Abstract
Twenty-six strains of 22 bacterial species were tested for growth on trypticase soy agar (TSA) or sea-salt agar (SSA) under hypobaric, psychrophilic, and anoxic conditions applied singly or in combination. As each factor was added to multi-parameter assays, the interactive stresses decreased the numbers of strains capable of growth and, in general, reduced the vigor of the strains observed to grow. Only Serratia liquefaciens strain ATCC 27592 exhibited growth at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. To discriminate between the effects of desiccation and hypobaria, vegetative cells of Bacillus subtilis strain 168 and Escherichia coli strain K12 were grown on TSA surfaces and simultaneously in liquid Luria-Bertani (LB) broth media. Inhibition of growth under hypobaria for 168 and K12 decreased in similar ways for both TSA and LB assays as pressures were reduced from 100 to 25 mbar. Results for 168 and K12 on TSA and LB are interpreted to indicate a direct low-pressure effect on microbial growth with both species and do not support the hypothesis that desiccation alone on TSA was the cause of reduced growth at low pressures. The growth of S. liquefaciens at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres was surprising since S. liquefaciens is ecologically a generalist that occurs in terrestrial plant, fish, animal, and food niches. In contrast, two extremophiles tested in the assays, Deinococcus radiodurans strain R1 and Psychrobacter cryohalolentis strain K5, failed to grow under hypobaric (25 mbar; R1 only), psychrophilic (0°C; R1 only), or anoxic (<0.1% ppO2; both species) conditions. Key Words: Habitable zone—Hypobaria—Extremophiles—Special regions—Planetary protection. Astrobiology 13, 115–131.
1. Introduction
Special regions on Mars (Beaty et al., 2006; COSPAR, 2008) are defined as those areas “within which terrestrial organisms are likely to propagate, or a region which is interpreted to have a high potential for the existence of extant martian life forms.” Examples of potential Special Regions on Mars include polar caps, subsurface brines, buried glaciers, recent gullies and gully-forming regions, pasted-on terrains, surficial ice in mid- and high-latitude craters, buried salt deposits, and areas of hydrothermal activity (Beaty et al., 2006; Committee on Preventing the Forward Contamination of Mars, 2006; COSPAR, 2008). Future life-detection missions to Mars are likely to target Special Regions because of the increased chances of finding an extant microbiota at these sites. To protect Special Regions on Mars from possible forward contamination from landed vehicles, research must be undertaken to characterize the limits of survival and growth of terrestrial microorganisms under conditions expected at the target landing sites.
Complicating efforts to predict habitable regions on Mars (see Stoker et al., 2010), there is a paucity of data on the interactive effects of biocidal or inhibitory factors on microbial growth under simulated martian conditions. Schuerger and coworkers (Schuerger, 2004; Schuerger and Nicholson, 2006; Schuerger et al., 2006, 2010; Berry et al., 2010), reviewing recent literature (Horneck et al., 1994, 2003; Newsom and Hagerty, 1997, 1999; Yen et al., 2000; Schuerger et al., 2003; Schuerger, 2004; Clark et al., 2005; Ming et al., 2006; Hecht et al., 2009), have proposed that a minimum of 14 biocidal or inhibitory factors are present on Mars, including (not in priority) [1] solar UV irradiation, [2] extreme desiccation, [3] low pressure (1–14 mbar), [4] anoxic CO2 atmosphere, [5] extremely low temperatures (global average of −61°C), [6] solar particle events, [7] galactic cosmic rays, [8] UV-glow discharge from blowing dust, [9] solar UV-induced volatile oxidants (e.g., 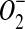 , O−, H2O2, NOx, O3), [10] globally distributed oxidizing soils, [11] extremely high salt levels (e.g., MgCl2, NaCl, FeSO4, and MgSO4) in surficial soils at some sites on Mars, [12] high concentrations of heavy metals in martian soils, [13] likely acidic conditions in martian regolith, and [14] perchlorates in at least some soils. Furthermore, we add here three additional constraints on microbial growth on the martian surface: [15] lack of defined energy sources free of UV irradiation, [16] no known sources of available nitrogen and carbon, and [17] no obvious redox couples for microbial metabolism (Beaty et al., 2006; Stoker et al., 2010). Factors [1] to [5] are ubiquitous over the surface or shallow subsurface of Mars and are likely to impact short-term microbial survival and growth rates the most. Factors [6] to [17] are likely to be more dispersed or intermittent in space or time and may depend on the local geochemistry of the regolith.
, O−, H2O2, NOx, O3), [10] globally distributed oxidizing soils, [11] extremely high salt levels (e.g., MgCl2, NaCl, FeSO4, and MgSO4) in surficial soils at some sites on Mars, [12] high concentrations of heavy metals in martian soils, [13] likely acidic conditions in martian regolith, and [14] perchlorates in at least some soils. Furthermore, we add here three additional constraints on microbial growth on the martian surface: [15] lack of defined energy sources free of UV irradiation, [16] no known sources of available nitrogen and carbon, and [17] no obvious redox couples for microbial metabolism (Beaty et al., 2006; Stoker et al., 2010). Factors [1] to [5] are ubiquitous over the surface or shallow subsurface of Mars and are likely to impact short-term microbial survival and growth rates the most. Factors [6] to [17] are likely to be more dispersed or intermittent in space or time and may depend on the local geochemistry of the regolith.
Further complicating estimates of the habitability of the martian surface is the fact that very little literature exists on the growth of microorganisms at pressures close to the conditions found on the surface of Mars (range 1–14 mbar). Three recent papers (Kanervo et al., 2005; Pokorny et al., 2006; Sakon and Burnap, 2006) examined microbial growth at pressures below an Earth-standard sea level pressure of 1013 mbar, but pressures were still much higher (>100 mbar) than those found on Mars (datum pressure=6.1 mbar). Schuerger and Nicholson (2006) reported that germination and subsequent growth were arrested at 35 mbar for endospores of seven Bacillus spp., while log-phase vegetative cells of several species were capable of minimal, but observable, growth down to 25 mbar. Nicholson et al. (2010) demonstrated both growth and evolution of Bacillus subtilis vegetative cells propagated at 50 mbar. Kral et al. (2011) reported methanogenesis by three archaea methanogens at 35°C in a Mars analog soil down to 50 mbar. And Berry et al. (2010) reported growth of Escherichia coli and Serratia liquefaciens down to 25 mbar in anoxic CO2-enriched atmospheres. It is apparent from these studies that some bacteria may be capable of cellular replication down to at least 25 mbar, while growth of other species is inhibited by pressures from 35 to 100 mbar.
In an effort to predict the potential of terrestrial microorganisms to grow and proliferate on Mars, a series of experiments were initiated to study the effects of three factors on growth of 26 strains of 22 bacteria under environmental conditions that begin to approach those found on the surface. The primary goal of the research was to discern whether terrestrial microorganisms typically recovered from spacecraft could grow in hydrated hypobaric environments in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Three key assumptions (A) adopted for the current work included the following: (A1) bacteria were shielded from biocidal UV irradiation, (A2) bacteria were continuously exposed to hydrated and carbon/nitrogen-rich substrates, and (A3) the habitable niche was protected from rapid evaporation typically encountered on the martian surface. Thus, the three environmental conditions tested here, and the three assumptions listed above, are proposed as a minimum set of conditions that must be met to support growth of terrestrial microorganisms on the martian surface. As will be examined in the Discussion, not all these conditions can be easily met on the surface of modern-day Mars. But for the current study, if terrestrial microorganisms proved to be unable to grow under hydrated and carbon/nitrogen-rich hypobaric conditions at 7 mbar, then further tests with some of the other 17 environmental factors listed above would likely prove unproductive. And lastly, a secondary goal of the current study was to model lapse rates for elevated gas-phase and lithographic pressures in the martian lithosphere in order to compare microbial growth at diverse pressures studied here to possible locations in the martian lithosphere where terrestrial microorganisms might grow and proliferate.
2. Materials and Methods
2.1. Microbiological procedures
Twenty-six strains of bacteria (Table 1) were chosen based on their recovery from spacecraft assembly facilities (SAF) (Venkateswaran et al., 2001; La Duc et al., 2007, 2009; Probst et al., 2010), the Mars Odyssey orbiter (La Duc et al., 2003), Kennedy Space Center (KSC), and lab surfaces (Schuerger, unpublished). Several strains were obtained from the American Type Culture Collection (ATCC). All bacteria were maintained on trypticase soy agar (TSA; Thermo Fisher Scientific, Inc., Pittsburg, PA, USA), except Psychrobacter cryohalolentis K5, which was maintained on a sea-salt agar (SSA) composed of 17 g sea salts, 5 g peptone, 1 g yeast extract, and 16.5 g agar per liter of medium. All chemicals and growth media were obtained from Sigma-Aldrich, Inc., St. Louis, Missouri, USA, or Thermo Fisher Scientific.
Table 1.
Sources and 16S Sequencing Results for 26 Bacteria
| 16S NCBI databasey | Source Strain # | Original sourcex | Gram Stain | Amplicon (bp) | NCBI closest match Accession number | NCBI Closest match (%ID) |
|---|---|---|---|---|---|---|
| (1)zAcinetobacter radioresistens | 50v1 | A. Baker | − | 1387 | JN669112 | 0.999 |
| (2) Bacillus megaterium | 25HS1 | A. Baker | + | 1123 | GQ199766 | 1.000 |
| (3) Bacillus pumilus | 40HS1 | A. Baker | + | 1080 | GU332599 | 1.000 |
| (4) Bacillus pumilus (B27F only) | 31v3 | A. Baker | + | 892 | HQ161778 | 0.999 |
| (5) Bacillus subtilis | HA101 | R. Mancinelli | + | 1296 | GQ280027 | 0.998 |
| (6) Bacillus subtilis subsp. subtilis | 168 | W.L. Nicholson | + | 1172 | JX120511 | 0.997 |
| (7) Burkholderia cepacia | ATCC 25608 | C. Cortes-Ramos | − | 1377 | AY741341 | 1.000 |
| (8) Curtobacterium flaccumfaciens | 48v2 | A. Baker | + | 1366 | NR025467 | 0.991 |
| (9) Deinococcus radiodurans (B27F only) | R1 | W.L. Nicholson | + | 840 | AE000513 | 0.992 |
| (10) Enterococcus faecalis | ATCC 29212 | C. Cortes-Ramos | − | 1326 | FJ804073 | 1.000 |
| (11) Escherichia coli | ATCC 35218 | C. Cortes-Ramos | − | 933 | CP000247 | 0.996 |
| (12) Escherichia coli | K12 | W.L. Nicholson | − | 1350 | CP000948 | 0.997 |
| (13) Microbacterium oleivorans | 27v1b | A. Baker | + | 1360 | FJ200410 | 0.997 |
| (14) Microbacterium oleivorans | 32v4 | A. Baker | + | 1422 | EF522131 | 0.997 |
| (15) Microbacterium testaceum | 27v1a | A. Baker | + | 1409 | HE681737 | 1.000 |
| (16) Micrococcus luteus (B27F only) | ACS-22 | KSC lab isolate | + | 956 | EU379295 | 1.000 |
| (17) Paenibacillus pabuli | ACS-6 | KSC lab isolate | + | 1355 | AB045094 | 0.998 |
| (18) Proteus mirabilis | ATCC 7002 | K. Venkateswaran | − | 1061 | AM942759 | 0.998 |
| (19) Pseudomonas aeruginosa | ATCC 27853 | C. Cortes-Ramos | − | 1254 | GQ339107 | 0.999 |
| (20) Pseudomonas fluorescens | ATCC 13525 | Fisher Scientific | − | 1174 | AF094725 | 0.997 |
| (21) Psychrobacter cryohalolentis | K5 | T.C. Onstott | − | 1382 | CP000323 | 0.999 |
| (22) Serratia liquefaciens (B1512R only) | ATCC 27592 | C. Cortes-Ramos | − | 964 | HQ335001 | 0.976 |
| (23) Sporosarcina aquamarina | SAFN-008 | K. Venkateswaran | + | 1186 | AY167819 | 0.993 |
| (24) Staphylococcus aureus | ATCC 29213 | C. Cortes-Ramos | + | 1448 | HE681097 | 0.999 |
| (25) Staphylococcus aureus | ATCC 33591 | C. Cortes-Ramos | + | 1386 | CP000730 | 1.000 |
| (26) Staphylococcus epidermidis | ATCC 14990 | C. Cortes-Ramos | + | 1406 | FJ768459 | 1.000 |
KSC lab isolates were recovered from lab benches within the Space Life Sciences Lab at the Kennedy Space Center (KSC), Florida. The new strains ACS-6 and ACS-22 were sequenced and entered into GenBank as accession numbers JX401570 and JX415537, respectively.
