Abstract
Knowledge of the stages that compose the seminiferous epithelium cycle (SEC) and determination of the duration of spermatogenic processes are fundamental for the accurate quantification of the dynamics of spermatogenesis. The aim of this study was to characterize the stages that compose the SEC of the bat Sturnira lilium, including evaluation of the average frequency of each of these stages throughout the year and calculation of the duration of the spermatogenic process. An ultrastructural characterization of the formation of the acrosomal cap was also performed. Testicular fragments were processed for morphological and immunohistochemical analysis as well as ultrastructural analysis using transmission electron microscopy. According to the tubular morphology method, the SEC in S. lilium is divided into eight stages, following the pattern found in other mammals. Primary spermatocytes were found at zygotene in stage 1 of the cycle. There was no variation in frequency of each of the stages over the seasons, with stage 1 being the most frequent, and stage 7 the least frequent. The duration of one seminiferous epithelium cycle was 3.45 days, and approximately 15.52 days were required for the development of sperm from spermatogonia. Ultrastructural characterization allowed the formation of the acrosomal cap in round spermatids to be monitored. In conclusion, the stages that compose the SEC in S. lilium are generally similar to those described for other mammals, but the duration of the spermatogenic process is shorter than previously recorded for mammals. The presence of primary spermatocytes at zygotene in stage 1 of the cycle is probably due to the longer duration of this stage.
Keywords: bromodeoxiuridine, reproduction in bats, spermatid, spermatocyte, spermatogonia
Introduction
The cells that compose the germinal epithelium are strictly organized in a series of cell associations, or stages (Leblond & Clermont, 1952). Knowledge of the stages that compose the seminiferous epithelium cycle (SEC) and determination of their frequency, associated with testicular morphometry, are fundamental in measuring and understanding the dynamics of spermatogenesis (Roosen-Runge & Giesel, 1950; Courot et al. 1970; Ortavant et al. 1977). The relative frequency of each phase of the SEC differs among species but is relatively constant between individuals of the same species (Amann, 1962; Courot et al. 1970; Clermont, 1972; Ortavant et al. 1977). The same is observed for the duration of the spermatogenic process, which requires about 4.5 cycles and lasts from 30 to 75 days in mammals (Clermont, 1972; França & Russell, 1998; Hess & França, 2007).
Over 1100 species of bats are currently known (Reis et al. 2007) and there are no data on the length of the SEC in any of these species yet. Among the small amount of existing information on the organization of the seminiferous epithelium and the frequency of the stages that compose the SEC in bats, Beguelini et al. (2009) showed that the organization of the stages in these animals appears to follow the pattern seen in other mammals.
Frugivorous bats have great ecological importance due to their role in regulating tropical ecosystems, such as the Atlantic Forest, where they are crucial for the dynamics and regeneration of tropical forests, including the establishment of pioneer species (Marinho-Filho & Vasconcellos-Neto, 1994; Cole & Wilson, 1996; Mikich & Bianconi, 2005; Mello et al. 2008). Abiotic factors such as temperature and photoperiod, have a great influence on the reproductive cycle of bats (Heideman et al. 1992; Mello et al. 2009). It has been demonstrated that the reproductive cycle of the bat Sturnira lilium can be directly influenced by factors such as temperature and food availability (Bronson, 1985; Mello et al. 2009). This species has great ecological importance for its role in the pollination and constitutes important seed dispersers of various tropical plants (Marinho-Filho & Vasconcellos-Neto, 1994; Vieira & Carvalho-Okano, 1996; Sazima et al. 2003).
Given the great importance of S. lilium for forest maintenance and recuperation (Mikich & Bianconi, 2005), and the lack of information about its reproductive cycle, specimens were collected in the different annual climatic seasons to enable characterization of the stages of the SEC in S. lilium, with an evaluation of the average frequency of each of these stages throughout the seasons, together with the duration of the SEC and an ultrastructural characterization of formation of the acrosomal cap in spermatids. Such information is essential for a better understanding of the dynamics and characterization of the spermatogenic process in this species, and is important for future work on the duration of the SEC in bats.
Material and methods
Study area
The animals were collected in the city of Viçosa, Minas Gerais State, Brazil (20º45′14″ S, 42º52′53″ W), which has an average altitude of 648.74 m. It is a mountainous region in the Atlantic Forest biome, with a Cwa-type climate (mesothermic, humid with rainy summers and dry winters; Golfari, 1975). The captures were authorized by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA-MG-139/06-NUFAS-MG) and by the State Institute of Forests in Minas Gerais (IEF-MG-121/06). The present study was evaluated and approved by the Ethics Commission of the Federal University of Viçosa, document number 93/011. The annual averages recorded during the experimental period (2009–2010) for rainfall, temperature and relative humidity were, respectively, 0.91 mm, 18 °C and 78% in the dry season (April–September) and 7.20 mm, 23 °C and 79% in the rainy season (October–March; Meteorological Station, Department of Agricultural Engineering, Federal University of Viçosa).
