Abstract
States of low perfusion pressure of the kidney associate with hyperplasia or expansion of renin-producing cells, but it is unknown whether hypoxia-triggered genes contribute to these changes. Here, we stabilized hypoxia-inducible transcription factors (HIFs) in mice by conditionally deleting their negative regulator, Vhl, using the Cre/loxP system with renin-1d promoter-driven Cre expression. Vhl −/−REN mice were viable and had normal BP. Deletion of Vhl resulted in constitutive accumulation of HIF-2α in afferent arterioles and glomerular cells and HIF-1α in collecting duct cells of the adult kidney. The preglomerular vascular tree developed normally, but far fewer renin-expressing cells were present, with more than 70% of glomeruli not containing renin cells at the typical juxtaglomerular position. Moreover, these mice had an attenuated expansion of renin-producing cells in response to a low-salt diet combined with an ACE inhibitor. However, renin-producing cells of Vhl −/−REN mice expressed the erythropoietin gene, and they were markedly polycythemic. Taken together, these results suggest that hypoxia-inducible genes, regulated by VHL, are essential for normal development and physiologic adaptation of renin-producing cells. In addition, deletion of Vhl shifts the phenotype of juxtaglomerular cells from a renin- to erythropoietin-secreting cell type, presumably in response to HIF-2 accumulation.
The number of renin-producing cells in afferent glomerular arterioles is an important determinant of renal renin secretion. Genetic defects1–3 or pharmacological inhibition4,5 of the renin-angiotensin system (RAS) during kidney development cause a massive compensatory increase in the number of renin-expressing cells associated with the development of multilayered preglomerular vessel walls. Indirect evidence suggests that a low kidney perfusion pressure associated with the aforementioned conditions could play a key role for the development of renin cell hyperplasia.1,6 In addition, renal artery stenosis in adult kidneys also induces an expansion of renin-producing cells along preglomerular vessels.7,8 It is not clear if these effects of insufficient kidney perfusion are transduced by mechanical signals or metabolic signals. Metabolic signals could be related to insufficient tissue oxygenation as a consequence of low kidney perfusion. Such a link to tissue oxygenation would also fit with models of vessel formation thought to be driven by tissue hypoxia.9 We were, therefore, interested to investigate if mimicking chronic hypoxia in renin-expressing cells during kidney development has influence on the number of renin-expressing cells and renal vascular development. To mimic chronic hypoxia, we stabilized hypoxia inducible transcription factors by the deletion of the von Hippel–Lindau protein (pVHL).10 pVHL mediates oxygen-dependent proteasomal degradation of hypoxia-inducible transcription factor-α (HIF-α) protein and thereby, acts as a negative regulator of HIF.11 Cell-specific deletion of Vhl, therefore, activates HIF-regulated pathways in these cells.10 It turned out that deletion of Vhl in renin-expressing cells and their descendants markedly downregulates rather than stimulates renin expression in juxtaglomerular cells. Instead, juxtaglomerular cells produce erythropoietin in this situation.
Results
HIF Protein Levels Are Increased by Targeted Deletion of Vhl in the Renin Cell Lineage
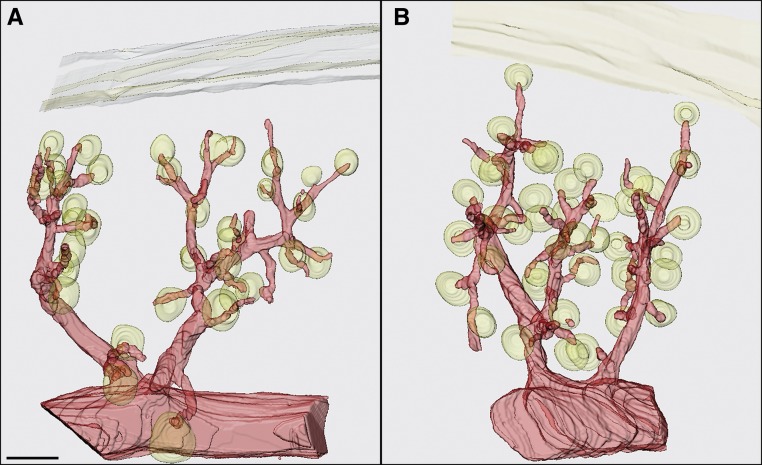
Vhl −/−REN mice (conditional knockout of Vhl in renin-producing cells) had similar body weights as Vhl fl/fl mice (23.7±1.6 g Vhl −/−REN versus 25.1±1.2 g Vhl fl/fl). Gross morphology of the preglomerular vascular tree displayed no major abnormalities in Vhl −/−REN kidneys (Figure 1). In particular, length, thickness, and branchings of afferent arterioles were not obviously different between Vhl −/−REN and Vhl fl/fl kidneys. However, the inner diameter of the afferent arterioles of Vhl −/−REN kidneys was widened by approximately 32% at their entrance into the glomerular capillary network compared with the control animals (18.9±0.5 μm Vhl −/−REN versus 14.4±0.8 μm Vhl fl/fl, P<0.001).
Figure 1.
The preglomerular vascular tree in Vhl −/−REN kidneys displays no major morphological abnormalities compared to control kidneys. Three-dimensional reconstructions show an isolated arcuate side branch with interlobular arteries and afferent arterioles of (A) a Vhl fl/fl and (B) a Vhl −/−REN mouse. Glomeruli and a section of the capsule are also shown. Scale bar, 100 µm.