All taxonomic affiliations are based on 16S sequencing in the NCBI nucleotide database. The BLAST program was used in NCBI with full contigs or with B27F (forward) or B1512R (reverse) sequences when full contigs were not available.
Taxonomic affiliations of all isolates were confirmed by 16S sequencing (Table 1). Vegetative cells of each strain were grown at 30°C for 48 h on either TSA or SSA, collected in sterile deionized water (18 MΩ), and then concentrated into 200 μL sterile deionized water. Cellular deoxyribonucleic acids (DNA) were extracted with Ultra Clean Microbial DNA Isolation Kits (Mo Bio Laboratories, Carlsbad, CA, USA). Bacterial DNA sequences were amplified by polymerase chain reactions with 16S universal primers B27F (5′-GAGTTTGATCM TTGGCTCAG-3′) and B1512R (5′-AAGGAGGTGATCCANCCRCA-3′), as described previously (Lueders et al., 2004). Sequencing was conducted by the Interdisciplinary Center for Biotechnology Research (University of Florida, Gainesville, FL, USA). Ribosomal DNA sequences were compared to the entire 16S nucleotide sequence database at the National Center for Biotechnology Information's (NCBI) BLAST server (http://www.ncbi.nlm.nih.gov/BLAST), and homology scores ≥97.5% were considered the same species (Stackebrandt et al., 2002).
2.2. Growth of bacteria under hypobaric conditions
Two separate assays were conducted to characterize the growth of bacteria under hypobaric conditions. First, 26 strains (Table 1) of spore-forming and non-spore-forming bacteria were incubated on TSA or SSA at 1013, 100, or 25 mbar under aerobic or anoxic conditions. To confirm that the growth responses for at least two of the bacterial species were due to pressure effects and not the potential desiccation of cells on the upper surfaces of the agar media, vegetative cells of Bacillus subtilis strain 168 and Escherichia coli strain K12 were grown in liquid media maintained at 1013 (Earth sea-level pressure), 75, 50, or 25 mbar under aerobic conditions.
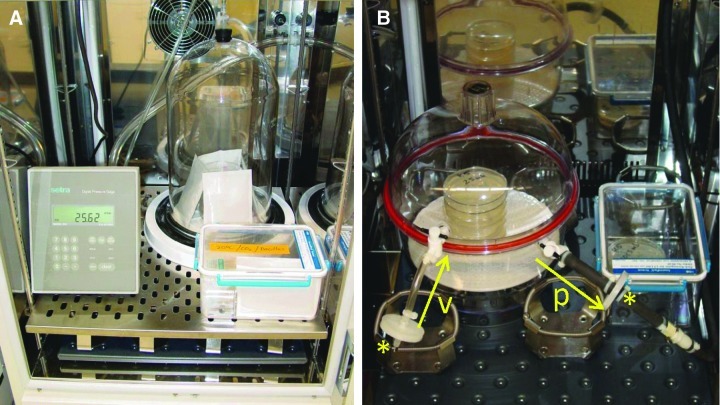
Assays were conducted in hypobaric systems (Fig. 1) capable of maintaining pressures down to 1 mbar (+/− 0.5 mbar). When required, the hypobaric chambers were fitted with CO2 generation pouches (R681001 AnaeroPacks, Remel, Thermo Fisher Scientific) to create anoxic atmospheres. Previous work by Van Horn et al. (1997) demonstrated that AnaeroPack sachets remove oxygen from low-volume closed containers to concentrations <0.1% within 1 h. Anaerobic indicator tablets (also from Remel) were placed within each hypobaric chamber to confirm the presence of an anoxic atmosphere. The hypobaric systems were maintained at 30°C for all bacteria, except P. cryohalolentis, which was incubated at 24°C. Pressures within the two hypobaric chambers were maintained with separate pumps and control units (model PU-842, KNF Neuberger, Trenton, NJ, USA). Vacuum and vent lines for the hypobaric chambers were fitted with 0.22 μm in-line air filters to prevent air contamination of the TSA, SSA, or Luria-Bertani (LB) media during pump cycling.
FIG. 1.
Hypobaric chambers used for creating low-pressure conditions reported in Tables 2, 3, and 6, and Figs. 2, 3, and 4. (A) One system was composed of a single glass bell jar attached to a high-density polyethylene stage through which vent holes permitted the connection to external pumps and controllers (model PU-842, KNF, Neuberger, Trenton NJ, USA). For anoxic conditions, AnaeroPack (Remel, Thermo Fisher Scientific, Chicago, IL, USA) sachets were placed around each stack of up to six Petri dishes of TSA or SSA media. (B) A second hypobaric system was developed to replace the single glass bell jar system in which two separate hypobaric chambers were maintained in separate microbial incubators. The newer hypobaric chambers were composed of clear polycarbonate desiccators (model #08-642-7, Thermo-Fisher Scientific, Rochester, NY, USA) connected to two separate KNF pump/controllers placed on the outsides of the microbial incubators. For each unit, in-line 0.22 μm filters (*) were used on the vent line (v) and the pump line (p) to prevent the introduction of room air into the hypobaric chamber during normal operations. Arrows designate the airflow into and out of the hypobaric desiccator system. Color images available online at www.liebertonline.com/ast
Vegetative cells of the 26 strains were grown overnight for 16 h on TSA or SSA and then streaked separately onto fresh media. The TSA and SSA plates were poured double-thick at a volume of 35 mL per plate (compared to standard TSA plates that used 15–18 mL per plate). Bacterial cells were streaked in 3 quadrants, and to avoid false positives, only colonies observed in the 2nd and 3rd quadrants were used to evaluate the growth of bacteria under test conditions. Endospores were not present in the 16 h old cultures of the spore-forming Bacillus spp. tested. The vegetative cells of all species were incubated in the hypobaric systems for 48 h at 1013, 100, or 25 mbar and maintained under aerobic or CO2-enriched anoxic atmospheres. Vegetative cells for all species were rated for growth on the semisolid media with a pair of 5× jeweler's glasses. The following rating system was used to rank the growth of all strains: 4=large robust colonies >5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies <0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. At the end of all assays, cultures were transferred to a lab incubator at 30°C, 1013 mbar, and 21% O2/78% N2 aerobic atmospheres and incubated an additional 48 h to determine whether the hypobaric conditions inactivated cells or were merely inhibitory to cell growth.
For the liquid-culture assay, bacterial strains B. subtilis 168 and E. coli K12 were grown in LB liquid medium alone (Miller, 1972) supplemented with or without (final concentrations) potassium nitrate (0.1% w/v) and glucose (0.25% w/v) (LBGN). For growth of cells at various pressures, triplicate cultures were grown in separate 10 mL of liquid media in 18 mm diameter flat-bottom screw-cap tubes with the caps loosened. Tubes were placed inside hypobaric chambers and incubated for 24 h at 30°C in 1013 mbar, 75, 50, or 25 mbar under aerobic conditions. Optical densities (ODs) were measured after 24 h with a Klett-Summerson colorimeter fitted with a red (660 nm) filter (Note: for purposes of comparison, 100 Klett units=1.0 OD660). Media levels in each tube at T=0 were marked with a felt-tip black marker. If the volume of LB or LBGN was observed to be lower after 24 h at 30°C (observed only for the 25 mbar samples), the media was returned to the original volume by the addition of sterile deionized water prior to reading in the Klett-Summerson colorimeter. Water loss was approximately 10–15% at 25 mbar in 24 h; and no loss of water was observed at 50, 75, or 1013 mbar.
2.3. Growth of bacteria under psychrophilic conditions
Bacteria were streaked on TSA or SSA as described above. Cultures were placed into polycarbonate containers (Remel AnaeroPack vessels) and sealed into an aerobic O2/N2 lab atmosphere or sealed into an anoxic environment in which the Remel AnaeroPack sachets (described above) were used. However, in the psychrophilic assays, the anaerobic containers were flushed for 30 s with ultrahigh purity carbon dioxide (CO2) gas prior to closing the lids. All containers were then incubated for 35 d at 0°C. In preliminary experiments, the AnaeroPack sachet system was able to maintain an anaerobic environment for 14 d before the indicator tablets turned from pink (anoxic conditions) to purple (aerobic conditions). Every 7 d all bacteria were removed from the polycarbonate containers and rated for bacterial growth. For CO2-enriched anoxic treatments, fresh AnaeroPack sachets and indicator tablets were inserted into the polycarbonate containers and allowed to equilibrate for 20 min prior to placing all cultures back into the 0°C incubator. After 35 d, cultures were transferred to lab conditions and incubated 48 h at 30°C, 1013 mbar, and aerobic O2/N2 atmosphere to determine whether the psychrophilic conditions inactivated cells or were merely inhibitory to cell growth.
2.4. Growth of two bacteria at 7 mbar, 0°C, and in a CO2-enriched anoxic atmosphere
Cells of Serratia liquefaciens (ATCC 27592) and Pseudomonas fluorescens (ATCC 13525) were grown on TSA for 16 h, streaked onto fresh TSA or TSA supplemented with (final concentrations) potassium nitrate (0.1% w/v) and glucose (0.25% w/v) (TSAGN) plates, and incubated in the polycarbonate desiccator system (Fig. 1B) maintained at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Controls were maintained at 1013 mbar, 0°C, and in CO2-enriched anoxic atmospheres. The TSA and TSAGN plates were poured with an above-average quantity of media at a volume of 35 mL per plate.
Every 7 d, the containers at 1013 and 7 mbar were opened, the growth of both species rated, and fresh AnaeroPack sachets placed within the sealed containers, as described above. Both the 7 mbar desiccator (Fig. 1B) and 1013 mbar polycarbonate containers were flushed with ultrahigh purity CO2 for 60 s before sealing the containers and adjusting the pressures. After 28 d, the plates were rated and transferred to a lab incubator for 48 h at 30°C, 1013 mbar, and aerobic O2/N2 atmosphere to determine whether the hypobaric, low-temperature, and anoxic conditions were biocidal to the vegetative cells of both species.
2.5. Spot-plate assays of bacterial strains grown under hypobaric, low-temperature, and anoxic conditions
A final confirmation for growth of S. liquefaciens under a 7 mbar, 0°C, and CO2-enriched anoxic atmosphere was performed by spot-plating all 26 strains of bacteria onto individual thick-poured TSA plates (35 mL per Petri dish) and then incubating the TSA spot-plates separately at one of the following sets of environmental conditions: (1) 48 h at 1013 mbar, O2/N2 atmosphere, and 30°C (Proteus mirabilis omitted, see below); (2) 35 d at 1013 mbar, O2/N2 atmosphere, and 0°C; (3) 35 d at 1013 mbar, CO2-enriched anoxic atmosphere, and 0°C; and (4) 35 d at 7 mbar, CO2-enriched anoxic atmosphere, and 0°C. Proteus mirabilis (strain #18 in Table 1) was omitted from the 30°C TSA plates because the aggressive growth of P. mirabilis would overgrow the entire TSA plates in 48 h; P. mirabilis was grown separately for the spot-plate assays incubated for 48 h at 30°C. Proteus mirabilis was included in all spot-plate assays incubated at 0°C. For all TSA spot-plate assays that were initially incubated 35 d at 0°C, bacteria were transferred to a lab incubator for an additional 48 h at 30°C, 1013 mbar, and aerobic O2/N2 atmosphere to document the reactivation of the environmentally inhibited cells at conducive mesophilic temperatures.
2.6. Statistical procedures
Statistical analyses were conducted with a PC-based Statistical Analysis System (SAS) version 9.2 (SAS Institute, Inc., Cary, NC, USA). Data on the growth experiments on TSA or SSA semisolid media were analyzed with analysis of variance (ANOVA) procedures (PROC GLM) followed by protected least-squares mean separation tests (P≤0.05). Data were 0.25-power transformed prior to analysis to induce homogeneity of variances. Data for the growth of B. subtilis 168 and E. coli K12 in liquid LB and LBGN media were arcsine transformed prior to being subjected to ANOVA procedures followed by protected least-squares mean separation tests (P≤0.05). Data are presented as untransformed values in all figures and tables.
2.7. Gas phase and lithographic pressure lapse rates for Mars
To predict locations in the subsurface on Mars where the pressure corresponds to the pressures used in our experiments, we modeled the increase in subsurface gas phase and lithographic pressures. Microorganisms located below the surface will experience one of two very different pressures, depending on how the subsurface niches are sealed off from the surface. If bacteria are located between regolith grains in void spaces, and if the void spaces are contiguous to the surface, the pressure is equal to that at the surface plus a contribution due to the gas column with a vertical extent equal to the depth. Almost any configuration of contiguous atmospheric conditions to the surface will result in this situation, even if the passage is very torturous and narrow because very little gas has to move through the passages to equilibrate the pressure. The void spaces in an unconsolidated matrix will exhibit the pressure behavior described above even if the grains are very fine because the percentage of void space is not a function of the size of the grains but is set only by their shape and packing configuration.
