Abstract
The Wnt signaling pathway plays an important role not only in embryonic development but also in the maintenance and differentiation of the stem cells in adulthood. In particular, Wnt signaling has been shown as an important regulatory pathway in the osteogenic differentiation of mesenchymal stem cells. Induction of the Wnt signaling pathway promotes bone formation while inactivation of the pathway leads to osteopenic states. Our current understanding of Wnt signaling in osteogenesis elucidates the molecular mechanisms of classic osteogenic pathologies. Activating and inactivating aberrations of the canonical Wnt signaling pathway in osteogenesis results in sclerosteosis and osteoporosis respectively. Recent studies have sought to target the Wnt signaling pathway to treat osteogenic disorders. Potential therapeutic approaches attempt to stimulate the Wnt signaling pathway by upregulating the intracellular mediators of the Wnt signaling cascade and inhibiting the endogenous antagonists of the pathway. Antibodies against endogenous antagonists, such as sclerostin and dickkopf-1, have demonstrated promising results in promoting bone formation and fracture healing. Lithium, an inhibitor of glycogen synthase kinase 3β, has also been reported to stimulate osteogenesis by stabilizing β catenin. Although manipulating the Wnt signaling pathway has abundant therapeutic potential, it requires cautious approach due to risks of tumorigenesis. The present review discusses the role of the Wnt signaling pathway in osteogenesis and examines its targeted therapeutic potential.
Keywords: Wnt signaling, bone formation, osteoporosis, fracture healing, bone tumors
Introduction
The Wnt signaling cascade was first discovered in the 1980s from mouse breast tumors induced by the mouse mammary tumor virus [Nusse and Varmus, 1982]. Since then, the Wnt signaling pathway has been investigated extensively and has been characterized as one of the most essential signaling cascades in multiple biological processes. The Wnt signaling pathway plays a critical role in embryonic development, postnatal development and adult tissue homeostasis. To coordinate these complex processes, the Wnt signaling regulates cell proliferation, cell fate determination, cell differentiation and cell polarity [Logan and Nusse, 2004]. A greater interest in the Wnt signaling pathway arose when the adenomatous polyposis coli (APC) protein was discovered to interact with β catenin in familial adenomatous polyposis (FAP) syndrome [Rubinfeld et al. 1993; Su et al. 1993]. The oncogenic association between APC and β catenin in FAP led to subsequent discoveries of associations between Wnt signaling and other human pathologies, ranging from cancers to metabolic disorders and developmental defects [Clevers and Nusse, 2012].
In recent years, the role of the Wnt signaling pathway in bone biology has gained considerable attention. Specific human pathologies of bone such as osteoporosis pseudoglioma syndrome, sclerosteosis and van Buchem’s disease have been associated with aberrant Wnt signaling; these discoveries have triggered further research of the Wnt signaling pathway in bone biology [Monroe et al. 2012]. Understanding the role of Wnt signaling in bone disorders has not only helped elucidate the pathogenesis of those disorders but has also led to the development of potential therapeutic avenues to treat the disorders [Hoeppner et al. 2009; Wagner et al. 2011]. In particular, the development of neutralizing antibodies targeting endogenous Wnt antagonists, such as sclerostin and dickkopf (Dkk) proteins, has shown considerable promise in early clinical trials. Other potential therapeutic targets are also currently undergoing preclinical investigation [Rachner et al. 2011]. The present review discusses the role of the Wnt signaling pathway in bone biology and osteogenic disorders and potential therapeutic targets in the Wnt signaling pathway for bone disorders. Finally, some of the oncogenic risks associated with therapeutics targeting the Wnt signaling pathway will be examined.
Overview of the Wnt signaling pathway
Canonical and noncanonical Wnt signaling pathways
The Wnt ligands are a group of 19 secreted glycoproteins that activate their cell surface receptors to induce specific intracellular signaling cascades controlling gene expression. The Wnt signaling pathway can be divided into the canonical and the noncanonical pathways. The canonical pathway mediates signaling through the stabilization of β catenin, whereas the noncanonical pathways work independently of β catenin. This review primarily focuses on the canonical Wnt signaling pathway as the canonical pathway has been better characterized regarding its role and therapeutic potential in bone disorders.
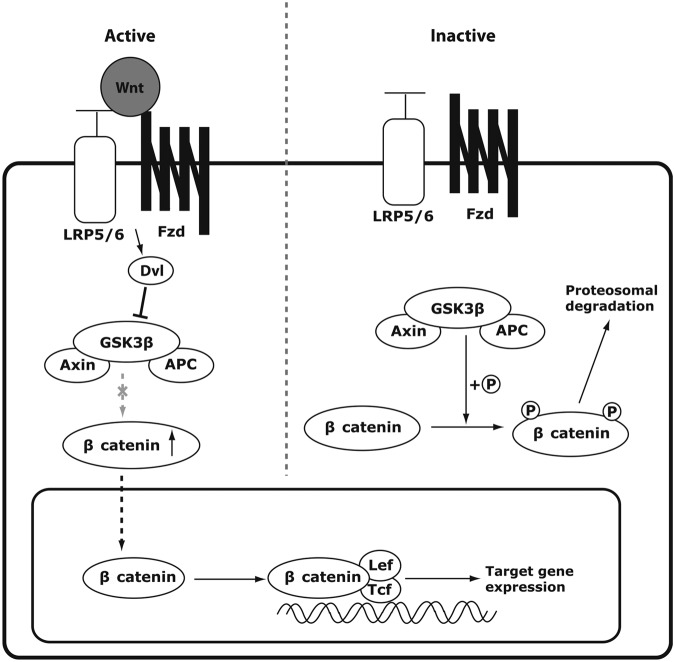
The hallmark of canonical Wnt signaling is the stabilization of β catenin in the cytoplasm upon activation (Figure 1). Without the Wnt ligands, cytoplasmic β catenin is phosphorylated by a protein degradation complex composed of glycogen synthase kinase 3β (GSK3β), axin and APC. GSK3β phosphorylates β catenin, tagging β catenin for ubiquitination and proteosomal degradation [Rao and Kühl, 2010]. However, when the canonical Wnt ligands, such as Wnt 1, 3a, and 8, are present, the ligands bind to the frizzled (Fzd) receptors and one of the coreceptors, either low-density lipoprotein receptor-related protein (LRP)-5 or LRP6, causing phosphorylation of the intracellular protein disheveled (Dvl). Phosphorylated Dvl inhibits GSK3β from phosphorylating β catenin in the cytoplasm. As a result, β catenin is stabilized and translocated into the nucleus. β catenin forms a transcriptional complex with T-cell factor (Tcf)/lymphoid enhancer-binding factor (Lef) to regulate the expression of target genes such as cyclin D1, axin2, c-Myc and peroxisome proliferator-activated receptor (PPARδ) [Logan and Nusse, 2004; MacDonald et al. 2009; Rao and Kühl, 2010].
Figure 1.
Diagrammatic representation of the canonical Wnt signaling pathway. In the presence of the Wnt ligand, the ligand binds to frizzled (Fzd) receptor and its coreceptor low-density lipoprotein receptor-related protein (LRP)-5/6. Disheveled (Dvl) is subsequently activated, which inhibits glycogen synthase kinase 3β (GSK3β) from phosphorylating β catenin. The cytoplasmic level of β catenin consequently rises, and β catenin translocates into the nucleus to bind with transcriptional factors T-cell factor (Tcf)/lymphoid enhancer-binding factor (Lef-1), upregulating the target gene expression. In the absence of the Wnt ligand, GSK3β constitutively phosphorylates β catenin which leads to ubiquitination and proteosomal degradation of β catenin. Thus, the target gene expression level remains low.
Two major noncanonical Wnt pathways are the calcium-dependent pathway and the planar cell polarity pathway. The calcium-dependent pathway is important in embryonic development, cell migration and cancer progression [De, 2011; Kühl, 2004]. It is initiated by Wnt ligands binding to their Fzd receptors followed by the activation of G protein and a signal cascade releasing intracellular calcium from the endoplasmic reticulum. The released calcium activates downstream mediators, such as protein kinase C, calcineurin and calcium calmodulin-dependent protein kinase II, which in turn activate transcription factors, such as nuclear factor κB, nuclear factor of activated T cells and cAMP response element binding protein [De, 2011; Rao and Kühl, 2010]. The cell polarity pathway is another noncanonical pathway important in cell planar polarity, migration, motility and division [Vladar et al. 2009]. This pathway regulates cytoskeletions by activating GTPases such as rho, rac and cdc42 through Fzd receptors and ultimately downstream kinases such as c-jun NH2 kinase [Rao and Kühl, 2010; Sugimura and Li, 2010]. However, the details of the noncanonical pathways are not well characterized in comparison to the canonical pathway.
