Abstract
Two recent Phase II results show opposing outcomes for the potential of activators of mGlu2 in the treatment of schizophrenia. The first outcome revealed that Eli Lilly’s mGlu2/3 agonist, pomaglumetad methionil (LY2140023), failed to meet the primary efficacy end point. The second report showed the mGlu2 positive allosteric modulator (PAM) from Addex (ADX71149) in conjunction with Janssen Research & Development met the primary objectives of safety, tolerability and demonstrated an effect on negative symptoms in patients.
Keywords: Agonist, mGlu2/3, mGlu2, positive allosteric modulator, PAM, Phase II
Schizophrenia (SZ) is a complex and chronic mental health disease that affects nearly 1% of the total adult population with more than 2 million Americans suffering from this disease a year. Because the disease normally presents itself in early adulthood (late teens/early twenties), the overall cost of this disease is staggering with costs well over $60 billion (based on estimates from 10 years ago).1 Although the name is derived from the Greek words for split (schizo) and mind (phrene), sufferers do not have split personalities, but suffer from unusual and/or disturbed thoughts, hallucinations, delusions, and lack of emotion and energy. The most prescribed medications for the treatment of SZ are so-called first- and second-generation antipsychotics (atypical antipsychotics) that work as antagonists of the dopamine 2 receptor (D2 antagonists). However, these compounds continue to be plagued by severe side effects such as significant weight gain and metabolic disorders. Due to these side effects, new and more effective treatments with fewer side effects have been the focus of significant research into this field of treatment.
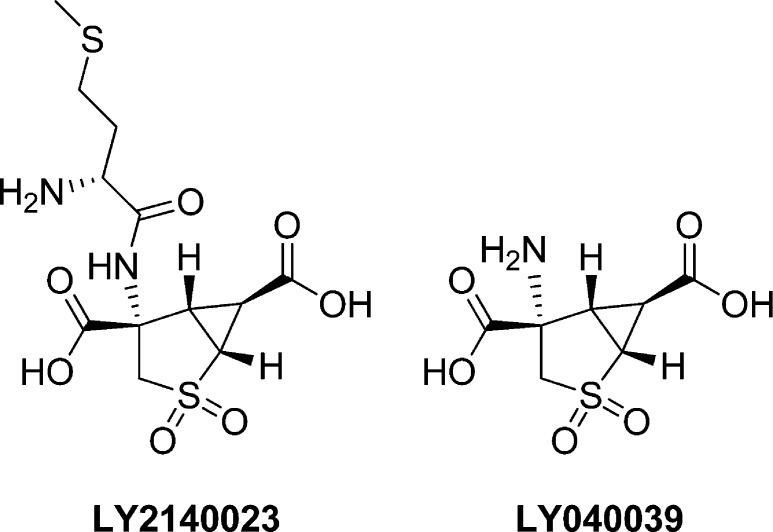
It has been 15 years since the first reports on the activation of metabotropic glutamate receptors and subsequent reversal of phencyclidine (PCP) induced cognitive deficits and psychosis.2 From that time, there have been multiple academic and industrial programs dedicated to the glutamate hypothesis of schizophrenia.3 Arguably the most advanced of these numerous projects is the activation of the Group 2 metabotropic glutamate receptors (mGlu2/3). Eli Lilly led the charge in this new area with their mGlu2/3 orthosteric agonist LY2140023 (a prodrug of LY404039), which showed efficacy in 2007 in a 4-week phase II trial (Figure 1).4 Although the efficacy was not as significant as a prototypical atypical Zyprexa, LY2140023 did not produce the weight gain as seen with Zyprexa. Unfortunately, the excitement over this initial phase II study has been buffered with newer results showing either inconclusive results5 or, more recently, results that are no better than placebo.6
Figure 1.
Structures of the Eli Lilly mGlu2/3 agonist (LY040039) and prodrug (LY2140023).
The first, inconclusive, result was from the phase II study HBBI comparing LY2140023 with placebo or olanzapine. Although LY2140023, at any dose, was not more efficacious than placebo, the results were considered inconclusive since the active control did not separate from placebo. The study was performed in patients with acutely exacerbated schizophrenia. In this study, LY2140023 monohydrate was well tolerated, though there were four cases of a serious adverse event (convulsion). In the more recent phase II HBBM study, LY2140023 was again tested against placebo and an active control; however, in this study, the active control was risperidone. Two doses were investigated (40 and 80 mg) in both an overall population and a predefined genetic subpopulation. In this study, LY2140023 (now known as pomaglumated methionil) did not show efficacy compared to placebo in either population or dose. However, the positive control risperidone was efficacious (compared to placebo) in both populations.6 Despite these results, Eli Lilly continues to study LY2140023 in a number of clinical trials as potential adjunct therapies, with new results expected in Spring 2013 of a Phase III trial.