Animals and tissue preparation
Fourteen specimens of adult male S. lilium were captured during 2010 and 2011, in the dry season (n = 7) and in the rainy season (n = 7). The animals were captured at nightfall, using mist nets near their roosts. Adult animals were identified based on the fusion of the epiphyseal cartilage of the fourth finger, at the metacarpal-phalangeal junction, according to Kunz & Anthony (1982).
The bats were placed in cages that were protected from light. A diet composed of fruits (papaya or banana) and water ad libitum was offered to the animals from the time of their capture up to their euthanasia in the laboratory, which occurred on the day after the night of the capture. The animals were weighted and anesthetized with sodium pentobarbital (Nembutal®) at a concentration of 40 mg kg−1 intraperitoneally, followed by euthanasia with a saturated solution of potassium chloride, and their testes were removed.
For morphological analysis under light microscopy, testes were fixed by immersion in Karnovsky solution (Karnovsky, 1965) for 24 h and transferred to 70% ethanol. Testicular fragments were dehydrated in an increasing ethanol series, and embedded in glycol methacrylate (Historesin©, Leica Microsystems, Nussloch, Germany) for morphometric analysis or in Paraplast® (Sigma-Aldrich, St. Louis, MO, USA) for determining the duration of SEC. The fragments embedded in resin were sectioned into 3-μm-thick slices in a sequential manner, using a rotating microtome (Leica RM2155©, Leica Microsystems), with a distance of 40 μm between each section. Preparations were stained with 1% toluidine blue/sodium borate (©Merck, Darmstadt, Germany).
For ultrastructural analysis, testicular fragments were fixed in Karnovsky solution for 1 h and then transferred to 2.5% glutaraldehyde solution (EMS, Hatfield, PA, USA) for 23 h. After washing in phosphate buffer (PBS) the samples were post-fixed in 1% osmium tetroxide (EMS) in the same buffer for 2 h. Dehydration was performed in ethanol and acetone, after which the samples were place in Epon 812 resin (EMS). Ultrathin sections were contrasted with 3% uranyl acetate (EMS) and lead citrate 3% (EMS) and observed in a transmission electron microscope (Jeol 1011, Munich, Germany), at the Center for Microscopy and Microanalysis, Federal University of Viçosa.
Stages of the seminiferous epithelium cycle and their relative frequencies
The stages of the SEC were characterized using the tubular morphology method (Berndtson, 1977). Eight stages were characterized based on the shape and location of the nucleus of spermatids and spermatocytes and the occurrence of figures of meiotic division. The relative frequency of the stages described was calculated based on the identification and occurrence of each stage in 200 cross-sections of seminiferous tubules in each animal.
Duration of the SEC
To calculate the duration of the SEC, two specimens of S. lilium were injected with 0.1 mL of commercial bromodeoxiuridine intratesticularly (Invitrogen BrdU Staining Kit Camarillo, USA). The duration of spermatogenesis can be determined via BrdU injection, since this substance is incorporated into the nucleus of germ cells that are synthesizing DNA at the time of application. The animals were euthanized after 1 h and 1 day after application of BrdU. After fixation in Karnovsky solution (Karnovsky, 1965), the testes were dehydrated in a series of increasing concentrations of ethanol and subsequently cleared in three consecutive baths of xylene before being embedded in Paraplast® (Sigma-Aldrich). Detection of BrdU was done by staining sections of 4 μm thickness with a monoclonal antibody. For this, sections were deparaffinized and rehydrated, washed in PBS, and peroxidase activity endogenously blocked with H2O2 and methanol. Then, the slides were washed in PBS for enzymatic pretreatment that was performed by incubation in trypsin solution. After washing in distilled water the slides were incubated with denaturing solution, followed by washing in PBS and incubation in blocking solution, which was not washed. Then, the material was incubated with biotinylated monoclonal mouse anti-BrdU (BrdU, Invitrogen), which was revealed with a streptavidin-peroxidase reaction (BrdU, Invitrogen). The estimation of the duration of the SEC was performed by observing the most advanced cell in the epithelium; the frequency of the stages that had been gone through after treatment with BrdU to its detection was then calculated. The frequency of the stages completed corresponds to the time spent, and the duration of a cycle of the seminiferous epithelium was calculated.
Statistical analysis
The results were subjected to descriptive statistical analysis, and the averages of the frequency of stages between the dry and rainy seasons were compared by Student's t-test for independent samples (statistica). The results were expressed as mean ± SD, with a significance level of 5% (P < 0.05).