Complexity of the Wnt signaling pathway
Given the many roles that Wnt signaling plays in development and stem cell maintenance, it is not surprising that Wnt signaling is under complex regulation. For instance, the binary classification of Wnt signaling into the canonical and the noncanonical pathways may be an oversimplistic view of reality. Classically, it has been viewed that the noncanonical Wnt ligands, such as Wnt 5a and Wnt 11, act through the noncanonical pathway while the canonical ligands, Wnt 1, 3a and 8, signal through the β-catenin signaling pathway [Rao and Kühl, 2010; van Amerongen et al. 2008]. However, cross activation of the counterpart pathway does not seem uncommon. For example, Wnt 5a, a classical noncanonical ligand, has been shown to activate the canonical β-catenin pathway in the presence of Fzd 5 [He et al. 1997] or Fzd 4 and LRP 5 [Mikels and Nusse, 2006]. In addition to cross activation, there are at least 19 Wnt ligands, 10 Fzd receptors and 2 LRP coreceptors that have been identified. Furthermore, Wnt ligands can also bind receptor tyrosine kinase and receptor tyrosine kinase-like orphan receptor 2 [Hoeppner et al. 2009; van Amerongen et al. 2008]. This multiplicity of Wnt ligands and receptors implies that numerous potential ligand–receptor combinations exist. In fact, the Wnt signaling pathway does not function as a single linear cascade of events. Rather, it appears to work within a tight regulatory network specific to the context of the receptors present and active on the cell membrane at the onset of activation [van Amerongen et al. 2008]. There is ample room for spatial and temporal regulation under the given context of the cell and its environment. Understanding the rich, complex regulation of Wnt signaling will prove to be crucial as we continue to explore potential therapeutic interventions within this signaling cascade.
Regulation of the Wnt signaling pathway
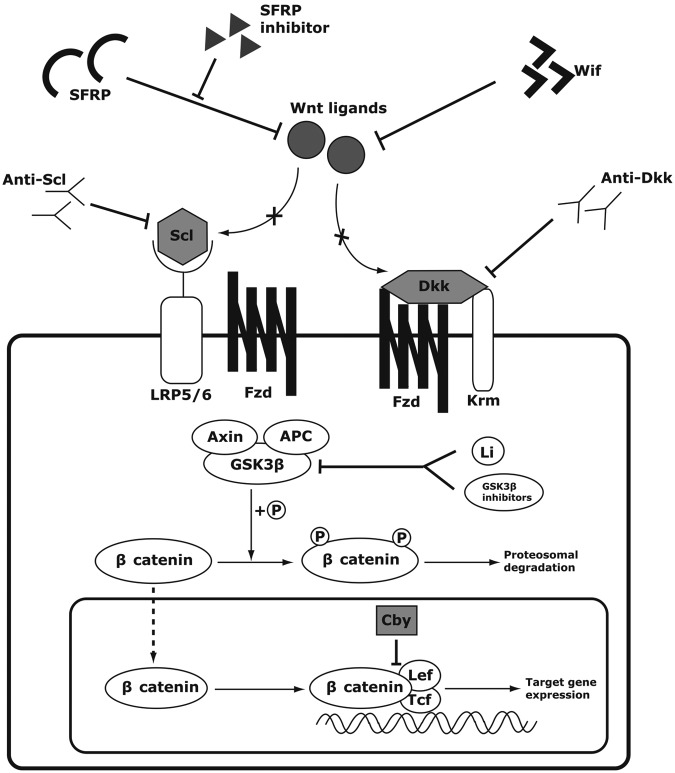
The endogenous regulators of the canonical Wnt signaling pathway can be largely divided into extracellular and intracellular antagonists based on their sites of action (Figure 2). Examples of extracellular inhibitors include sclerostin, Dkks, Wnt inhibitory factor 1/2 (Wif-1/2) and secreted frizzled-related proteins (SFRPs). Most extracellular antagonists are secreted factors that interact with either Wnt receptors or Wnt ligands. For example, sclerostin binds the coreceptor LRP5/6 to competitively inhibit the binding of Wnt ligands to LRP5/6 [Li et al. 2005; Semënov et al. 2005]. Dkk-1 also forms a complex with LRP6 and another transmembrane protein, Kremen1/2 (Krm1/2). This complex formation leads to the internalization and degradation of LRP, lowering the availability of LRP for Wnt ligand binding [Bafico et al. 2001; Mao et al. 2002]. Meanwhile, SFRPs and Wif-1 directly bind Wnt ligands to prevent their binding to Fzd and LRP5/6 [Hsieh et al. 1999; Kawano and Kypta, 2003].
Figure 2.
Potential therapeutic targets of the Wnt signaling pathway. In the presence of sclerostin or dickkopf (Dkk) proteins, the ligands cannot bind their receptors to activate the Wnt signaling. The inhibitory effects of sclerostin and Dkk proteins can be neutralized by their respective antibodies, thus activating the Wnt signaling pathway downstream. Secreted Frizzled-related proteins (SFRPs) and Wif inhibit the Wnt signaling by binding the Wnt ligands, but SFRP inhibitors such as diphenylsulfonyl sulfonamide can inhibit SFRPs from binding the Wnt ligands. Glycogen synthase kinase 3β (GSK3β) normally targets β catenin for degradation by phosphorylation. However, lithium or other pharmacological GSK3β-inhibiting compounds can inhibit the GSK3β activity, thus allowing β catenin to accumulate in the cytoplasm and the nucleus expressing the target genes. Chibby (Cby) is an endogenous antagonist, which interrupts the binding of β catenin to transcriptional factors T-cell factor (Tcf)/lymphoid enhancer-binding factor (Lef-1).
However, the intracellular antagonists disrupt the Wnt signaling cascade in the cytoplasm or nucleus. GSK3β, axin and APC form the degradation complex which tags β catenin for degradation in the inactive state [Kimelman and Xu, 2006]. Chibby (Cby) works inside the nucleus to antagonize the interaction between β catenin and Tcf/Lef-1 [Takemaru et al. 2003].
These endogenous antagonists deserve attention because they highlight the key points of regulation in the Wnt signaling pathway and help us to better understand the diseases caused by aberrant Wnt signaling. Thus, the endogenous antagonists have become popular targets of potential therapeutic approaches which manipulate the Wnt signaling pathway to treat bone disorders.
Mesenchymal stem cells and the Wnt signaling pathway
Wnt-mediated osteogenic differentiation of mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent progenitors that retain the capacity to differentiate into multiple types of tissues, including bone, cartilage, fat, tendon and muscle [Bianco et al. 2008]. MSCs carry vast therapeutic potential in regenerative medicine because a substantial amount of these cells can be harvested from various locations of the patient’s body, especially bone marrow [Zhang et al. 2012]. Induction of MSCs along the osteogenic lineage may serve as an effective therapy to promote bone formation in osteogenic disorders.
Commitment of MSCs into a single cell lineage is regulated by a variety of growth factors [Zhang et al. 2012], yet the current understanding of this process is still limited. Nonetheless, it is relatively well established that the Wnt signaling pathway plays an important role in promoting the osteogenic differentiation of MSCs [Cawthorne et al. 2012; Krishnan et al. 2006]. It has been shown that β catenin promotes the progression of MSCs from osteoblastic precursor cells into more mature osteoblasts while suppressing differentiation into adipogenic and chondrogenic lineages [Case and Rubin, 2010; Glass et al. 2005]. Specifically, the canonical Wnt pathway inhibits the expression of the major adipogenic inducers PPARγ and CCAAT/enhancer binding protein α to suppress adipogenic differentiation while upregulating the osteogenic regulators Runx2, Dlx5 and Osterix [Bennett et al. 2005; Kang et al. 2007]. In addition, the noncanonical Wnt pathway has also been shown to induce osteogenic differentiation, albeit it through a different mechanism. The noncanonical ligand Wnt 5a was shown to suppress PPARγ through the inactivation of chromatins rather than through β-catenin action [Takada et al. 2007]. Though the interplay between the two independent mechanisms induced by Wnt ligands is still unclear, it is evident that Wnt signaling regulates the osteogenic differentiation of MSCs.
Crosstalk with other signaling pathways during the osteogenic differentiation of mesenchymal stem cells
It is important to understand that crosstalk between the Wnt pathways and other signaling pathways exists to regulate the osteogenic differentiation of MSCs. For example, bone morphogenic proteins (BMPs) have been shown to either enhance or antagonize the osteogenic differentiation induced by Wnt signaling [Chen et al. 2012; Lin and Hankenson, 2011]. BMPs, particularly BMP2, 6 and 9, are major osteogenic growth factors that induce osteogenic differentiation in MSCs [Luu et al. 2007]. Studies have demonstrated that Wnt signaling and the BMP signaling pathways have common targets which induce the osteogenic differentiation of MSCs; one such target is connective growth tissue factor [Luo et al. 2004; Si et al. 2006]. Furthermore, functional Wnt signaling was demonstrated to be required for the BMP-induced osteogenic differentiation of MSCs. Wnt 3a enhanced the osteogenic effects of BMP9 while β-catenin knockdown or overexpression of a Fzd antagonist, FrzB, inhibited the osteogenic effects of BMP9 [Tang et al. 2009]. Similarly, conditional β-catenin knockout or Dkk-1 overexpression inhibited BMP2-induced ectopic bone formation [Chen et al. 2007b]. It was suggested that BMP2 stimulates LRP5 expression and downregulates β-Trcp, leading to stabilization of β catenin and its signaling to promote osteogenic differentiation [Zhang et al. 2009].