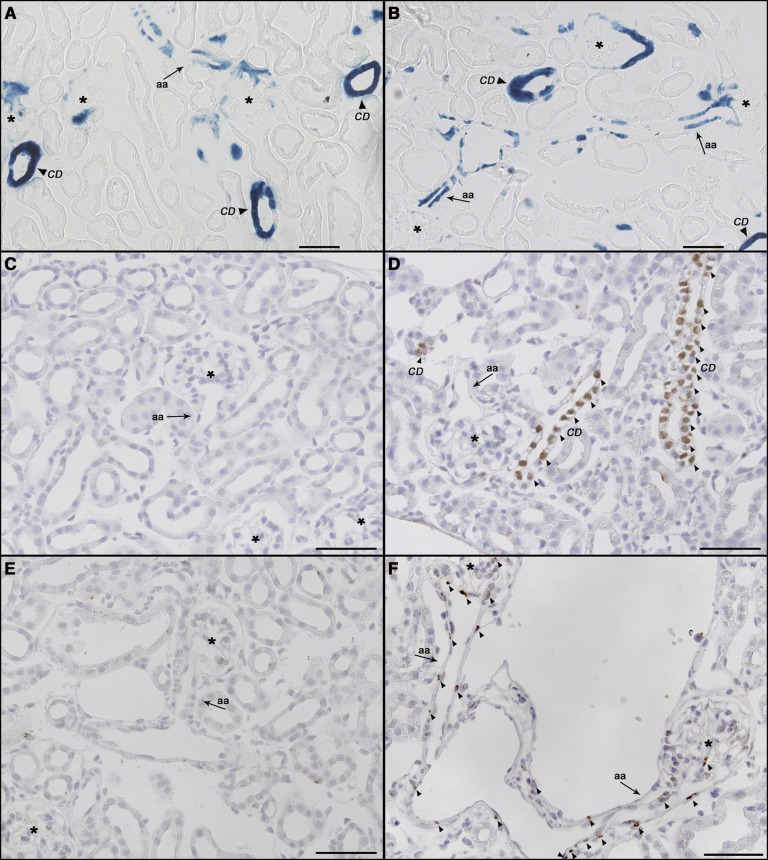
Lineage tracing of renin-expressing cells and their descendants as indicated by LacZ staining also showed no apparent difference between Vhl knockout and wild-type (wt) kidneys. LacZ staining was mainly found in the media layer of preglomerular vessels and collecting duct cells. In addition, juxtaglomerular cells and individual intraglomerular cells stained for LacZ (Figure 2, A and B). In accordance with previous reports,12 kidneys of adult wt mice showed no staining for HIF-1α or HIF-2α. In contrast, Vhl −/−REN kidneys showed constitutive nuclear HIF-1α staining in collecting duct cells (Figure 2D). In addition, in Vhl −/−REN kidneys, HIF-2α was detectable by immunohistochemistry in cells along the afferent arterioles, including the glomerular vascular poles, as well as individual intraglomerular cells (Figure 2F).
Figure 2.
HIF-1α and HIF-2α are stabilized by targeted deletion of Vhl in the renin cell lineage. (A and B) Lineage tracing of renin-expressing cells and their descendants in kidney sections of (A) a wt and (B) a Vhl −/−REN mouse by performance of LacZ staining. LacZ expression was found mainly in afferent arterioles (aa; arrow) and collecting duct (CD; arrowhead) cells. There is no obvious difference in LacZ staining between the two genotypes. (C–F) Immunohistochemistry for (C and D) HIF-1α and (E and F) HIF-2α in kidney sections of Vhl fl/fl and Vhl −/−REN mice. Positive staining for HIF-1α and HIF-2α (highlighted by arrowheads) are found in (D and F) Vhl −/−REN mice only: HIF-1α staining in CD cells and HIF-2α staining in cells along the aa (arrow), including the glomerular vascular poles, as well as in individual intraglomerular cells. Kidney sections of (C and E) Vhl fl/fl mice were negative for HIF staining. Asterisks indicate glomeruli. Scale bar, 50 µm.
Vhl Deletion in the Renin Cell Lineage Inhibits Renin Expression in the Kidney
Renin immunohistochemistry of Vhl fl/fl kidneys confirmed the typical localization of renin-expressing cells at the vascular poles of all glomeruli (Figure 3A). In Vhl −/−REN kidneys, however, more than 70% of the glomeruli did not contain renin-positive cells at all at their vascular poles (Figure 3B). Others contained a few cells with faint renin immunostaining. In contrast to Vhl fl/fl kidneys, Vhl −/−REN kidneys frequently showed some renin-positive cells at the branching sites of interlobular arteries and afferent arterioles.
Figure 3.
Targeted Vhl deletion in the renin cell lineage inhibits renin expression in the kidney and changes the expression site. (A–D) Immunohistochemistry for renin (green) and α-SMA (red) in kidney sections of (A and C) Vhl fl/fl and (B and D) Vhl −/−REN mice under (A and B) normal salt diet and (C and D) after treatment with low-salt diet and the ACE inhibitor enalapril (LS+Enal) for 3 weeks. Scale bar, 50 µm. Asterisks indicate glomeruli, and arrowheads mark renin-producing cells. (E) Kidney renin mRNA levels and (F) plasma renin concentrations in Vhl fl/fl and Vhl −/−REN mice under normal salt diet and after treatment with LS+Enal for 3 weeks. Values are means ± SEM of 10 mice in each group. *P<0.005 by t test.
Kidney renin mRNA levels and plasma renin concentrations were markedly lower in Vhl −/−REN mice relative to Vhl fl/fl mice (Figure 3, E and F). However, systolic BP in Vhl −/−REN mice (133.3±1.3 mmHg; n=6) was not different from systolic BP of Vhl fl/fl mice (134.0±2.9 mmHg; n=6). Challenge of the RAS by a combined treatment of low-salt diet and an angiotensin-I converting enzyme (ACE) inhibitor for 3 weeks produced a strong increase in the number of renin-expressing cells in the media layer of afferent arterioles in Vhl fl/fl mice (Figure 3C), which was paralleled by 16- and 10-fold increases of renin mRNA abundance and plasma renin concentrations, respectively (Figure 3, E and F). In contrast, in Vhl −/−REN mice, chronic stimulation of renin synthesis by low-salt diet combined with an ACE inhibitor only moderately increased renin mRNA levels and plasma renin concentrations (Figure 3, E and F). A marked difference in renin expression between Vhl fl/fl and Vhl −/−REN kidneys remained (Figure 3), which was also confirmed by in situ hybridization for renin mRNA (Figure 4). Despite the stimulating conditions, a high percentage of glomeruli in Vhl −/−REN mice did not contain renin-expressing cells at their vascular poles; others showed faint renin expression only. Renin expression along afferent arterioles was scarce in Vhl −/−REN kidneys, even during challenge of the RAS (Figure 3D). Analysis of serial sections stained for either renin or HIF-2α revealed no coexpression of both proteins at least at the level of detection limits of immunohistochemistry (not shown).
