLA-DRB1*15:01 Epitope Is α3136–146, with Four Critical Residues
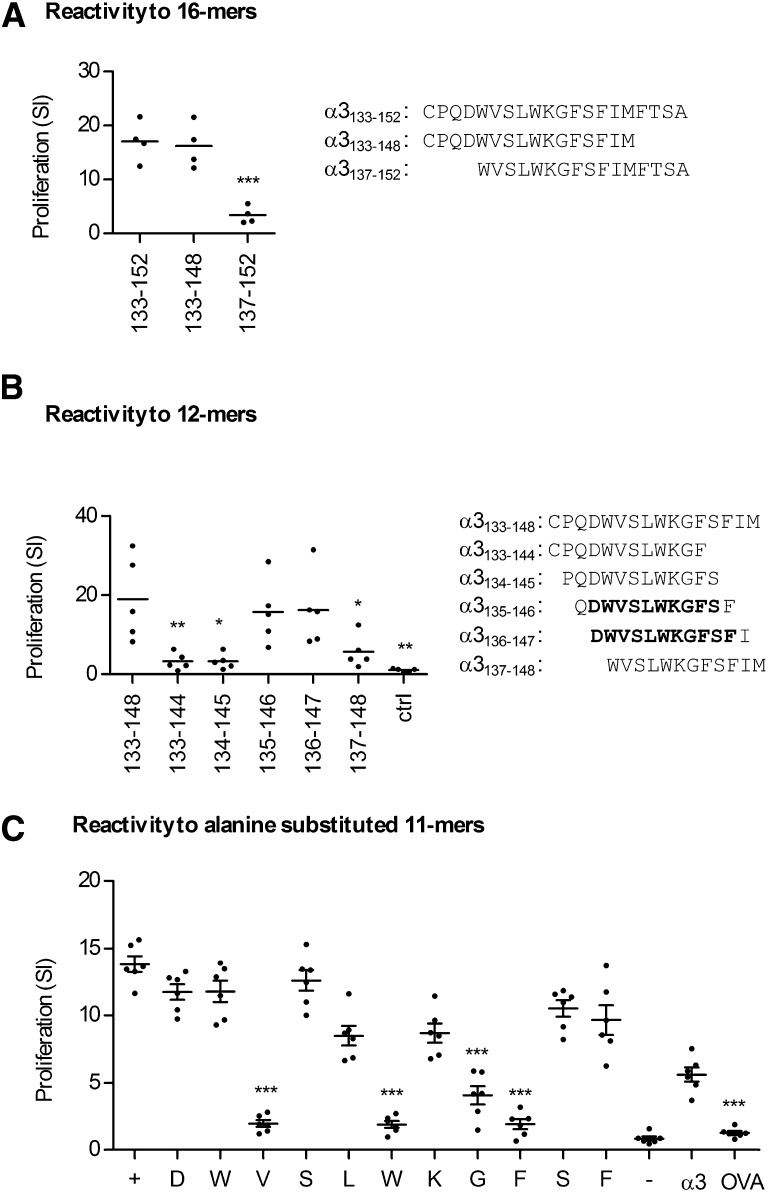
We identified the core immunogenic region within α3133–152 by immunizing DRB1*15:01 Tg mice with α3133–152, and then stimulating draining lymph node cells at 10 days with shortened 16-mers. The 4 aa N-terminal end of α3133–152 (α3133–136) was required but the C-terminal 4 aa (α3149–152) was redundant (Figure 5A). Immunization with the 16-mer α3133–148 and re-stimulation with sequential 12-mers overlapping by 11 aa defined the nonredundant sequence DWVSLWKGFSF (α3136–146) (Figure 5B). The critical residues within α3136–146 were delineated by immunizing DRB1*15:01 Tg mice with DWVSLWKGFSF and re-stimulating lymphocytes with individual alanine substituted 11-mers, with each peptide having one residue substituted for alanine in sequential order. Valine138, tryptophan141, glycine143, and phenylalanine144 were most critical for responses (Figure 5C).
Figure 5.
Defining the T cell HLA-DRB1*15:01–restricted Goodpasture’s epitope minimum length and critical residues. (A) 1501 Tg mice (n=4) are immunized with the 20-mer α3133–152 and re-stimulated with 16-mers truncated at either the carboxyl (α3133–148) or amino (α3137–152) terminals, showing that the carboxyl end sequence, FTSA, is not required for reactivity (***P<0.001 compared with control: α3133–152). (B) 1501 Tg mice are then immunized (n=5) with the 16-mer α3133–148 and lymphocytes are re-stimulated with sequentially aligned 12-mers, with the results showing that the 11-mer α3136–146 (DWVSLWKGFSF) is critical for reactivity (*P<0.0.5; **P<0.01 compared with control: α3133–148). (C) 1501 Tg mice (n=6) are immunized with α3136–146 and re-stimulated with individual alanine substituted 11-mers, with each peptide having one residue substituted for alanine in sequential order. Valine138, tryptophan141, and phenylalanine144 are the aa most critical for responses, with glycine143 also being important. + represents re-stimulation with α3136–146; − with a nonstimulatory peptide (α3161–180); α3 with recombinant murine α3(IV)NC1, OVA with ovalbumin (***P<0.001 compared with control: α3136–146 (+)).
