Abstract
Background: Alport syndrome (AS) is a genetically heterogeneous disorder that is characterized by hematuria, progressive renal failure typically resulting in end-stage renal disease, sensorineural hearing loss, and variable ocular abnormalities. Only 15% of cases with AS are autosomal recessive and are caused by mutations in the COL4A3 or COL4A4 genes, encoding type IV collagen. Methods: Clinical data in a large consanguineous family with four affected members were reviewed, and genomic DNA was extracted. For mapping, 15 microsatellite markers flanking COL4A3, COL4A4, and COL4A5 in 16 family members were typed. For mutation screening, all coding exons of COL4A3 were polymerase chain reaction- amplified and Sanger-sequenced from genomic DNA. Results: The disease locus was mapped to chromosome 2q36.3, where COL4A3 and COL4A4 reside. Sanger sequencing revealed a novel mis-sense mutation (c.2T>C; p.M1T) in exon 1 of COL4A3. The identified nucleotide change was not found in 100 healthy ethnicity-matched controls via Sanger sequencing. Conclusions: We present a large consanguineous Turkish family with AS that was found to have a COL4A3 mutation as the cause of the disease. Although the relationship between the various genotypes and phenotypes in AS has not been fully elucidated, detailed clinical and molecular analyses are helpful for providing data to be used in genetic counseling. It is important to identify new mutations to clarify their clinical importance, to assess the prognosis of the disease, and to avoid renal biopsy for final diagnosis.
Introduction
Alport syndrome (AS) (Mendelian Inheritance in Man [MIM] 104200, 203780, and 301050) is a genetically heterogeneous disorder that is characterized by hematuria, progressive renal failure typically resulting in end-stage renal disease (ESRD), sensorineural hearing loss, and variable ocular abnormalities (Levy and Feingold, 2000). It affects 1 in 50,000 live births (Rana et al., 2007). AS is caused by mutations in several genes encoding type IV collagen, which is a major structural component of the basement membrane (van der Loop et al., 2000). X-linked inheritance is observed in ∼85% of the cases, which is caused by mutations in COL4A5 (MIM 303630) coding the α5-chain of type IV collagen (Feingold et al., 1985; Barker et al., 1990). About 15% of the cases show autosomal-recessive inheritance and are caused by homozygous or compound heterozygous mutations in COL4A3 (MIM 120070) or COL4A4 (MIM 120131) (Lemmink et al., 1994; Mochizuki et al., 1994). Autosomal-dominant inheritance is rare, and is usually caused by heterozygous COL4A3 or COL4A4 mutations (Jefferson et al., 1997). The COL4A3 and COL4A4 genes code for type IV collagen α3- and α4-chains, respectively. To date, 52 COL4A3 mutations have been determined as the cause of AS. These include mis-sense, nonsense, deletion, insertion, and splice-site changes, all of which are predicted to result in loss of a functional protein.
A clear-cut genotype–phenotype correlation for AS is not available. Therefore, it is important to identify new mutations and their associated phenotypes to predict the prognosis of the disease (Hoefele et al., 2010). The aim of this study was to identify the causative mutations in a family with autosomal-recessive AS.
Materials and Methods
Subjects
A 29-year-old woman presented to our department with a history of hemodialysis starting at age 15 years and renal transplantation at age 25 years (V-5 in Fig. 1). She had mild sensorineural hearing loss and bilateral cataracts. The family history was remarkable for the presence of other individuals with similar health concerns and was suggestive of autosomal-recessive inheritance (Fig. 1). Three of her relatives also developed end-stage renal failure, and two of whom (VI-4 and VI-6) had already undergone renal transplantation. For each affected individual, a detailed clinical examination was obtained, including ophthalmologic and otologic evaluations, pure tone audiometry, and retroperitoneal ultrasonography besides routine blood and urine tests. Audiological investigations showed mild sensorineural hearing loss; eye exams showed various ocular abnormalities such as cataracts and anterior lenticonus (Table 1). Family members of affected individuals also received regular clinical evaluations to detect any symptoms that may be potentially associated with the disease or heterozygosity for the disorder. All unaffected individuals were clinically normal, and none of them had renal findings. This study was approved by the Ankara University Ethics Committee and by the Institutional Review Board at the University of Miami, and signed informed consent was obtained from each participant.