Results
According to the tubular morphology method, eight stages were described in the S. lilium testis. The distribution of these stages is presented segmentally along the length of the seminiferous tubule, and there is usually therefore only one stage per tubular cross-section. Besides the Sertoli cells and spermatogonia, which were present at all stages, different generations of germ cells were observed in cross-sections of the seminiferous epithelium and were compartmentalized. Descriptions of the stages are given below:
Stage 1
Primary spermatocytes of three types were found to be present at this stage, in the pre-leptotene to leptotene or already in zygotene phase, close to the tunica propria, and at pachytene, in an intermediate location in the epithelium. Round spermatids were seen near the tubular lumen, distributed in 3 or 4 layers of cells (Fig. 1a).
Fig. 1.
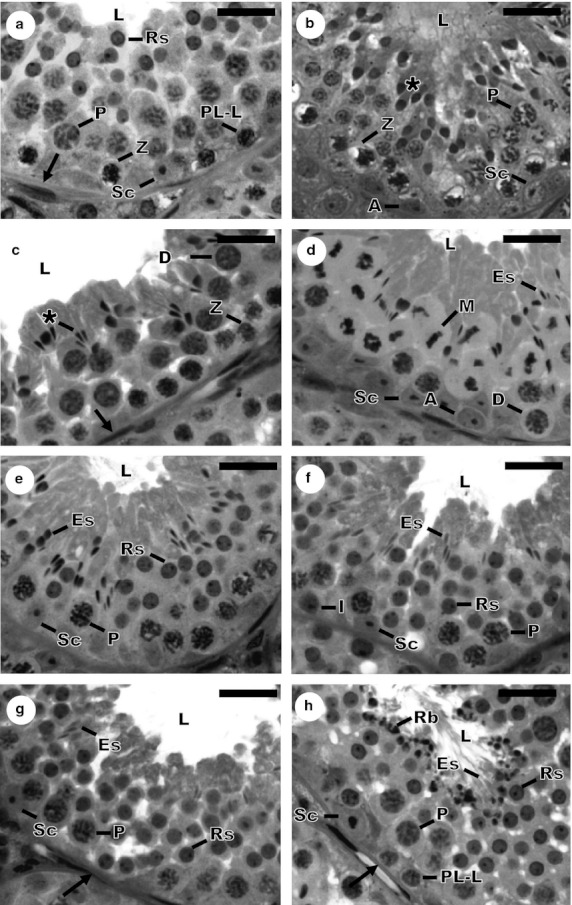
Histological cross-sections of seminiferous tubules showing the eight stages of the seminiferous epithelium cycle in Sturnira lilium, according to the tubular morphology method. (a) Stage 1; (b) Stage 2; (c) Stage 3; (d) Stage 4; (e) Stage 5; (f) Stage 6; (g) Stage 7; (h) Stage 8. Sc, Sertoli cell; A, type A spermatogonia; I, intermediate spermatogonia; PL-L, primary spermatocyte in Pre-leptotene to leptotene; Z, primary spermatocyte in zygotene; P, primary spermatocyte in pachytene; D, primary spermatocyte in diplotene; M, metaphase figure; Rs, round spermatid; *, elongating spermatid; Es, elongated spermatid; →, tunica propria; L, lumen of the seminiferous tubule; Rb, residual body. Scale bars: 10 μm.
Stage 2
At this stage, primary spermatocytes at zygotene near the basal lamina and primary spermatocytes at pachytene in the intermediate region of the epithelium were observed. The most remarkable aspect of this stage was the beginning of elongation of the nuclei of round spermatids (Fig. 1b).
Stage 3
Two generations of primary spermatocytes were present at this stage, these being the spermatocytes at zygotene and in the transition from pachytene to diplotene, which have a characteristically large nucleus. The nuclei of spermatids continued to elongate and were grouped into bunches, deeply inserted into the seminiferous epithelium, with their heads oriented towards the basal environment (Fig. 1c).
Stage 4
Figures of meiotic metaphase are typically observed in this stage, characterizing the transition from primary spermatocytes in diplotene to secondary spermatocytes, and from these to round spermatids. As in the preceding stage, bunches of spermatids were observed, now even more elongated (Fig. 1d).
Stage 5
At this stage, there was only one generation of primary spermatocytes in the transition from zygotene to pachytene, since the generation of spermatocytes later originated a generation of round spermatids. Thus, there were two distinct spermatid generations from this stage to stage 8. The generation of elongated spermatids is found in compact bunches more deeply embedded in the seminiferous epithelium, in the crypts of the Sertoli cells (Fig. 1e).
Stage 6
In this stage, spermatogonia are observed, originating from type A spermatogonia but with smaller and darker nuclei. The present generation of primary spermatocytes at this stage is in pachytene, and the bunches of spermatids have become more superficial in the epithelium compared with the previous stage. All generations of germ cells were very similar to those observed in the preceding stage (Fig. 1f).