The gas-phase pressure in subsurface regolith or rock void spaces, and contiguous to the martian surface, is given by the barometric equation:
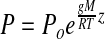 |
(1) |
where P=gas-phase pressure at depth z below the surface (in mbar); Po=pressure at the surface (mbar); g=gravity (3.71 m/s2 for Mars); M=molecular weight of the atmosphere (44 kg/kmol); R=ideal gas constant (8313 kg m2/s2 kg-mole K); T=surface temperature (K); z=depth below surface (m). The exponential relationship between depth and pressure is due to the compressibility of the gas phase. Formula 1 was used to estimate the gas-phase lapse rate for void space niches within the martian lithosphere.
If the microorganisms are sealed off from the overall atmosphere by being located within rock or ice inclusions, then the pressures within the inclusions are potentially much greater than the surrounding gases and are related to the weight of the solid overburden. The densities of ices and rocks span a range from 1000 to 5000 kg/m3, while the density of the martian atmosphere at the surface is only 0.017 kg/m3. This 5 order of magnitude difference is reflected in the much higher pressures in the lithographic phase than in the gas phase. Since the compressibility of rock and ice is very low, the lithographic pressure below the surface increases linearly with depth:
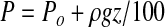 |
(2) |
where P=lithographic pressure (mbar) at depth z below the surface; Po=pressure at the surface (mbar); ρ=density of overburden (kg/m3); g=gravity (3.71 m/s2); z=depth below surface (m); and 100 converts pascals (Pa) to millibar (mbar). The lithographic lapse rate is ρg/100 mbar/m and represents the linear pressure increase per meter of depth. Formula 2 was used to estimate the lithographic lapse rate for rock or ice inclusions buried within the martian lithosphere.
3. Results
3.1. Growth of bacterial species in hypobaric, psychrophilic, and anoxic conditions
All bacteria exhibited vigorous growth at 1013 and 100 mbar when incubated at 30°C under aerobic O2/N2 conditions, but most were inhibited at 25 mbar (Table 2). Of the strains that grew at 25 mbar, visible colonies for Bacillus subtilis 168, Escherichia coli ATCC 35218, E. coli K12, Proteus mirabilis, P. cryohalolentis, and Serratia liquefaciens were consistently observed but reduced in size compared to growth at either 1013 mbar (control) or 100 mbar. In contrast, incubation under CO2-enriched anoxic atmospheres (Table 3) indicated that 12 of the 26 strains were unable to grow at any pressure tested, likely due to their obligate aerobic physiologies. Seven non-spore-forming species (including two strains of E. coli) and two spore-forming species (B. subtilis strains 168 and HA101) were able to grow in CO2 atmospheres down to 25 mbar. The most robust growth at 25 mbar under CO2 was observed for one strain of E. coli (ATCC 35218) and S. liquefaciens (ATTC 27592), in which both strains exhibited similar growth at all three pressures tested (Table 3; P>0.05). Other species that exhibited growth on TSA at 25 mbar in CO2 atmospheres were Enterococcus faecalis, Paenibacillus pabuli, P. mirabilis, and Staphylococcus aureus (ATCC 29213), although in all cases growth was much weaker than anoxic controls incubated at 1013 mbar. Several species (B. pumilus 31v3, B. pumilus 40hs1, S. aureus ATCC 33591, and S. epidermidis) exhibited weak but consistent growth at either 1013 or 100 mbar under CO2 but failed to extend growth to 25 mbar under anoxic conditions (Table 3). In all cases, rating values <0.1 were considered equivocal and resulted from averaging zero growth in some repetitions of the experiments (i.e., 0 rating) with minimal ratings of 0.1 in other repetitions.
Table 2.
Growth of Vegetative Cells of 26 Bacteria under Hypobaric O2/N2 Atmospheres
| |
O2/N2 at 30°Cy |
|
||
|---|---|---|---|---|
| Bacteria (strain number)x | 1013 mb | 100 mb | 25 mb | 1013 mb O2/N2z |
| Acinetobacter radioresistens (50v1) | 3.3 a | 2.0 b | 0 c | + |
| Bacillus megaterium (25hs1) | 3.5 a | 2.6 b | 0 c | + |
| Bacillus pumilus (31v3) | 3.5 a | 3.0 a | 0 b | + |
| Bacillus pumilus (40hs1) | 3.7 a | 3.0 a | 0 b | + |
| Bacillus subtilis (168) | 4.0 a | 2.5 b | 0.17 c | + |
| Bacillus subtilis (HA101) | 4.0 a | 3.3 b | 0 c | + |
| Burkholderia cepacia (ATCC 25608) | 2.8 a | 1.8 b | 0 c | + |
| Curtobacterium flaccumfaciens (48v2) | 3.3 a | 2.3 b | 0 c | + |
| Deinococcus radiodurans (R1) | 2.5 a | 2.0 a | 0 b | + |
| Enterococcus faecalis (ATCC 29212) | 2.8 a | 2.2 b | 0 c | + |
| Escherichia coli (ATCC 35218) | 2.8 a | 2.5 b | 0.5 c | + |
| Escherichia coli (K12) | 3.5 a | 3.0 b | 0.13 c | + |
| Microbacterium oleivorans (27v1b) | 3.5 a | 2.3 b | 0 c | + |
| Microbacterium oleivorans (32v4) | 2.3 a | 1.7 a | 0 b | + |
| Microbacterium testaceum (27v1a) | 1.8 a | 1.5 a | 0 b | + |
| Micrococcus luteus (ACS-22) | 2.8 a | 2.0 a | 0 b | + |
| Paenibacillus pabuli (ACS-6) | 4.0 a | 3.2 b | 0 c | + |
| Proteus mirabilis (ATCC 7002) | 3.0 a | 2.5 b | 0.5 c | + |
| Pseudomonas aeruginosa (ATCC 27853) | 3.8 a | 2.5 b | 0 c | + |
| Pseudomonas fluorescens (ATCC 13525) | 3.1 a | 1.7 b | 0 c | + |
| Psychrobacter cryohalolentis (K5) | 2.0 a | 1.5 a | 0.42 b | + |
| Serratia liquefaciens (ATCC 27592) | 3.3 a | 2.3 b | 0.67 c | + |
| Sporosarcina aquamarina (SAFN 008) | 3.1 a | 2.5 a | 0 b | + |
| Staphylococcus aureus (ATCC 29213) | 3.0 a | 2.0 b | 0 c | + |
| Staphylococcus aureus (ATCC 33591) | 2.5 a | 1.8 a | 0 b | + |
| Staphylococcus epidermidis (ATCC 14990) | 2.3 a | 2.0 a | 0 b | + |
All bacteria were grown 48 h on trypticase soy agar (TSA) at 30°C, except Psychrobacter cryohalolentis K5, which was grown on sea-salt agar (SSA) at 24°C. Bacteria were incubated under an aerobic O2/N2 (21%/78%) atmosphere and pressures of 1013, 100, or 25 mbar.
Bacterial growth for each strain was rated using a 5×pair of jeweler's magnifying glasses. Growth was rated in only the 2nd or 3rd quadrants of three-quadrant streaked plates; the 1st quadrant was ignored in order to avoid false positives. Rating scale: 4=large robust colonies>5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies<0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. Letters in rows designate significant differences among pressure treatments for individual species based on ANOVA and protected least-squares mean separation tests (P≤0.05; n=3).
At the end of the assays, all cultures were transferred to lab conditions for an additional 48 h of incubation at 30°C, 1013 mbar, and O2/N2 atmosphere. In general, bacteria inhibited at low pressures grew normally under lab conditions and were not killed by exposure to hypobaric and aerobic conditions.
Table 3.
Growth of Vegetative Cells of 26 Bacteria under Hypobaric CO2 Atmospheres
| |
CO2 at 30°Cy |
|
||
|---|---|---|---|---|
| Bacteria (strain number)x | 1013 mb | 100 mb | 25 mb | 1013 mb O2/N2z |
| Acinetobacter radioresistens (50v1) | 0 | 0 | 0 | + |
| Bacillus megaterium (25hs1) | 0.03 | 0 | 0 | + |
| Bacillus pumilus (31v3) | 0.1 a | 0 b | 0 b | + |
| Bacillus pumilus (40hs1) | 0.3 a | 0.03 ab | 0 b | + |
| Bacillus subtilis (168) | 0.7 a | 0.6 a | 0.25 b | + |
| Bacillus subtilis (HA101) | 0.6 a | 0.4 b | 0.07 c | + |
| Burkholderia cepacia (ATCC 25608) | 0 | 0 | 0 | + |
| Curtobacterium flaccumfaciens (48v2) | 0 | 0 | 0 | + |
| Deinococcus radiodurans (R1) | 0 | 0 | 0 | + |
| Enterococcus faecalis (ATCC 29212) | 1.7 ab | 2.2 a | 0.2 c | + |
| Escherichia coli (ATCC 35218) | 2.0 a | 2.0 a | 2.0 a | + |
| Escherichia coli (K12) | 1.2 a | 0.9 ab | 0.5 b | + |
| Microbacterium oleivorans (27v1b) | 0 | 0 | 0 | + |
| Microbacterium oleivorans (32v4) | 0 | 0 | 0 | + |
| Microbacterium testaceum (27v1a) | 0 | 0 | 0 | + |
| Micrococcus luteus (ACS-22) | 0 | 0 | 0 | + |
| Paenibacillus pabuli (ACS-6) | 1.2 a | 1.3 a | 0.2 b | + |
| Proteus mirabilis (ATCC 7002) | 1.7 a | 1.1 a | 1.0 a | + |
| Pseudomonas aeruginosa (ATCC 27853) | 0 | 0 | 0 | + |
| Pseudomonas fluorescens (ATCC 13525) | 0 | 0 | 0 | + |
| Psychrobacter cryohalolentis (K5) | 0 | 0 | 0 | + |
| Serratia liquefaciens (ATCC 27592) | 2.0 a | 1.7 a | 1.8 a | + |
| Sporosarcina aquamarina (SAFN 008) | 0 | 0 | 0 | + |
| Staphylococcus aureus (ATCC 29213) | 1.1 a | 1.0 a | 0.12 b | + |
| Staphylococcus aureus (ATCC 33591) | 1.5 a | 0.7 b | 0 c | + |
| Staphylococcus epidermidis (ATCC 14990) | 0.8 a | 0.35 b | 0 c | + |
All bacteria were grown 48 h on trypticase soy agar (TSA) at 30°C, except Psychrobacter cryohalolentis K5, which was grown on sea-salt agar (SSA) at 24°C. Bacteria were incubated under an anaerobic CO2 atmosphere and low pressures of 1013, 100, or 25 mbar.
Bacterial growth for each strain was rated using a 5×pair of jeweler's magnifying glasses. Growth was rated in only the 2nd or 3rd quadrants of three-quadrant streaked plates; the 1st quadrant was ignored in order to avoid false positives. Rating scale: 4=large robust colonies>5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies<0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. Letters in rows designate significant differences among pressure treatments for individual species based on ANOVA and protected least-squares mean separation tests (P≤0.05; n=3).
At the end of the assays, all cultures were transferred to lab conditions for an additional 48 h of incubation at 30°C, 1013 mbar, and O2/N2 atmosphere. In general, bacteria inhibited at low pressures under CO2 atmospheres grew normally under lab conditions and were not killed by exposure to hypobaric and anoxic conditions.
After the completion of the 48 h hypobaric assays, all cultures from both O2/N2 and CO2 conditions were incubated an additional 48 h at 1013 mbar, 30°C, and under lab O2/N2 atmospheres in order to determine whether the hypobaric conditions had killed the bacteria. In general, bacteria that were returned to lab conditions resumed vigorous growth (columns 4, Tables 2 and 3) and appeared similar to colony morphotypes in size, number, and color as observed for the assays performed at 1013 mbar reported in column one in Tables 2 and 3.