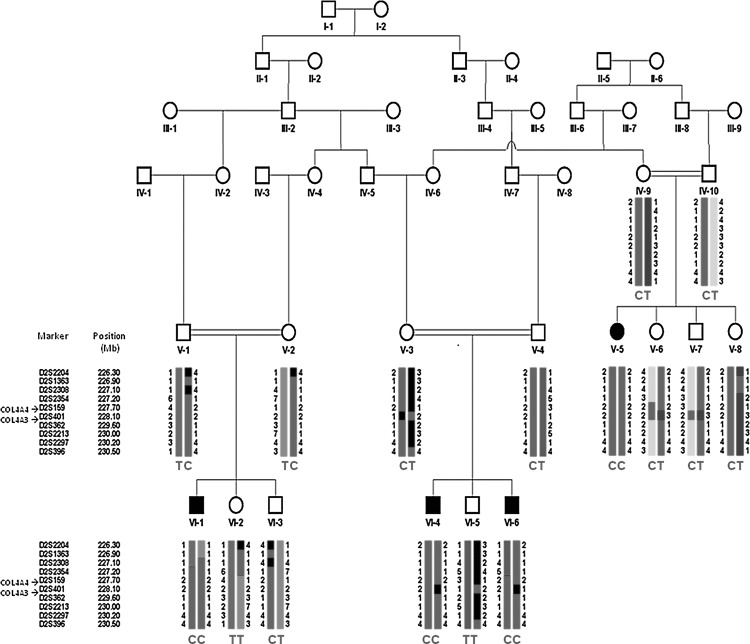
FIG. 1.
Pedigree, haplotypes flanking the COL4A3 and COL4A4 genes, and COL4A3 mutation results in the studied family. C and T are mutant and wild-type genotypes, respectively.
Table 1.
Phenotypic Characteristics of the Patients
| V-5 | VI-1 | VI-4 | VI-6 | |
|---|---|---|---|---|
| Gender | Female | Male | Male | Male |
| Hemodialysis starting at age | 15 | 13 | 14 | 14 |
| Age at diagnosis | 11 | 10 | 9 | 11 |
| End-stage renal failure | + | + | + | + |
| Renal transplantation | + | + | + | − |
| Audiometric results (left/right) | 53/57 | 55/59 | 50/48 | 52/55 |
| Ophthalmic findings | Bilateral anterior subcapsular cataract | Bilateral anterior subcapsular cataract | Bilateral anterior lenticonus and bilateral anterior subcapsular cataract | Bilateral anterior subcapsular cataract |
Genotyping and mutation analysis
Genomic DNA from affected (4) and unaffected (12) family members was extracted from peripheral blood by a phenol–chloroform method. Fifteen microsatellite markers (D2S401, D2S362, D2S2213, D2S2297, D2S396, D2S2204, D2S1363, D2S2308, D2S2354, D2S159, DXS8048, DXS8097, DXS6797, DXS1105, and DXS1210) from chromosomes 2 and X flanking the three known genes for AS (COL4A3, COL4A4, and COL4A5) were studied in all available samples from the family. Microsatellite (STR) markers tightly linked to the candidate genes for the disease were selected for linkage analysis from marshfieldclinic.org. The microsatellite markers were amplified by polymerase chain reaction (PCR), and the fragments were analyzed on an automated sequencer (ABI 3730xl).
The coding 52 exons of COL4A3 (NM_000091.4) were PCR-amplified using primer sets designed by primer 3.0. The list of primers is available on request. PCRs were run in a 25-mL volume applying a touch-down protocol and annealing temperatures between 65°C and 57°C. PCR products were visualized on agarose gels and cleaned over Sephadex columns, and BigDye reactions were performed following the manufacturer's recommendations (Applied Biosystems, Inc.). A DNA Sequencer (ABI 3730xl) was used to detect mutations. Results were visualized with Sequencher 4.7 software (Gene Codes Corporation).
Results
Results of mapping studies showed a haplotype flanking COL4A3 that cosegregates in the entire family as an autosomal-recessive trait (Fig. 1).