Stage 7
Type B spermatogonia were found at this stage, the nuclei of which had an ovoid or rounded shape. Primary spermatocytes in pachytene, round and elongated spermatids were also present. The bunched groups of the ES were dissociated from one another and located close to the tubular lumen (Fig. 1g).
Stage 8
At this stage, the type B spermatogonia originated the primary spermatocytes in pre/leptotene. Primary spermatocytes in pachytene and round spermatids were also observed, present in the mid-region of the seminiferous epithelium. The most characteristic feature of this stage was the location of elongated spermatids in close proximity to the tubular lumen, at a developmental stage ready to be delivered from the seminiferous epithelium. Their tails were well exposed and there were also residual bodies lying in the luminal border of the seminiferous epithelium (Fig. 1h).
There were no differences in the relative frequency of each of the eight stages between the seasons: stage 1 was the most frequent, with an estimated duration of 3.3 days, and stage 7 was the least frequent, with an estimated duration of 0.9 days. The pre-meiotic, meiotic and post-meiotic phases of spermatogenesis of S. lilium represented 46.1, 7.3 and 46.6% of the cycle, respectively (Fig. 2).
Fig. 2.
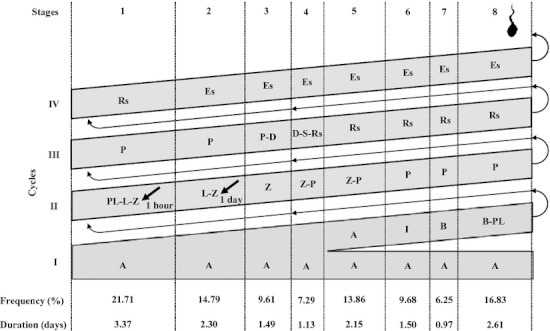
Diagram of the spermatogenesis process in Sturnira lilium, with the mean frequency (%) and the duration in days of each one of the eight stages of the seminiferous epithelium cycle. Each line corresponds to a generation of spermatogenic cells and each column corresponds to one stage. The Roman numerals indicate the spermatogenic cycles. A, type A spermatogonia; I, intermediate spermatogonia; B, type B spermatogonia; PL-L, primary spermatocyte, in preleptotene to leptotene; Z, in zygotene; P, in pachytene; D, in diplotene; S, secondary spermatocyte; Rs, round spermatid; Es, elongated spermatid. The labeled germinative cell, which was more advanced (arrow) at the eight stages of the cycle, 1 day after treatment with bromodeoxiuridine, was the primary spermatocyte in leptotene to zygotene, at stage 2.
The stained cells, which were most advanced in the seminiferous epithelium after 1 day of BrdU application, were primary spermatocytes in transition from leptotene to zygotene at stage 2 of the seminiferous epithelium cycle (Fig. 3a). At the beginning of stage 3, the cells were no longer stained and showed no further marking (Fig. 3b). Spermatogonia, which were present at all stages of the SEC, were BrdU-positive at all stages of the cycle due to their constant mitotic activity.
Fig. 3.
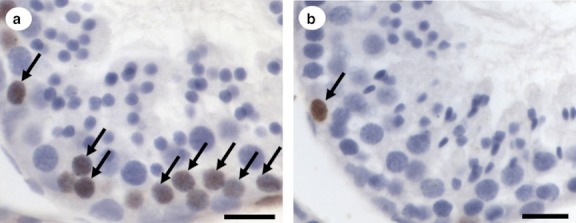
The most advanced labelled germ cell types found after intratesticular bromodeoxiuridine injections in Sturnira lilium. Leptotene to Zygotene cells in stage 2 (a) and type A spermatogonia in stage 3 (b) of the seminiferous epithelium cycle; arrows indicate marking with bromodeoxiuridine. Scale bar: 20 μm.
Within 1 day, there was a progression of 29% (average frequency of the stages 1 until the middle of stage 2) in the SEC, a cycle was therefore 3.45 days on average. Considering that 4.5 seminiferous epithelium cycles are necessary for all spermatogenic processes to be completed, the total length of spermatogenesis in S. lilium was estimated as 15.52 days.
Figure 4 illustrates the nuclei of different types of spermatids, from the initial round phase (Fig. 4a) in the initial stage (Fig. 4b–d) and intermediate stage (Fig. 4e–g) of formation of the acrosome, to the elongated spermatid in which the acrosomal cap covers much of the nuclear surface (Fig. 4h,i). The head line of microtubules and the early stages of formation of the flagellum (Fig. 4e,h) can be seen. The spermatogenic phase shown corresponds to the second half of the third spermatogenic cycle, when the round spermatids appear, until the end of the fourth cycle, when the elongated spermatids appear, culminating in the release of spermatozoa (Fig. 2).