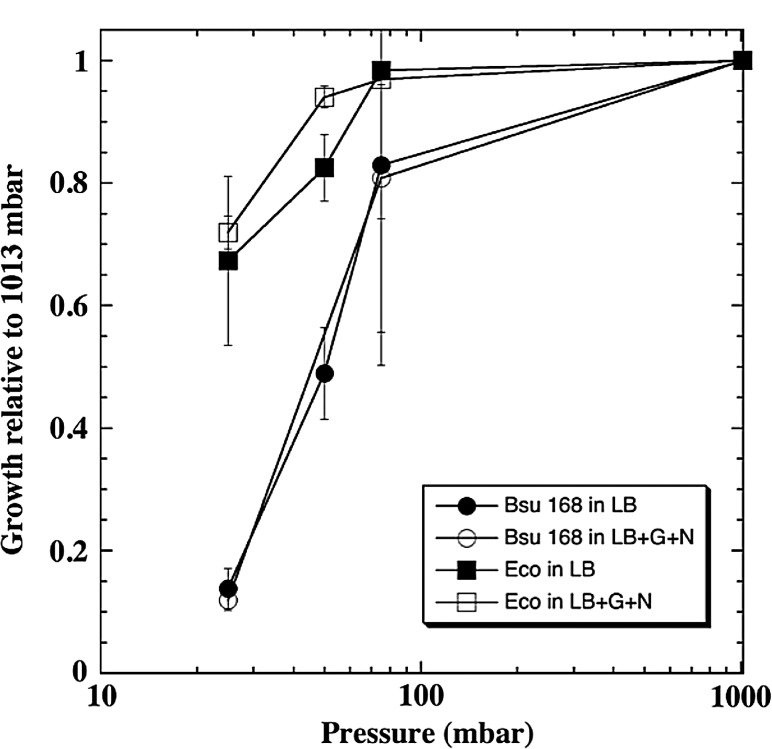
Conducted in parallel with the TSA and SSA assays described above (Tables 2 and 3), growth of vegetative cells of B. subtilis 168 and E. coli K12 suspended in liquid LB or LBGN media showed that, below 100 mbar, growth was progressively inhibited for both species with low pressure inhibiting the growth rates for 168 more than for K12 (Fig. 2). It was postulated that a reduction in the concentration of dissolved oxygen at low pressures may have been the cause for inhibiting growth of both bacteria. To test this hypothesis, LB media was supplemented with glucose (to allow fermentation) and nitrate (to permit anaerobic respiration) (LBGN medium), and the liquid-culture-based hypobaric assays were repeated with 168 and K12. The LBGN growth rates were similar to those observed for the LB alone assays for all pressures tested (Fig. 2; P>0.10). Growth of B. subtilis 168 at 25 mbar was near the lower limit of detection in the LB liquid assay. The sensitivities to low pressures of both species were very similar between the TSA solid media (Table 2) and LB liquid media (Fig. 2) assays, except that the growth rates for 168 were significantly more reduced than for K12 at low pressures in the liquid LB assays than in the TSA semisolid agar assays. We interpret these responses to be evidence of a direct pressure effect on B. subtilis 168 and E. coli K12 under hypobaric conditions.
FIG. 2.
Growth of Bacillus subtilis 168 (Bsu) and Escherichia coli K12 (Eco) in liquid culture under hypobaric conditions. Vegetative cells of both species were grown for 24 h at 30°C in Luria-Bertani (LB) liquid media with and without added glucose (0.25%) and nitrate (0.1%) (LB+G+N). Bacteria were grown at pressures of 1013, 75, 50, or 25 mbar. Data were normalized to growth rates at an Earth-normal sea-level pressure of 1013 mbar (n=3; P≤0.05; bars=standard deviations).
In a second series of experiments, all 26 strains of bacteria were tested for growth at 0°C under either aerobic (O2/N2) or anoxic (CO2-enriched) atmospheres in order to investigate the interactive effects of low-ppO2 and low-temperature conditions. Only eight of 26 strains exhibited the ability to grow at 0°C under aerobic conditions (Table 4), and only two strains demonstrated growth when incubated for 35 d under the combination of 0°C and CO2-enriched anoxic atmospheres (Table 5). Serratia liquefaciens ATCC 27592 was the only strain capable of replicative growth under separately tested hypobaric (down to 25 mbar; Table 2), psychrophilic (0°C; Table 4), and anoxic (CO2-enriched atmospheres; Tables 3 and 5) conditions. Vigorous growth was observed at 0°C for the strains Pseudomonas fluorescens, P. cryohalolentis, S. liquefaciens, and Sporosarcina aquamarina when cultures were incubated for 35 d under aerobic conditions (Table 4), but only P. fluorescens and S. liquefaciens could tolerate both anoxic and psychrophilic conditions (Table 5). Interestingly, P. fluorescens (considered an obligate aerobe; Holt et al., 1994) yielded very small but observable colonies when incubated for 35 d under anoxic conditions at 0°C (Table 5). In earlier short-term 48 h tests (Table 3), P. fluorescens failed to grow under a CO2 atmosphere at 1013 mbar. Although some strains of P. fluorescens can use nitrate as a terminal electron acceptor (Holt et al., 1994), the addition of 0.1% potassium nitrate failed to enhance growth at 7 mbar (Table 6). And finally, four strains (E. coli ATCC 35218, Micrococcus luteus, Paenibacillus pabuli, and S. aureus ATCC 33591) exhibited weak or inconsistent growth at 0°C under aerobic conditions (Table 4), but all failed to yield observable microcolonies when the concomitant factors of 0°C and anoxia were tested together (Table 5).
Table 4.
Growth of Vegetative Cells of 26 Bacteria at 0°C for 35 d under Aerobic O2/N2 Atmospheres
| |
O2/N2 at 0°Cy |
|
|||
|---|---|---|---|---|---|
| Bacteria (strain number)x | 7 days | 14 days | 28 days | 35 days | 30°C in O2/N2z |
| Acinetobacter radioresistens (50v1) | 0y | 0 | 0 | 0 | 3.4 |
| Bacillus megaterium (25hs1) | 0 | 0 | 0 | 0 | 3.3 |
| Bacillus pumilus (31v3) | 0 | 0 | 0 | 0 | 3.7 |
| Bacillus pumilus (40hs1) | 0 | 0 | 0 | 0 | 3.2 |
| Bacillus subtilis (168) | 0 | 0 | 0 | 0 | 3.3 |
| Bacillus subtilis (HA101) | 0 | 0 | 0 | 0 | 3.2 |
| Burkholderia cepacia (ATCC 25608) | 0 | 0 | 0 | 0 | 3.3 |
| Curtobacterium flaccumfaciens (48v2) | 0 | 0 | 0 | 0 | 3.3 |
| Deinococcus radiodurans (R1) | 0 | 0 | 0 | 0 | 2.0 |
| Enterococcus faecalis (ATCC 29212) | 0 | 0 | 0 | 0 | 2.5 |
| Escherichia coli (ATCC 35218) | 0.07 b | 0.13 b | 0.13 b | 0.27 b | 4.0 a |
| Escherichia coli (K12) | 0 | 0 | 0 | 0 | 3.8 |
| Microbacterium oleivorans (27v1b) | 0 | 0 | 0 | 0 | 2.5 |
| Microbacterium oleivorans (32v4) | 0 | 0 | 0 | 0 | 2.3 |
| Microbacterium testaceum (27v1a) | 0 | 0 | 0 | 0 | 2.5 |
| Micrococcus luteus (ACS-22) | 0 b | 0 b | 0 b | 0.07 b | 2.7 a |
| Paenibacillus pabuli (ACS-6) | 0 b | 0 b | 0.10 b | 0.25 b | 4.0 a |
| Proteus mirabilis (ATCC 7002) | 0 | 0 | 0 | 0 | 4.0 |
| Pseudomonas aeruginosa (ATCC 27853) | 0 | 0 | 0 | 0 | 4.0 |
| Pseudomonas fluorescens (ATCC 13525) | 0.6 d | 1.6 c | 2.7 b | 3.7 a | 4.0 a |
| Psychrobacter cryohalolentis (K5) | 0.7 d | 1.5 c | 2.0 bc | 2.8 b | 3.3 a |
| Serratia liquefaciens (ATCC 27592) | 0.4 c | 0.7 c | 1.6 bc | 2.8 b | 4.0 a |
| Sporosarcina aquamarina (SAFN 008) | 0.07 e | 0.5 d | 1.4 c | 2.6 b | 4.0 a |
| Staphylococcus aureus (ATCC 29213) | 0 | 0 | 0 | 0 | 3.5 |
| Staphylococcus aureus (ATCC 33591) | 0 b | 0 b | 0.07 b | 0.07 b | 2.8 a |
| Staphylococcus epidermidis (ATCC 14990) | 0 | 0 | 0 | 0 | 2.8 |
Bacteria were grown for 35 d on trypticase soy agar (TSA) at 0°C, except Psychrobacter cryohalolentis K5, which was grown on sea-salt agar (SSA). Bacteria were incubated under an aerobic O2/N2 (21%/78%) atmosphere and rated on 7, 14, 28, and 35 d.
Bacterial growth for each strain was rated using a 5×pair of jeweler's magnifying glasses. Growth was rated in only the 2nd or 3rd quadrants of three-quadrant streaked plates; the 1st quadrant was ignored in order to avoid false positives. Rating scale: 4=large robust colonies>5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies<0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. Letters in rows designate significant differences among time steps for individual species based on ANOVA and protected least-squares mean separation tests (P≤0.05; n=3).
At the end of the assays, all cultures were transferred to lab conditions for an additional 48 h of incubation at 30°C, 1013 mbar, and O2/N2 atmosphere. In general, bacteria grew normally under lab conditions and were not killed by exposure to low-temperature aerobic atmospheres.
Table 5.
Growth of Vegetative Cells of 26 Bacteria at 0°C for 35 d under Anoxic CO2 Atmospheres
| |
CO2 at 0°Cy |
|
|||
|---|---|---|---|---|---|
| Bacteria (strain number)x | 7 days | 14 days | 28 days | 35 days | 30°C in O2/N2z |
| Acinetobacter radioresistens (50v1) | 0 | 0 | 0 | 0 | 2.6 |
| Bacillus megaterium (25hs1) | 0 | 0 | 0 | 0 | 3.3 |
| Bacillus pumilus (31v3) | 0 | 0 | 0 | 0 | 3.7 |
| Bacillus pumilus (40hs1) | 0 | 0 | 0 | 0 | 4.0 |
| Bacillus subtilis (168) | 0 | 0 | 0 | 0 | 4.0 |
| Bacillus subtilis (HA101) | 0 | 0 | 0 | 0 | 3.3 |
| Burkholderia cepacia (ATCC 25608) | 0 | 0 | 0 | 0 | 2.9 |
| Curtobacterium flaccumfaciens (48v2) | 0 | 0 | 0 | 0 | 2.3 |
| Deinococcus radiodurans (R1) | 0 | 0 | 0 | 0 | 2.2 |
| Enterococcus faecalis (ATCC 29212) | 0 | 0 | 0 | 0 | 2.2 |
| Escherichia coli (ATCC 35218) | 0 | 0 | 0 | 0 | 4.0 |
| Escherichia coli (K12) | 0 | 0 | 0 | 0 | 3.6 |
| Microbacterium oleivorans (27v1b) | 0 | 0 | 0 | 0 | 1.7 |
| Microbacterium oleivorans (32v4) | 0 | 0 | 0 | 0 | 2.2 |
| Microbacterium testaceum (27v1a) | 0 | 0 | 0 | 0 | 2.7 |
| Micrococcus luteus (ACS-22) | 0 | 0 | 0 | 0 | 1.7 |
| Paenibacillus pabuli (ACS-6) | 0 | 0 | 0 | 0 | 3.7 |
| Proteus mirabilis (ATCC 7002) | 0 | 0 | 0 | 0 | 4.0 |
| Pseudomonas aeruginosa (ATCC 27853) | 0 | 0 | 0 | 0 | 4.0 |
| Pseudomonas fluorescens (ATCC 13525) | 0 c | 0 c | 0.10 b | 0.22 b | 3.3 a |
| Psychrobacter cryohalolentis (K5) | 0 | 0 | 0 | 0 | 2.0 |
| Serratia liquefaciens (ATCC 27592) | 0 d | 0.07 c | 0.25 c | 0.62 b | 3.5 a |
| Sporosarcina aquamarina (SAFN 008) | 0 | 0 | 0 | 0 | 3.2 |
| Staphylococcus aureus (ATCC 29213) | 0 | 0 | 0 | 0 | 3.3 |
| Staphylococcus aureus (ATCC 33591) | 0 | 0 | 0 | 0 | 2.3 |
| Staphylococcus epidermidis (ATCC 14990) | 0 | 0 | 0 | 0 | 2.3 |
Bacteria were grown for 35 d on trypticase soy agar (TSA) at 0°C, except Psychrobacter cryohalolentis K5, which was grown on sea-salt agar (SSA). Bacteria were incubated under an anaerobic CO2 atmosphere and rated on 7, 14, 28, and 35 d.
Bacterial growth was rated using a 5×pair of jeweler's magnifying glasses. Growth was rated in only the 2nd or 3rd quadrants of three-quadrant streaked plates; the 1st quadrant was ignored in order to avoid false positives. Rating scale: 4=large robust colonies>5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies<0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. All bacteria were grown on trypticase soy agar (TSA). Letters in rows designate significant differences among time steps for individual species based on ANOVA and protected least-squares mean separation tests (P≤0.05; n=3).