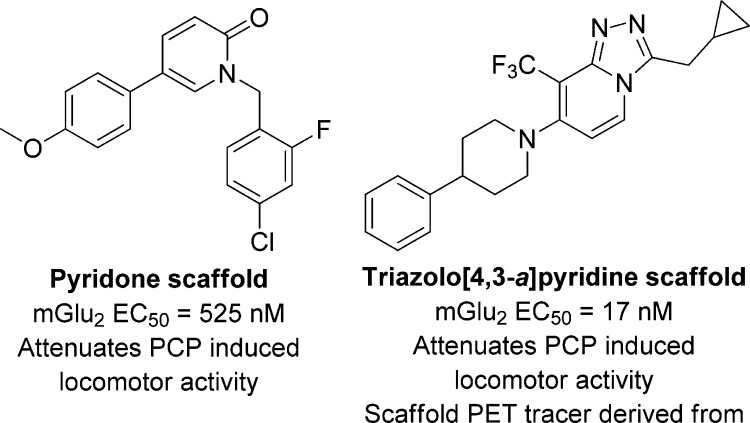
Contrasting to the orthosteric mGlu2/3 agonist, there has been significant effort in finding novel allosteric modulators of mGlu2. These positive allosteric modulators (PAMs) potentially provide more subtype selective molecules than traditional orthosteric agonists, and since the PAMs only act in the presence of endogenous ligands, they are thought to overcome the receptor desensitization that often plagues orthosteric agonists. The most advanced mGlu2 PAM is from Addex, in partnership with Janssen R&D, ADX71149. Although the structure of ADX71149 has yet to be released, Addex has published extensively on two separate scaffolds as mGluR2 PAMs: disubstituted pyridine/isoquinolines7 and imidazo[1,2-a]- and triazolo[4,3-a]pyridines8 which were derived from a scaffold hopping campaign (Figure 2). In addition to publications in the primary literature, patent applications have published around these two scaffolds: pyridones9 and triazolo[4,3-a]pyridines.10 Although it can be difficult to ascertain information regarding the structure of ADX71149, these publications shed light into medicinal chemistry campaigns for mGlu2 PAMs. Lastly, the most recent publication from these groups disclose an effort to identify potential tracers for positron emission tomography (PET) imaging.11 The Addex/Janssen groups have disclosed novel chemical matter that have shown excellent efficacy in preclinical models of SZ.
Figure 2.
Representative mGlu2 scaffolds from Addex/Janssen in the primary literature.
However, and more excitingly, Addex has recently reported a successful Part B of the first-in-patient Phase IIa clinical study of ADX71149 in SZ.12 The Phase IIa study was separated into two parts which were run concurrently with Part A looking at the safety, tolerability, and efficacy as monotherapy in patients with subacute psychosis and Part B was as adjunctive therapy to antipsychotics. The data that was reported in late 2012 showed that ADX71149 met the primary objectives of safety and tolerability and demonstrated an effect in patients with residual negative symptoms.
This promising data is welcome news for the millions of patients and caregivers that suffer from SZ. However, as was highlighted with LY2140023, this excitement must be tempered with the knowledge that there is a long road to go before ADX71149 or any similar therapeutic is available to the community. Will these positive allosteric modulators succeed where the orthosteric agonists have not? Only time, and more clinical data, will tell. However, with so many pharmaceutical companies fleeing psychiatry as a therapeutic area, it is welcome news that there are those still progressing potential drugs forward for this devastating and not-well-treated disease.
The authors declare no competing financial interest.
References
- Wu E. Q.; Birnbaum H. G.; Shi L.; Ball D. E.; Kessler R. C.; Moulis M.; Aggarwal J. (2005) The economic burden of schizophrenia in the United States in 2002. J. Clin. Psychiatry 66, 1122–1129. [DOI] [PubMed] [Google Scholar]
- Adams B.; Moghaddam B. (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 18, 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B.; Javitt D. (2012) From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharm. Rev. 37, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. T.; Zhang L.; Martenyi F.; Lowe S. L.; Jackson K. A.; Andreev B. V.; Avedisova A. S.; Bardenstein L. M.; Gurovich I. Y.; Morozova M. A.; Mosolov S. N.; Neznanov N. G.; Reznik A. M.; Smulevich A. B.; Tochilov V. A.; Johnson B. G.; Monn J. A.; Schoepp D. D. (2007) Activation of mGlu2/3 receptors as a new approach to treat Schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 13, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Kinon B. J.; Zhang L.; Millen B. A.; Osuntokun O. O.; Williams J. E.; Kollack-Walker S.; Jackson K. A.; Kryzhanovskaya L.; Jarkova N. (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychiatry 31, 349–355. [DOI] [PubMed] [Google Scholar]
- http://newsroom.lilly.com/releasedetail.cfm?releaseid=690836.