Fig. 4.
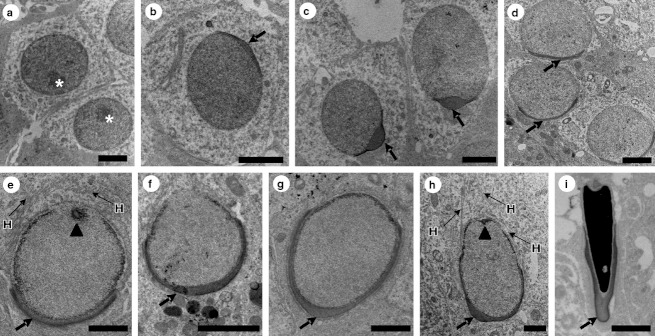
Nuclear ultrastructure of round and elongated spermatids and acrosome formation in Sturnira lilium. (a) Round spermatid without acrosome at stage 5; (b–d) round spermatids with different degrees of nuclear occupation by acrosome; (e–h) elongating spermatids with different degrees of nuclear occupation by acrosome; (i) elongated spermatid with complete formation of acrosome; *, nucleolus; →, acrosomal cap; H, head line of microtubules; ▴, beginning of flagellum formation. Scale bars: (a–d) 2 μm; (e) 1 μm; (f) 2 μm; (g–i) 1μm.
A single generation of round spermatids is present at stage 1 of the SEC. At this stage, the acrosomal cap covering part of the nuclear surface was visible (Fig. 4d). From stage 2 this generation of spermatids begins to elongate, and the acrosomal cap gradually extends under the nuclear surface (Fig. 4e–h). From stage 3 (Fig. 4h) the same generation of elongated spermatids will gradually reach its maximum degree of elongation and nuclear condensation, as observed at stage 8 (Fig. 4i). The second division of meiosis, which occurs in stage 4, produces from the stage 5 two well defined generations of spermatids, comprising the initial round spermatids, which do not have an acrosomal cap (Fig. 4a), and the completely elongated spermatid (Fig. 4i). These two generations of spermatids are present until stage 8, when spermiation occurs.
Discussion
The results of this study are of great importance as they provide, for the first time, despite the large number of bats known, information on the duration of the seminiferous epithelium cycle of a bat species.
Morphologically, the seminiferous epithelium of S. lilium follows the pattern observed in other bat species (Singwi & Lall, 1983; Saidapur & Patil, 1992; Morigaki et al. 2001; Beguelini et al. 2009; Oliveira et al. 2009), and in other mammal species (Paula et al. 1999; Lloyd et al. 2008; Soares et al. 2009; Balarini et al. 2011; Costa et al. 2011), with distinct arrangements between the different generations of germ cells. The eight stages of the SEC, designated in S. lilium, according to the tubular morphology method, also followed the pattern found in other bats (Beguelini et al. 2009; Oliveira et al. 2009), as well as in other non-primate mammals (Paula et al. 1999; Bittencourt et al. 2007; Lloyd et al. 2008; Balarini et al. 2011; Costa et al. 2011).
As the relative frequency of the stages of the SEC is constant between individuals of the same species (Amann, 1962; Courot et al. 1970; Clermont, 1972; Ortavant et al. 1977), this study confirmed the absence of variation in the average frequency of each stage between different seasons. Stage 1 has been reported as one of the most frequent, and stage 7 as one of the least frequent (Bittencourt et al. 2007; Lloyd et al. 2008; Costa et al. 2010a, 2011; Balarini et al. 2011). The higher frequency of a certain stage indicates that this has a longer duration within the SEC. In S. lilium, the higher frequency of stage 1 allowed the appearance of primary spermatocytes at zygotene, whereas in most species already studied this cell type appears from stage 2 (Paula et al. 1999; Bittencourt et al. 2004; Beguelini et al. 2009; Costa et al. 2010a; Balarini et al. 2011).
Due to the large variations in frequency of stages between different species, allowing comparative analyses, Courot et al. (1970) suggested the grouping of frequencies into pre-meiotic (stages 1–3), meiotic (stage 4) and post-meiotic phases (stages 5–8). In many mammals, the pre- or post-meiotic phases had a longer or shorter duration in comparison with each other (Nakai et al. 2004; Guião-Leite et al. 2006; Costa et al. 2008, 2010a,b, 2011). Beguelini et al. (2009), studying the frequencies of stages among different bat species, found the pre-meiotic phases shorter than the post-meiotic phases. However, in S. lilium such phases had similar frequencies within the SEC, as observed in the oncilla (Balarini et al. 2011).