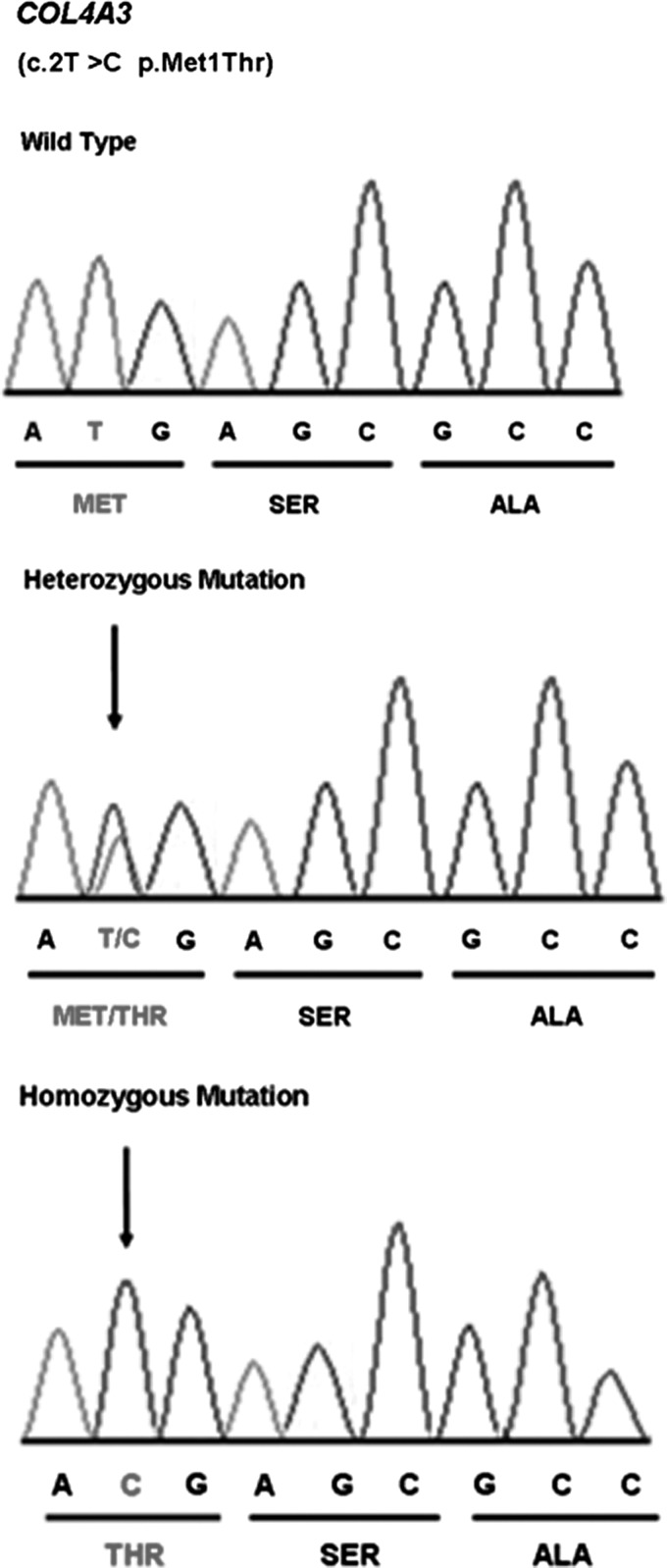
DNA sequencing showed a novel homozygous mutation c.2T>C resulting in p.M1T in the COL4A3 gene (Fig. 2). This variant segregated in the entire family as a completely penetrant autosomal-recessive trait. The identified nucleotide change was not found in 100 healthy ethnicity-matched controls via Sanger sequencing.
FIG. 2.
Electropherograms showing the c.2T>C mutation resulting in p.Met1Thr in COL4A3.