α3136–146–Specific CD4+ Cell Clones Induce Anti-GBM GN in DRB1*15:01 Tg Mice
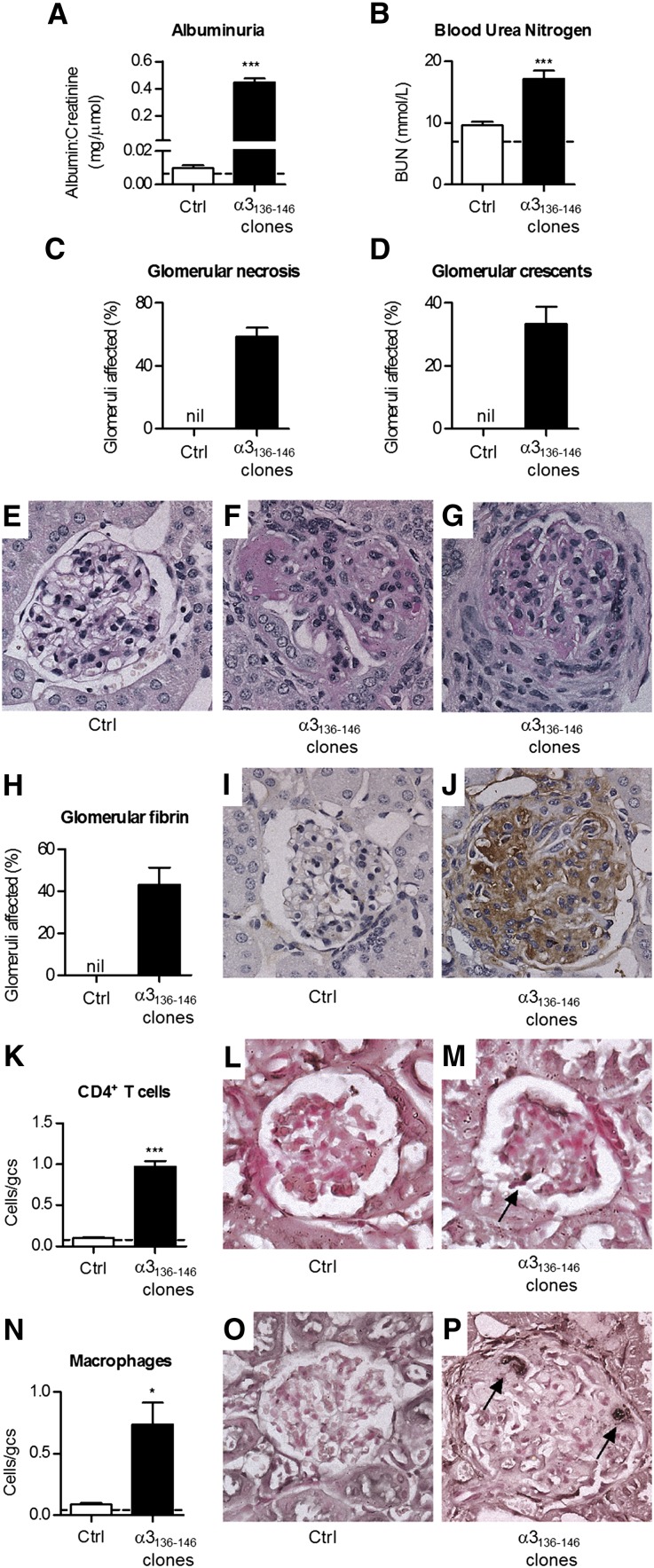
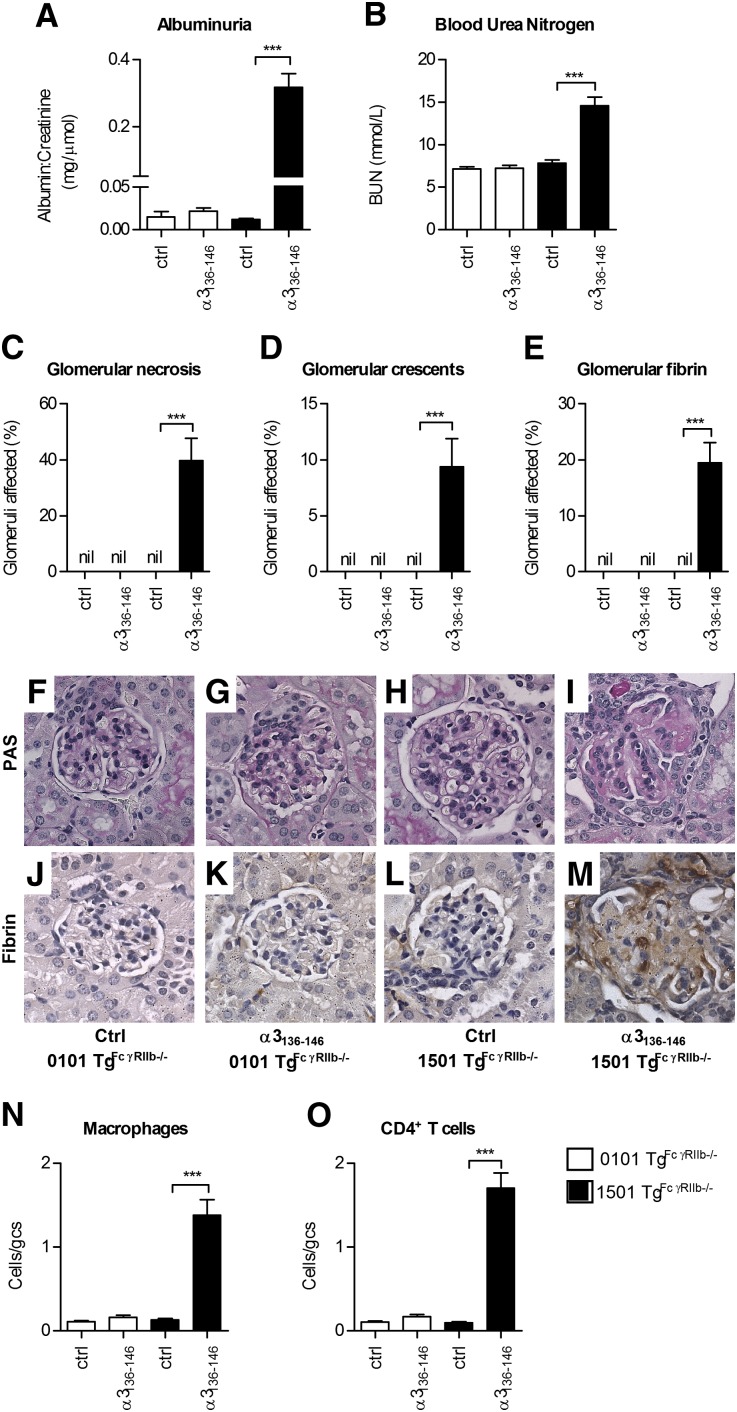
To determine whether α3136–146–specific T cells mediate GN in the context of HLA-DRB1*15:01, α3136–146–specific IFN-γ–secreting CD4+ T cell clones were generated from DRB1*15:01 Tg mice and expanded in vitro. We transferred α3136–146–specific T cells into naïve DRB1*15:01 Tg mice, with 0.5 μg/g of LPS. Control mice received LPS and 107 CD4+ cells derived from a DRB1*15:01 Tg mouse immunized with Freund’s complete adjuvant alone. By 14 days, α3136–146–specific clone recipients had developed albuminuria (baseline, normal mice 0.01±0.00, day 14 control cell recipients 0.01±0.00, day14 α3136–146–specific clone recipients 0.57±0.08 mg albumin/μmol creatinine; P<0.001), continuing until the end of experiments (35 days; Figure 6A). Analyses at 35 days showed GN only in the recipients of α3136–146–specific clones, with renal impairment (Figure 6B), necrotizing and crescentic GN (Figure 6, C–G), and features of delayed type hypersensitivity (DTH), including glomerular fibrin deposition (Figure 6, H–J) with CD4+ cells and macrophages in glomeruli (Figure 6, K–P). Glomerular neutrophil recruitment was not a feature (control cell recipients 0.1±0.0, α3136–146–specific clone recipients 0.1±0.0 cells/glomerular cross-section). Glomerular pathology was prominent in recipients of α3136–146–specific clones, but tubulointerstitial infiltrates and disease were relatively modest.
Figure 6.