Like the relative frequency of each stage, the duration of spermatogenesis is a species-specific constant, since both are controlled by the genotype of germ cells (França et al. 1998). A spermatogenic cycle of 3.45 days in S. lilium represents the shortest duration recorded in mammals. The shortest previously recorded duration was for the rodent Cletheriomys glareolus, at 6.7 days, and the longest was for the opossum Didelphis albiventris, at 17.3 days (Grocock & Clark, 1976; Queiroz & Nogueira, 1992). The average duration recorded for mammals ranges from 7 to 14 days (França & Russell, 1998). Similarly, the total duration of spermatogenesis in S. lilium (15.52 days) was far below the average previously recorded for mammals, ranging from 30 to 75 days (França & Russell, 1998; Leal & França, 2006; Hess & França, 2007; Soares et al. 2009).
The ultrastructural study of spermatids demonstrated the formation of the acrosomal cap. The acrosome is formed from pro-acrosomal vesicles of the Golgi apparatus, which coalesce and adhere to the nuclear surface, occupying about two-thirds of the anterior nucleus in mature spermatozoa in mammals in general (Bishop & Smiles, 1963; Christensen & Fawcett, 1966; Holt & Moore, 1984). Head lines were observed in the cytoplasm of elongated spermatids, these being structures formed by microtubules and actin filaments. These are routes of intracellular transport, including the transport of the pro-acrosomal vesicles, and are responsible for determining the polarity of the spermatids. They also participate in elongation and determining the shape of the nucleus of spermatids during spermiogenesis (Kierszenbaum & Tres, 2004; Kierszenbaum et al. 2011). The early stages of the formation of the flagellum were also apparent in the region that will originate the sperm tail. The cytoskeletal organization of S. lilium is typical of the cytoplasm of elongated spermatids, and the characteristics of the spermatids were similar to those described in other species of bats (Beguelini et al. 2011, 2012).
Conclusions
The organization of stages that compose the SEC in S. lilium, and the characteristics of the seminiferous epithelium, are generally similar to those described for other mammals. However, the duration of each spermatogenic cycle, and the total duration of spermatogenesis in S. lilium, were the lowest ever recorded for mammals.
Acknowledgments
To the CAPES Foundation, Ministry of Education of Brazil, for the scholarships provided.
Conflict of interest
The authors have no conflict of interest.
Authors' contributions
D. B. Morais: animal collection, histological processing, immunohistochemical and ultrastructural analysis, data analysis, interpretation and manuscript writing. T. A. R. Paula: data collection and interpretation. M. S. Barros: animal collection. M. K. Balarini: histological processing and immunohistochemical analysis. M. B. D. Freitas: manuscript revision. S. L. P. da Matta: data analysis and interpretation, manuscript revision.
References
- Amann R. Reproductive capacity of dairy bulls. IV. Spermatogenesis and testicular germ cell degeneration. Am J Anat. 1962;110:69–78. doi: 10.1002/aja.1001100107. [DOI] [PubMed] [Google Scholar]
- Balarini MK, Paula TAR, da Matta SLP, et al. Stages and duration of the cycle of the seminiferous epithelium in oncilla (Leopardus tigrinus, Schreber, 1775) Theriogenology. 2011;77:873–880. doi: 10.1016/j.theriogenology.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Beguelini MR, Moreira PRL, Faria KC, et al. Morphological characterization of the testicular cells and seminiferous epithelium cycle in six species of neotropical bats. J Morphol. 2009;270:943–953. doi: 10.1002/jmor.10731. [DOI] [PubMed] [Google Scholar]
- Beguelini MR, Puga CCI, Taboga SR, et al. Ultrastructure of spermatogenesis in the white-lined broad-nosed bat, Platyrrhinus lineatus (Chiroptera: Phyllostomidae) Micron. 2011;42:586–599. doi: 10.1016/j.micron.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Beguelini MR, Taboga SR, Morielle-Versute E. Ultrastructural characteristics of spermatogenesis in Pallas's Mastiff Bat, Molossus molossus (Chiroptera: Molossidae) Micros Res Tech. 2012;75:856–868. doi: 10.1002/jemt.22005. [DOI] [PubMed] [Google Scholar]
- Berndtson WE. Methods for quantifying mammalian spermatogenesis: a review. J Anim Sci. 1977;44:818–883. doi: 10.2527/jas1977.445818x. [DOI] [PubMed] [Google Scholar]