Discussion
In 1927, Alport described a syndrome that bears his name. Passwell et al. (1981) described an autosomal-recessive form of the disease in a 1-year-old girl who presented with failure to thrive, nephritis, and deafness and was born to first-cousin parents. Subsequently, Mochizuki et al. (1994) reported four unrelated families with autosomal-recessive AS. In 1997, Colville et al. and Rhys et al. reported on the ocular manifestations of autosomal-recessive AS. They found that anterior lenticonus, dot-and-fleck retinopathy, and recurrent corneal erosions are seen in patients with autosomal-recessive AS more frequently (or severe) than those observed in age-matched controls. In 1994, Mochizuki et al. identified a homozygous deletion of five nucleotides in exon 5 of COL4A3, resulting in a truncated protein. Subsequently, Lemmink et al. (1994) identified compound heterozygous mutations in COL4A3 in a patient with autosomal-recessive AS. To date, 71 COL4A3 variants that include mis-sense, nonsense, deletion, insertion, and splice-site changes have been determined, 52 of which are related with autosomal-recessive or autosomal-dominant AS. The other COL4A3 mutations have been found to be related with hematuria, focal segmental glomerulosclerosis, microhematuria, and proteinuria, and one mutation has been found to be related with chronic obstructive pulmonary disease (Lemmink et al., 1994; Ding et al., 1995; Knebelmann et al., 1995; Van Der Loop et al., 2000; Heidet et al., 2001; Badenas et al., 2002; Longo et al., 2002; Tazon et al., 2003; Pescucci et al., 2004; Wang et al., 2004; Nagel et al., 2005; Longo et al., 2006; Hou et al., 2007; Slajpah et al., 2007; Voskarides et al., 2007; Hou et al., 2008; Kim et al., 2008, Hoefele et al., 2010; Zhang et al., 2011).
Autosomal-recessive AS can be caused by homozygous or compound heterozygous mutations in COL4A3 (120070) or COL4A4 (120131), both of which map to chromosome 2q (Finielz et al., 1998). In a very recent study, including 17 unrelated Chinese patients with autosomal-recessive AS, it was determined that the COL4A3 and COL4A4 mutations are present in 82% and 18% patients, respectively (Zhang et al., 2012).
The c.2T>C mutation identified in this study affects the initiation codon of the COL4A3 gene and is likely, therefore, to affect the initiation of translation from COL4A3 mRNA. Methionine at codon 1 is completely conserved in all species, as expected. When there is a mutation in the initiation codon, it is possible that an alternative AUG within the transcript may be used as an aberrant translation initiation site. There is an ATG triplet (which can function as an initiation start site) in exon 11, at nucleotide positions 625–627, 621 bp downstream from the authentic initiation codon. It is possible that this downstream ATG acts as an initiation codon. Even if that ATG was used as an initiation codon, though the resulting truncated protein would lack 208 amino acids from the N-terminus of the precursor protein, including the entire signal peptide spanning from amino acid residues 1 to 26. This sequence motif is thought to play a critical role in the targeting of proteins, and it is therefore unlikely that the mutant protein will be functional.
In this study, the ages of patients at first clinical presentation were similar and ranged from 9 to 11 years. On average, each individual required hemodialysis within 3.75 years after their first clinical diagnosis. End-stage renal failure occurred earlier when compared with some of the previous reports. According to the previous studies, patients with autosomal-recessive AS develop ESRD at variable ages. Mochizuki et al. (1994) reported a case with deletion in the COL4A3 gene resulting in ESRD by age 9. Finielz et al. (1998) reported four families with an insertion in the COL4A3 gene; two of the cases resulted in ESRD by ages 26 and 28. In another family in the same study that had the same mutation, end-stage renal failure occurred earlier (ages from 14 to 18 years). In 2001, 60 patients affected with AS were reported, 73% of whom reached ESRD. Age at end-stage renal failure was 21.8 years on average, ranging from 10 to 44 years (Heidet et al., 2001). Based on the limited variability observed in our four patients, we suggest that the identified mutation has a profound impact on the protein, likely to be completely absent, and is sufficient to produce a uniform severe phenotype.
In this report, audiometric results showed pure-tone average hearing loss level of 52.5 and 54.75 for the left and right ears, respectively. All four patients had bilateral anterior subcapsular cataracts. Only one patient (VI-4) had bilateral anterior lenticonus. In 35 patients with autosomal-recessive AS reported in 2001, hearing loss was detected in 27 (77%); however, 8 patients who did not have hearing loss were young (8.3 years on average). Sixteen out of 26 (62%) patients tested for ocular symptoms were found to have positive findings; again, the average age of the 10 others who did not have eye findings was 10.0 years (Heidet et al., 2001). In a series reported in 2012, 7 out of 12 patients had hearing loss, and only 1 out of 10 patients who underwent ocular examination had ocular lesions (Zhang et al., 2012).