At the end of the assays, all cultures were transferred to lab conditions for an additional 48 h of incubation at 30°C, 1013 mbar, and O2/N2 atmosphere. In general, bacteria grew normally under lab conditions and were not killed by exposure to low-temperature anoxic atmospheres.
Table 6.
Growth of Vegetative Cells of Pseudomonas fluorescens ATCC 13525 and Serratia liquefaciens ATCC 27592 for 28 d at 7 mbar, 0°C, and CO2-Enriched Anoxic Atmospheres
| |
|
Growthy |
|
|||
|---|---|---|---|---|---|---|
| Bacteriax | Pressure (mbar) | 7 days | 14 days | 21 days | 28 days | 30°C in O2/N2z |
| TSA | ||||||
| Pseudomonas fluorescens | 1013 | 0 c | 0 c | 0.10 b | 0.13 b | 1.9 a |
| 7 | 0 | 0 | 0 | 0 | 2.1 | |
| Serratia liquefaciens | 1013 | 0 d | 0.10 c | 0.20 bc | 0.20 b | 2.5 a |
| 7 | 0 d | 0.17 c | 0.26 c | 0.37 b | 2.6 a | |
| TSAGN | ||||||
| Pseudomonas fluorescens | 1013 | 0 c | 0 c | 0.10 b | 0.15 b | 1.9 a |
| 7 | 0 | 0 | 0 | 0 | 2.1 | |
| Serratia liquefaciens | 1013 | 0 d | 0.10 c | 0.20 bc | 0.23 b | 2.4 a |
| 7 | 0 d | 0.20 c | 0.20 c | 0.37 b | 2.6 a | |
Bacteria were grown for 28 d on trypticase soy agar (TSA) or TSA supplemented with 0.25% glucose and 0.1% potassium nitrate (TSAGN) at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Bacteria were rated on 7, 14, 21, and 28 d.
Bacterial growth was rated using a 5×pair of jeweler's magnifying glasses. Growth was rated in only the 2nd or 3rd quadrants of three-quadrant streaked plates; the 1st quadrant was ignored in order to avoid false positives. Rating scale: 4=large robust colonies>5 mm in diameter; 3=colonies 2–4 mm in diameter; 2=colonies ∼1 mm in diameter; 1=colonies ∼0.5 mm in diameter; 0.50=colonies<0.5 mm in diameter; 0.1=smallest visually discernible colonies (pin-prick-sized colonies at ∼0.1 mm in diameter); 0=no growth. All bacteria were grown on double-thick trypticase soy agar (TSA; 35 mL per plate). Data were 0.25-power transformed prior to analysis to induce homogeneity of variances. Letters in rows designate significant differences among time steps for individual species based on ANOVA and protected least-squares mean separation tests (P≤0.05; n=3).
At the end of the assays, all cultures were transferred to lab conditions for an additional 48 h of incubation at 30°C, 1013 mbar, and O2/N2 atmosphere. In general, bacteria grew normally under lab conditions and were not killed by exposure to hypobaric, low-temperature, or anoxic atmospheres.
When cultures were returned to lab conditions of 1013 mbar, 30°C, and O2/N2 atmospheres, all bacteria exhibited near-normal growth. Results suggest that zero ratings observed in Tables 4 and 5 during the 35 d experiments were not due to inactivation of vegetative cells but instead demonstrated that growth of the bacteria were inhibited, which is consistent with earlier observations (Schuerger and Nicholson, 2006; Nicholson et al., 2010).
3.2. Growth of Serratia liquefaciens in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres
Growth was observed for S. liquefaciens, but not P. fluorescens, when cells were maintained for 28 d in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres (Table 6). The growth of S. liquefaciens was slightly higher at 7 than at 1013 mbar when rated at 28 d under the experimental conditions tested. Cells of both bacteria grew vigorously when cultures were returned to conditions of 1013 mbar, 30°C, and O2/N2 atmospheres for 48 h (Table 6). However, the final rating values assigned to microbial growth of both species after 48 h at 1013 mbar, 30°C, and O2/N2 atmospheres were slightly lower than similar assays for the experiments presented in Table 5, indicating that the additional stress of hypobaria might have impaired the recovery of metabolic activity and cellular growth. For both species, there were no effects noted for microbial growth between cultures grown on TSA versus TSAGN.
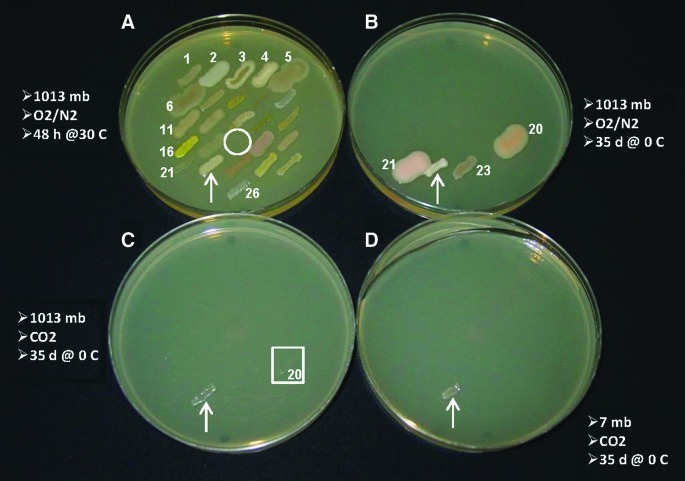
Spot-plate assays were used to confirm that only S. liquefaciens was capable of growth in a 7 mbar, 0°C, and CO2-enriched anoxic atmosphere (Fig. 3D; arrow). No other bacterial strains exhibited visually discernible growth on the TSA spot-plate assays incubated at 7 mbar. Consistent with the data in Tables 5 and 6, cells of S. liquefaciens (Fig. 3C, arrow) and P. fluorescens (Fig. 3C, box) were observed to grow on TSA incubated in a 1013 mbar, 0°C, and CO2-enriched atmosphere, although the growth of P. fluorescens was very weak (i.e., barely observed in Fig. 3C, square). Furthermore, the spot-plate assays of all bacterial strains grown at 1013 mbar, 0°C, but in an O2/N2 atmosphere were consistent with data presented in Table 4, in which P. fluorescens, P. cryohalolentis, S. liquefaciens, and Sporosarcina aquamarina exhibited strong and clear visible growth under similar conditions (Fig. 3B). Although not discernible in the digital image in Fig. 4B, direct viewing with the 5× jeweler's glasses revealed weak but observable growth for E. coli ATCC 35218 and P. pabuli, incubated in a 1013 mbar, 0°C, and O2/N2 atmosphere. In contrast to the results presented above, spot-plate assays of all strains incubated 48 h in 1013 mbar, 30°C, and O2/N2 atmospheres (Fig. 3A) demonstrated normal growth for all strains consistent with the results given in Table 2 (column 1), and for Tables 4, 5, and 6 (column 5).
FIG. 3.
Growth of 26 strains of 22 bacterial species under hypobaric, psychrophilic, and anoxic conditions. Spot-plate assays with bacterial strains were laid out in 5×5 grids with strain 26 placed below the grids; numbers (#s) coincide with bacterial strains listed in Table 1. Arrows indicate locations of Serratia liquefaciens (strain #22). (A) Lab controls were incubated for 48 h at 1013 mbar, 30°C, in a standard 21%/78% O2/N2 atmosphere. The circle denotes the location where Proteus mirabilis was omitted from the lab conditions because the bacterium would normally overgrow the TSA plates in 48 h when incubated at 30°C. Proteus mirabilis was included in the spot-plate assays maintained 35 d at 0°C under nonstandard conditions (B), (C), and (D). Environmental conditions for assays are given adjacent to the TSA plates. Only S. liquefaciens exhibited obvious growth in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres (D). The box labeled #20 (C) includes subtle but observable growth of Pseudomonas fluorescens. Results presented here, and Fig. 4, confirm the results presented in Tables 4, 5, and 6. Color images available online at www.liebertonline.com/ast
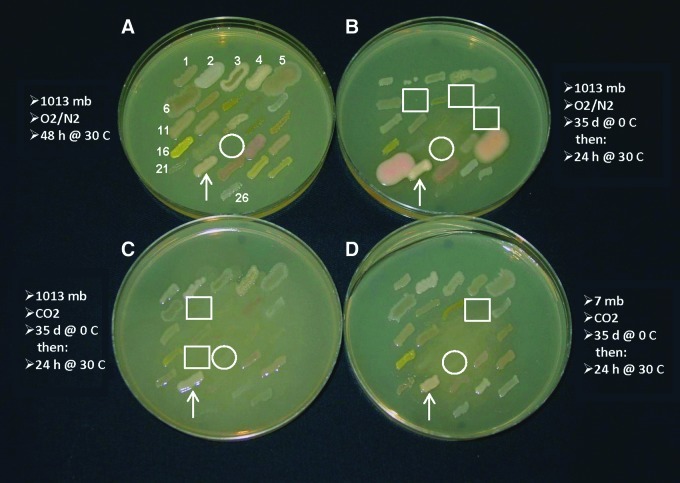
FIG. 4.
Growth of 26 strains of 22 bacterial species previously exposed to hypobaric, psychrophilic, and anoxic conditions (Fig. 3) were transferred to lab conditions and incubated an additional 48 h at 1013 mbar, 30°C, and an Earth-normal O2/N2 atmosphere. Arrows indicate the location of Serratia liquefaciens. Circles indicate the locations of the omitted Proteus mirabilis for the 30°C (A), and the included P. mirabilis for the 0°C assay plates (i.e., B, C, and D). Note how P. mirabilis overgrows the other bacterial strains when spot-plates were transferred from 0°C to 30°C for only 24 h (current image). Boxes indicate bacterial strains in which growth was not observed after 24 h incubation at 30°C, but in all cases bacterial growth was observed for the box-marked strains when the TSA plates were incubated an additional 24 h (data not shown). The spot-plates shown here are the exact same plates presented in Fig. 3. Results presented here, and Fig. 3, confirm the results presented in Tables 4, 5, and 6. Color images available online at www.liebertonline.com/ast
Following incubation for 35 d at various conditions maintained at 0°C, spot-plate TSA assays were transferred to a 1013 mbar, 30°C, and O2/N2 atmosphere for an additional 48 h of incubation (Fig. 4). In all cases, bacterial growth recovered by the end of the 48 h incubation period and appeared to produce normal-looking colonies. The digital image in Fig. 4 was taken after only 24 h of incubation at 30°C to minimize the overgrowth of the TSA spot-plate assays by P. mirabilis (circles in Fig. 4). The boxes in Fig. 4 represent bacterial strains that exhibited growth at 48 h but were obscured by P. mirabilis at 24 h, or strains that were not yet reactivated at 24 h.
3.3. Atmospheric and lithographic lapse rates for pressure
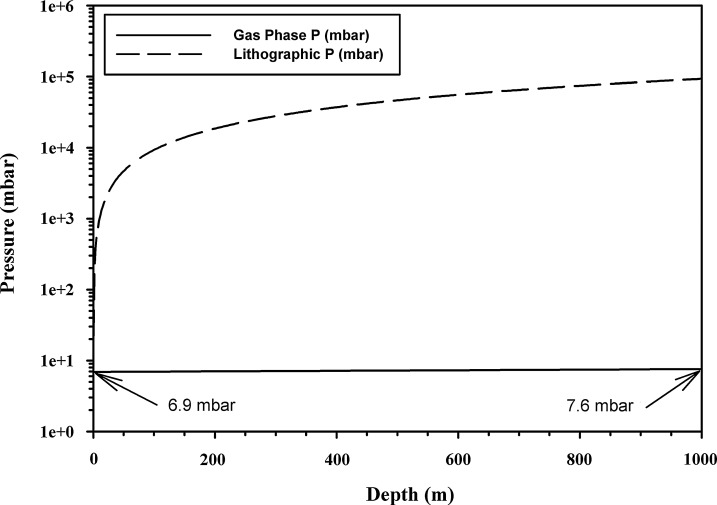
Equations 1 and 2 were used to estimate the gas-phase and the lithographic lapse rates, respectively, encountered as depth increases in the martian lithosphere. The gas-phase lapse rate changes very slowly and was estimated as an increase of 1 mbar per 1450 m descent in the upper 2 km of the lithosphere (Fig. 5). The gas-phase lapse rate is an exponential function; thus, with a surface pressure of 6.9 mbar, 13.8 km depth is required to reach 25 mbar. In contrast, the lithographic lapse rate changes much more quickly (Fig. 5). When using a density of 2500 kg/m2 and Mars gravity, the lithographic lapse rate is 92.8 mbar/m; the most rapid increase in lithographic pressure occurs from the surface down to 200 m, after which it increases more slowly with increased depth. The depth for the lithographic pressure to reach 25 mbar is only 19.5 cm as opposed to 13.8 km for the gas-phase pressure. Also using Eq. 1, we estimate that the pressure range on the exposed surface of Mars is 0.95 mbar at the top of Mt. Olympus and 14.8 mbar at the deepest point in Hellas Basin. Changes in lithographic pressure are mostly independent of gas pressure; thus, lithographic pressures of 25 mbar would occur at approximately 20 cm depth at each of the two extremes on Mars given here, and for any location in between.