Transfer of CD4+ cells specific for α3136–146 induces crescentic GN in HLA-DRB1*15:01 Tg mice. Naïve DRB1*15:01 Tg mice are injected with 0.5 μg/g of LPS and either with 107 CD4+ cells of a clone specific for α3136–146 or nonspecific CD4+ cells derived from adjuvant immunized mice. Additional exogenous antigen or peptide is not administered. After 14 days, mice receiving the α3136–146–specific clone develop functional renal injury, manifested by pathologic albuminuria (A) and a raised serum BUN (B) (dotted lines represent values in normal mice). Mice receiving naïve cells are not affected. The majority of glomeruli in α3136–146–specific clone show areas of segmental necrosis (C and F) and approximately 30% of glomeruli are affected by crescent formation (D and G), but recipients of naive CD4+ cells have normal histology (E). (H–J) Glomerular fibrin deposition is present only in mice that received the α3136–146–specific CD4+ clone. Within glomeruli, CD4+ cells (K–M) and macrophages (N–P) are present only in recipients of the α3136–146–specific CD4+ clone. Cells/gcs represents cells per glomerular cross-section, dotted lines represent mean values in normal mice (n=3), and arrows represent positively stained cells (black reaction product). *P<0.05; ***P<0.001 versus control cell recipients.
α3133–152–Immunized HLA-DRB1*15:01 Tg Mice Develop Nephritogenic Autoimmunity
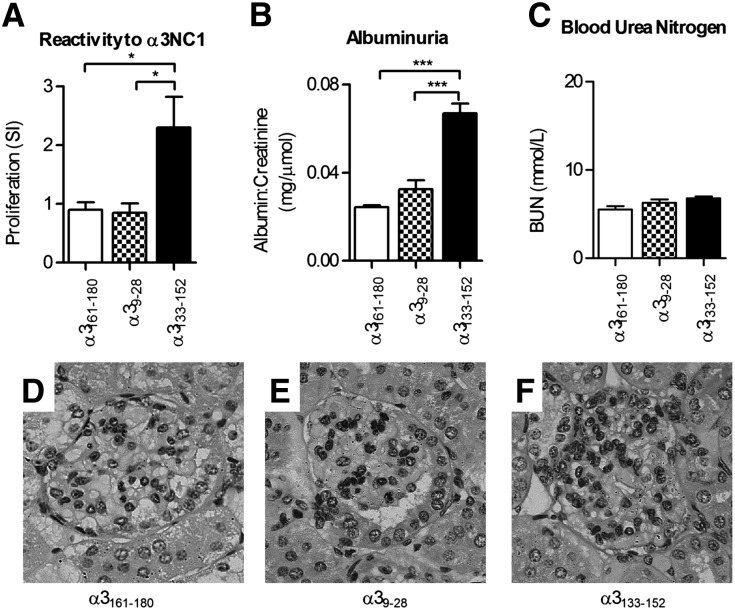
DRB1*15:01 Tg mice were immunized with α3161–180 (a control peptide, not shown to induce responses), α39–28 (which overlaps with the EA region), or α3133–152. After 42 days, mice immunized with either control peptide or α39–28 did not develop lymphocyte proliferative responses to the immunogen (Figure 7A), and they did not develop albuminuria (Figure 7B). However, mice immunized with α3133–152 developed both reactivity to α3133–152 and significant albuminuria, but not renal impairment (Figure 7C). Glomerular changes were mild in all groups of mice with occasional proliferation and accumulation of periodic acid–Schiff (PAS)-positive material (Figure 7, D–F).
Figure 7.
Immunization of HLA-DRB1*15:01 Tg mice with α3133–152 induces autoreactivity to α3(IV)NC1 but only mild disease. Groups of DRB1*15:01 Tg mice (n=4 per group) are immunized with one of three 20-mers: an irrelevant peptide from within α3(IV)NC1 (α3161–180), a peptide from within the Hudson EA epitope α39–28, or the α3133–152 sequence. After 42 days, mice immunized with α3133–152, but not the two other peptides, develop T cell reactivity to α3(IV)NC1 (A). All mice immunized with α3133–152 develop albuminuria (B), indicating some renal injury, but mice immunized with the control peptide or α39–28 are unaffected. However, α3133–152 does not induce renal impairment (C). There is no histologic evidence of glomerular disease in control peptide and α39–28 immunized mice (D and E), and alterations in glomerular histology induced by α3133–152 are modest, with no areas of segmental necrosis or crescent formation (F). *P<0.05; ***P<0.001.
Anti-GBM GN Is Induced by α3136–146 in DRB1*15:01, but not DRB1*01:01 Transgenic Mice
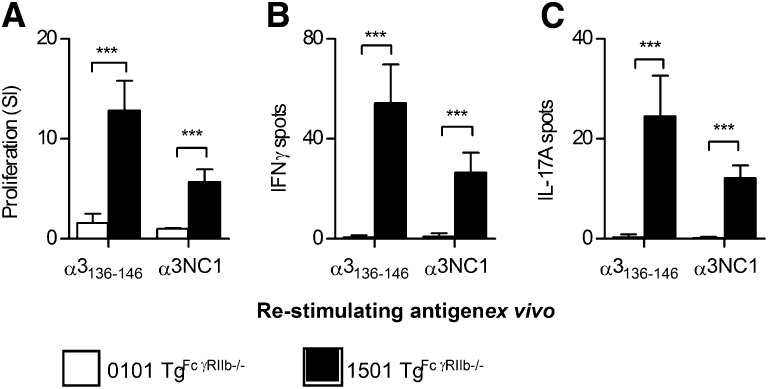
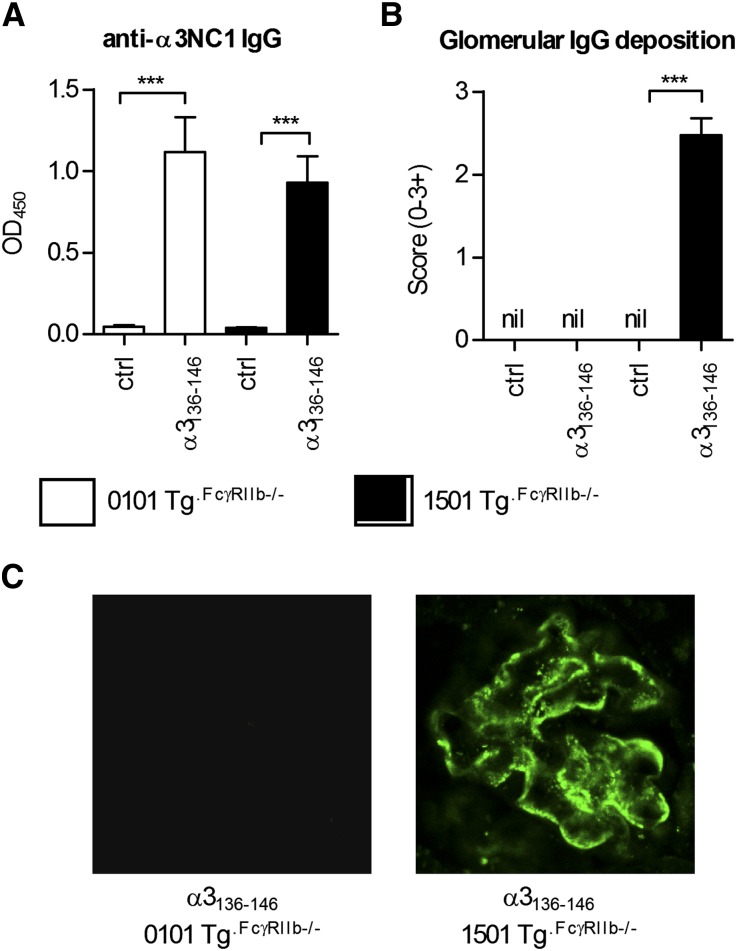
Although mice do not easily develop severe autoimmune anti-GBM GN, the WKY rat is susceptible. This susceptibility is not MHC mediated, but several genetic elements have been identified, including FcγR abnormalities.23,24 We introduced an additional susceptibility element to MHCII−/− DRB1*15:01 Tg and DRB1*01:01 Tg mice by intercrossing them with mice that lack the inhibitory FcγR, FcγRIIb. After immunization of both strains with α3136–146, mice expressing DRB1*15:01 (mouse MHC−/−.DRB1*15:01 Tg.FcgRIIb−/− mice) developed measurable T cell autoimmunity to α3136–146 and to rmα3(IV)NC1 at 10 days (Figure 8), but mice expressing DRB1*01:01 (mouse MHC−/−.DRB1*01:01 Tg.FcgRIIb−/− mice) did not. After repeated immunization, significant anti-GBM GN including albuminuria and renal impairment, with focal glomerular necrosis, crescent formation, fibrin deposition, and glomerular CD4+ cell and macrophage accumulation had developed in DRB1*15:01-expressing mice at 42 days, but glomeruli in immunized DRB1*01:01-expressing mice were normal (Figure 9). In DRB1*15:01-expressing mice, there were serum anti-α3(IV)NC1 autoantibodies and IgG deposition in glomeruli (Figure 10) that included both linear and granular components, as recently described.25 Although DRB1*01:01-expressing mice exhibited comparable serum anti-α3(IV)NC1 autoantibody titers to DRB1*15:01-expressing mice, IgG was not detected in glomeruli of α3136–146–immunized DRB1*01:01-expressing mice. Furthermore, mice expressing DRB1*15:01 that were immunized with full-length rmα3(IV)NC1 developed GN, but DRB1*01:01-expressing mice did not (Supplemental Figure 2).