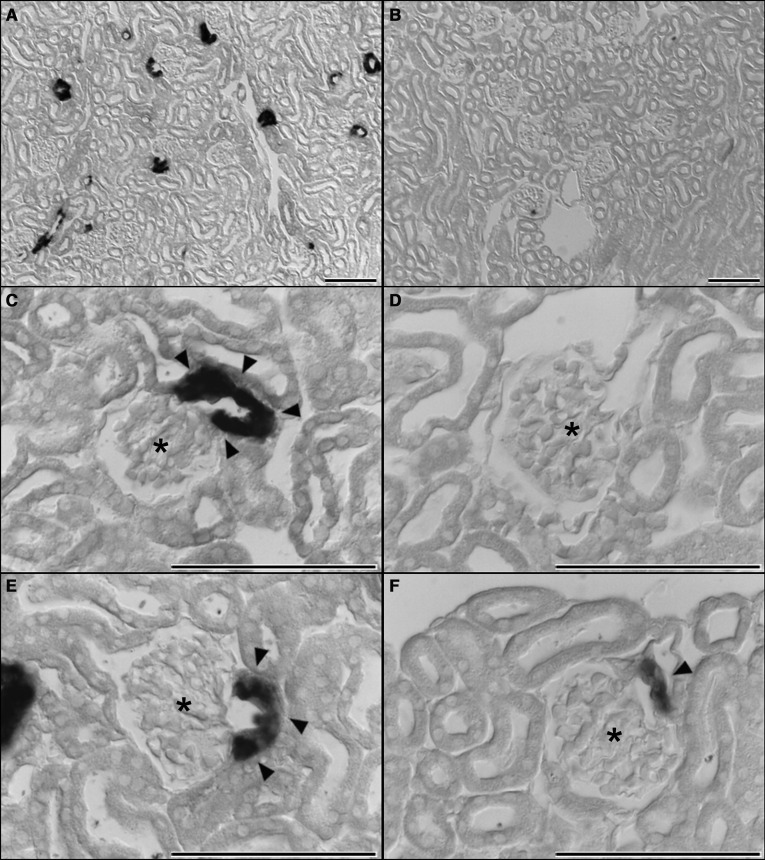
Figure 4.
Vhl deletion in the renin cell lineage inhibits renin expression at the typical expression site in the kidney. (A, C, and E) In situ hybridization detects renin mRNA expression (highlighted by arrowheads) at the typical juxtaglomerular position in kidney sections of Vhl fl/fl mice. (B, D, and F) Despite the stimulating conditions, a high percentage of glomeruli in Vhl −/−REN mice did not contain renin-expressing cells at their vascular poles; only a few glomeruli showed a faint signal for renin mRNA expression. Scale bar, 100 µm. Asterisks indicate glomeruli, and arrowheads show renin-expressing cells.
Vhl Deletion in the Renin Cell Lineage Activates Erythropoietin Expression in the Kidney
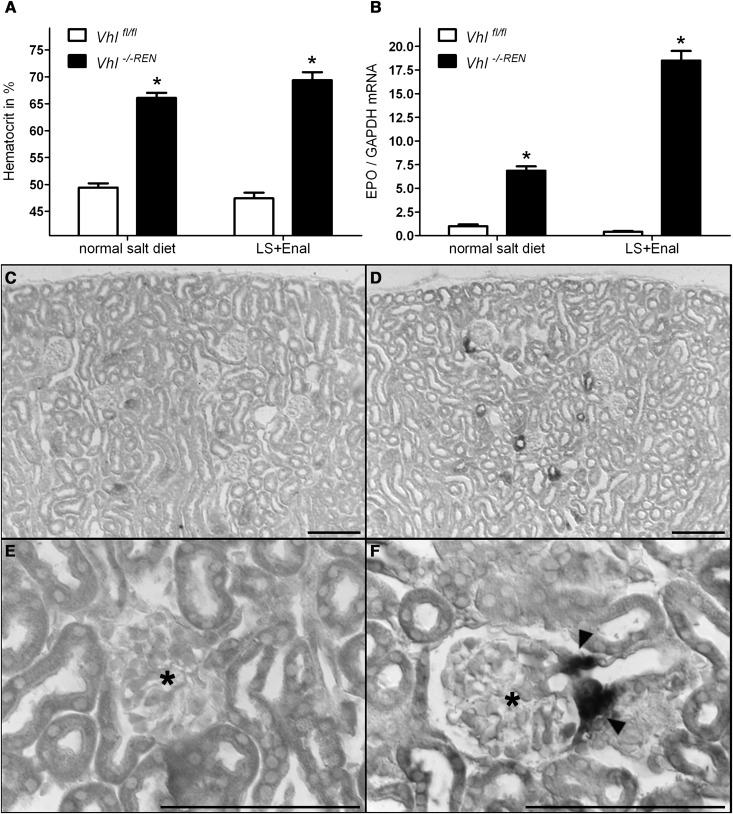
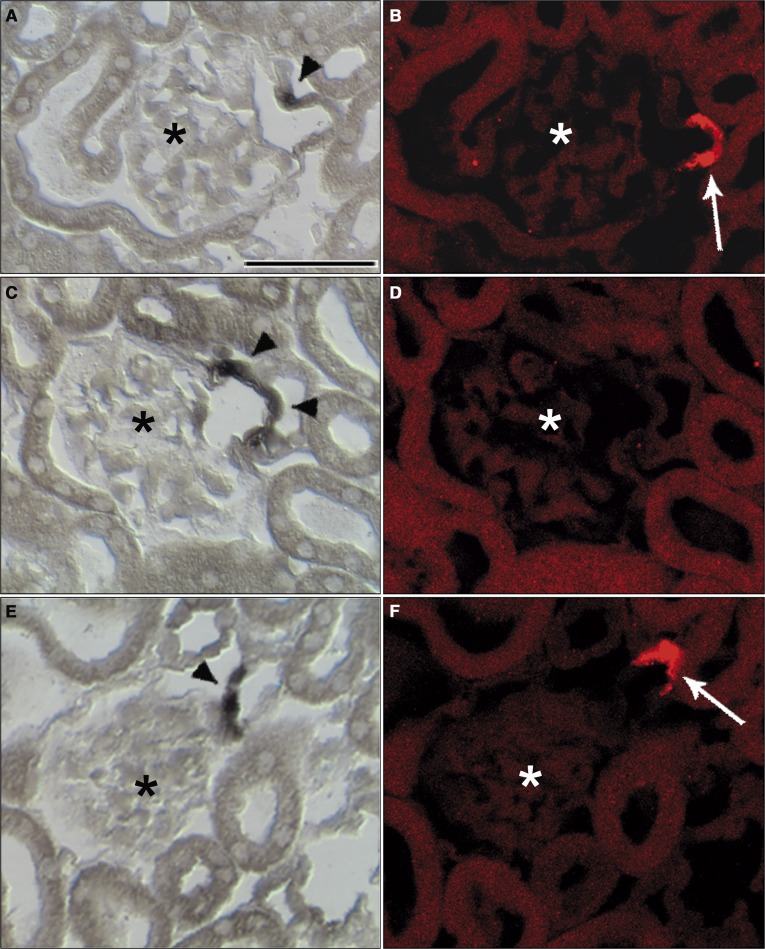
We noticed that Vhl −/−REN mice were clearly polycythemic (Figure 5A). Red blood cell counts were higher by 34% in Vhl −/−REN mice compared with control mice (Table 1). This observation raised the question of if Vhl deletion resulted in an increased production of erythropoietin (EPO). In fact, EPO mRNA levels were increased approximately 7-fold under baseline conditions and approximately 46-fold after RAS stimulation in Vhl −/−REN kidneys relative to Vhl fl/fl kidneys (Figure 5B). In accordance with the elevated mRNA levels, EPO plasma concentrations were also elevated in the renin cell-specific Vhl knockout mice compared with their controls (Table 1). In situ hybridization localized EPO mRNA expression to afferent arterioles and more pronounced to their juxtaglomerular portions (Figure 5, D and F). Analysis of serial sections subjected to in situ hybridization for EPO mRNA and immunohistochemistry for renin revealed that EPO expression and renin production were exclusive in most instances (Figure 6). Only in 10% of cells, which were positive for either EPO or renin, both signals could be detected.