Interestingly, Wnt5a, a classical noncanonical Wnt, was recently reported as a critical component of BMP2-mediated osteogenic differentiation [Nemoto et al. 2012]. Others have also shown that BMPs can downregulate Wnt signaling in osteogenic differentiation via sclerostin and Dkk-1. Knocking out BMP receptor type 1 in osteoblasts led to downregulation of sclerostin and Dkk-1 and an increased bone mass phenotype in mice [Kamiya et al. 2008, 2010]. As an explanation for the Wnt-antagonizing effects of BMP, it was suggested that Smad1 may form a complex with Dvl, thereby sequestering Dvl from the canonical Wnt pathway [Liu et al. 2006]. However, these seemingly conflicting findings on the crosstalk between BMPs and Wnts remain unresolved.
In addition to BMPs, the Wnt signaling pathway crosstalks with multiple signaling pathways, including the Notch signaling and Hedgehog (Hh) pathways. Activation of the Notch pathway has been shown to inhibit the osteogenic differentiation induced by the Wnt/β-catenin signaling. Overexpression of Notch intracellular domain, both in vivo and in vitro, led to the downregulation of Wnt signaling and impaired osteoblastogenesis [Deregowski et al. 2006; Sciaudone et al. 2003; Zanotti et al. 2008]. The Hh pathway has been reported to work upstream of Wnt signaling in a sequential manner to induce osteogenic differentiation of MSCs. Inhibition of Wnt signaling was shown to reduce Hh-induced osteogenic activity both in vitro [Rawadi et al. 2003] and in vivo [Mak et al. 2006]. It has been suggested that Hh signaling regulates the early stages of osteogenic differentiation of MSC followed by the Wnt signaling further downstream [Lin and Hankenson, 2011; Mak et al. 2006; Rodda and McMahon, 2006].
Wnt signaling also crosstalks with inflammatory signaling processes during bone formation. Notably, tumor necrosis factor (TNF)-α has been shown to induce Dkk-1, an endogenous regulator of Wnt signaling, thus blocking osteoblast differentiation [Diarra et al. 2007; Heiland et al. 2010]. Overexpression of TNFα in mice leads to joint destruction without proper bone repair, mimicking rheumatoid arthritis. However, neutralizing Dkk-1 with anti-Dkk-1 antibodies in TNFα transgenic mice inhibited the joint destruction and resulted in osteophyte formation, indicative of active bone repair [Diarra et al. 2007]. In other words, the intricate balance between bone formation and bone resorption is maintained by the crosstalk between the Wnt signaling and the TNFα-induced inflammatory process.
Knowledge of the crosstalk between Wnt and other signaling pathways continues to expand. Recently, it was shown that the Wnt signaling pathway reciprocally regulates progranulin growth factor in frontotemporal dementia [Rosen et al. 2011; Wexler et al. 2011]. Meanwhile, progranulin, also known as proepithelin, is one of the newly identified growth factors that promotes chondrogenic differentiation of MSCs and endochondral ossification [Feng et al. 2010; Wu et al. 2011]. More details of the crosstalk between Wnts and progranulin in bone formation will need to be elucidated by further investigation.
MicroRNA (miRNA) represents another category of elements that interact with Wnt signaling to regulate osteogenic differentiation. Numerous miRNAs have been reported to either promote or inhibit the osteogenic differentiation of MSCs. Different miRNAs intervene at different locations and stages of osteogenic differentiation, interacting with extracellular growth factors and intracellular transcriptional factors such as Runx2 and osterix [Vimalraj and Selvamurugan, 2012]. Several miRNAs have been reported to specifically interact with Wnt signaling molecules to affect osteogenesis. miR-27 inhibits APC, thus activating canonical Wnt signaling to promote bone formation [Wang and Xu, 2010]. miR-335-5p was shown to downregulate Dkk-1, thus enhancing osteogenic differentiation [Zhang et al. 2011].
Overall, a complex regulatory network exists between the Wnt signaling pathway and other signaling pathways during osteogenic differentiation. A complete characterization of all the interactions between Wnts and these other pathways are far from complete. Nevertheless, a better understanding of the intricate crosstalks between Wnt and other signaling pathways in osteogenesis will prove to be essential in discovering therapeutic interventions to effectively manipulate these signaling pathways and treat osteogenic disorders.
The Wnt signaling pathway in osteogenic pathologies
Since Wnt signaling plays such a critical role in the osteogenic differentiation of MSCs, it is not surprising that aberrations in the Wnt signaling pathway may lead to failures of proper bone formation. Indeed, studies of animal models and human pathologies have shown that aberrant Wnt signaling results in various osteogenic disorders. The effects of these altered Wnt signaling pathways not only highlight the crucial role that the Wnt signaling plays in normal bone physiology but also point to the potential of the Wnt signaling pathway as a target for the development of therapeutic targets in treating bone disorders (Figure 2).
Extracellular components of the Wnt signaling pathway in osteogenic pathologies
The most noteworthy example of altered Wnt signaling causing an osteogenic pathology is the inactivating mutation of LRP5 leading to osteoporosis pseudoglioma syndrome (OPPG). OPPG is an autosomal recessive disorder characterized by early-onset osteoporosis, low bone mineral density (BMD) and blindness [Levasseur et al. 2005]. A loss-of-function mutation of LRP5 has been linked to the pathogenesis of OPPG through genetic analyses [Gong et al. 2001] and later confirmed by animal studies. Inactivating mutations of LRP5 in mice recapitulated the phenotypes of OPPG in human patients [Cui et al. 2011; Kato et al. 2002], and LRP5 knockout resulted in impaired fracture healing in comparison to wild-type mice [Komatsu et al. 2010]. On the contrary, researchers have also identified a gain-of-function missense mutation of LRP5 (G171V) that results in high-bone-mass (HBM) phenotypes in humans [Boyden et al. 2002; Little et al. 2002]. Transgenic mice with the same G171V mutation again recapitulated the HBM phenotypes, confirming that an activating mutation of LRP5 leads to HBM [Babij et al. 2003].
In contrast to LRP5, there is no well-defined human osteogenic pathology associated with an LRP6 mutation. Nevertheless, genetic analyses and animal studies demonstrate that disruption of LRP6 in the Wnt signaling pathway severely affects osteogenic development. A missense mutation of LRP6 mutation in humans led to osteoporosis with some features of the metabolic syndrome [Mani et al. 2007]. In addition, certain polymorphisms of LRP6 were significantly associated with low BMD [Riancho et al. 2011]. In mice, an LRP6 null mutation led to neonatal death, severe axial skeleton defects and limb abnormalities, mimicking the phenotypes of various Wnt gene mutations [Pinson et al. 2000]. Similarly, a spontaneous missense mutation of LRP6 in mice (named ringelswanz) was also discovered to affect development and osteogenic homeostasis. Mice homozygous for ringelswanz showed skeletal abnormalities, delayed ossification during development [Kokubu et al. 2004] and low bone density as adults due to reduced canonical Wnt signaling [Kubota et al. 2008]. Lastly, LRP6 deletion was shown to disrupt bone density synergistically with LRP5 deletion during the osteogenic development of mice, illustrating the critical interplay between LRP6 and LRP5 in skeletal development and bone formation [Holmen et al. 2004].
Osteogenic pathologies can also result from the disruption of secreted extracellular antagonists of Wnt signaling. Sclerostin is one of the best characterized Wnt antagonists. As the product of SOST gene, sclerostin is secreted by osteocytes during bone remodeling. Sclerostin binds to LRP5/6 to inhibit the Wnt signaling pathway during bone formation, completing a negative feedback loop of osteogenesis [van Bezooijen et al. 2005] (Figure 2). Two related pathologies have been linked to specific mutations of SOST: sclerosteosis and van Buchem’s disease. Both pathologies are autosomal recessive disorders characterized by progressive bone growth, increased bone density, volume and strength. Mutations within the SOST gene were shown to be responsible for sclerosteosis [Balemans et al. 2001], whereas van Buchem’s disease is associated with a 52 kb deletion in the noncoding region containing the enhancer element of SOST [Balemans et al. 2002; Loots et al. 2005]. The phenotypes of both diseases were recapitulated in SOST knockout mice which exhibited higher bone mass with increased bone density, volume and strength [Li et al. 2008]. As expected, overexpression of SOST in mice conversely led to osteopenia [Winkler et al. 2003]. The Wnt inhibitory effects of sclerostin can be also reproduced by certain LRP5 mutations. The LRP5 mutations causing HBM, such as the G171V mutant, have been shown to render LRP5 unable to bind sclerostin, thus making it resistant to the inhibitory effects of sclerostin [Ellies et al. 2006; Semenov and He, 2006]. Therefore, sclerostin is a key endogenous regulator of the Wnt signaling pathway in bone formation and has abundant potential as a therapeutic target in modulating the Wnt pathway either to promote or reduce bone formation.