Figure 8.
The T cell Goodpasture epitope induces T cell reactivity to itself and to α3(IV)NC1 in HLA-DRB1*1501 Tg mice, but not HLA-DRB1*01:01 Tg mice deficient in FcγRIIb (1501 Tg.FcγRIIb−/− and 0101 Tg.FcγRIIb−/−, respectively) immunized with α3136–146. As expected, 1501 Tg mice develop reactivity to both α3136–146 and α3(IV)NC1 (proliferation A, IFN-γ ELISPOT B, IL-17A ELISPOT C). However, despite the presence of further autoimmunity susceptibility genes, lymphocytes from 0101 Tg mice are unresponsive to α3136–146 and α3(IV)NC1. ***P<0.001.
Figure 9.
The T cell Goodpasture epitope induces anti–GBM GN in mice with human HLA-DRB1*15:01. DRB1*15:01 Tg or DRB1*01:01 Tg mice deficient in FcγRIIb (1501 Tg.FcγRIIb−/− and 0101 Tg.FcγRIIb−/−, respectively) are immunized (n=8 per group) with α3136–146. After 42 days, 1501 Tg.FcγRIIb−/− mice immunized with α3136–146 develop pathologic albuminuria (A) and a raised BUN (B), but α3136–146 immunized 0101 Tg.FcγRIIb−/− mice, or nonimmunized mice (n=8 per group) of either strain, have normal urinary albumin and normal renal function. Similarly, only α3136–146 immunized 1501 Tg.FcγRIIb−/− mice show glomeruli with areas of segmental necrosis (C), crescent formation (D), or glomerular fibrin deposition (E). (F–I) Representative PAS-stained glomeruli from the four groups of mice. (J–M) Photomicrographs of glomerular fibrin deposition. Significant numbers of glomerular CD4+ T cells (N) and macrophages (O) are present only in α3136–146 immunized 1501 Tg.FcγRIIb−/− mice. Results are representative of two independent experiments. ***P<0.001.
Figure 10.
The development of anti-α3(IV)NC1 autoantibodies in DRB1*15:01 Tg or DRB1*01:01 Tg mice deficient in FcγRIIb (1501 Tg.FcγRIIb−/− and 0101 Tg.FcγRIIb−/−, respectively). (A) Sera from α3136–146 immunized and control mice (n=8 per group) are assayed for anti-murine α3(IV)NC1 antibodies, which are present to a similar titer in both strains of mice. However, only α3136–146 immunized 1501 Tg.FcγRIIb−/− mice developed IgG deposition in glomeruli (B), which when assessed by direct immunofluorescence is not present in α3136–146 immunized 0101 Tg.FcγRIIb−/− mice or nonimmunized mice (ctrl) of either strain. (C) IgG deposition is observed in glomeruli of all 8 α3136–146 immunized 1501 Tg.FcγRIIb−/− mice. Direct immunofluorescence reveals IgG staining of the glomerular basement membrane in a linear pattern with granular components in all mice (×1000). ***P<0.001.
Discussion
HLA-DRB1*15:01 is a key susceptibility element in anti-GBM disease.10 By using mice expressing this risk allele or the protective HLA-DRB1*01:01 allele (but no mouse MHC II),19 we have defined an immunodominant CD4+ T cell epitope in α3(IV)NC1 (α3136–146) that also triggers autoantibody production. Importantly, α3136–146–specific CD4+ cells induce GN in naïve DRB1*15:01 mice and when additional FcγR-related susceptibility elements are introduced, active autoimmunity and disease is markedly enhanced.
Our initial comparative studies in DRB1*15:01 Tg, DRB1*01:01 Tg, and C57BL/6 mice showed several α3(IV)NC1 peptide sequences corresponding to reactivity in previous rat studies, and in some humans.12,15–17,26 However, only HLA-DRB1*15:01 Tg mice responded to one region spanning α3129–156 (later shortened to α3136–146). Immunization with α3(IV)NC1 induced a similar, but somewhat more restricted, pattern of responses. The nonresponsiveness of HLA-DRB1*01:01 Tg mice was not a quirk of the DR1 allele, because C57BL/6 mice (I-Ab) were also nonresponsive to this peptide. Because this region overlaps a B cell epitope (EB), we used human α3/α1 chimeric domains. Findings were consistent with a dominant initial role for α3136–146, because HLA-DRB1*15:01 Tg mice immunized with the EB chimera, but not the EA chimera, developed T and B cell responses similar to mice immunized with α3(IV)NC1. There is evidence for destructive processing within the α3136–146 epitope, because the leucine140 to tryptophan141 (murine numbering) bond is cleaved by cathepsin D.27 However, that HLA-DRB1*15:01 Tg mice seem to present peptides derived from the EB chimera implies that destructive processing may not be a dominant tolerogenic mechanism in DRB1*15:01-positive individuals. It is unclear whether antigen-presenting cells have similar levels of processing enzymes and their inhibitors as other tissues, although inflammatory stimuli, such as LPS used in our clone transfer studies, can influence the net activity of these systems.28 The role of destructive processing in tolerance to α3(IV)NC1 requires further study.