We conclude that although the relationship between various genotypes and phenotype in AS has not been fully elucidated, detailed family studies are helpful for genetic counseling, prediction of prognosis, the assessment of the risk for kidney transplantation, and for the follow-up and therapy of AS.
Acknowledgments
The authors want to acknowledge the family members for their participation in the study. This work was supported by the National Institutes of Health grants R01DC009645 to M.T.
Author Disclosure Statement
No competing financial interests exist.
References
- Alport AC. Hereditary familial congenital haemorrhagic nephritis. Br Med J. 1927;1:504–506. doi: 10.1136/bmj.1.3454.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenas C. Praga M. Tazón B, et al. Mutations in the COL4A4 and COL4A3 genes cause familial benign hematuria. J Am Soc Nephrol. 2002;13:1248–1254. doi: 10.1681/ASN.V1351248. [DOI] [PubMed] [Google Scholar]
- Barker DF. Hostikka SL. Zhou J, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Colville D. Savige J. Morfis M, et al. Ocular manifestations of autosomal recessive Alport syndrome. Ophthalmic Genet. 1997;18:119–128. doi: 10.3109/13816819709057125. [DOI] [PubMed] [Google Scholar]
- Ding J. Stitzel J. Berry P, et al. Autosomal recessive Alport syndrome: mutation in the COL4A3 gene in a woman with Alport syndrome and posttransplant antiglomerular basement membrane nephritis. J Am Soc Nephrol. 1995;5:1714–1717. doi: 10.1681/ASN.V591714. [DOI] [PubMed] [Google Scholar]
- Feingold J. Bois E. Chompret A, et al. Genetic heterogeneity of Alport syndrome. Kidney Int. 1985;27:672–677. doi: 10.1038/ki.1985.63. [DOI] [PubMed] [Google Scholar]
- Finielz P. Cartault F. Chuet C, et al. Alport syndrome in Reunion Island: phenotypic heterogeneity of the recessive-autosomal form. (Letter) Nephron. 1998;79:237. doi: 10.1159/000045039. [DOI] [PubMed] [Google Scholar]
- Heidet L. Arrondel C. Forestier L, et al. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J Am Soc Nephrol. 2001;12:97–106. doi: 10.1681/ASN.V12197. [DOI] [PubMed] [Google Scholar]
- Hoefele J. Lange-Sperandio B. Ruessmann D, et al. Novel heterozygous COL4A3 mutation in a family with late-onset ESRD. Pediatr Nephrol. 2010;25:1539–1542. doi: 10.1007/s00467-010-1467-4. [DOI] [PubMed] [Google Scholar]
- Hou P. Chen Y. Ding J, et al. A novel mutation of COL4A3 presents a different contribution to Alport syndrome and thin basement membrane nephropathy. Am J Nephrol. 2007;27:538–544. doi: 10.1159/000107666. [DOI] [PubMed] [Google Scholar]
- Hou P. Lü JC. Chen YQ, et al. Approaches to detect the gene mutations in autosomal recessive Alport's syndrome: analysis of a family. Zhonghua Yi Xue Za Zhi. 2008;88:573–575. [PubMed] [Google Scholar]
- Jefferson JA. Lemmink HH. Hughes AE, et al. Autosomal dominant Alport syndrome linked to the type IV collagen a3 and a4 genes (COL4A3 and COL4A4) Nephrol Dial Transplant. 1997;12:1595–1599. doi: 10.1093/ndt/12.8.1595. [DOI] [PubMed] [Google Scholar]
- Kim KM. Park SH. Kim JS, et al. Polymorphisms in the type IV collagen alpha3 gene and the risk of COPD. Eur Respir J. 2008;32:35–41. doi: 10.1183/09031936.00076207. [DOI] [PubMed] [Google Scholar]
- Knebelmann B. Forestier L. Drouot L, et al. Splice-mediated insertion of an Alu sequence in the COL4A3 mRNA causing autosomal recessive Alport syndrome. Hum Mol Genet. 1995;4:675–679. doi: 10.1093/hmg/4.4.675. [DOI] [PubMed] [Google Scholar]