Dkk proteins are another example of extracellular antagonists of the Wnt signaling pathway. Dkk-1 has been proposed to form a ternary complex with Krm and LRP5/6 on the plasma membrane, thus preventing LRPs from binding Wnt ligands [Mao et al. 2002] (Figure 2). It is controversial whether or not LRP6 is internalized and degraded upon Dkk-1 binding [Li et al. 2010b; Mao et al. 2002]. Some researchers have argued that LRP6 disrupts the Wnt-induced formation of Fzd-LRP6 complex without internalizing the receptors [Semënov et al. 2008; Wang et al. 2008]. Regardless, the substantial impact that Dkk-1 has on bone development and homeostasis is evident from genetic and animal studies. Several mutations of LRP5 causing the HBM phenotype in humans were found to have resistance to inhibition by Dkk-1 [Ai et al. 2005; Balemans et al. 2007; Boyden et al. 2002]. This is consistent with findings from mouse models deficient of Dkk-1. A single allele deletion of Dkk-1 in mice showed increased bone mass and greater bone formation activity [Morvan et al. 2006], as did mice with hypomorphic alleles (doubleridge) of Dkk-1 [MacDonald et al. 2007]. However, transgenic overexpression of Dkk-1 in osteoblasts of mice led to osteopenia and skeletal defects [Li et al. 2006]. Thus, Dkk-1 plays an important regulatory role in bone tissue development and homeostasis and causes osteogenic disruptions when altered from its physiologic function.
SFRP is another antagonist of the Wnt signaling pathway. SFRP directly binds Wnt ligands to prevent their binding to Fzd and LRP5/6 [Kawano and Kypta, 2003] (Figure 2). A deficiency of SFRP has been shown to disrupt proper osteogenesis in mice. SFRP-1 knockout mice showed higher trabecular bone volume, density and mass indicative of increased bone formation [Bodine et al. 2004; Gaur et al. 2005]. More specifically, deleting SFRP-1 enhanced endochondral ossification [Gaur et al. 2006] and even led to accelerated fracture healing in animal models [Gaur et al. 2009]. This target can potentially serve as a therapeutic avenue for improving fracture healing in humans. Additionally, certain polymorphisms of SFRP3 in humans have been associated with hip osteoarthritis [Loughlin et al. 2004], making SFRP an attractive therapeutic target in the treatment of arthritis.
The Wnt ligands themselves can also lead to abnormalities in osteogenesis when overexpressed or mutated. Wnt 10b overexpression in mice caused increased postnatal bone mass compared with wild-type mice [Bennett et al. 2005, 2007]. Conversely, Wnt 10b deficient mice exhibited lower bone mass, fewer trabeculae [Bennett et al. 2005] and progressive bone loss after 1 month of age [Stevens et al. 2010]. In addition, Wnt 7b deficiency led to defective osteoblastogenesis and thus ineffective bone formation in the embryos of mice [Tu et al. 2007].
These examples of osteogenic abnormalities associated with aberrant Wnt signaling illustrate potential targets for therapeutic interventions in treating osteogenic disorders. Both inhibition of endogenous antagonists and mimicry of the functions of endogenous agonists of the Wnt signaling should be pursued as potential therapeutic approaches for osteogenic disorders.
Intracellular mediators of the Wnt signaling pathway in osteogenic pathologies
Similar to their extracellular counterparts, intracellular mediators of the Wnt signaling pathway can cause aberrant osteogenesis when altered from their physiologic states. For example, the functional activity of β catenin directly affects the osteogenic differentiation of MSCs. Conditional deletion of β catenin in early osteogenic progenitors prevented terminal differentiation into osteoblasts and instead resulted in chondrocyte formation [Day et al. 2005; Hill et al. 2005; Rodda and McMahon, 2006]. Conversely, stabilization of β catenin led to high bone mass and premature ossification without chondrocyte formation [Glass et al. 2005; Rodda and McMahon, 2006]. Interestingly, the osteopenic state caused by β-catenin inactivation was accompanied by osteoclast activation while stabilization of β catenin was associated with decreased osteoclasts [Holmen et al. 2005]. This suggests that β catenin is involved in the regulation of both osteoblasts and osteoclasts, thus controlling overall bone formation.
Deficiency of Axin-GSK3β-APC complex or Tcf/Lef-1 can also lead to the disruption of normal osteogenic development. Axin2 deletion in mice has been shown to cause overactivation of the canonical Wnt signaling pathway. Phenotypes of Axin2-deficient mice include increased bone mass, increased osteoblast proliferation, increased chondrocyte maturation and craniosynostosis [Dao et al. 2010; Yan et al. 2009; Yu et al. 2005]. Similarly, as expected from its physiologic interaction with β catenin, haploinsufficiency of GSK3β in mice resulted in increased bone formation with enhanced Runx2 activity [Kugimiya et al. 2007]. However, alteration of APC has been shown to both promote and disrupt osteogenensis. APC deletion in osteoblasts led to increased bone mass through overactivating the β-catenin-dependent Wnt signaling [Holmen et al. 2005]. Another study showed that conditional deletion of APC in Col2a1-expressing cells increased β-catenin level as expected, but caused skeletal defects from the failure of proper osteogenic and chondrogenic differentiation [Miclea et al. 2009]. Thus, it appears that a tight regulation of β-catenin level by APC is necessary before commitment into the osteogenic lineage for proper osteogenesis. Lastly, Tcf/Lef-1 transcriptional factors have also been shown to be essential for proper osteogenesis. Lef-1+/– mice showed significantly reduced bone volume during the early years of life [Noh et al. 2009] while Tcf–/– mice exhibited increased bone resorption, thus leading to a lower bone mass phenotype [Glass et al. 2005].
When the Wnt signaling pathway is altered in progenitor cells, proper osteogenic differentiation is compromised leading to osteogenic pathologies/abnormalities. Thus, it is conceivable that the mediators of Wnt signaling may be appealing targets for potential therapeutic interventions that aim to either restore proper osteogenic signaling or manipulate Wnt signaling in favor of bone formation.
Potential therapeutic targets in the Wnt signaling pathway
Given the numerous findings showing that the Wnt signaling pathway regulates osteogenesis, this pathway has arisen as an attractive therapeutic target for treating several osteogenic disorders. Common osteogenic disorders of interest include osteoporosis, fracture healing, nonunion fractures and critical-sized defects. These disorders represent either systemic or local failures of proper bone formation. Thus, inducing the Wnt signaling pathway to promote bone formation is a plausible therapeutic approach to treat these disorders. As reviewed in the previous sections, Wnt signaling contains multiple levels of regulation that can be manipulated to modulate the pathway of osteogenesis: Wnt receptors, secreted Wnt antagonists and intracellular mediators (see Figure 2 and Table 1). Therefore, researchers have been working to develop drugs that target specific components of Wnt signaling to induce osteogenesis. Recently, a few agents have shown promising results and are currently undergoing clinical trials. Other drugs continue to be investigated at the preclinical stages.
Table 1.
Potential therapeutic targets in the Wnt signaling pathway and their therapeutic agents.
| Component | Physiological role | Therapeutic agent | Mechanism of intervention | Therapeutic effects | References |
|---|---|---|---|---|---|
| Sclerostin | Inhibits β-catenin signaling via binding LRP5/6 | Anti-sclerostin antibody (AMG 785) | Neutralizes sclerostin | Increased bone mass, density, and strength in OVX rats, aged male rats, and cynomolgus monkeys | [Li et al. 2009, 2010; Ominsky et al. 2010] |
| Phase I clinical trial: Dose-dependent increase in bone formation without adverse events | [Padhi et al. 2011] | ||||
| Phase II clinical trial: compares AMG 785 with alendronate and teriparatide in postmenopausal women with low BMD | [ClinicalTrials.gov identifier: NCT00896532] | ||||
| Dkk-1 | Inhibits β-catenin signaling via binding (and potentially internalizing) LRP | Anti-Dkk-1 antibody (BHQ 880) | Neutralizes Dkk-1 | Reduced bone loss from rheumatoid arthritis in mice | [Diarra et al. 2007] |
| Enhanced fracture healing and orthopedic implant fixation in mice | [Agholme et al. 2011a; Komatsu et al. 2010; Li et al. 2011] | ||||
| Reduced osteolytic lesions in myeloma mouse models | [Fulciniti et al. 2009; Heath et al. 2009; Yaccoby et al. 2007] | ||||
| Phase I/II clinical trial: Examines the safety and the efficacy of BHQ 880 in combination with zoledronic acid in refractory myeloma | [ClinicalTrials.gov identifier: NCT00741377] | ||||
| SFRP | Inhibits β-catenin signaling via binding the Wnt ligands | Diphenylsulfonyl sulfonamide | Inhibits SFRPs | Inhibits SFRP-1 in vitro and induces bone formation ex vivo | [Bodine et al. 2009; Moore et al. 2009, 2010] |
| GSK3β | Inhibits β catenin via targeting β catenin for proteosomal degradation | Lithium | Inhibits GSK3β | Restores bone mass in LRP5 KO mice and increases bone mass in WT mice | [Clément-Lacroix et al. 2005] |
| Enhances fracture healing and prevents tumor growth in myeloma mouse model | [Edwards et al. 2008] | ||||
| GSK3β inhibitors | Increases bone mass in normal mice | [Gambardella et al. 2011] | |||
| Restores bone loss in OVX mice | [Kulkarni et al. 2006, 2007] | ||||
| β catenin | Binds Tcf/Lef-1 and upregulates target gene expression | Deoxycholic acid | Activates β catenin | Upregulates target gene expression levels of β catenin | [Pai et al. 2004] |
BMD, bone mineral density; Dkk, dickkopf; GSK3β, glycogen synthase kinase 3β; KO, knockout; Lef-1, lymphoid enhancer-binding factor; LRP, low-density lipoprotein receptor-related protein; OVX, ovariectomized; SFRP, secreted frizzled-related protein; Tcf, T-cell factor; WT, wild type.