We defined the 11 aa peptide α3136–146 as the core immunogenic region, and identified the amino acids critical for immunoreactivity, with findings similar to a previous study.27 The absence of α3136 reduced ex vivo recall responses in the core region mapping experiments. However, α3137–156 induced strong recall responses after immunization with either α3137–156 or α3129–148, suggesting that additional flanking residues on the carboxy-terminal end of α3137–156 help to stabilize the peptide-DRB1*15:01 complex. Whereas HLA-DRB1*15:01 Tg mice express human MHC II, they express murine α3(IV)NC1. However, the murine and human α3133–152 sequences are well conserved, with only two differences between the cross-reactive human and murine α3136–146 sequences. The murine aspartic acid136 is a glycine (not a critical amino acid) and valine138 is an isoleucine, a similar branched chain aa.29 The core sequence being α3136–146 helps explain why responses occurred when mice were immunized with the EB chimeric domain. Although the critical Goodpasture’s T cell epitope is α3136–146, the EB chimeric domain includes only the human α3127–141 sequence (homologous to murine α3128–142), with the α1 sequence comprising the remaining 4 aa. However, in these C-terminal aa, the α1 and α3 sequences are similar (α1: GYSF, α3: GFSF), with the single difference being a tyrosine for phenylalanine, amino acids that possess very similar properties.
The α3(IV)NC1 protein is present within the thymus,30 so one mechanism by which HLA-DRB1*0101 mice do not respond to α3136–146 may be thymic deletion of CD4+ cells with high affinity to DR1-α3136–146. However, because peripheral tolerance is also important in Goodpasture’s disease,31 regulatory cells could also be relevant. Studies using a B cell transgenic mice specific for a mouse α3(IV)NC1 B cell epitope suggest that bone marrow expression of α3(IV)NC1 (or a related protein) is relevant to B cell tolerance.32,33 The critical α3136–146 epitope identified in our studies was not identified as an important T cell epitope in WKY rats. This may be due to CD4+ cell thymic selection being guided by the binding of α3-derived peptides to the human anti-GBM disease susceptibility gene HLA-DRB1*15:01, not the MHC II RT.1Bl expressed by the WKY rat. The likely consequence of this is that because they were derived in the context of different MHC II molecules, the CD4+ T cell repertoires in the two rodents are different.
Whereas anti-GBM antibodies, especially when eluted from kidneys,34 are pathogenic in this autoimmune disease,34,35 cellular immunity also plays a role.5,6,9,18 We generated α3136–146–specific CD4+ Th1 clones from HLA-DRB1*15:01 Tg mice and showed that transfer of clones to naïve HLA-DRB1*15:01 Tg mice induced necrotizing crescentic GN. Whereas T cell lines from an α3(IV)NC1 immunized WKY rat have transferred disease in a previous study,8 these studies use a clone generated in mice containing the most relevant human MHC II, reacting to a MHC II restricted peptide. The IFN-γ–producing α3136–146–specific clone in our studies induced a significant DTH-like glomerular lesion, consistent with its Th1 phenotype. Whereas Th1 IFN-γ–producing cells act as effectors in experimental crescentic GN,36,37 models involving autoimmunity to α3(IV)NC1 show a more prominent role for IL-23/Th17 responses and a regulatory role for IFN-γ in loss of tolerance.38,39 Our studies reinforce an interpretation that although IFN-γ–producing Th1 cells are powerful mediators of DTH-like macrophage-mediated glomerular injury, maintenance of tolerance itself may be regulated by IFN-γ.
Our studies focused on defining the relevance of the α3136–146 epitope detected only in HLA-DRB1*15:01 transgenic mice (the human susceptibility MHC II). However, there are other epitopes, including α397–124 and α3193–212 (found after immunization with peptide pools or whole α3(IV)NC1) and α31–28 and α365–92 (reactive after peptide pool but not α3(IV)NC1 immunization), that could be nephritogenic. With the exception of α39–28, tested due to its proximity to the EA region, which did not induce autoimmunity in HLA-DRB1*15:01 Tg mice, testing other possible epitopes in further studies could define their pathogenicity in the context of HLA-DRB1*15:01. Supplemental Table 2 contains a comparison of our studies with those of Cairns et al.12 in six humans with anti-GBM disease. Importantly, although there were notable similarities (and some differences), human peptides within the α3129–156 region examined in our studies overlapped with the core epitope, bound with high affinity to DR15, and in the case of the human α3(IV)NC1 IPPCPHGWISLWKGFSFIMF sequence contained the entire core sequence and stimulated response ex vivo in all six patients tested. The identification of a key epitope paves the way for studies that examine the nature of the intra- and intermolecular epitope spreading seen in this disease.2,16,40,41
Although autoimmune anti-GBM GN has been modeled in a number of animal species,5,6,42,43 the most commonly used model injects human α3(IV)NC1 or rat α3(IV)NC1 peptides into WKY rats7,15,16 that carry genetic susceptibilities rendering them vulnerable to various aspects of experimental GN.23,24 Although mice develop autoimmunity to α3(IV)NC1 and may develop disease after approximately 10 weeks,9,39,44 severe disease in mice has been more difficult to establish. Immunization of HLA-DRB1*15:01 Tg mice with α3136–146 broke tolerance to α3(IV)NC1 and induced mild albuminuria, but α39–28 immunization did not. Although HLA-DRB1*15:01 is an important susceptibility factor in Goodpasture’s disease, this allele is common in human populations, so other factors must also be involved in autoimmunity to α3(IV)NC1. Because autoimmune diseases involve failures of multiple checkpoints,45 we bred another susceptibility element, deficiency of an inhibitory FcγR, FcγRIIb, into DRB1*15:01 Tg and DRB1*01:01 Tg mice. We chose FcγRs because alterations in this system are associated with glomerular disease in rodents and humans.23,25,46,47 This additional susceptibility enhanced injury in HLA-DRB1*15:01–expressing mice after α3136–146 immunization, without promoting initial T cell autoimmune responses or injury in HLA-DRB1*01:01 Tg mice. By 42 days after α3136–146 immunization, FcγRIIb-deficient HLA-DRB1*01:01 Tg mice did develop serum anti-α3(IV)NC1 antibodies (suggesting also some T cell autoreactivity), but these were not deposited in the kidney. Possible explanations for this observation include that the autoantibodies from 0101 Tg mice may have lower affinity, or different, nonpathogenic epitopes, or that the lack of cell-mediated injury in 0101 Tg mice means that native α3(IV)NC1 is not conformationally accessible to the anti-α3(IV)NC1 autoantibodies and binding does not occur. Previous studies have suggested that both are possible: certainly autoantibodies binding to the GBM eluted from kidneys are much more potent that those found in the serum,34 suggesting the presence of some anti-GBM antibodies that are nonpathogenic (or only marginally injurious). In addition, several reports have suggested that in vivo, injury to the GBM (by chemical [experimentally by reactive oxygen species]48 or immunologic [autoreactive T cells]26 means) may alter the structure of type IV collagen hexamers, exposing B cell α3(IV)NC1 epitopes, thus allowing and enhancing antibody binding.