Figure 5.
Vhl deletion in the renin cell lineage activates EPO expression in the kidney at a site that is typical for renin expression. (A) Hematocrit values of Vhl fl/fl and Vhl −/−REN mice show that Vhl −/−REN mice are markedly polycythemic: on normal salt diet, 66% were polycythemic, and on low-salt diet combined with the ACE inhibitor enalapril (LS+Enal) for 3 weeks, 69% were polycythemic versus Vhl fl/fl mice (49% and 47%, respectively). (B) Kidney EPO mRNA levels were increased in Vhl −/−REN mice: approximately 7-fold under baseline conditions and approximately 46-fold after RAS stimulation (LS+Enal) versus Vhl fl/fl mice. (C–F) In situ hybridization in kidney cortex of (D and F) Vhl −/−REN mice (LS+Enal for 3 weeks) localized EPO mRNA expression to afferent arterioles, which was more pronounced to their juxtaglomerular portions. Afferent arterioles in kidney sections of (C and E) Vhl fl/fl mice (LS+Enal for 3 weeks) were negative for EPO signal. Data are means ± SEM of 10 mice in each group. *P<0.005 by t test. Scale bar, 100 µm. Asterisks indicate glomeruli, and arrowheads show EPO-producing cells.
Table 1.
Red blood cell counts and EPO plasma concentrations in control (Vhl fl/fl) and Vhl knockout mice (Vhl −/−REN)
| Parameter | Unit | Vhl fl/fl (n=6) | Vhl −/− REN (n=6) | P | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Red blood cell count | ×106/µl | 8.0 | 0.6 | 10.8 | 0.3 | <0.01 |
| EPO plasma concentration | pg/ml | 201.5 | 71.9 | 970.4 | 185.9 | <0.05 |
Figure 6.
EPO mRNA expression and renin production in the kidney are exclusive in most instances. (A, C, and E) In situ hybridization for EPO mRNA and (B, D, and F) immunostaining for renin revealed that EPO mRNA expression and renin production were exclusive in most instances. Scale bar, 50 µm. Asterisks indicate glomeruli, and arrowheads indicate EPO-producing cells; renin-producing cells are marked by arrows.
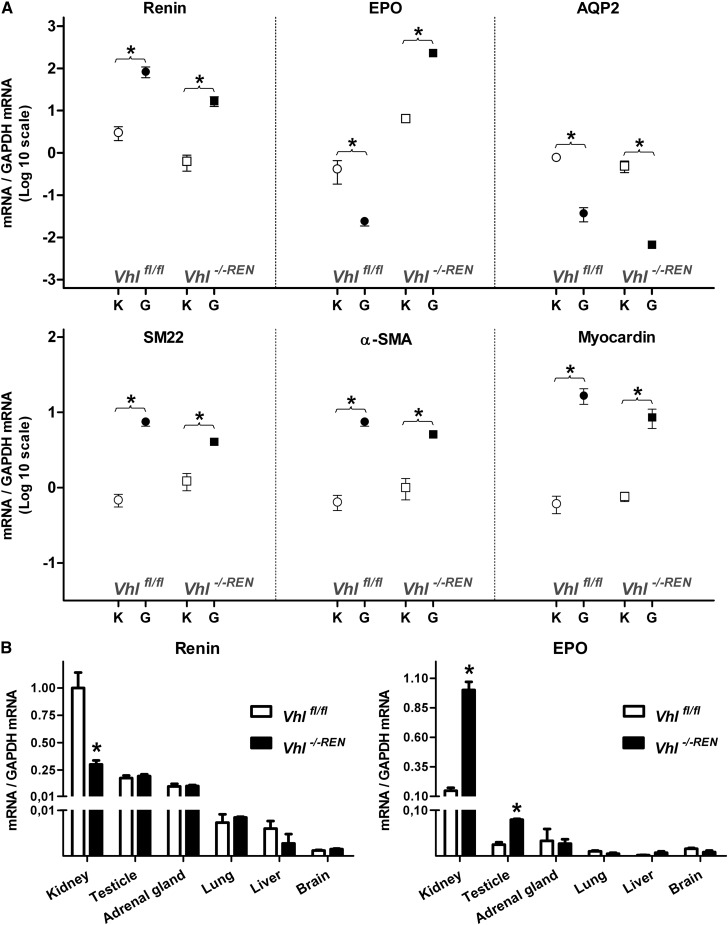
Physiologic changes in the number of renin-producing cells in the kidney are caused by reversible phenotype switches between preglomerular vascular smooth muscle cells and renin-producing cells.8 We, therefore, performed gene expression studies to investigate if the decrease of renin-producing cells in afferent arterioles of Vhl −/−REN kidneys was associated with an increase of gene expression characteristic for vascular smooth muscle cells. Comparisons of gene expression between isolated glomeruli containing attached afferent arterioles and whole kidneys confirmed strong enrichment of renin mRNA as expected and a markedly reduced expression of aquaporin-2 (AQP2) mRNA, indicating the absence of significant contamination of the preparation with collecting duct cells in both Vhl fl/fl and Vhl −/−REN kidneys (Figure 7A). EPO mRNA showed a strong enrichment in isolated glomeruli/arterioles of Vhl −/−REN kidneys and a clear depletion in glomeruli/arterioles of Vhl fl/fl kidneys (Figure 7A), confirming the expression of EPO in preglomerular vessels of Vhl −/−REN kidneys as opposed to expression outside the glomeruli/arterioles in Vhl fl/fl kidneys. The smooth muscle cell markers SM22, α-smooth muscle actin (α-SMA), myocardin, and smooth muscle myosin heavy chain (not shown) displayed lower rather than higher enrichments in glomeruli/afferent arterioles of Vhl −/−REN kidneys compared with Vhl fl/fl kidneys (Figure 7A), thus not supporting the idea of a phenotype shift to smooth muscle cells in afferent arterioles of Vhl −/−REN kidneys.