FIG. 5.
Atmospheric gas-phase (i.e., for void spaces) and lithographic (i.e., for salt, or ice inclusions) pressure lapse rates for Mars. The atmospheric gas-phase pressure increases very slowly with increasing depth in the martian lithosphere and reaches 25 mbar at a depth of 13.8 km below the martian datum. In contrast, the lithographic pressure for salt or ice inclusions in the lithosphere can achieve 25 mbar at 19.5 cm of overburden depth. The lithographic pressure is entirely dependent upon the microbial niche being completely (i.e., 100%) sealed from outgassing; otherwise, the niche would equilibrate to the atmospheric pressure predicted by the gas-phase lapse rate.
4. Discussion
4.1. Growth of Serratia liquefaciens at 7 mbar
With the experiments outlined herein, we systematically examined the growth of 26 strains of 22 bacterial species under three physical conditions within the many environmental factors found at the surface of Mars, that is, low pressure (7 mbar), low temperature (0°C), and CO2-dominated anoxic atmosphere. Most bacterial species were selected for their previously described occurrence in spacecraft assembly facilities (Venkateswaran et al., 2001; La Duc et al., 2007, 2009; Newcombe et al., 2008; Ghosh et al., 2010; Probst et al., 2010), Mars spacecraft (Puleo et al., 1977; La Duc et al., 2003, 2004a), or human-rated space vehicles (Puleo et al., 1973; Pierson et al., 1993; Novikova, 2004). Deinococcus radiodurans R1 and Psychrobacter cryohalolentis K5 were included as extremophilic species that have been previously tested for survival or growth under partially simulated martian conditions (Pogoda de la Vega et al., 2007; Smith et al., 2009). The primary objective was to determine whether any of the plausible spacecraft contaminants or extremophiles might grow under concomitant conditions of hypobaria, low temperature, and anoxia similar to the ranges periodically encountered on the martian surface.
Of the 26 bacterial strains tested, only Serratia liquefaciens ATCC 27592 was found to be capable of growth under the concomitant conditions of 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. As each stress condition was added to the assays, fewer strains and species were observed to grow. Thus, the interactive conditions of hypobaria, low temperature, and CO2-enriched anoxic atmospheres worked additively or synergistically to prohibit growth of all strains tested except S. liquefaciens. The three stress factors of 7 mbar, 0°C, and CO2-enriched anoxic atmosphere were chosen in the current study because it was postulated that growth of terrestrial microorganisms must be possible under at least these three simultaneous conditions to plausibly permit growth of terrestrial bacteria on Mars.
Although S. liquefaciens was shown here to grow in hypobaric environments down to 7 mbar, there were several assumptions in the current study that are not easily met on current-day Mars. The assumptions adopted for the current work included the following: (A1) bacteria were shielded from biocidal UV irradiation, (A2) bacteria were continuously exposed to stable hydrated substrates rich in carbon and nitrogen sources, and (A3) the habitable niche was protected from rapid evaporation typically encountered on the martian surface. Thus, S. liquefaciens required a stable hydrated growth matrix maintained continuously at 7 mbar and 0°C for at least 14 d before obvious growth of microcolonies was observed (Table 6). The thermodynamics of liquid water on the martian surface (Haberle et al., 2001; Sears and Moore, 2005) argue against such a long-term stable environment on current-day Mars, and carbon- and nitrogen-rich media have not yet been observed for the martian surface (Stoker et al., 2010).
Based on the data presented here, we propose that future research on the growth of terrestrial microorganisms under conditions relevant to the surface of Mars be conducted using, at minimum, the three concomitant parameters tested here: namely, 7 mbar (median surface pressure on Mars), 0°C (temperature required for stable liquid water at 7 mbar), and CO2-enriched anoxic atmospheres (95.5% CO2 in the martian atmosphere). As terrestrial microorganisms are identified that can grow on hydrated carbon- and nitrogen-rich media under at least these three environmental conditions, subsequent experiments with other biocidal or inhibitory factors become more relevant for Mars. For example, in an earlier study in our lab (Smith et al., 2009) the salt-tolerant psychrophile Psychrobacter cryohalolentis K5 was used to explore the survival of an extremophile under simulated martian UV fluence rates, pressures, and temperatures. The work was initiated as a set of preliminary experiments that were intended to be a prelude to microbial evolution studies under hydrated martian conditions. However, in the current study, P. cryohalolentis K5 was observed to be incapable of anoxic growth (Tables 3 and 5; Fig. 3). Thus, although the Smith et al. (2009) study was interesting in the sense that it did examine microbial survival in salt encrustations under partially simulated martian conditions, it was not an effective preliminary study for microbial growth experiments under martian conditions because the bacterium failed to grow under any of the anoxic conditions tested here. Furthermore, the extremophile Deinococcus radiodurans R1 has been suggested as a candidate species for astrobiology experiments relevant to Mars (Diaz and Schulze-Makuch, 2006; Pogoda de la Vega et al., 2007) but was found here to be incapable of growth at pressures below 100 mbar (Table 2; Fig. 3), at 0°C (Table 4; Fig. 3), or under CO2-enriched anoxic atmospheres (Tables 3 and 5; Fig. 3). Thus, two extremophiles that might be predicted to survive, grow, and evolve better than non-extremophilic species on Mars have in fact been shown here to be incapable of growth under individual or interacting combinations of three environmental conditions found on the martian surface.
What is also intriguing from the current study is that the non-extremophilic bacterium Serratia liquefaciens was observed to possess the metabolic range to carry out growth in 7 mbar, 0°C, and CO2-enriched anaerobic atmospheres, even though it logically evolved as an environmental microorganism under mesophilic temperatures and at pressures closer to 1013 mbar. The terrestrial ecology of Serratia liquefaciens indicates that it is present in diverse ecosystems including plant rhizospheres, fruits, grasses, and vegetables (Grimont et al., 1981; Lalande et al., 1989; Ashelford et al., 1999); processed foods (Lindberg et al., 1998; Oh et al., 2001); meats (Stiles and Ng, 1981; Lindberg et al., 1998); as opportunistic pathogens in fish (Aydin et al., 2001) and the human bloodstream (Grohskopf et al., 2001); and in drinking water on the International Space Station (La Duc et al., 2004b). Heretofore, a significant percentage of microbial astrobiology literature relevant to Mars (see Gilmour et al., 2003; Horneck and Baumstark-Khan, 2002) has emphasized research on extremophilic microorganisms that have adapted to harsh environmental conditions found in deserts, polar regions, salts, hydrothermal vents, and acidic environments. But based on the success of S. liquefaciens cells growing at 7 mbar, 0°C, and anoxic conditions, perhaps species that are not tightly constrained ecologically but persist in a wide range of diverse ecosystems should be considered along with extremophiles for growth and evolution research relevant to Mars.
Adaptation to narrow ecological environments has likely forced microbial evolution along ever-tightening sets of environmentally constrained conditions that facilitated the occurrence of extremophiles (i.e., specialists) and their capacity for growth under precise sets of harsh conditions but did not permit them to easily adapt to new conditions. In contrast, species capable of broad-based ecological adaptation (i.e., generalists) may have developed the genomic and metabolic pathways to immediately thrive in new and unusual conditions without requiring the specialization of extremophiles. In support of the concept that generalist species with broad ecological ranges may exhibit greater adaptive advantage to hypobaric, psychrophilic, and anoxic conditions than specialist extremophilic species are the following observations: (1) besides S. liquefaciens, only P. fluorescens (soil and plant aerobe) exhibited growth under the concomitant conditions of 0°C and a CO2-enriched anoxic atmosphere (Table 5); (2) E. coli ATCC 35218 (human gut microbe), Proteus mirabilis (human pathogen), and S. liquefaciens exhibited similar growth ratings from 1013 to 25 mbar in CO2-enriched anoxic atmospheres, whereas the other facultative anaerobic species exhibited reduced vigor at lower pressures (Table 3); (3) the extremophilic species of Deinococcus radiodurans and Psychrobacter cryohalolentis failed to grow in CO2-enriched anoxic atmospheres (Tables 3 and 5); and (4) the generalist Bacillus subtilis has been shown to be capable of rapid evolution under hypobaric conditions (Nicholson et al., 2010). The unexpected success of S. liquefaciens at 7 mbar suggests that ecological breadth, and not narrowness, might be a key factor in modeling the potential growth of terrestrial microorganisms on Mars.
4.2. Other unexpected results for microorganisms grown under hypobaric conditions
Several unexpected results were observed in the current study. First, of the species that grew at 25 mbar under both O2/N2 and CO2 atmospheres, B. subtilis 168, E. coli ATCC 35218, E. coli K12, Proteus mirabilis, and S. liquefaciens exhibited higher growth rates under CO2 than under O2/N2 atmospheres (Tables 2 and 3), which suggests that the metabolic machinery that permits facultative anaerobic growth in these species may be more conducive to supporting growth at low pressures under CO2 atmospheres than in hypobaric aerobic conditions. A similar response was observed for seven Bacillus spp. grown under decreasing pressures (i.e., 1013, 100, 50, 35, or 25 mbar) in either O2/N2 or CO2 atmospheres (Schuerger and Nicholson, 2006).
Second, the addition of 0.1% potassium nitrate or 0.25% glucose to either TSA or LB media failed to enhance growth in B. subtilis 168 and E. coli K12 (Fig. 2) or in P. fluorescens and S. liquefaciens (Table 6). The addition of potassium nitrate and glucose also failed to enhance microbial growth at low pressures by seven Bacillus spp. tested down to 25 mbar (Schuerger and Nicholson, 2006). Glucose and nitrate have been shown to enhance anaerobic growth in some species by permitting fermentation and anaerobic respiration, respectively (reviewed in Nakano and Zuber, 2002). However, it appears that both glucose and nitrate did not enhance growth when multiple stressing agents were used in the hypobaric assays.
Third, another unexpected result was the observation that P. fluorescens failed to grow in CO2-enriched anoxic atmospheres at 30°C during 48 h assays (Table 3) but did develop clearly discernible microcolonies on TSA when grown in CO2-enriched anoxic atmospheres at 0°C for 35 d assays (Table 5; Fig. 3). Pseudomonas fluorescens is generally considered an obligate aerobe (Holt et al., 1994), but some strains can grow at low ppO2 if nitrate is available as a final electron acceptor. However, the addition of 0.1% potassium nitrate had no observable beneficial effect on growth of P. fluorescens under anoxic conditions. Thus, the ability of P. fluorescens to replicate in 0°C and anoxic atmospheres implies that long-term growth studies of other microorganisms previously considered obligate aerobes might identify additional species capable of growth under anoxic low-temperature conditions.
And fourth, no species were killed by exposure to any of the hypobaric, low-temperature, and anoxic conditions of the assays described herein. When cultures exhibiting zero growth in all assays (Tables 2–6; Fig. 3) were transferred to a lab incubator for an additional 48 h at 30°C, 1013 mbar, and standard 21%/78% O2/N2 atmospheres, all species grew vigorously. Results confirm earlier work (Schuerger and Nicholson, 2006; Nicholson et al., 2010) and indicate that the stressing factors tested herein did not inactivate cells but reversibly inhibited their growth.
4.3. Hypobaria versus desiccation effects
The triple point of water on Mars occurs at 6.1 mbar and 0.1°C (Haberle et al., 2001). As pressure increases above 6.1 mbar but temperature is held constant at 0°C (as described herein), the increase in pressure adds to the stability of liquid water. Thus, liquid water is stable at 25 mbar if temperature is held at 0°C (Fig. 1; Haberle et al., 2001). Pure water appears to be stable on Mars up to 12.4 mbar and 283 K (10°C) (Haberle et al., 2001). However, even though pure water might be stable near the triple point of water on Mars, evaporation will occur unless the immediate headspace above the liquid water is under saturated conditions (Sears and Moore, 2005).
In the current work, only two hypobaric assays on agar plates were conducted above 10°C (Tables 2 and 3). All other assays were conducted in either liquid LB broth or at 0°C. For the hypobaria assays reported in Tables 2 and 3, it is thus likely that suppression of growth was due to the concomitant effects of both hypobaria and desiccation. However, during all other agar-based assays, the TSA and SSA surfaces remained hydrated over the course of the experiments. At 24–30°C, the double-thick TSA and SSA plates were stable for 72–96 h (Schuerger and Nicholson, 2006); thus, assays at 30°C were run in the current study no longer than 48 h. At 0°C, the double-thick TSA plates were stable for at least 49 d (data not shown); thus, assays were run no longer than 35 d.