- Bishop MW, Smiles J. Differentiation of the acrosome in living mammalian spermatozoa and spermatids by fluorescence microscopy. J Reprod Fertil. 1963;6:297–303. doi: 10.1530/jrf.0.0060297. [DOI] [PubMed] [Google Scholar]
- Bittencourt VL, Paula TAR, da Matta SLP, et al. Avaliação da população celular do epitélio seminífero e índices indicativos da produção espermática, através de biópsia testicular em lobo-Guará (Chrysocyon brachyurus, Illiger, 1811) adulto. Rev Bras Reprod Anim. 2004;28:108–113. [Google Scholar]
- Bittencourt VL, Paula TAR, da Matta SLP, et al. The seminiferous epithelium cycle and daily spermatic production in the adult maned wolf (Chrysocyon brachyurus, Illiger, 1811) Micron. 2007;38:584–589. doi: 10.1016/j.micron.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Christensen AK, Fawcett DW. The fine structure of testicular interstitial cells in mice. Am J Anat. 1966;118:551–571. doi: 10.1002/aja.1001180214. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Cole FR, Wilson DE. Mammalian diversity and natural history. In: Wilson DE, Cole FR, Nichols JD, Rudran R, Foster MS, editors. Measuring and Monitoring Biological Diversity. Standard Methods for Mammals. Washington, DC: Smithsonian Institution Press; 1996. pp. 9–39. [Google Scholar]
- Costa GMJ, Chiarini-Garcia H, Morato RG, et al. Duration of spermatogenesis and daily sperm production in the jaguar (Panthera onca. Theriogenology. 2008;70:136–1146. doi: 10.1016/j.theriogenology.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Costa GM, Leal MC, Ferreira AC, et al. Duration of spermatogenesis and spermatogenic efficiency in two large neotropical rodent species: the agouti (Dasyprocta leporina) and paca (Agouti paca. J Androl. 2010a;31:489–499. doi: 10.2164/jandrol.109.009787. [DOI] [PubMed] [Google Scholar]
- Costa GMJ, Leal MC, Silva JV, et al. Spermatogenic cycle length and sperm production in a feral pig species (Collared Peccary, Tayassu tajacu. J Androl. 2010b;31:221–230. doi: 10.2164/jandrol.109.008524. [DOI] [PubMed] [Google Scholar]
- Costa KLC, da Matta SLP, Gomes MLM, et al. Histomorphometric evaluation of the neotropical brown brocket deer Mazama gouazoubira testis, with an emphasis on cell population indexes of spermatogenic yield. Anim Reprod Sci. 2011;127:202–212. doi: 10.1016/j.anireprosci.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Courot M, Hochereau-De-Reviers MT, Ortavant R. Spermatogenesis. In: Johnson AD, Gomer WR, Vandemark NL, editors. The Testis. New York: Academic Press; 1970. pp. 399–432. [Google Scholar]
- França LR, Russell LD. The testis of domestic animals. In: Martínez F, Regadera J, editors. Male Reproduction. A Multidisciplinary Overview. 1st edn. Madrid: Churchill Livingstone; 1998. pp. 197–219. [Google Scholar]
- França LR, Ogawa T, Avarbock MR, et al. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Golfari L. Belo Horizonte: PRODEPEF/PNUD/FAO/IBDF/Bra-45; 1975. Zoneamento ecológico do Estado de Minas Gerais para reflorestamento. Série técnica 3. [Google Scholar]
- Grocock CA, Clark JR. Duration of spermatogenesis in the vole (Microtus agrestis) and the bank vole (Cleterionomys glareolus. J Reprod Fertil. 1976;47:133–135. doi: 10.1530/jrf.0.0470133. [DOI] [PubMed] [Google Scholar]
- Guião-Leite FL, Paula TAR, da Matta SLP, et al. Cycle and duration of the seminiferous epithelium in puma (Puma concolor. Anim Reprod Sci. 2006;91:307–316. doi: 10.1016/j.anireprosci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Heideman PD, Deoraj P, Bronson FH. Seasonal reproduction of a tropical bat, Anoura geoffroyi, in relation to photoperiod. J Reprod Fertil. 1992;96:765–773. doi: 10.1530/jrf.0.0960765. [DOI] [PubMed] [Google Scholar]
- Hess RA, França LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. New York: Landes Bioscience; 2007. pp. 1–15. [Google Scholar]
- Holt WV, Moore HDM. Ultrastructural aspects of spermatogenesis in the common marmoset (Callithrix jacchus. J Anat. 1984;138:175–188. [PMC free article] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137. [Google Scholar]
- Kierszenbaum AL, Tres LL. The acrosome–acroplaxome–manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL, Rivkin E, Tres LL. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis. 2011;1:221–230. doi: 10.4161/spmg.1.3.18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz TH, Anthony ELP. Age estimation and post-natal growth in the bat Myotis lucifugus. J Mammal. 1982;63:23–32. [Google Scholar]