Figure 7.
The decrease of renin-producing cells is not associated with a phenotype switch into vascular smooth muscle cells. (A) Abundance of renin, EPO, AQP2, α-SMA, SM22, and myocardin mRNAs in isolated glomeruli with attached afferent arterioles and their original whole kidneys from Vhl fl/fl and Vhl −/−REN mice. mRNA abundance is given as ratio over GAPDH mRNA, which was considered as a standard. Ratio values are depicted on a logarithmic scale to allow the estimation of both accumulation and depletion of mRNAs in the isolated afferent arterioles relative to their original kidneys. G and K indicate glomeruli with afferent arterioles and kidney, respectively. (B) Distribution of renin and EPO mRNA in kidneys and extrarenal tissues of Vhl fl/fl and Vhl −/−REN mice. Data are means ± SEM of five mice in each group. *P<0.05 by t test.
Extrarenal Effects of Vhl Deletion in the Renin Cell Lineage
Apart from the kidney, renin is also expressed to a lesser extent in the adrenal gland, lung, and gonads.13–15 We, therefore, investigated if deletion of Vhl in extrarenal renin cells has an impact on renin and EPO expression as well. In adrenal glands, brain, heart, liver, and lung of Vhl −/−REN mice, neither renin nor EPO expression was changed. In the testicles, however, EPO expression was significantly higher in Vhl −/−REN mice compared with Vhl fl/fl animals (Figure 7B).
Developmental Changes of Renin and EPO Expression
To obtain information on if and how the kidney phenotype induced by deletion of Vhl from the renin cell lineage changes with kidney development, we also analyzed mice at the day of birth. Hematocrit of newborn Vhl −/−REN mice was already increased relative to litter controls (44.5±1.9% Vhl −/−REN versus 36.8±1.4% Vhl fl/fl, n=6 in each group, P<0.05). Kidney renin mRNA levels were decreased to 59±5% (P<0.05), and kidney EPO mRNA levels were increased to 286±35% in Vhl −/−REN relative to the respective mean values of Vhl fl/fl litter controls.
Discussion
Using a conditional knockout mouse model targeting deletion of Vhl within renin-producing cells and their descendants, we found that pVHL plays an essential role for normal embryonic development of renin-producing cells in the juxtaglomerular apparatus and their adaptive increase within the afferent arteriole on RAS stimulation. Although more than one renin gene exists in mice, we believe that the expression pattern of the mouse renin-1d gene, which was used as a reporter in this study, is very similar to the expression of the human renin gene.16,17
As anticipated, pVHL knockdown resulted in constitutive stabilization of HIF in renin-expressing cells in afferent arterioles and collecting ducts. The activation of the renin promoter in the collecting duct during kidney development is a known but unexplained phenomenon.17,18 These data, thus, do not support the hypothesis that hypoxia and HIF-dependent gene expression promote the phenotypic switch of vascular smooth muscle cells to renin-producing cells. In contrast, these findings suggest that HIF target genes and in particular, HIF-2–dependent genes suppress renin production.
In addition to a marked reduction in the number of renin-producing cells, their location in Vhl −/−REN mice was atypical. Although renin cells are normally located in juxtaglomerular position in mammals and each glomerulus in mice contains a few of those cells,19,20 in Vhl −/−REN mice, more than 70% of the glomeruli did not contain renin cells in juxtaglomerular position. Instead, some renin-expressing cells appeared in the walls of afferent arterioles more distant to the vascular pole. Salt reduction combined with ACE inhibition, which typically causes a strong retrograde recruitment of renin-expressing cells along afferent arterioles, exerted only a weak stimulatory effect on renin expression in Vhl −/−REN mice, and the juxtaglomerular areas remained mostly free of renin-expressing cells, even under these stimulatory conditions. Analysis of serial sections stained for HIF-2α and renin in Vhl −/−REN kidneys further revealed that HIF-2α–positive cells did not express renin, suggesting that HIF-2 somehow leads to inhibition of renin synthesis. Likely, this negative regulatory effect of HIF-2 is not caused by a direct inhibitory action of HIF-2 on the renin gene, because adrenal renin expression was unaffected in Vhl −/−REN mice; also, no direct inhibitory transcriptional activity has been described for HIFs.21
Our findings add to the concept that pVHL is important for tissue development and differentiation, a role that seems highly conserved during evolution.22 Although global Vhl knockout results in embryonic mortality because of aberrant formation of the placental vasculature23 and endothelial Vhl knockout also results in early developmental defects,24 Vhl knockout in nonendothelial cells leads to variable, cell-dependent pathology.10 In the kidney, the interest in the role of VHL has so far focused on tubular cells given that biallelic inactivation of Vhl can result in the formation of cysts and tumors in patients with the VHL syndrome and spontaneous clear cell renal carcinoma.25 In addition, conditional knockout of Vhl in podocytes was found to induce severe glomerular pathology resembling rapidly progressive GN.26
Apart from smaller solitary cysts, we found no evidence for abnormal proliferation of Vhl-deficient tubular and nontubular cells, and we did not observe the induction of inflammation. Remarkably, however, Vhl deletion in renin-producing cells resulted in aberrant derepression of the EPO gene with increased circulating EPO concentrations, causing secondary marked polycythemia. In fact, it seemed as if renin-producing cells underwent a shift to EPO-producing cells. EPO mRNA expression was found in cells in the juxtaglomerular portion and media layer of afferent arterioles in Vhl −/−REN kidneys, typical locations for renin-producing cells. Serial sections revealed that EPO and renin expression were reciprocally expressed, and only a minor portion of cells revealed coexpression of both EPO and renin, compatible with a transitional phenotype. One possible explanation for this finding could be that the Cre/loxP recombination was not equally effective in all cells. In fact, not all renin-positive cells showed immunoreactivity for HIF-2α, suggesting that the Vhl gene was not effectively deleted in all cells. Additional evidence for a link between characteristics of renin-producing cells and EPO production was added by the observation that salt restriction combined with ACE inhibition (physiologic stimuli of renin production) resulted in a significant increase in EPO mRNA expression. Our data also show that the reciprocal changes of renin and EPO expression are visible already at the day of birth but become more pronounced during postnatal development.