These studies used HLA humanized mice to identify a key pathogenic epitope in Goodpasture’s disease. This epitope is important in the initial autoreactivity to α3(IV)NC1 as well as GN. Our studies provide the basis for a mechanistic understanding of how the strongest genetic risk factor in anti-GBM disease confers risk, based on new humanized models of cell-mediated autoimmune anti-GBM GN. These studies assist in moving toward more specific therapies by developing relevant preclinical models.
Concise Methods
α3(IV)NC1 Peptides and Proteins
The murine α3(IV)NC1 peptide library used to determine immunoreactivity was synthesized as a PepSet (Mimotopes, Clayton, Australia). There were 28 peptides synthesized according to the published sequence.49 Each peptide was 20 aa long and overlapped by 12 aa (Supplemental Table 1). Control peptide, OVA323–339, was used in some experiments. Individual peptide immunization and re-stimulation assays were performed with 11–20 aa >90% pure peptides by HPLC (Mimotopes or AusPep, West Melbourne, Australia), confirmed for some peptides by further HPLC analysis at Monash University. Recombinant murine α3(IV)NC1 was generated using a baculovirus system as previously described.22 Recombinant FLAG-tagged human α1(IV)NC1, α3(IV)NC1, α2(IV)NC1, and α3/α1(IV)NC1 chimeric proteins were expressed in HEK 293 cells as previously described.11
Mice
Mouse MHC class II−/−, HLA-DRB1*15:01 Tg mice, and mouse MHC class II−/−, HLA-DRB1*01:01 Tg mice were generated by intercrossing mouse MHC class II−/−, HLA DRB1*15:01 Tg mice50 with mouse MHC class II+/+, HLA DRB1*01:01 Tg mice.51 The expression of mouse MHC class II was screened using Alexa Fluor 488 conjugated anti-mouse IAk (clone 10–3.6, which cross-reacts with murine I-Af; Biolegend, San Diego, CA). The expression of human MHC class II was screened using anti-human HLA-DR-APC (clone L243; BD Biosciences, North Ryde, Australia) as well as biotin conjugated DR1-specific (DRB1*01:01) and DR2-specific (DRB1*15:01) antibodies (One Lambda Inc, Canoga Park, CA) and streptavidin-FITC (BD Biosciences). Genotyping primers (Life Technologies, Mulgrave, Australia) were used to determine hereditary transmission of the HLA DRA1*01:01 Tg (forward primer, 5′GGGGGATCCTGCCCTAAGTGTATTTAATGCCCGG-3′; reverse primer, 5′-GGGGAATTCCTATGGATTCCCGTGTCCAGGAGT-3′),52 the HLA DRB1*15:01 Tg (forward primer, 5′-GTGCGGTTCCTGGACAG-3′; reverse primer, 5′-TAGGTGTCCACCGCG-3′), and the HLA DRB1*01:01 Tg (forward primer, 5′GTTGCTGGAAAGATGCATC-3′; reverse primer, 5′-CTCGCCCGTGCACTGT-3′). C57BL/6 mice were obtained from Monash Animal Services, Monash University. The 0101 Tg.FcγRIIb−/− and 1501 Tg.FcγRIIb−/− mice were generated by crossing DRB1*01:01 Tg and DRB1*15:01 Tg mice (described in this article) with FcγRIIb−/− mice on a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME).53 Mice were screened by flow cytometry for murine MHC class II using anti-murine I-A/I-E-PE antibodies (clone M5/114.15.2; BD Biosciences,), for human MHC class II using anti-human HLA-DR-APC as well as biotin conjugated DR1-specific (DRB1*01:01) and DR2-specific (DRB1*15:01) antibodies and streptavidin-FITC, for FcγRIIB by gating on anti-murine B220-PE (clone RA3–6B2; BD Biosciences) and anti-murine CD16/CD32-FITC antibodies (clone 2.4G2; BD Biosciences).53 Hereditary transmission of the HLA-DRA1*01:01, HLA-DRB1*15:01, and HLA-DRB1*01:01 Tg was detected using genotyping primers. Mice that were mouse MHC class II−/−, HLA-DRA1*01:01+/+ and expressed either HLA-DRB1*15:01 or HLA-DRB1*01:01 but not both were selected as founding breeders and bred to homozygosity. Male mice, aged 6–8 weeks, were used for experiments and kept in specific pathogen-free conditions at Monash Medical Centre Animal Facilities. Animal studies were approved by the Monash University Animal Ethics Committee.
Reactivity to α3(IV)NC1 T Cell Epitopes
Mice were immunized subcutaneously with peptide pools (containing α3 aa 1–92, 81–164, or 153–233; 10 µg/peptide per mouse), individual peptides, or whole recombinant proteins in Freund’s complete adjuvant (Sigma, Sydney, Australia). Draining LN cells were harvested 10 days after immunization. Reactivity to individual peptides and to whole proteins was compared by [3H]-thymidine proliferation and IFN-γ and IL-17A ELISPOTs. IFN-γ and IL-17A secretion was detected by ELISPOT (BD Biosciences, and R&D systems, Minneapolis, MN). Lymphocytes were cultured for 18 hours (37°C, 5% CO2) at 5×105 cells/well with or without peptide or murine α3(IV)NC1 (10 μg/ml), in supplemented RPMI media (10% FCS, 2 mM l-glutamine, 2-ME, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; Sigma) in triplicate. Spots were developed according to the manufacturer’s instructions, enumerated by an automated ELISPOT reader (ELR06; AID, Strassberg, Germany) and results expressed as the mean number spots minus baseline (media alone). For lymphocyte proliferation, lymphocytes were cultured in triplicate for 72 hours at 5×105 cells/well, [3H]-thymidine, (0.5 μCi/well, PerkinElmer, Glen Waverley, Australia), was added to culture for the last 16 hours and results expressed as a stimulation index (SI). To control for the potential immunogenicity of the FLAG component within the recombinant proteins in re-stimulation proliferation assays, mice were re-stimulated with α2(IV)NC1 with and without the FLAG component (re-stimulation with media alone or α2(IV)NC1 without FLAG both showed an SI of 1).