Targeting the extracellular antagonists
Inhibition of the extracellular antagonists with monoclonal antibodies has been a popular approach to modify the Wnt signaling pathway and promote bone formation. Sclerostin-neutralizing antibody is one of the most prominent examples. Given the physiologic role of sclerostin as a negative regulator of the Wnt pathway, neutralization of sclerostin is expected to upregulate the Wnt signaling pathway, promoting bone formation (see Figure 2 and Table 1). Indeed, studies have shown that subcutaneous injection of sclerostin-neutralizing antibody markedly increased bone formation in ovariectomized rats after 5 weeks of treatment [Li et al. 2009]. In fact, the anabolic effect of antisclerostin antibody was so effective that it not only reversed bone loss by estrogen deficiency, but it actually increased the bone mass compared with nonovariectomized rats. The bone-forming effects of antisclerostin antibody were similarly observed in aged male rats after 5 weeks of treatment [Li et al. 2010a] and in ovary-intact cynomolgus monkeys after 2 months [Ominsky et al. 2010]. Moreover, the osteogenic effect of antisclerostin antibody was persistent regardless of mechanical loading [Agholme et al. 2011b] or presence of fracture [Ominsky et al. 2011]. In humans, polymorphisms of the SOST promotor have been associated with osteoporosis, further making antisclerostin antibody an appealing therapeutic agent for treating osteoporosis [Huang et al. 2009]. Recently, a phase I clinical trial of antisclerostin antibody was completed. Single injections of antisclerostin antibody into healthy men and postmenopausal women were well tolerated and resulted in a dose-dependent increase in bone formation markers and a dose-dependent decrease in bone resorption markers over the 85-day study period [Padhi et al. 2011]. Currently, a phase II trial is ongoing, and it investigates the efficacy of antisclerostin antibody in comparison to alendronate and teriparatide as treatment for osteoporosis in postmenopausal women with low BMD [ClinicalTrials.gov identifier: NCT00896532].
Neutralization of Dkk-1 with antibody is another therapeutic approach under extensive investigation (see Figure 2 and Table 1). Although still at the preclinical stage, animal studies have demonstrated promising results. Similar to antisclerostin antibody, anti-Dkk-1 antibodies increase trabecular bone mass and density in mice [Glantschnig et al. 2010] and restore bone density in osteopenic mice and rhesus macaques [Glantschnig et al. 2011]. The use of anti-Dkk-1 antibody has been shown to be useful in several specific pathologies. First, anti-Dkk-1 antibodies reduced bone loss in a rheumatoid arthritis mouse model [Diarra et al. 2007] as well as in systemic inflammatory states [Heiland et al. 2010]. Furthermore, antibody-mediated blockade of Dkk-1 stimulated bone formation during healing from traumatic injury, such as fracture repair and orthopedic implant fixation [Agholme et al. 2011a; Komatsu et al. 2010; Li et al. 2011]. Lastly, Dkk-1 blockade has been reported to prevent the suppression of osteoblasts in multiple myeloma mouse models while decreasing osteoclasts, thus reducing osteolytic lesions [Fulciniti et al. 2009; Heath et al. 2009; Yaccoby et al. 2007]. Furthermore, the neutralization of Dkk-1 with antibody helped control the growth of myeloma cells [Fulciniti et al. 2009], and active immunization with Dkk-1 was shown to protect mice from developing multiple myeloma [Qian et al. 2012]. Currently, a phase I/II clinical trial is ongoing to test the safety and the efficacy of anti-Dkk-1 antibody in combination with zoledronic acid in refractory multiple myeloma [ClinicalTrials.gov identifier: NCT00741377].
In comparison to sclerostin and Dkk-1, therapies targeting SFRP are at early stages of investigation. By screening multiple compounds, one group has identified a compound, diphenylsulfonyl sulfonamide, which inhibits SFRP-1 activity and induces bone formation ex vivo [Bodine et al. 2009; Moore et al. 2009, 2010] (see Figure 2 and Table 1). No study has explicitly studied the in vivo osteogenic activity of SFRP antibodies or inhibitors. However, commercially available polyclonal antibodies to SFRP-1 were demonstrated to reduce inflammation-induced periodontal bone loss and osteoclastogenesis [Li and Amar, 2007]. Thus, the therapeutic potential of antagonizing SFRP in bone formation remains worthy of further investigation.
Targeting the intracellular mediators
Directly manipulating the intracellular mediators of the Wnt signaling pathway is another potential approach to promote osteogenesis. For instance, inhibiting GSK3β from phosphorylating β catenin would stabilize the cytoplasmic level of β catenin, allowing further progression through the Wnt signaling pathway downstream. Lithium, a commonly used medication for bipolar disorder, is a well characterized example of a GSK3β inhibitor (see Figure 2 and Table 1). Animal studies have shown that the administration of lithium chloride for 4 weeks in LRP5 knockout mice restored bone mass to normal levels and increased the bone mass of wild-type mice [Clément-Lacroix et al. 2005]. Additionally, mice treated with lithium after bone injury exhibited enhanced fracture healing [Chen et al. 2007a]. Furthermore, lithium chloride activated osteoblast differentiation and prevented tumor growth in myeloma mouse models [Edwards et al. 2008]. The association between lithium and enhanced bone formation in mouse models is consistent with epidemiological data in humans. It has been shown that lithium use is associated with reduced fracture risks [Vestergaard et al. 2005] and increased bone mass and density [Zamani et al. 2009]. Yet, a more extensive mechanistic study of lithium and its effect on bone formation is necessary because there are some reports that argue against the pharmacologic benefit of lithium reducing fracture risks [Wilting et al. 2007].
Similar effects of GSK3β can be found with the use of pharmacologic interventions to inhibit GSK3β (see Figure 2 and Table 1). An orally active GSK3α/β inhibitor, 603281-31-8, has demonstrated the ability to induce osteoblastogenesis in vitro and enhance bone formation with greater bone density, thickness and strength in vivo after 60 days [Kulkarni et al. 2006]. The compound 603281-31-8 was also able to reverse trabecular bone volume loss from estrogen deficiency in ovariectomized rats and restore the adipogenicity of bone marrow down to the normal level after 60 days of treatment [Kulkarni et al. 2007]. The effect of GSK3β inhibition was recapitulated with the administration of another GSK3β inhibitor, AR28, which increased osteogenesis while decreasing adipogenicity in mice after 14 days of treatment [Gambardella et al. 2011]. Despite the promising osteogenic benefits of GSK3β inhibitors including lithium and other pharmacologic agents, it is important to note that the GSK3β activity is not limited to bone formation but also involved in other intracellular processes. Thus, caution needs to be taken in overinhibiting GSK3β due to oncogenic risks which will be discussed in the subsequent section.
Interventions on other downstream intracellular mediators carry abundant therapeutic potential for bone disorders. For example, modulation of the interaction between β catenin and Tcf/Lef-1 is a theoretically plausible approach to control the Wnt signaling pathway. Some compounds have been identified to exert activating or inhibitory effects on the interaction between β catenin and Tcf/Lef-1. For example, deoxycholic acid, a secondary bile acid, has been shown to increase the activation of β catenin and expression of its target genes [Pai et al. 2004]. Yet, explicit data on deoxycholic acid promoting bone formation is lacking. Cby, a conserved nuclear protein, has been reported to antagonize β catenin by competing with Lef-1 for binding β catenin [Takemaru et al. 2003] (Figure 2). As a result, Cby is considered an important factor that promotes adipogenic differentiation while inhibiting downstream β-catenin signaling [Li et al. 2007]. Thus, either utilizing its adipogenic properties or developing an intervention to antagonize Cby could provide another therapeutic avenue to manipulate Wnt signaling.