Although previous studies have also observed the development of EPO-dependent secondary polycythemia in Vhl knockout models, the mechanisms are likely different. Loss of pVHL has so far not been reported to be sufficient for EPO induction in a cell type that does not normally produce the hormone27 (e.g., the polycythemia in the Vhl knockout model using phosphoenolpyruvate carboxykinase-driven Cre recombinase results from EPO production in the liver, where hepatocytes are a physiologic site of EPO synthesis, whereas EPO production is not induced in renal tubular cells, despite marked HIF accumulation).28 Also, Vhl deletion in keratinocytes does not directly induce EPO but stimulates renal EPO synthesis through complex hemodynamic mechanisms.29 In the present study, we provide evidence that renin-producing cells devoid of Vhl directly produce EPO. Recently, it has been reported, that deletion of Vhl from osteoblasts induced EPO expression in the bone and stimulated erythropoiesis.30 Again, evidence was provided that osteoblasts also could be a so-far unrecognized physiologic EPO production site.
Although there has been early speculation about links between renin and EPO production in the kidney,31 a direct relation between renin- and EPO-producing cells in the kidney had not been obvious so far. EPO-producing cells have been suggested to originate from the neural crest,32 whereas renin-producing cells are likely derived from FoxD1-mesenchymal cells,33 suggesting different developmental roots. Although renal EPO production has so far only been localized to peritubular interstitial cells,34–36 we cannot exclude that the juxtaglomerular cells producing renin may express the EPO gene during selected periods of development or under very selected circumstances.
Of interest, HIF-2α, and not HIF-1α, was found to accumulate in renin-producing cells after Vhl deletion, whereas HIF-1α, but not HIF-2α, was induced in collecting duct cells of knockout animals. This result corresponds to the normally observed distribution of both HIF isoforms in tubular epithelial cells and cells of mesenchymal origin in the kidney under hypoxic conditions or after pharmacological inhibition of prolyl-hydroxylases.37,38 Mechanisms of the differential expression of HIF-1 and HIF-2 have not been resolved so far. In tubular cells, biallelic inactivation of Vhl can result in accumulation of HIF-2α in addition to HIF-1α, suggesting that Vhl may specifically suppress HIF-2.39 The observations in the current and other Vhl knockout models40,41 suggest, however, that Vhl deletion is not always sufficient to overcome cell-specific expression of HIF isoforms.
HIF-2 expression in juxtaglomerular cells is presumably highly relevant for the polycythemic phenotype, because multiple levels of evidence indicate that HIF-2 rather than HIF-1 regulates EPO. Cells physiologically producing EPO express HIF-2 rather than HIF-1.38,40,42 In EPO-expressing cell lines expressing both HIF isoforms, HIF-2 deletion rather than HIF-1 deletion inhibits EPO,43 and also, in in vivo models, including models of Vhl knockout, EPO synthesis is sensitive to HIF-2 deletion rather than HIF-1 deletion.40,44
Although the evidence for the role of HIF-2 in EPO production in renin-producing cells deficient for Vhl is compelling, one of the limitations of our study is that we cannot yet distinguish between HIF-dependent and -independent effects in inhibiting the development and expansion of renin-producing cells. Previous studies have consistently shown that developmental abnormalities caused by Vhl deficiency are partially reversible in double knockout animals with HIF deletion.28,45,46 Nevertheless, pVHL exerts important HIF-independent effects on, for example, matrix metabolism and cytoskeletal function.10,47
Our study also sheds some light on the role of the functional consequences of disturbed development of renin-producing cells, which were overall surprisingly minor. Renal vascular development was not disturbed, indicating that residual renin expression was sufficient for normal development of the glomerular tree. Also, we did not observe an effect on BP, but the increase in hematocrit and blood viscosity might have counterbalanced a BP-lowering effect of reduced renin synthesis.
In conclusion, we have identified renin-producing cells as a new cell type in which pVHL suppresses EPO synthesis and proposes the ability of these specialized endocrine cells to switch their functional capacity from renin to EPO production.
Concise Methods
Animals
The conditional knockout mice (Vhl −/−REN) were developed from two mouse strains: mice with loxP-flanked Vhl alleles48 and mice with targeted insertion of Cre recombinase into the renin-1d locus.16 Animals were kept under standard housing conditions with a 12:12-hour light:dark cycle and food (NaCl 0.6%; Ssniff, Soest, Germany) and water ad libitum. Stimulation of the RAS was induced by feeding a low-salt diet (NaCl 0.02%; Ssniff) combined with the ACE inhibitor enalapril (on average, 10 mg/kg per day) added to the drinking water for 3 weeks. Furthermore, mice were generated to examine the lineage tracing of renin-expressing cells and their descendants: Vhl −/−REN;Rosa26-LacZ (Vhl knockout) and Vhl +/+REN;Rosa26-LacZ (Vhl wt) mice. From the Rosa26-LacZ–positive mice, only 1 mouse was homozygous for the loxP-flanked Vhl allele (Vhl fl/fl), whereas 48 mice were heterozygous (Vhl fl/+). Animals used in the study were males between 8 and 12 wk of age. All animal experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the local ethics committee.
BP Measurements
Systolic BP was measured in conscious mice noninvasively by tail-cuff manometry. Mice were put in a steel cover on a 30°C prewarmed platform and habituated to the experimental procedure for 5 subsequent days. BP was determined on 7 subsequent days.
Determination of Hematocrit Levels and Plasma Renin Concentration
Blood samples (75 μl) were taken from the tail vein into capillary tubes containing 1 μl 125 mM EDTA to prevent clotting. After centrifugation (13,000×g, 7 minutes, room temperature), hematocrit values were calculated, and renin concentration in plasma samples was measured on the basis of the generation of angiotensin-I after the addition of plasma from bilaterally nephrectomized male rats as excess renin substrate. The generated angiotensin-I (nanograms per milliliter per hour) was determined by radioimmunoassay (Byk & DiaSorin Diagnostics, Dietzenbach, Germany).49
EPO ELISA
EPO protein in blood plasma was determined using the Quantikine Mouse EPO ELISA kit (R&D Systems) according to manufacturer’s protocol.