The primary finding of the current study is that Serratia liquefaciens ATCC 27592 was capable of growth at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres, and is based on the results presented in Table 6 and Fig. 3. Both data sets were conducted at pressure and temperature conditions in which we can rule out desiccation as a primary factor and instead conclude that S. liquefaciens is a hypobarophile capable of growth under hydrated low-pressure, low-temperature, and anoxic conditions. In addition, our conclusion that hypobaria can have a direct effect on bacterial growth is based on the nearly identical results on TSA surfaces (Table 2) or in LB broth (Fig. 2) for the growth of B. subtilis 168 and E. coli K12 at low pressures. Had desiccation alone been the primary factor involved in suppressing bacterial growth under 7 mbar and 0°C conditions on TSA, then the growth of 168 and K12 at 25 mbar in LB would have remained similar across all pressures tested.
4.4. Contamination of habitable zones on Mars
Habitable zones (HZs), by definition, are capable of supporting metabolism and growth of living microorganisms with capabilities similar to those of terrestrial microbes (Stoker et al., 2010). Terrestrial microorganisms have demonstrated remarkable abilities to adapt to nearly all niches on Earth in which liquid water is stable, and in the field of astrobiology, liquid water is considered the primary condition necessary for life. Thus, planetary protection protocols for spacecraft are in essence an effort to protect liquid water niches on Mars in order to prevent the forward contamination of HZ sites. The protection of potential habitable niches on Mars takes on increased importance if spacecraft microorganisms are shown to be capable of metabolism and growth under environmental conditions found at plausible HZ locations near the surface.
On the surface of Mars, the most dominant biocidal factor remains the high solar UV flux with biocidal photons reaching the surface down to 190 nm (Kuhn and Atreya, 1979; Moores et al., 2007). As long as inadvertent terrestrial contamination remains on spacecraft surfaces exposed to solar UV, the contamination is likely to be inactivated within a few hours to a few sols on equatorial Mars (Schuerger et al., 2003, 2006; Smith et al., 2009) and only slightly longer for midlatitude and polar regions (Moores et al., 2007). However, solar UV irradiation is attenuated by <0.5 mm of fine-grained dust (Mancinelli and Klovstad, 2000; Schuerger et al., 2003), and buried niches will offer significantly improved conditions for growth of hypobarophiles on Mars. If protected from solar UV irradiation, microbial growth in hydrated HZ niches on Mars will next depend upon tolerance to hypobaria (1–14 mbar range on the martian surface), low temperatures (global average of −61°C), anoxic atmospheres (95.5% CO2 atmosphere), and desiccation.
The successful growth of Serratia liquefaciens on hydrated surfaces of TSA maintained at 7 mbar, 0°C, and CO2-enriched anoxic atmospheres suggests that active metabolism and growth are plausible in hydrated sites on Mars that contain available carbon and nitrogen sources. However, at least 17 biocidal and inhibitory factors have been proposed for the martian surface (see Introduction), and some of these factors might yet preclude the successful growth of S. liquefaciens on Mars. For example, Berry et al. (2010) demonstrated that S. liquefaciens was sensitive to desiccation and salt concentrations above 5% (w/v) for MgCl2 and NaCl and above 10% for MgSO4. Shallow subsurface liquid brines have been invoked as possible explanations for recurring slope lineae (RSL) features on crater slopes on Mars (McEwen et al., 2011). If brines are composed of excessively high concentrations of salts, dispersal of S. liquefaciens onto RSL sites may not lead to growth of the bacterium within the RSL niches even though liquid water may be present. Furthermore, we have only demonstrated growth of S. liquefaciens in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Since the average global temperature on Mars is −61°C, and most places remain well below 0°C during the martian year (Haberle, et al., 2001), temperatures below 0°C might also preclude the growth of S. liquefaciens in liquid brines. Research efforts are ongoing with regard to anoxic growth minima for S. liquefaciens at lower pressures and temperatures than were tested here.
Predicting contamination scenarios for hydrated HZ niches also requires a model that addresses how pressure might increase with depth. We estimated increasing pressures within the martian lithosphere, giving consideration to both gas-phase and lithographic lapse rates. The hypothesis was that, as pressure increased with depth, the range of microbial species capable of metabolism and growth in the HZ niches would increase. Results indicate that for gas-phase lapse rates alone, the pressure within void spaces in rocks and regolith that remain contiguous to the surface of Mars would experience a very slow increase in pressure at the rate of 1 mbar per 1450 m. Such a slow increase in gas-phase pressure suggests that only species capable of metabolism and growth near 7 mbar would be capable of contaminating hydrated niches at the surface under current rover and lander technology. Based on a gas-phase lapse rate of only 1 mbar per 1450 m, the depth required to achieve 25 mbar would be 13.8 km. However, the lithographic lapse rate suggests that, if drilling or digging operations emplace spacecraft microorganisms into niches that become fully sealed off from the gas phase (e.g., ice or salt inclusions), then the internal pressures within the inclusions would equilibrate to the overburden pressure acting on the ice or salt matrix surrounding the inclusions. Overburden pressure increases very quickly with depth, and a pressure of 25 mbar within inclusions can be reached by as little as 19.5 cm of overburden, opening up a much larger pressure range within which terrestrial microorganisms could grow. Although historical spacecraft operations on Mars do not appear to have created ice or salt inclusions in the materials accessed by trenching, digging, or drilling activities, emplacement of spacecraft microorganisms into salt or ice inclusions with subsequent burial by regolith will remain a significant risk for future surface operations on Mars.
5. Conclusions
Of 26 bacterial strains tested, only S. liquefaciens was found capable of growth in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Results demonstrate that at least one terrestrial microorganism can grow under hypobaric conditions relevant to the surface of Mars. In a parallel study, Nicholson et al. (2012) demonstrated growth of eight type strains and two Siberian permafrost field strains in the genus Carnobacterium in 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Thus, at least 11 species of bacteria in two genera appear capable of growth under hypobaric conditions relevant to Mars. However, results of both studies do not demonstrate growth of any of the species under highly desiccated conditions that are present on current-day Mars. The logic followed in both studies was to identify strains of bacteria capable of growth at 7 mbar such that follow-on experiments on the effects of desiccation, regolith geochemistry, hypobaria or temperature minima, et cetera, on microbial growth would use bacterial species already proven capable of growth in the pressure range found on Mars.
Although the success of S. liquefaciens at 7 mbar suggests that some spacecraft microorganisms may be capable of growth in hydrated surface or shallow subsurface niches on Mars, several caveats are in order. First, there are at least 17 biocidal or inhibitory factors present on the martian surface, and the addition of other factors might still preclude the growth of S. liquefaciens or Carnobacterium spp. in potential martian HZ niches. Other key factors that must be added to the three stressing agents tested here include at least the following: (1) What are the interactive effects of salts on microbial growth under martian conditions? (2) What carbon and nitrogen sources are available for microbial growth on the martian surface, and can terrestrial microorganisms utilize these nutrient sources while under concomitant stresses by hypobaria, low temperature, and anoxia? (3) What are the lower thresholds of hypobaria and temperature for microbial growth under realistic hydrated martian conditions? (4) How will extreme diurnal temperature and desiccation cycles affect growth of terrestrial microorganisms under simulated martian conditions?
One unexpected finding of the current work was the observation that the generalist bacterium, S. liquefaciens, possessed a broad enough metabolic range to grow at 7 mbar, while the extremophilic species, D. radiodurans and P. cryohalolentis, failed to do so. Much of the astrobiology literature is predicated on the assumption that studying the behavior of extremophilic microbial species and communities will yield insights into how terrestrial microorganisms might proliferate in other planetary environments. In contrast, the results presented here suggest that generalist species like S. liquefaciens also might be good microbial models for astrobiology research relevant to Mars.
Acknowledgments
The authors would like to thank Jordan Barney and Christopher DeFraia for technical assistance during some of the experiments and Christopher P. McKay and one anonymous reviewer for their comments and suggestions during the review process. The research was supported by grants from the NASA Planetary Protection Office (NNA05CS68G and NNX08AQ81A), the NASA Astrobiology: Exobiology and Evolutionary Biology program (NNX08AO15G), and the Florida Space Grant Consortium at the University of Central Florida (UCF20040009).
Abbreviations
ANOVA, analysis of variance; ATCC, American Type Culture Collection; HZ, habitable zone; LB, Luria-Bertani; LBGN, Luria-Bertani liquid medium supplemented with potassium nitrate and glucose; NCBI, National Center for Biotechnology Information; RSL, recurring slope lineae; SAF, spacecraft assembly facilities; SSA, sea-salt agar; TSA, trypticase soy agar; TSAGN, trypticase soy agar supplemented with potassium nitrate and glucose.
References
- Ashelford K.E. Fry J.C. Bailey M.J. Jeffries A.R. Day M.J. Characterization of six bacteriophages of Serratia liquefaciens CP6 isolated from the sugar beet phytosphere. Appl Surf Sci. 1999;65:1959–1965. doi: 10.1128/aem.65.5.1959-1965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin S. Erman Z. Bilgin O.C. Investigation of Serratia liquefaciens infection in rainbow trout (Oncorhynchus mykiss Walbaum) Turkish Journal of Veterinary and Animal Sciences. 2001;25:643–650. [Google Scholar]
- Beaty D.W. Buxbaum K. Meyer M. Boynton W.V. Clark B.C. Deming J.W. Doran P.T. Edgett K.S. Hecht M.H. Hipkin V. Kieft T. McDonald E. McKay C.P. Mellon M.T. Newsom H.E. Ori G. Paige D. Schuerger A.C. Sogin M. Spry A. Steele A. Tanaka K.L. Voytek M.A. Findings of the Mars Special Regions Science Analysis Group. Astrobiology. 2006;6:677–732. doi: 10.1089/ast.2006.6.677. [DOI] [PubMed] [Google Scholar]
- Berry B.J. Jenkins D.G. Schuerger A.C. Inhibition of Escherichia coli and Serratia liquefaciens under high-salt, low-pressure, and low-temperature environments that approach surface conditions on Mars. Appl Environ Microbiol. 2010;76:2377–2386. doi: 10.1128/AEM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B.C. Morris R.V. McLennan S.M. Gellert R. Jolliff B. Knoll A.H. Squyres S.W. Lowerstein T.K. Ming D.W. Tosca N.J. Yen A. Christensen P.R. Gorevan S. Brückner J. Calvin W. Dreibus G. Farrand W. Klingelhoefer G. Waenke H. Zipfel J. Bell J.F., III Grotzinger J. McSween H.Y. Rieder R. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet Sci Lett. 2005;240:73–94. [Google Scholar]
- Committee on Preventing the Forward Contamination of Mars. Preventing the Forward Contamination of Mars. National Academies Press; Washington, DC: 2006. [Google Scholar]
- COSPAR. COSPAR Planetary Protection Policy. Committee on Space Research; Paris: 2008. COSPAR/IAU 1-4-A1-A7. and World Space Council, Houston. [Google Scholar]
- Diaz B. Schulze-Makuch D. Microbial survival rates of Escherichia coli and Deinococcus radiodurans under low temperature, low pressure, and UV-irradiation conditions, and their relevance to possible martian life. Astrobiology. 2006;6:332–347. doi: 10.1089/ast.2006.6.332. [DOI] [PubMed] [Google Scholar]