- Lemmink HH. Mochizuki T. van den Heuvel LP, et al. Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum Mol Genet. 1994;3:1269–1273. doi: 10.1093/hmg/3.8.1269. [DOI] [PubMed] [Google Scholar]
- Levy M. Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 2000;58:925–943. doi: 10.1046/j.1523-1755.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Longo I. Porcedda P. Mari F, et al. COL4A3/COL4A4 mutations: from familial hematuria to autosomal-dominant or recessive Alport syndrome. Kidney Int. 2002;61:1947–1956. doi: 10.1046/j.1523-1755.2002.00379.x. [DOI] [PubMed] [Google Scholar]
- Longo I. Scala E. Mari F, et al. Autosomal recessive Alport syndrome: an in-depth clinical and molecular analysis of five families. Nephrol Dial Transplant. 2006;21:665–671. doi: 10.1093/ndt/gfi312. [DOI] [PubMed] [Google Scholar]
- Mochizuki T. Lemmink HH. Mariyama M, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- Nagel M. Nagorka S. Gross O. Novel COL4A5, COL4A4, and COL4A3 mutations in Alport syndrome. Hum Mutat. 2005;26:60. doi: 10.1002/humu.9349. [DOI] [PubMed] [Google Scholar]
- Passwell JH. David R. Boichis H, et al. Hereditary nephritis with associated defects in proximal renal tubular function. J Pediatr. 1981;98:85–87. doi: 10.1016/s0022-3476(81)80545-8. [DOI] [PubMed] [Google Scholar]
- Pescucci C. Mari F. Longo I, et al. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 2004;65:1598–1603. doi: 10.1111/j.1523-1755.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- Rana K. Tonna S. Wang YY, et al. Nine novel COL4A3 and COL4A4 mutations and polymorphisms identified in inherited membrane diseases. Pediatr Nephrol. 2007;22:652–657. doi: 10.1007/s00467-006-0393-y. [DOI] [PubMed] [Google Scholar]
- Rhys C. Snyers B. Pirson Y. Recurrent corneal erosion associated with Alport's syndrome. Kidney Int. 1997;52:208–211. doi: 10.1038/ki.1997.321. [DOI] [PubMed] [Google Scholar]
- Slajpah M. Gorinsek B. Berginc G, et al. Sixteen novel mutations identified in COL4A3, COL4A4, and COL4A5 genes in Slovenian families with Alport syndrome and benign familial hematuria. Kidney Int. 2007;71:1287–1295. doi: 10.1038/sj.ki.5002221. [DOI] [PubMed] [Google Scholar]
- Van der Loop FTL. Heidet L. Timmer EDJ, et al. Autosomal dominant Alport syndrome caused by a COL4A3 splice site mutation. Kidney Int. 2000;58:1870–1875. doi: 10.1111/j.1523-1755.2000.00358.x. [DOI] [PubMed] [Google Scholar]
- Vorechovsky I. Transposable elements in disease-associated cryptic exons. Hum Genet. 2009;127:135–154. doi: 10.1007/s00439-009-0752-4. [DOI] [PubMed] [Google Scholar]
- Voskarides K. Damianou L. Neocleous V, et al. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18:3004–3016. doi: 10.1681/ASN.2007040444. [DOI] [PubMed] [Google Scholar]
- Wang YY. Rana K. Tonna S, et al. COL4A3 mutations and their clinical consequences in thin basement membrane nephropathy (TBMN) Kidney Int. 2004;65:786–790. doi: 10.1111/j.1523-1755.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- Zhang H. Ding J. Wang F, et al. Mutation detection of COL4An gene based on mRNA of peripheral blood lymphocytes and prenatal diagnosis of Alport syndrome in China. Nephrology (Carlton) 2011;16:377–380. doi: 10.1111/j.1440-1797.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Wang F. Ding J, et al. Genotype-phenotype correlations in 17 Chinese patients with autosomal recessive Alport syndrome. Am J Med Genet A. 2012;158A:2188–2193. doi: 10.1002/ajmg.a.35528. [DOI] [PubMed] [Google Scholar]