- a Cid J. M.; Duvey G.; Cluzeau P.; Nhem V.; Macary K.; Raux A.; Poirier N.; Muller J.; Boléa C.; Finn T.; Poli S.; Epping-Jordan M.; Chamelot E.; Derouet F.; Girard F.; Macdonald G. J.; Vega J. A.; de Lucas A. I.; Matesanz E.; Lavreysen H.; Linares M. L.; Oehlrich D.; Oyarzábal J.; Tresadern G.; Trabanco A. A.; Andrés J. I.; Le Poul E.; Imogai H.; Lutjens R.; Rocher J.-P. (2010) Discovery of 1,5-disubstituted pyridones: a new class of positive allosteric modulators of the metabotropic gluatamate 2 receptor. ACS Chem. Neurosci. 1, 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Trabanco A. A.; Duvey G.; Cid J. M.; Macdonald G. J.; Cluzeau P.; Nhem V.; Furnari R.; Behaj N.; Poulain G.; Finn T.; Poli S.; Lavreysen H.; Raux A.; Thollon Y.; Poirier N.; D’Addona D.; Andrés J. I.; Lutjens R.; Le Poul E.; Imogai H.; Rocher J.-P. (2011) New positive allosteric modulators of the metabotropic glutamate receptor 2 (mGluR2). Identification and synthesis of N-propyl-5-substituted isoquinolines. Med. Chem. Commun. 2, 132–139. [DOI] [PubMed] [Google Scholar]; c Trabanco A. A.; Duvey G.; Cid J. M.; Macdonald G. J.; Cluzeau P.; Nhem V.; Furnari R.; Behaj N.; Poulain G.; Finn T.; Lavreysen H.; Poli S.; Raux A.; Thollon Y.; Poirier N.; D’Addona D.; Andrés J. I.; Lutjens R.; Le Poul E.; Imogai H.; Rocher J.-P. (2011) New positive allosteric modulators of the metabotropic glutamate receptor 2 (mGluR2): identification and synthesis of N-propyl-8-chloro-6-substituted isoquinolines. Bioorg. Med. Chem. Lett. 21, 971–976. [DOI] [PubMed] [Google Scholar]
- a Tresadern G.; Cid J. M.; Macdonald G. J.; Vega J. A.; de Lucas A. I.; García A.; Matesanz E.; Linares M. L.; Oehlrich D.; Lavreysen H.; Biesmans I.; Trabanco A. A. (2010) Scaffold hopping from pyridones to imidazo[1,2-a]pyridines. New positive allosteric modulators of metabotropic glutamate 2 receptor. Bioorg. Med. Chem. Lett. 20, 175–179. [DOI] [PubMed] [Google Scholar]; b Trabanco A. A.; Tresadern G.; Macdonald G. J.; Vega J. A.; De Lucas A. I.; Matesanz E.; García A.; Linares M. L.; Alonso de Diego S. A.; Alonso J. M.; Oehlrich D.; Ahnaou A.; Drinkenburg W.; Mackie C.; Andrés J. I.; Lavreysen H.; Cid J. M. (2012) Imidazo[1,2-a]pyridines: orally active positive allosteric modulators of the metabotropic glutamate 2 receptor. J. Med. Chem. 55, 2688–2701. [DOI] [PubMed] [Google Scholar]; c Cid J. M.; Tresadern G.; Vega J. A.; de Lucas A. I.; Matesanz E.; Iturrino L.; Linares M. L.; Garcia A.; Andrés J. I.; Macdonald G. J.; Oehlrich D.; Lavreysen H.; Megens A.; Ahnaou A.; Drinkenburg W.; Mackie C.; Pype S.; Gallacher D. J.; Trabanco A. A. (2012) Discovery of 3-cyclopropylmethyl-7-(4-phenylpiperidin-1-yl)-8-trifluoromethyl[1,2,4]triazolo[4,3-a]pyridine (JNJ-42153605): a positive allosteric modulator of the metabotropic glutamate 2 receptor. J. Med. Chem. 55, 8770–8789. [DOI] [PubMed] [Google Scholar]
- Imogai H. J., Cid-Núñex J. M., Duvey G. A. J., Bolea C. M., Nhem V., Finn T. P., Le Poul E. C., Rocher J.-P., F. C., and Lutjens R. J. (2006) Novel pyridinone derivatives and their use as positive allosteric modulators of mGluR2-receptors. WO2006/030032.
- Cid-Núñex J. M., Oehlrich D., Trabanco-Suárez A. A., Tresadern G. J., Vega Ramiro J. A., and Macdonald G. J. (2010) 1,2,3-Triazolo[4–3a]pyridine derivatives and their use for the treatment or prevention of neurological and psychiatric diseases. WO2010/130424.
- Andrés J. I.; Alcázar J.; Cid J. M.; De Angelis M.; Iturrino L.; Langlois X.; Lavreysen H.; Trabanco A. A.; Celen S.; Bormans G. (2012) Synthesis, evaluation, and radiolabeling of new potent positive allosteric modulators of the metabotropic glutamate receptor 2 as potential tracers for positron emission tomography imaging. J. Med. Chem. 55, 8685–8699. [DOI] [PubMed] [Google Scholar]
- http://www.addextherapeutics.com/investors/press-releases/news-details/article/addex-reports-top-line-data-from-a-successful-phase-2a-clinical-study-with-adx71149-in-schizophrenia/.