Tumorigenic risks associated with Wnt-targeted therapies
Despite the therapeutic potential of manipulating the Wnt signaling pathway for osteogenic disorders, caution must be taken. The potential therapeutics involve stimulation of the Wnt signaling pathway to promote osteogenesis. Given that the Wnt signaling pathway is not specific to bone tissue, any overactivation of Wnt signaling inevitably leads to consequences outside of bone. More specifically, promoting the proliferation and renewal of stem cells via activation of the Wnt signaling pathway carries the risk of inducing cancer. In fact, these oncogenic risks have been well documented with mutations of Wnt signaling mediators such as APC, Axin, GSK3β, β catenin and LRP [Polakis, 2012]. APC mutation is strongly associated with FAP syndrome and colorectal cancers [Kinzler et al. 1991; Kinzler and Vogelstein, 1996; Nishisho et al. 1991]. Mutations of Axin proteins have also been linked to an increased risk of colorectal cancer [Lammi et al. 2004]. Tcf4 is commonly mutated in colorectal cancers as well [Cuilliere-Dartigues et al. 2006]. Mutations of β catenin and its associated transcription factors can lead to several types of cancer, including hepatoblastoma, hair follicle tumors, sebaceous tumors and leukemia [Chan et al. 1999; Jamieson et al. 2004; Polakis 2007; Takeda et al. 2006].
Therefore, any therapeutic intervention that modulates the Wnt signaling pathway should take into account the potential oncogenic risks associated with exogenous manipulation of the pathway. Deoxycholic acid is an example of exogenous stimulation of the β catenin, which has been reported to induce tumor progression [Pai et al. 2004]. In the context of bone, close attention must be paid to the risk of osteosarcoma development. It has been reported that Wif-1, an endogenous antagonist of Wnt signaling, is downregulated in 76% of human osteosarcoma [Rubin et al. 2010]. More specifically, Wif-1 was epigenetically silenced via promotor hypermethylation, thus overactivating the Wnt signaling pathway and promoting the proliferation of tumor cells [Kansara et al. 2009]. This is consistent with findings that reveal high β-catenin levels in the cytoplasm and nucleus of osteosarcoma cells indicative of high Wnt signaling activity [Iwaya et al. 2003]. Conversely, it is conceivable that any therapeutic intervention inhibiting Wnt signaling can be utilized as an anticancer therapy. Recently, an antibody targeting Fzd receptors, OMP-18R5, was reported to inhibit the growth of human breast, pancreatic, colon and lung tumors in mouse models [Gurney et al. 2012]. Nevertheless, most therapeutic interventions being investigated for the treatment of bone disorders aim to stimulate the Wnt signaling pathway to promote bone formation. Future studies need to carefully examine the optimal level of modulation of the Wnt signaling pathway in treating osteogenic disorders so that oncogenic risks are minimized.
In addition to oncogenic risks, therapeutics involving Wnt signaling should take into account several other considerations [discussed in Hoeppner et al. 2009]. One particularly relevant point in the context of bone disorders is the potential development of osteoarthritic symptoms. Prolonged exogenous stimulation of the Wnt signaling pathway appears to lead to osteoarthritic symptoms. For example, anti-Dkk-1 antibodies, despite reducing bone loss in the rheumatoid arthritis mouse model, produced osteophytes characteristic of osteoarthritis [Diarra et al. 2007]. This observation is consistent with animal studies showing that either upregulating β catenin or inhibiting GSK3β led to osteoarthritic phenotypes with osteophytes [Miclea et al. 2011; Zhu et al. 2009]. Therefore, when utilizing anti-Dkk-1 antibody to prevent bone loss from rheumatoid inflammation or any other therapeutics to activate Wnt signaling for a prolonged period, a careful calibration of the dose and duration of treatment is required to minimize osteoarthritic side effects.
Conclusion and future directions
Our current understanding of the Wnt signaling pathway in osteogenic disorders has broadened considerably over the past decade. We now not only better understand the pathogenesis of these bone disorders caused by aberrant Wnt signaling, but tremendous therapeutic avenues for treating these bone disorders by targeting the Wnt signaling pathway are being thoroughly explored. Antisclerostin antibody exemplifies translation of our knowledge of the Wnt signaling into clinical application. Other agents, such as anti-Dkk-1 antibody and SFRP inhibitors, will continue to undergo clinical investigation and may soon become available for clinical use.
Nevertheless, a considerable amount of work remains. The oncogenic risks associated with exogenous modulation of the Wnt signaling pathway must always be taken into consideration when developing a therapy that targets any component of the Wnt signaling pathway. Finding the optimal balance between the osteogenic effects and the oncogenic risks of Wnt-targeted therapies will be critical and must be explored carefully and extensively. Furthermore, as Wnt signaling is not limited to bone tissue, finding a method to directly target bone tissues without affecting other tissues may be ideal to maximize therapeutic benefits while minimizing oncogenic risks. Further studies clearly defining the best therapeutic targets in the Wnt signaling pathway will be critical in the development of future treatments. Despite current obstacles to the development of new Wnt-targeted therapies, targeting the Wnt signaling pathway has emerged as an attractive strategy to treat many of the most common bone disorders and thus holds great promise as a future therapeutic approach.
Footnotes
Funding: The reported work was supported in part by research grants from the National Institutes of Health (HHL, RCH and TCH) and Orthopaedic Research and Education Foundation (HHL, RCH and TCH).
Conflict of interest statement: The authors declare no conflicts of interest.
Contributor Information
Jeong Hwan Kim, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA.
Xing Liu, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA The Children’s Hospital of Chongqing Medical University, Chongqing, China.
Jinhua Wang, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Xiang Chen, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA The Affiliated Tangdu Hospital of the Fourth Military Medical University, Xi’an, China.
Hongyu Zhang, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Stephanie H. Kim, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago,IL, USA
Jing Cui, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Ruidong Li, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Wenwen Zhang, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Yuhan Kong, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Jiye Zhang, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Wei Shui, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Joseph Lamplot, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA.
Mary Rose Rogers, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA.
Chen Zhao, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA School of Laboratory Medicine and the Affiliated Southwest Hospital, Third Military Medical University, Chongqing, China.
Ning Wang, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA School of Laboratory Medicine and the Affiliated Southwest Hospital, Third Military Medical University, Chongqing, China.
Prashant Rajan, Miami University, Oxford, OH, USA.
Justin Tomal, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA.
Joseph Statz, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA.
Ningning Wu, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA Chongqing Medical University, Chongqing, China.
Hue H. Luu, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA
Rex C. Haydon, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, Chicago, IL, USA
Tong-Chuan He, Molecular Oncology Laboratory, Department of Orthopaedic Surgery, University of Chicago Medical Center, 5841 South Maryland Avenue, MC 3079, Chicago, IL 60637, USA.