- Ghosh S. Osman S. Vaishampayan P. Venkateswaran K. Recurrent isolation of extremotolerant bacteria from the clean room where the Phoenix spacecraft components were assembled. Astrobiology. 2010;10:325–335. doi: 10.1089/ast.2009.0396. [DOI] [PubMed] [Google Scholar]
- Gilmour I. Sephton M.A. Conway A. Jones B.W. Rothery D.A. Zarnecki J.C. An Introduction to Astrobiology. Cambridge University Press; New York: 2003. [Google Scholar]
- Grimont P.A.D. Grimont F. Starr M.P. Serratia species isolated from plants. Curr Microbiol. 1981;5:317–322. [Google Scholar]
- Grohskopf L.A. Roth V.R. Feikin D.R. Arduino M.J. Carson L.A. Tokars J.I. Holt S.C. Jensen B.J. Hoffman R.E. Jarvis W.R. Serratia liquefaciens bloodstream infection from contamination of epoetin alfa at a hemodialysis center. N Engl J Med. 2001;344:1491–1497. doi: 10.1056/NEJM200105173442001. [DOI] [PubMed] [Google Scholar]
- Haberle R.M. McKay C.P. Schaeffer J. Cabrol N.A. Grin E.A. Zent A.P. Quinn R. On the possibility of liquid water on present-day Mars. J Geophys Res. 2001;106:23317–23326. [Google Scholar]
- Hecht M.H. Kounaves S.P. Quinn R.C. West S.J. Young M.M. Ming D.W. Catling D.C. Clark B.C. Boynton W.V. Hoffman J. DeFlores L.P. Gospodinova K. Kapit J. Smith P.H. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science. 2009;325:64–67. doi: 10.1126/science.1172466. [DOI] [PubMed] [Google Scholar]
- Holt J.G. Krieg N.R. Sneath P.H.A. Staley J.T. Williams S.T. Bergey's Maunal of Determinative Bacteriology. 9th. Lippincott Williams & Wilkins; New York: 1994. [Google Scholar]
- Horneck G. Baumstark-Khan C. Astrobiology: The Quest for the Conditions of Life. Springer-Verlag; Köln, Germany: 2002. [Google Scholar]
- Horneck G. Bücker H. Reitz G. Long-term survival of bacterial spores in space. Adv Space Res. 1994;14:41–45. doi: 10.1016/0273-1177(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Horneck G. Reitz G. Rettberg P. Baumstark-Khan C. Gerzer R. Critical issues in connection with human missions to Mars: protection of and from the martian environment. Adv Space Res. 2003;31:87–95. doi: 10.1016/s0273-1177(02)00662-2. [DOI] [PubMed] [Google Scholar]
- Kanervo E. Lehto K. Stahle K. Lehto H. Maenpaa P. Characterization of growth and photosynthesis of Synechocystis sp. PCC 6803 cultures under reduced atmospheric pressures and enhanced CO2 levels. International Journal of Astrobiology. 2005;4:97–100. [Google Scholar]
- Kral T.A. Altheide T.S. Lueders A.E. Schuerger A.C. Low pressure and desiccation effects on methanogens: implications for life on Mars. Planet Space Sci. 2011;59:264–270. [Google Scholar]
- Kuhn W.R. Atreya S.K. Solar radiation incident on the martian surface. J Mol Evol. 1979;14:57–64. doi: 10.1007/BF01732367. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Nicholson W.L. Kern R. Venkateswaran K. Microbial characterization of the Mars Odyssey spacecraft and its encapsulation facility. Environ Microbiol. 2003;5:977–985. doi: 10.1046/j.1462-2920.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Kern R. Venkateswaran K. Microbial monitoring of spacecraft and associated environments. Microb Ecol. 2004a;47:150–158. doi: 10.1007/s00248-003-1012-0. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Sumner R. Pierson D.L. Venkat P. Venkateswaran K. Evidence of pathogenic microbes in the International Space Station drinking water: reasons for concern? Habitation. 2004b;10:39–48. doi: 10.3727/154296604774808883. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Dekas A. Osman S. Moissl C. Newcombe D. Venkateswaran K. Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean room environments. Appl Environ Microbiol. 2007;73:2600–2611. doi: 10.1128/AEM.03007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Duc M.T. Osman S. Vaishampayan P. Piceno Y. Andresen G. Spry J.A. Venkateswaran K. Comprehensive census of bacteria in clean rooms by using DNA microarray and cloning methods. Appl Environ Microbiol. 2009;75:6559–6567. doi: 10.1128/AEM.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalande R. Bissonnette N. Coutlee D. Antoun H. Identification of rhizobacteria from maize and determination of their plant-growth promoting potential. Plant Soil. 1989;115:7–11. [Google Scholar]
- Lindberg A.-M. Ljungh A. Löfdahl S. Molin G. Enterobacteriaceae found in high numbers in fish, minced meat, and pasteurized milk or cream and the presence of toxin encoding genes. Int J Food Microbiol. 1998;39:11–17. doi: 10.1016/s0168-1605(97)00104-9. [DOI] [PubMed] [Google Scholar]
- Lueders T. Manefield M. Friedrich M.W. Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol. 2004;6:73–78. doi: 10.1046/j.1462-2920.2003.00536.x. [DOI] [PubMed] [Google Scholar]
- Mancinelli R.L. Klovstad M. Martian soil and UV radiation: microbial viability assessment on spacecraft surfaces. Planet Space Sci. 2000;48:1093–1097. [Google Scholar]
- McEwen A.S. Ojha L. Dundas C.M. Mattson S.S. Byrne S. Wray J.J. Cull S.C. Murchie S.L. Thomas N. Gulick V.C. Seasonal flows on warm martian slopes. Science. 2011;333:740–743. doi: 10.1126/science.1204816. [DOI] [PubMed] [Google Scholar]
- Miller J. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Ming D.W. Mittlefehldt D.W. Morris R.V. Golden D.C. Gelhert R. Yen A. Clark B.C. Squyres S.W. Farrand W.H. Ruff S.W. Arvidson R.E. Klingelhöfer G. McSween H.Y. Rodionov D.S. Schröder C. de Souza P.A., Jr. Wang A. Geochemical and mineralogical indicators for aqueous processes in the Columbia Hills of Gusev Crater, Mars. J Geophys Res. 2006;111 doi: 10.1029/2005JE002560. [DOI] [Google Scholar]
- Moores J.E. Smith P.H. Tanner R. Schuerger A.C. Venkateswaran K. The shielding effect of small-scale martian surface geometry on ultraviolet flux. Icarus. 2007;192:417–433. [Google Scholar]
- Nakano M.M. Zuber P. Anaerobiosis. In: Sonenshein A.L., editor; Hoch J.A., editor; Losick R., editor. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. ASM Press; Washington, DC: 2002. pp. 393–404. [Google Scholar]
- Newcombe D.A. La Duc M.T. Vaishampayan P. Venkateswaran K. Impact of assembly, testing and launch operations on the airborne bacterial diversity within a spacecraft assembly facility cleanroom. International Journal of Astrobiology. 2008;7:223–236. [Google Scholar]
- Newsom H.E. Hagerty J.J. Chemical components of the martian soil: soil degassing, hydrothermal alteration, and chondritic debris. J Geophys Res. 1997;102:19345–19355. [Google Scholar]
- Newsom H.E. Hagerty J.J. Mixed hydrothermal fluids and the origin of the martian soil. J Geophys Res. 1999;104:8717–8728. [Google Scholar]
- Nicholson W.L. Fajardo-Cavazos P. Fedenko J. Ortiz-Lugo J.L. Rivas-Castillo A. Waters S.M. Schuerger A.C. Exploring the low-pressure growth limit: evolution of Bacillus subtilis in the laboratory to enhanced growth at 5 kilopascals. Appl Environ Microbiol. 2010;76:7559–7565. doi: 10.1128/AEM.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W.L. Krivushin K. Gilichinsky D. Schuerger A.C. Growth of Carnobacterium spp. isolated from Siberian permafrost under simulated Mars conditions of pressure, temperature and atmosphere. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1209793110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova N.D. Review of the knowledge of microbial contamination of the Russian manned spacecraft. Microb Ecol. 2004;47:127–132. doi: 10.1007/s00248-003-1055-2. [DOI] [PubMed] [Google Scholar]
- Oh H.I. Kim Y.J. Chang E.J. Kim J.Y. Antimicrobial characteristics of chitosans against food spoilage microorganisms in liquid media and mayonnaise. Biosci Biotechnol Biochem. 2001;65:2378–2383. doi: 10.1271/bbb.65.2378. [DOI] [PubMed] [Google Scholar]
- Pierson D.L. Bassinger V.J. Molina T.C. Gunter E.G. Groves T.O. Cioletti L.A. Mishra S.K. SAE Technical Paper Paper 932139. SAE International; Warrendale, PA: 1993. Preflight and postflight microbiological results from 25 Space Shuttle crews. [Google Scholar]
- Pogoda de la Vega U. Rettberg P. Reitz G. Simulation of the environmental climate conditions on martian surface and its effect on Deinococcus radiodurans. Adv Space Res. 2007;40:1672–1677. [Google Scholar]
- Pokorny N.J. Boulter-Bitzer J.I. Hart M.M. Storey L. Lee H. Trevors J.T. Hypobaric bacteriology: growth, cytoplasmic membrane polarization and total cellular fatty acids in Escherichia coli and Bacillus subtilis. International Journal of Astrobiology. 2006;4:187–193. [Google Scholar]
- Probst A. Vaishampayan P. Osman S. Moissl-Eichinger C. Andersen G.L. Venkateswaran K. Diversity of anaerobic microbes in spacecraft assembly clean rooms. Appl Environ Microbiol. 2010;76:2837–2845. doi: 10.1128/AEM.02167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo J.R. Oxborrow G.S. Fields N.D. Herring C.M. Smith L.S. Microbiological profiles of four Apollo spacecraft. Appl Microbiol. 1973;26:838–845. doi: 10.1128/am.26.6.838-845.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo J.R. Fields N.D. Berstrom S.L. Oxborrow G.S. Stabekis P.D. Koukol R.C. Microbiological profiles of the Viking spacecraft. Appl Environ Microbiol. 1977;33:379–384. doi: 10.1128/aem.33.2.379-384.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon J.J. Burnap R.L. An analysis of potential photosynthetic life on Mars. International Journal of Astrobiology. 2006;5:171–180. [Google Scholar]
- Schuerger A.C. Microbial ecology of the surface exploration of Mars with human-operated vehicles. In: Cockell C.S., editor. Martian Expedition Planning, AAS Publication 03-322. Univelt Publishers; Escondido, CA: 2004. pp. 363–386. [Google Scholar]
- Schuerger A.C. Nicholson W.L. Interactive effects of hypobaria, low temperature, and CO2 atmosphere inhibit the growth of mesophilic Bacillus spp. under simulated martian conditions. Icarus. 2006;185:143–152. [Google Scholar]
- Schuerger A.C. Mancinelli R.L. Kern R.G. Rothschild L.J. McKay C.P. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus. 2003;165:253–276. doi: 10.1016/s0019-1035(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Schuerger A.C. Berry B.J. Nicholson W.L. Terrestrial bacteria typically recovered from Mars spacecraft do not appear able to grow under simulated martian conditions [abstract 1397]. In 37th Lunar and Planetary Science Conference; Houston. Lunar and Planetary Institute; 2006. [Google Scholar]
- Schuerger A.C. Ming D.C. Golden D.C. Biotoxicity of Mars analog soils: microbial dispersal in desiccated soils versus emplacement in salt or ice inclusion fluids [abstract 5336]. Astrobiology Science Conference 2010: Evolution and Life: Surviving Catastrophes and Extremes on Earth and Beyond; Houston. Lunar and Planetary Institute; 2010. [Google Scholar]
- Sears D.W.G. Moore S.R. On laboratory simulations and the evaporation rate of water on Mars. Geophys Res Lett. 2005;32 doi: 10.1029/2005GL023443. [DOI] [Google Scholar]
- Smith D.J. Schuerger A.C. Davidson M.M. Pacala S.W. Bakermans C. Onstott T.C. Survivability of Psychrobacter cryohalolentis K5 under simulated martian surface conditions. Astrobiology. 2009;9:221–228. doi: 10.1089/ast.2007.0231. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E. Fredrickson J.K. Garrity G.M. Grimont P.A.D. Kämpfer P. Maiden C.J. Nesme X. Roselló-Mora R. Swings J. Trüper H.G. Vauterin L. Ward A.C. Whitman W.B. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol. 2002;52:1043–1047. doi: 10.1099/00207713-52-3-1043. [DOI] [PubMed] [Google Scholar]
- Stiles M.E. Ng L.-K. Biochemical characteristics and identification of Enterobacteriaceae isolated from meats. Appl Environ Microbiol. 1981;41:639–645. doi: 10.1128/aem.41.3.639-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker C.R. Zent A.P. Catling D.C. Douglas S. Marshall J.R. Archer D., Jr. Clark B.C. Kounaves S.P. Lemmon M.T. Quinn R. Renno N.O. Smith P.H. Young S.M.M. Habitability of the Phoenix landing site. J Geophys Res. 2010;115 doi: 10.1029/2009JE003421. [DOI] [Google Scholar]
- Van Horn K.G. Warren K. Baccaglini E.J. Evaluation of the AnaeroPack System for growth of anaerobic bacteria. J Clin Microbiol. 1997;35:2170–2173. doi: 10.1128/jcm.35.8.2170-2173.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran K. Satomi M. Chung S. Kern R. Koukol R. Basic C. White D. Molecular microbial diversity of a spacecraft assembly facility. Syst Appl Microbiol. 2001;24:311–320. doi: 10.1078/0723-2020-00018. [DOI] [PubMed] [Google Scholar]
- Yen A.S. Kim S.S. Hecht M.H. Frant M.S. Evidence that the reactivity of the martian soil is due to superoxide ions. Science. 2000;289:1909–1912. doi: 10.1126/science.289.5486.1909. [DOI] [PubMed] [Google Scholar]