T Cell Clone Transfer Model
α3136–146–specific CD4+ clones were generated by immunizing DRB1*15:01 Tg mice with 10 μg of α3136–146 subcutaneously in Freund’s complete adjuvant. Draining LN cells were harvested after 10 days, and then cultured at a concentration of 3×106 cells/well in 6-well plates with 10 μg/ml of α3136–146 in supplemented RPMI media. Well isolated colonies of proliferating cells were identified by light microscopy and by repeated micromanipulation. Single cells were transferred into individual wells in a 96-well plate with 106 splenocytes/well from naïve DRB1*15:01 Tg mice. Splenocytes were CD4+ depleted (by mouse CD4 [L3T4] MACS Microbeads with LD columns; Miltenyi Biotec, North Ryde, Australia) and mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan) treated (5×106 cells/ml with 50 μg/ml mitomycin C in PBS for 30 minutes at 37°C). Cells were cultured in supplemented RPMI media with 50 U/ml recombinant murine IL-2 (Jomar Bioscience, Kensington, Australia) and 50 μg/ml α3136–146, changed weekly, and cell viability assessed at 4 weeks by [3H]-thymidine proliferation assay. Viable clones were expanded in 24-well plates with weekly media changes, fortnightly division and addition of CD4+ depleted, mitomycin C–treated splenocytes. The T cell clone used for experiments was selected based on consistent IFN-γ secretion. As control, nonspecific CD4+ T cells from a mouse immunized with Freund’s complete adjuvant were selected and expanded in vitro by the same method. The TCR Vβ expression was screened by flow cytometry using the Mouse Vβ TCR Screening Panel (BD Biosciences). The TCR Vα expression was screened by flow cytometry using anti-Vα2, 3.2 and 8 specific antibodies (Biolegend). In a pilot experiment (n=4 per group), recipient DRB1*15:01 Tg mice received 107 clones intravenously, 100 µg of α3136–146 subcutaneously in Freund’s complete adjuvant, and 0.5 µg/g of LPS (Sigma) intraperitoneally, and renal disease was assessed 2 weeks later. Recipient DRB1*15:01 Tg mice that received α3136–146–specific clones developed GN as well as dermal DTH, measured by the change in footpad thickness 24 hours after injecting 10 µg of rmα3(IV)NC1 in the footpad. In the second experiment (Figure 6), recipient DRB1*15:01 Tg mice received 107 clones intravenously and 0.5 µg/g of LPS on day 0 and were killed 5 weeks later.
Murine Model of Induced Autoimmune Anti-GBM GN
Mice were immunized with 100 µg of peptide or rmα3(IV)NC1 subcutaneously on days 0, 7, and 14; first in Freund’s complete, and then Freund’s incomplete adjuvant. Mice were killed on day 42.
Assessment of Renal Injury and Immune Cell Infiltration
Glomerular necrosis and crescent formation were assessed on 3 μm thick, PAS-stained, formalin fixed, paraffin-embedded sections (≥50 glomeruli/mouse). Glomerular necrosis was defined as accumulation of PAS-positive material in the glomerulus with hypocellularity and a glomerular crescent as ≥2 layers of cells in Bowman’s space. Fibrin deposition was assessed on 3-μm thick paraffin-embedded sections (≥50 glomeruli/mouse), using a rabbit anti-murine fibrinogen antibody (R-4025) with an ABC kit (DAKO) using DAB (Sigma). Urine was collected 24 hours before the end of experiment using metabolic cages. Urinary albumin was assessed by ELISA (Bethyl Laboratories, Montgomery, TX) and expressed as milligrams per micromoles of creatinine. BUN was measured using standard laboratory methods. CD4+ T cells, macrophages, and neutrophils were detected by immunoperoxidase staining of 6 μm thick, periodate lysine paraformaldehyde– fixed, frozen kidney sections. The primary mAbs used were GK1.5 (anti-murine CD4; American Type Culture Collection, Manassas, Virginia), FA/11 (macrophages, anti-murine CD68; from Dr. Gordon L Koch, Cambridge, England), and RB6–8C5 (neutrophils, anti-Gr-1). A minimum of 20 consecutively viewed glomeruli were assessed per animal. Ig staining of the GBM was assessed on 6 μm thick, snap-frozen tissue sections using FITC-sheep anti-murine Ig (1:1000; Silenus, Hawthorn, Australia) antibodies. Images were captured using a NikonC1 confocal laser (488 nm) attached to a Nikon Ti-E inverted microscope.
Detection of Anti-α3(IV)NC1–Specific IgG
ELISA plates were coated with 5 µg/ml of recombinant human or murine α3(IV)NC1, and then blocked with 2% casein in PBS. Mouse serum diluted 1:200 was added in duplicate and incubated for 1 hour followed by HRP conjugated sheep anti-murine IgG-specific antibodies (GE Healthcare, Sydney, Australia) diluted 1:2000.
Statistical Analyses
Where there were ≥3 groups, one-way ANOVA followed by Tukey’s post test was used to assess differences. Where there were only two groups, a t test was used. Means and SEM are shown.
Disclosures
None.
Acknowledgments
We thank Dennis Zaller (Merck, Cambridge, MA) for providing HLA DRB1*0101 Tg mice. This study was supported by grants from the National Health and Medical Research Council of Australia (Program 334067 to A.R.K and S.R.H) and the National Institute of Diabetes and Digestive and Kidney Diseases (B.G.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070705/-/DCSupplemental.
References
- 1.Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514–2527, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Neale TJ, Tipping PG, Carson SD, Holdsworth SR: Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet 2: 421–424, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Bolton WK, Tucker FL, Sturgill BC: New avian model of experimental glomerulonephritis consistent with mediation by cellular immunity. Nonhumorally mediated glomerulonephritis in chickens. J Clin Invest 73: 1263–1276, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Danoff TM, Okada H, Neilson EG: Susceptibility to anti-glomerular basement membrane disease and Goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest 100: 2263–2275, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds J, Tam FW, Chandraker A, Smith J, Karkar AM, Cross J, Peach R, Sayegh MH, Pusey CD: CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest 105: 643–651, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Hicks J, Borillo J, Glass WF, 2nd, Lou YH: CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest 109: 517–524, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean EG, Wilson GR, Li M, Edgtton KL, O’Sullivan KM, Hudson BG, Holdsworth SR, Kitching AR: Experimental autoimmune Goodpasture’s disease: A pathogenetic role for both effector cells and antibody in injury. Kidney Int 67: 566–575, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Phelps RG, Rees AJ: The HLA complex in Goodpasture’s disease: A model for analyzing susceptibility to autoimmunity. Kidney Int 56: 1638–1653, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, Langeveld JP, Hudson BG: The goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17-31 and 127-141 of the NC1 domain. J Biol Chem 274: 11267–11274, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Cairns LS, Phelps RG, Bowie L, Hall AM, Saweirs WW, Rees AJ, Barker RN: The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture’s disease. J Am Soc Nephrol 14: 2801–2812, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Luo AM, Fox JW, Chen L, Bolton WK: Synthetic peptides of Goodpasture’s antigen in antiglomerular basement membrane nephritis in rats. J Lab Clin Med 139: 303–310, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Borillo J, Glass WF, Hicks J, Ou CN, Lou YH: T-cell epitope of alpha3 chain of type IV collagen induces severe glomerulonephritis. Kidney Int 64: 1292–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Robertson J, Wu J, Arends J, Glass WF, 2nd, Southwood S, Sette A, Lou YH: Characterization of the T-cell epitope that causes anti-GBM glomerulonephritis. Kidney Int 68: 1061–1070, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Bolton WK, Chen L, Hellmark T, Wieslander J, Fox JW: Epitope spreading and autoimmune glomerulonephritis in rats induced by a T cell epitope of Goodpasture’s antigen. J Am Soc Nephrol 16: 