Red Blood Cell Count
Blood samples were diluted in 0.9% NaCl, and erythrocytes were counted manually using the Neubauer counting chamber.
Determination of the Inner Diameter of Afferent Arterioles
For each genotype and mouse (Vhl fl/fl and Vhl −/−REN, each n=6), the inner diameter of 50 arterioles at their entrance into the glomerular capillary network was determined using AxioVision LE software (Zeiss).
Magnetic Isolation of Glomeruli
The procedure of isolating glomeruli was very similar to the procedure described by Takemoto et al.50 Mice were anesthetized by an intraperitoneal injection of 12 mg/kg xylazine and 80 mg/kg ketamine⋅HCl and perfused with 2×109 Dynabeads M-450 EPOXY (diluted in 20 ml PBS) through the abdominal aorta. The kidneys were removed, and the left kidney was cut in half. One-half was snap-frozen in liquid nitrogen for subsequent mRNA analysis. One-half of the left kidney plus the right kidney were cut into small pieces of approximately 0.75 mm3 and digested in collagenase A (1 mg/ml in HBSS) at 37°C for 20 minutes. After digestion, tissue was gently pressed through a 100-μm cell strainer using a flattened pestle. The filtered cells were then transferred in a falcon tube, which was placed at a magnet (BD Becton Dickinson GmbH, Heidelberg, Germany). In this way, glomeruli containing Dynabeads could be captured. After several washing procedures, glomeruli were snap-frozen in liquid nitrogen for mRNA analysis.
Determination of mRNA Expression by Real-Time PCR
Total RNA was isolated from kidneys, glomeruli, testicles, and adrenal glands as described by Chomczynski and Sacchi51 and quantified by a photometer. The cDNA was synthesized by MMLV reverse transcription (Superscript; Invitrogen) and amplified with following primers: 5′-atgaagggggtgtctgtgggg-3′ (sense) and 5′-atgtcggggagggtgggcacct-3′ (antisense) for renin; 5′-aatggaggtggaagaacagg-3′ (sense) and 5′-acccgaagcagtgaagtga-3′ (antisense) for EPO; 5′-actgggacgacatggaaaag-3′ (sense) and 5′-catctccagagtccagcaca-3′ (antisense) for α-SMA; 5′-ggagtcatcaagactgacatg-3′ (sense) and 5′-cagttggctgtctgtgaagtc-3′ (antisense) for SM22; 5′-acccatggactctgcctatg-3′ (sense) and 5′-aggggtattgctcagtggtg-3′ (antisense) for myocardin; 5′-ctggctgtcaatgctctccac-3′ (sense) and 5′-ttgtcactgcggcgctcatc-3′ (antisense) for AQP2; 5′-caccagggctgccatttgca-3′ (sense) and 5′-gctccacccttcaagtgg-3′ (antisense) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). For quantification of mRNA expression, real-time PCR was performed using a Light Cycler Instrument and the LightCycler 480 SYBR Green I Master Kit (Roche Diagnostics) and GAPDH as a control.
LacZ Staining
Kidneys from Vhl −/−REN;Rosa26-LacZ (Vhl knockout in renin-expressing cells) and Vhl +/+REN;Rosa26-LacZ (wt) mice were perfusion-fixed with 3% paraformaldehyde, embedded in Tissue-Tec, frozen on dry ice, and sectioned (5 μm) on a cryostat for LacZ staining as described previously.52 Sections were rinsed three times in LacZ washing buffer (0.1 M phosphate buffer, pH 7.4, 1.25 mM MgCl2, 5 mM EGTA, 0.02% Nonidet P-40, and 0.01% sodium deoxycholate) and stained in LacZ substrate buffer (LacZ washing buffer supplemented with 0.25 mg/ml X-Gal, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide) overnight at 37°C. After washing in PBS, sections were mounted with DakoCytomation Glycergel mounting medium and viewed with an Axiovert Microscope (Zeiss, Jena, Germany).
Immunohistochemistry of Renin and α-SMA
As described previously,53 kidneys were perfusion-fixed with 3% paraformaldehyde, dehydrated, and embedded in paraffin. Immunolabeling was performed on 5-μm sagittal paraffin sections. After blocking with 10% horse serum and 1% BSA in PBS, sections were incubated with chicken anti-mouse renin (generated by Davids Immunotechnologie, Regensburg, Germany) and α-SMA (Beckman Coulter, Immunotech, Marseille, France) antibodies overnight at 4°C followed by incubation with Cy2 and TRITC secondary antibodies. Slices were mounted with DakoCytomation Glycergel mounting medium and viewed with an Axiovert Microscope.
Three-Dimensional Reconstruction
Digitalization of the antibody-stained serial sections (100 sections) was performed using an AxioCam MRm camera (Zeiss) mounted on an Axiovert 200M microscope (Zeiss) with fluorescence filters for renin and α-SMA (TRITC, filter set 43; Cy2, filter set 38 HE; Zeiss). After acquisition, a stack of equal-size images was built using the graphic tool ImageJ (Wayne Rasband; National Institutes of Health, Bethesda, MD). The equalized data were then imported into the Amira 4.1 visualization software (Mercury Computer Systems, Chelmsford, MA) on a Dell Precision 690 computer system (Dell) and split into the renin and α-SMA channels. After this step, the renin and α-SMA channels were aligned. In the segmentation step, the α-SMA and renin datasets served as a scaffold and were spanned manually or automatically using grayscale values. Matrices, volume surfaces, and statistics were generated from these segments.53
Immunohistochemistry of HIF-1α and HIF-2α
Staining of kidney sections for both HIFs was performed as described by Schley et al.41 Paraffin-embedded kidney sections (4-µm thick) were incubated with the following primary antibodies: mouse anti-human HIF-1α (α67; Novus Biologicals) and rabbit anti-mouse Hif-2α (PM9).37 Detection of primary antibodies was performed by using biotinylated secondary anti-mouse or anti-rabbit antibodies and a catalyzed signal amplification system (Dako, Hamburg, Germany) based on the streptavidin-biotin-peroxidase reaction according to the instructions provided by the manufacturer.