- Leal MC, França LR. The seminiferous epithelium cycle length in the black tufted-ear marmoset (Callithrix penicillata) is similar to humans. Biol Reprod. 2006;74:616–624. doi: 10.1095/biolreprod.105.048074. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–584. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Carrick F, Hall L. Unique features of spermiogenesis in the Musky Rat-kangaroo: reflection of a basal lineage or a distinct fertilization process? J Anat. 2008;212:257–274. doi: 10.1111/j.1469-7580.2008.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho-Filho J, Vasconcellos-Neto J. Dispersão de sementes de Vismia cayennensis (Jacq.) Pers. (Guttiferae) por morcegos na região de Manaus, Amazonas. Acta Bot Bras. 1994;8:87–96. [Google Scholar]
- Mello MAR, Kalko EKV, Silva WR. Diet and abundance of the bat Sturnira lilium (Chiroptera) in a Brazilian montane Atlantic Forest. J Mammal. 2008;89:485–492. [Google Scholar]
- Mello MAR, Kalko EKV, Silva WR. Ambient temperature is more important than food availability in explaining reproductive timing of the bat Sturnira lilium (Mammalia: Chiroptera) in a montane Atlantic forest. Can J Zool. 2009;87:239–245. [Google Scholar]
- Mikich SB, Bianconi GV. Potencializando o papel dos morcegos frugívoros na recuperação de áreas degradadas. Pesqui Florest Bras. 2005;51:155–164. [Google Scholar]
- Morigaki T, Kurohmaru M, Kanai Y, et al. Cycle of the seminiferous epithelium in the Java fruit bat (Pteropus vampyrus) and the Japanese lesser horseshoe bat (Rhinolophus cornutus. J Vet Med Sci. 2001;63:773–779. doi: 10.1292/jvms.63.773. [DOI] [PubMed] [Google Scholar]
- Nakai M, Van Cleeff JK, Bahr JM. Stages and duration of spermatogenesis in the domestic ferret (Mustela putorius furo. Tissue Cell. 2004;36:439–446. doi: 10.1016/j.tice.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Oliveira RL, Oliveira AG, Mahecha GAB, et al. Distribution of estrogen receptors (ERα and ERβ) and androgen receptor in the testis of big fruit-eating bat Artibeus lituratus is cell- and stage-specific and increases during gonadal regression. Gen Comp Endocrinol. 2009;161:283–292. doi: 10.1016/j.ygcen.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Ortavant R, Courot M, Hochereau-De-Reviers MT. Spermatogenesis in domestic mammals. In: Cole HH, Cupps PT, editors. Reproduction in Domestic Animals. New York: Academic Press; 1977. pp. 203–227. [Google Scholar]
- Paula TA, Chiarini-Garcia H, França LR. Seminiferous epithelium cycle and its duration in capybaras (Hydrochoerus hydrochaeris. Tissue Cell. 1999;31:327–334. doi: 10.1054/tice.1999.0039. [DOI] [PubMed] [Google Scholar]
- Queiroz GF, Nogueira JC. Duration of the cycle of the seminiferous epithelium and quantitative histology of the testis of the South American white-belly opossum (Didelphis albiventris), Marsupialia. Reprod Fertil Dev. 1992;4:213–222. doi: 10.1071/rd9920213. [DOI] [PubMed] [Google Scholar]
- Reis NR, Peracchi AL, Pedro WA, et al. Morcegos do Brasil. 1st edn. Londrina: Editora da Universidade Estadual de Londrina; 2007. [Google Scholar]
- Roosen-Runge EC, Giesel LO., Jr Quantitative studies on spermatogenesis in the albino rat. Am J Anat. 1950;87:1–30. doi: 10.1002/aja.1000870102. [DOI] [PubMed] [Google Scholar]
- Saidapur SK, Patil SB. Kinetics of spermatogenesis in megachiropteran bat, Rousettus leschenaulti (Desmarset): seminiferous epithelial cycle, frequency of stages, spermatogonial renewal and germ cell degeneration. Indian J Exp Biol. 1992;30:1037–1044. [PubMed] [Google Scholar]
- Sazima M, Buzato S, Sazima I. Dyssochroma viridiflorum (Solanaceae): a reproductively bat-dependent epiphyte from the Atlantic rainforest in Brazil. Ann Bot. 2003;92:725–730. doi: 10.1093/aob/mcg190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singwi MS, Lall SB. Spermatogenesis in the non-scrotal bat-Rhinopoma kinneari Wroughton (Microchiroptera: Mammalia) Acta Anat (Basel) 1983;116:136–145. [PubMed] [Google Scholar]
- Soares JM, Avelar GF, França LR. The seminiferous epithelium cycle and its duration in different breeds of dog (Canis familiaris. J Anat. 2009;215:462–471. doi: 10.1111/j.1469-7580.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira MF, Carvalho-Okano RM. Pollination biology of Mabea fistulifera (Euphorbiaceae) in Southeastern Brazil. Biotropica. 1996;28:61–68. [Google Scholar]