References
- Agholme F., Isaksson H., Kuhstoss S., Aspenberg P. (2011a) The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone 48: 988–996 [DOI] [PubMed] [Google Scholar]
- Agholme F., Isaksson H., Li X., Ke H., Aspenberg P. (2011b) Anti-sclerostin antibody and mechanical loading appear to influence metaphyseal bone independently in rats. Acta Orthop 82: 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M., Holmen S., Van Hul W., Williams B., Warman M. (2005) Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol 25: 4946–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P., Zhao W., Small C., Kharode Y., Yaworsky P., Bouxsein M., et al. (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18: 960–974 [DOI] [PubMed] [Google Scholar]
- Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S. (2001) Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol 3: 683–686 [DOI] [PubMed] [Google Scholar]
- Balemans W., Devogelaer J., Cleiren E., Piters E., Caussin E., Van Hul W. (2007) Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling. J Bone Miner Res 22: 708–716 [DOI] [PubMed] [Google Scholar]
- Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., et al. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543 [DOI] [PubMed] [Google Scholar]
- Balemans W., Patel N., Ebeling M., Van Hul E., Wuyts W., Lacza C., et al. (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39: 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C., Longo K., Wright W., Suva L., Lane T., Hankenson K., et al. (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102: 3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C., Ouyang H., Ma Y., Zeng Q., Gerin I., Sousa K., et al. (2007) Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res 22: 1924–1932 [DOI] [PubMed] [Google Scholar]
- Bianco P., Robey P., Simmons P. (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2: 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine P., Stauffer B., Ponce-de-Leon H., Bhat R., Mangine A., Seestaller-Wehr L., et al. (2009) A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone 44: 1063–1068 [DOI] [PubMed] [Google Scholar]
- Bodine P., Zhao W., Kharode Y., Bex F., Lambert A., Goad M., et al. (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 18: 1222–1237 [DOI] [PubMed] [Google Scholar]
- Boyden L., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M., et al. (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521 [DOI] [PubMed] [Google Scholar]
- Case N., Rubin J. (2010) Beta-catenin – a supporting role in the skeleton. J Cell Biochem 110: 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn W., Bree A., Yao Y., Du B., Hemati N., Martinez-Santibañez G., et al. (2012) Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50: 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E., Gat U., McNiff J., Fuchs E. (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 21: 410–413 [DOI] [PubMed] [Google Scholar]
- Chen G., Deng C., Li Y. (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8: 272–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Whetstone H., Lin A., Nadesan P., Wei Q., Poon R., et al. (2007a) Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Whetstone H., Youn A., Nadesan P., Chow E., Lin A., et al. (2007b) Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem 282: 526–533 [DOI] [PubMed] [Google Scholar]
- Clément-Lacroix P., Ai M., Morvan F., Roman-Roman S., Vayssière B., Belleville C., et al. (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102: 17406–17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149: 1192–1205 [DOI] [PubMed] [Google Scholar]
- Cui Y., Niziolek P., MacDonald B., Zylstra C., Alenina N., Robinson D., et al. (2011) Lrp5 functions in bone to regulate bone mass. Nat Med 17: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuilliere-Dartigues P., El-Bchiri J., Krimi A., Buhard O., Fontanges P., Fléjou J., et al. (2006) TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene 25: 4441–4448 [DOI] [PubMed] [Google Scholar]
- Dao D., Yang X., Flick L., Chen D., Hilton M., O’Keefe R. (2010) Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res 28: 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T., Guo X., Garrett-Beal L., Yang Y. (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8: 739–750 [DOI] [PubMed] [Google Scholar]
- De A. (2011) Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin 43: 745–756 [DOI] [PubMed] [Google Scholar]
- Deregowski V., Gazzerro E., Priest L., Rydziel S., Canalis E. (2006) Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem 281: 6203–6210 [DOI] [PubMed] [Google Scholar]
- Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M., Dwyer D., et al. (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156–163 [DOI] [PubMed] [Google Scholar]
- Edwards C.M., Edwards J.R., Lwin S.T., Esparza J., Oyajobi B.O., McCluskey B., et al. (2008) Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111(5): 2833–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies D., Viviano B., McCarthy J., Rey J., Itasaki N., Saunders S., et al. (2006) Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21: 1738–1749 [DOI] [PubMed] [Google Scholar]
- Feng J., Guo F., Jiang B., Zhang Y., Frenkel S., Wang D., et al. (2010) Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J 24: 1879–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M., Tassone P., Hideshima T., Vallet S., Nanjappa P., Ettenberg S., et al. (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella A., Nagaraju C., O’Shea P., Mohanty S., Kottam L., Pilling J., et al. (2011) Glycogen synthase kinase-3α/β inhibition promotes in vivo amplification of endogenous mesenchymal progenitors with osteogenic and adipogenic potential and their differentiation to the osteogenic lineage. J Bone Miner Res 26: 811–821 [DOI] [PubMed] [Google Scholar]
- Gaur T., Lengner C., Hovhannisyan H., Bhat R., Bodine P., Komm B., et al. (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280: 33132–33140 [DOI] [PubMed] [Google Scholar]
- Gaur T., Rich L., Lengner C., Hussain S., Trevant B., Ayers D., et al. (2006) Secreted frizzled related protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol 208: 87–96 [DOI] [PubMed] [Google Scholar]
- Gaur T., Wixted J., Hussain S., O’Connell S., Morgan E., Ayers D., et al. (2009) Secreted frizzled related protein 1 is a target to improve fracture healing. J Cell Physiol 220: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H., Hampton R., Lu P., Zhao J., Vitelli S., Huang L., et al. (2010) Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285: 40135–40147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H., Scott K., Hampton R., Wei N., McCracken P., Nantermet P., et al. (2011) A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J Pharmacol Exp Ther 338: 568–578 [DOI] [PubMed] [Google Scholar]
- Glass D., II, Bialek P., Ahn J., Starbuck M., Patel M., Clevers H., et al. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764 [DOI] [PubMed] [Google Scholar]
- Gong Y., Slee R., Fukai N., Rawadi G., Roman-Roman S., Reginato A., et al. (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523 [DOI] [PubMed] [Google Scholar]
- Gurney A., Axelrod F., Bond C., Cain J., Chartier C., Donigan L., et al. (2012) Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA 2 July (Epub ahead of print). DOI: 10.1073/pnas.1120068109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Saint-Jeannet J., Wang Y., Nathans J., Dawid I., Varmus H. (1997) A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275: 1652–1654 [DOI] [PubMed] [Google Scholar]
- Heath D., Chantry A., Buckle C., Coulton L., Shaughnessy J., Jr, Evans H., et al. (2009) Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24: 425–436 [DOI] [PubMed] [Google Scholar]
- Heiland G., Zwerina K., Baum W., Kireva T., Distler J., Grisanti M., et al. (2010) Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis 69: 2152–2159 [DOI] [PubMed] [Google Scholar]
- Hill T., Später D., Taketo M., Birchmeier W., Hartmann C. (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8: 727–738 [DOI] [PubMed] [Google Scholar]
- Hoeppner L., Secreto F., Westendorf J. (2009) Wnt signaling as a therapeutic target for bone diseases. Exp Opin Ther Targets 13: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen S., Giambernardi T., Zylstra C., Buckner-Berghuis B., Resau J., Hess J., et al. (2004) Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19: 2033–2040 [DOI] [PubMed] [Google Scholar]
- Holmen S., Zylstra C., Mukherjee A., Sigler R., Faugere M., Bouxsein M., et al. (2005) Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 280: 21162–21168 [DOI] [PubMed] [Google Scholar]
- Hsieh J., Kodjabachian L., Rebbert M., Rattner A., Smallwood P., Samos C., et al. (1999) A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398: 431–436 [DOI] [PubMed] [Google Scholar]
- Huang Q., Li G., Kung A. (2009) The -9247 T/C polymorphism in the SOST upstream regulatory region that potentially affects C/EBPalpha and FOXA1 binding is associated with osteoporosis. Bone 45: 289–294 [DOI] [PubMed] [Google Scholar]
- Iwaya K., Ogawa H., Kuroda M., Izumi M., Ishida T., Mukai K. (2003) Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis 20: 525–529 [DOI] [PubMed] [Google Scholar]
- Jamieson C., Ailles L., Dylla S., Muijtjens M., Jones C., Zehnder J., et al. (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 351: 657–667 [DOI] [PubMed] [Google Scholar]
- Kamiya N., Kobayashi T., Mochida Y., Yu P., Yamauchi M., Kronenberg H., et al. (2010) Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res 25: 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N., Ye L., Kobayashi T., Mochida Y., Yamauchi M., Kronenberg H., et al. (2008) BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135: 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Bennett C., Gerin I., Rapp L., Hankenson K., Macdougald O. (2007) Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem 282: 14515–14524 [DOI] [PubMed] [Google Scholar]
- Kansara M., Tsang M., Kodjabachian L., Sims N., Trivett M., Ehrich M., et al. (2009) Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119: 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Patel M., Levasseur R., Lobov I., Chang B., Glass D., II, et al. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157: 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627–2634 [DOI] [PubMed] [Google Scholar]
- Kimelman D., Xu W. (2006) beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25: 7482–7491 [DOI] [PubMed] [Google Scholar]
- Kinzler K., Nilbert M., Su L., Vogelstein B., Bryan T., Levy D., et al. (1991) Identification of FAP locus genes from chromosome 5q21. Science 253: 661–665 [DOI] [PubMed] [Google Scholar]
- Kinzler K., Vogelstein B. (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Kokubu C., Heinzmann U., Kokubu T., Sakai N., Kubota T., Kawai M., et al. (2004) Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development 131: 5469–5480 [DOI] [PubMed] [Google Scholar]
- Komatsu D., Mary M., Schroeder R., Robling A., Turner C., Warden S. (2010) Modulation of Wnt signaling influences fracture repair. J Orthop Res 28: 928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Bryant H., Macdougald O. (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Michigami T., Sakaguchi N., Kokubu C., Suzuki A., Namba N., et al. (2008) Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J Bone Miner Res 23: 1661–1671 [DOI] [PubMed] [Google Scholar]