2657–2666, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Reynolds J, Haxby J, Juggapah JK, Evans DJ, Pusey CD: Identification of a nephritogenic immunodominant B and T cell epitope in experimental autoimmune glomerulonephritis. Clin Exp Immunol 155: 311–319, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Arends J, Borillo J, Zhou C, Merszei J, McMahon J, Lou YH: A self T cell epitope induces autoantibody response: Mechanism for production of antibodies to diverse glomerular basement membrane antigens. J Immunol 172: 4567–4574, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L: Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A 96: 10338–10343, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregersen JW, Kranc KR, Ke X, Svendsen P, Madsen LS, Thomsen AR, Cardon LR, Bell JI, Fugger L: Functional epistasis on a common MHC haplotype associated with multiple sclerosis. Nature 443: 574–577, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Attfield KE, Dendrou CA, Fugger L: Bridging the gap from genetic association to functional understanding: The next generation of mouse models of multiple sclerosis. Immunol Rev 248: 10–22, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR: The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods 308: 167–178, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT: Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Reynolds J, Cook PR, Behmoaras J, Smith J, Bhangal G, Tadros S, Tee J, Salama AD, Evans DJ, Aitman TJ, Cook HT, Pusey CD: Genetic susceptibility to experimental autoimmune glomerulonephritis in the Wistar Kyoto rat. Am J Pathol 180: 1843–1851, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp PE, Martin-Ramirez J, Boross P, Mangsbo SM, Reynolds J, Moss J, Pusey CD, Cook HT, Tarzi RM, Verbeek JS: Increased incidence of anti-GBM disease in Fcgamma receptor 2b deficient mice, but not mice with conditional deletion of Fcgr2b on either B cells or myeloid cells alone. Mol Immunol 50: 49–56, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Robertson J, Wu J, Arends J, Zhou C, McMahon J, Torres L, Lou YH: Activation of glomerular basement membrane-specific B cells in the renal draining lymph node after T cell-mediated glomerular injury. J Am Soc Nephrol 16: 3256–3263, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Zou J, Henderson L, Thomas V, Swan P, Turner AN, Phelps RG: Presentation of the Goodpasture autoantigen requires proteolytic unlocking steps that destroy prominent T cell epitopes. J Am Soc Nephrol 18: 771–779, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES: Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307: 1630–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Dayhoff MO, Schwartz RM, Orcutt BC: A model of evolutionary change in proteins. In: Atlas of Protein Sequence and Structure, edited by Dayhoff MO, Washington, DC, National Biomedical Research Foundation, 1978, pp 345–352 [Google Scholar]
- 30.Wong D, Phelps RG, Turner AN: The Goodpasture antigen is expressed in the human thymus. Kidney Int 60: 1777–1783, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Salama AD, Chaudhry AN, Holthaus KA, Mosley K, Kalluri R, Sayegh MH, Lechler RI, Pusey CD, Lightstone L: Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int 64: 1685–1694, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Su SC, Hecox DB, Brady GF, Mackin KM, Clark AG, Foster MH: Central tolerance regulates B cells reactive with Goodpasture antigen alpha3(IV)NC1 collagen. J Immunol 181: 6092–6100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark AG, Mackin KM, Foster MH: Genetic elimination of α3(IV) collagen fails to rescue anti-collagen B cells. Immunol Lett 141: 134–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerner RA, Glassock RJ, Dixon FJ: The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med 126: 989–1004, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohda T, Okada S, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y: High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int 66: 177–186, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kitching AR, Holdsworth SR, Tipping PG: IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol 10: 752–759, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Timoshanko JR, Holdsworth SR, Kitching AR, Tipping PG: IFN-gamma production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol 168: 4135–4141, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, Timoshanko JR, Hudson BG, Holdsworth SR: Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: A protective role for IFN-gamma. J Am Soc Nephrol 15: 1764–1774, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ooi JD, Phoon RK, Holdsworth SR, Kitching AR: IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol 20: 980–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Hellmark T, Pedchenko V, Hudson BG, Pusey CD, Fox JW, Bolton WK: A nephritogenic peptide induces intermolecular epitope spreading on collagen IV in experimental autoimmune glomerulonephritis. J Am Soc Nephrol 17: 3076–3081, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH: Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int 76: 1108–1115, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Steblay RW, Rudofsky UH: Experimental autoimmune glomerulonephritis induced by anti-glomerular basement membrane antibody. II. Effects of injecting heterologous, homologous, or autologous glomerular basement membranes and complete Freund’s adjuvant into sheep. Am J Pathol 113: 125–133, 1983 [PMC free article] [PubMed] [Google Scholar]
- 43.Sado Y, Okigaki T, Takamiya H, Seno S: Experimental autoimmune glomerulonephritis with pulmonary hemorrhage in rats. The dose-effect relationship of the nephritogenic antigen from bovine glomerular basement membrane. J Clin Lab Immunol 15: 199–204, 1984 [PubMed] [Google Scholar]
- 44.Hopfer H, Holzer J, Hünemörder S, Paust HJ, Sachs M, Meyer-Schwesinger C, Turner JE, Panzer U, Mittrücker HW: Characterization of the renal CD4+ T-cell response in experimental autoimmune glomerulonephritis. Kidney Int 82: 60–71, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Goodnow CC: Multistep pathogenesis of autoimmune disease. Cell 130: 25–35, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Smith KG, Clatworthy MR: FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat Rev Immunol 10: 328–343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boross P, Arandhara VL, Martin-Ramirez J, Santiago-Raber ML, Carlucci F, Flierman R, van der Kaa J, Breukel C, Claassens JW, Camps M, Lubberts E, Salvatori D, Rastaldi MP, Ossendorp F, Daha MR, Cook HT, Izui S, Botto M, Verbeek JS: The inhibiting Fc receptor for IgG, FcγRIIB, is a modifier of autoimmune susceptibility. J Immunol 187: 1304–1313, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Kalluri R, Cantley LG, Kerjaschki D, Neilson EG: Reactive oxygen species expose cryptic epitopes associated with autoimmune goodpasture syndrome. J Biol Chem 275: 20027–20032, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Miner JH, Sanes JR: Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: Sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127: 879–891, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, Burrows GG, Fugger L, Vandenbark AA: Myelin oligodendrocyte glycoprotein-35-55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol 34: 1251–1261, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Rosloniec EF, Brand DD, Myers LK, Whittington KB, Gumanovskaya M, Zaller DM, Woods A, Altmann DM, Stuart JM, Kang AH: An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med 185: 1113–1122, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woods A, Chen HY, Trumbauer ME, Sirotina A, Cummings R, Zaller DM: Human major histocompatibility complex class II-restricted T cell responses in transgenic mice. J Exp Med 180: 173–181, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV: Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379: 346–349, 1996 [DOI] [PubMed] [Google Scholar]