In Situ Hybridization
Distribution of EPO and renin mRNA synthesis was studied by nonradioactive mRNA in situ hybridization using a probe covering base pairs 312–602 of the rat EPO cDNA (NM_0171001)38 and a 300-bp PSTI/KPNI fragment of rat renin cDNA subcloned in PGEM3 vector for renin.54 Digoxygenin-labeled UTP and T7 or SP6 RNA polymerase (Roche Applied Science, Mannheim, Germany) were used to generate sense and antisense RNA probes. In situ hybridization was performed as previously described,55,56 and hybridized RNA probes were visualized using 4-nitroblue tetrazolium chloride. Sections were examined using a Leica DMRB microscope equipped with an interference contrast module (Leica, Wetzlar, Germany). Bright-field images were acquired using a Moticam 2300 digital camera and the Motic images 2.0 imaging system (Motic, Xiamen, China). EPO in situ hybridization and subsequent Cy3 renin immunofluorescence was used to determine the extent of colocalization of EPO mRNA and renin protein. Fluorescence signal was evaluated using a Zeiss exciter 5 confocal microscope (Zeiss, Jena, Germany).
Statistical Analyses
All data are presented as means ± SEM. Differences between groups were analyzed by t test. P values less than 0.05 were considered statistically significant.
Disclosures
None.
Acknowledgments
The expert technical assistance provided by Regine Volkmann, Andrea Dengler, and Katharina Gerl is gratefully acknowledged.
This work was financially supported by the German Research Foundation (DFG; SFB 699).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC: Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K: Angiotensinogen-deficient mice with hypotension. J Biol Chem 269: 31334–31337, 1994 [PubMed] [Google Scholar]
- 3.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O: Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gubler MC, Antignac C: Renin-angiotensin system in kidney development: Renal tubular dysgenesis. Kidney Int 77: 400–406, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Martinovic J, Benachi A, Laurent N, Daikha-Dahmane F, Gubler MC: Fetal toxic effects and angiotensin-II-receptor antagonists. Lancet 358: 241–242, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Pryde PG, Sedman AB, Nugent CE, Barr M, Jr: Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol 3: 1575–1582, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Graham PC, Stewart HV, Downie I, Lindop GB: The distribution of renin-containing cells in kidneys with renal artery stenosis—an immunocytochemical study. Histopathology 16: 347–355, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y: Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney. An ultrastructural-cytochemical and autoradiographic study. Am J Pathol 87: 581–602, 1977 [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell PH, Ratcliffe PJ: Oxygen sensors and angiogenesis. Semin Cell Dev Biol 13: 29–37, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Kapitsinou PP, Haase VH: The VHL tumor suppressor and HIF: Insights from genetic studies in mice. Cell Death Differ 15: 650–659, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D: HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15: 2445–2453, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF: Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci U S A 83: 7552–7556, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekker M, Tronik D, Rougeon F: Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci U S A 86: 5155–5158, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field LJ, McGowan RA, Dickinson DP, Gross KW: Tissue and gene specificity of mouse renin expression. Hypertension 6: 597–603, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA: Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Castrop H, Oppermann M, Weiss Y, Huang Y, Mizel D, Lu H, Germain S, Schweda F, Theilig F, Bachmann S, Briggs J, Kurtz A, Schnermann J: Reporter gene recombination in juxtaglomerular granular and collecting duct cells by human renin promoter-Cre recombinase transgene. Physiol Genomics 25: 277–285, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, Hurley MI, Black TA, Kane CM, Pan L, Pruitt SC, Gross KW: Expression of a renin/GFP transgene in mouse embryonic, extra-embryonic, and adult tissues. Physiol Genomics 4: 75–81, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Taugner R, Hackenthal E, Nobiling R, Harlacher M, Reb G: The distribution of renin in the different segments of the renal arterial tree: Immunocytochemical investigation in the mouse kidney. Histochemistry 73: 75–88, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Machura K, Steppan D, Neubauer B, Alenina N, Coffman TM, Facemire CS, Hilgers KF, Eckardt KU, Wagner C, Kurtz A: Developmental renin expression in mice with a defective renin-angiotensin system. Am J Physiol Renal Physiol 297: F1371–F1380, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Haase VH: Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adryan B, Decker HJ, Papas TS, Hsu T: Tracheal development and the von Hippel-Lindau tumor suppressor homolog in Drosophila. Oncogene 19: 2803–2811, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM: Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A 94: 9102–9107, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang N, Mack F, Haase VH, Simon MC, Johnson RS: pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol 26: 2519–2530, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim WY, Kaelin WG: Role of VHL gene mutation in human cancer. J Clin Oncol 22: 4991–5004, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Weidemann A, Johnson RS: Nonrenal regulation of EPO synthesis. Kidney Int 75: 682–688, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS: Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 133: 223–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, Giaccia AJ: The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149: 63–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fyhrquist F, Rosenlöf K, Grönhagen-Riska C, Hortling L, Tikkanen I: Is renin substrate an erythropoietin precursor? Nature 308: 649–652, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Obara N, Suzuki N, Kim K, Nagasawa T, Imagawa S, Yamamoto M: Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 111: 5223–5232, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Sequeira Lopez ML, Gomez RA: Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koury ST, Bondurant MC, Koury MJ: Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 71: 524–527, 1988 [PubMed] [Google Scholar]
- 35.Bachmann S, Le Hir M, Eckardt KU: Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ: Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, Flippin LA, Arend M, Klaus SJ, Bachmann S: Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int 77: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Theilig F, Enke AK, Scolari B, Polzin D, Bachmann S, Koesters R: Tubular deficiency of von Hippel-Lindau attenuates renal disease progression in anti-GBM glomerulonephritis. Am J Pathol 179: 2177–2188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH: Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schley G, Klanke B, Schödel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C: Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22: 2004–2015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chavez JC, Baranova O, Lin J, Pichiule P: The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 26: 9471–9481, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU: Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS: The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest 119: 3373–3383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH: Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 29: 4527–4538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH: Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene 27: 5354–5358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaelin WG, Jr: The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8: 865–873, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Haase VH, Glickman JN, Socolovsky M, Jaenisch R: Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A 98: 1583–1588, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweda F, Wagner C, Krämer BK, Schnermann J, Kurtz A: Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol 284: F770–F777, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Kurt B, Kurtz L, Sequeira-Lopez ML, Gomez RA, Willecke K, Wagner C, Kurtz A: Reciprocal expression of connexin 40 and 45 during phenotypical changes in renin-secreting cells. Am J Physiol Renal Physiol 300: F743–F748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C: Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Djavidani B, Sander M, Kreutz R, Zeh K, Bader M, Mellon SH, Vecsei P, Peters J, Ganten D: Chronic dexamethasone treatment suppresses hypertension development in the transgenic rat TGR(mREN2)27. J Hypertens 13: 637–645, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S: Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R694–R700, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S: Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005 [DOI] [PubMed] [Google Scholar]