- Kugimiya F., Kawaguchi H., Ohba S., Kawamura N., Hirata M., Chikuda H., et al. (2007) GSK-3beta controls osteogenesis through regulating Runx2 activity. PLoS One 2: e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl M. (2004) The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci 9: 967–974 [DOI] [PubMed] [Google Scholar]
- Kulkarni N., Onyia J., Zeng Q., Tian X., Liu M., Halladay D., et al. (2006) Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21: 910–920 [DOI] [PubMed] [Google Scholar]
- Kulkarni N., Wei T., Kumar A., Dow E., Stewart T., Shou J., et al. (2007) Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem 102: 1504–1518 [DOI] [PubMed] [Google Scholar]
- Lammi L., Arte S., Somer M., Jarvinen H., Lahermo P., Thesleff I., et al. (2004) Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 74: 1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur R., Lacombe D., de Vernejoul M. (2005) LRP5 mutations in osteoporosis-pseudoglioma syndrome and high-bone-mass disorders. Joint Bone Spine 72: 207–214 [DOI] [PubMed] [Google Scholar]
- Li C., Amar S. (2007) Inhibition of SFRP1 reduces severity of periodontitis. J Dent Res 86: 873–877 [DOI] [PubMed] [Google Scholar]
- Li F., Singh A., Mofunanya A., Love D., Terada N., Moon R., et al. (2007) Chibby promotes adipocyte differentiation through inhibition of beta-catenin signaling. Mol Cell Biol 27: 4347–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sarosi I., Cattley R., Pretorius J., Asuncion F., Grisanti M., et al. (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39: 754–766 [DOI] [PubMed] [Google Scholar]
- Li X., Grisanti M., Fan W., Asuncion F., Tan H., Dwyer D., et al. (2011) Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res 26: 2610–2621 [DOI] [PubMed] [Google Scholar]
- Li X., Ominsky M., Niu Q., Sun N., Daugherty B., D’Agostin D., et al. (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869 [DOI] [PubMed] [Google Scholar]
- Li X., Ominsky M., Warmington K., Morony S., Gong J., Cao J., et al. (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24: 578–588 [DOI] [PubMed] [Google Scholar]
- Li X., Warmington K., Niu Q., Asuncion F., Barrero M., Grisanti M., et al. (2010a) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25: 2647–2656 [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., et al. (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887 [DOI] [PubMed] [Google Scholar]
- Li Y., Lu W., King T., Liu C., Bijur G., Bu G.(2010b) Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One 5: e11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Hankenson K. (2011) Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem 112: 3491–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R., Carulli J., Del Mastro R., Dupuis J., Osborne M., Folz C., et al. (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Tang Y., Qiu T., Cao X., Clemens T. (2006) A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem 281: 17156–17163 [DOI] [PubMed] [Google Scholar]
- Logan C., Nusse R. (2004) The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Loots G., Kneissel M., Keller H., Baptist M., Chang J., Collette N., et al. (2005) Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15: 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J., Dowling B., Chapman K., Marcelline L., Mustafa Z., Southam L., et al. (2004) Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA 101: 9757–9762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Kang Q., Si W., Jiang W., Park J., Peng Y., et al. (2004) Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 279: 55958–55968 [DOI] [PubMed] [Google Scholar]
- Luu H., Song W., Luo X., Manning D., Luo J., Deng Z., et al. (2007) Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25: 665–677 [DOI] [PubMed] [Google Scholar]
- MacDonald B., Joiner D., Oyserman S., Sharma P., Goldstein S, He X., et al. (2007) Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41: 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B., Tamai K., He X. (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K., Chen M., Day T., Chuang P., Yang Y.(2006) Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 133: 3695–3707 [DOI] [PubMed] [Google Scholar]
- Mani A., Radhakrishnan J., Wang H., Mani A., Mani M., Nelson-Williams C., et al. (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315: 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B., et al. (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417: 664–667 [DOI] [PubMed] [Google Scholar]
- Miclea R., Karperien M., Bosch C., van der Horst G., van der Valk M., Kobayashi T., et al. (2009) Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev Biol 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclea R., Siebelt M., Finos L., Goeman J., Löwik C., Oostdijk W., et al. (2011) Inhibition of Gsk3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthritis Cartilage 19: 1363–1372 [DOI] [PubMed] [Google Scholar]
- Mikels A., Nusse R. (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe D., McGee-Lawrence M., Oursler M., Westendorf J. (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene 492: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W., Kern J., Bhat R., Bodine P., Fukyama S., Krishnamurthy G., et al. (2010) Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides: part II. Bioorg Med Chem 18: 190–201 [DOI] [PubMed] [Google Scholar]
- Moore W., Kern J., Bhat R., Commons T., Fukayama S., Goljer I., et al. (2009) Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides. J Med Chem 52: 105–116 [DOI] [PubMed] [Google Scholar]
- Morvan F., Boulukos K., Clément-Lacroix P., Roman-Roman S., Suc-Royer I., Vayssière B., et al. (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21: 934–945 [DOI] [PubMed] [Google Scholar]
- Nemoto E., Ebe Y., Kanaya S., Tsuchiya M., Nakamura T., Tamura M., et al. (2012) Wnt5a signaling is a substantial constituent in bone morphogenetic protein-2-mediated osteoblastogenesis. Biochem Biophys Res Commun 422: 627–632 [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., et al. (1991) Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253: 665–669 [DOI] [PubMed] [Google Scholar]
- Noh T., Gabet Y., Cogan J., Shi Y., Tank A., Sasaki T., et al. (2009) Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One 4: e5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- Ominsky M., Li C., Li X., Tan H., Lee E., Barrero M., et al. (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26: 1012–1021 [DOI] [PubMed] [Google Scholar]
- Ominsky M., Vlasseros F., Jolette J., Smith S., Stouch B., Doellgast G., et al. (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25: 948–959 [DOI] [PubMed] [Google Scholar]
- Padhi D., Jang G., Stouch B., Fang L., Posvar E. (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26: 19–26 [DOI] [PubMed] [Google Scholar]
- Pai R., Tarnawski A., Tran T. (2004) Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell 15: 2156–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson K., Brennan J., Monkley S., Avery B., Skarnes W. (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535–538 [DOI] [PubMed] [Google Scholar]
- Polakis P. (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17(1): 45–51 [DOI] [PubMed] [Google Scholar]
- Polakis P. (2012) Wnt signaling in cancer. Cold Spring Harbor Perspect Biol 4 DOI: 10.1101/cshperspect.a00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Zheng Y., Zheng C., Wang L., Qin H., Hong S., et al. (2012) Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood 119: 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner T., Khosla S., Hofbauer L. (2011) Osteoporosis: now and the future. Lancet 377: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T., Kühl M. (2010) An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106: 1798–1806 [DOI] [PubMed] [Google Scholar]
- Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18: 1842–1853 [DOI] [PubMed] [Google Scholar]
- Riancho J., Olmos J., Pineda B., García-Ibarbia C., Pérez-Núñez M., Nan D., et al. (2011) Wnt receptors, bone mass, and fractures: gene-wide association analysis of LRP5 and LRP6 polymorphisms with replication. Eur J Endocrinol 164: 123–131 [DOI] [PubMed] [Google Scholar]
- Rodda S., McMahon A. (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133: 3231–3244 [DOI] [PubMed] [Google Scholar]
- Rosen E., Wexler E., Versano R., Coppola G., Gao F., Winden K., et al. (2011) Functional genomic analyses identify pathways dysregulated by progranulin deficiency, implicating Wnt signaling. Neuron 71: 1030–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E., Guo Y., Tu K., Xie J., Zi X., Hoang B. (2010) Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol Cancer Ther 9: 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S., Masiarz F., et al. (1993) Association of the APC gene product with beta-catenin. Science 262: 1731–1734 [DOI] [PubMed] [Google Scholar]
- Sciaudone M., Gazzerro E., Priest L., Delany A., Canalis E. (2003) Notch 1 impairs osteoblastic cell differentiation. Endocrinology 144: 5631–5639 [DOI] [PubMed] [Google Scholar]
- Semënov M., He X. (2006) LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem 281: 38276–38284 [DOI] [PubMed] [Google Scholar]
- Semënov M., Tamai K., He X. (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280: 26770–26775 [DOI] [PubMed] [Google Scholar]
- Semënov M., Zhang X., He X. (2008) DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem 283: 21427–21432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W., Kang Q., Luu H., Park J., Luo Q., Song W., et al. (2006) CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol 26: 2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J., Miranda-Carboni G., Singer M., Brugger S., Lyons K., Lane T. (2010) Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res 25: 2138–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Johnson K., Smith K., Hill D., Vogelstein B., Kinzler K. (1993) Association between wild type and mutant APC gene products. Cancer Res 53: 2728–2731 [PubMed] [Google Scholar]
- Sugimura R., Li L. (2010) Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today 90: 243–256 [DOI] [PubMed] [Google Scholar]
- Takada I., Mihara M., Suzawa M., Ohtake F., Kobayashi S., Igarashi M., et al. (2007) A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9: 1273–1285 [DOI] [PubMed] [Google Scholar]
- Takeda H., Lyle S., Lazar A., Zouboulis C., Smyth I., Watt F. (2006) Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med 12: 395–397 [DOI] [PubMed] [Google Scholar]
- Takemaru K., Yamaguchi S., Lee Y., Zhang Y., Carthew R., Moon R. (2003) Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422: 905–909 [DOI] [PubMed] [Google Scholar]
- Tang N., Song W., Luo J., Luo X., Chen J., Sharff K., et al. (2009) BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 13: 2448–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X., Joeng K., Nakayama K., Nakayama K., Rajagopal J., Carroll T., et al. (2007) Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell 12: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Mikels A., Nusse R. (2008) Alternative Wnt signaling is initiated by distinct receptors. Sci Signal 1: re9. [DOI] [PubMed] [Google Scholar]
- van Bezooijen R., ten Dijke P., Papapoulos S., Löwik C. (2005) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16: 319–327 [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. (2005) Reduced relative risk of fractures among users of lithium. Calcif Tissue Int 77: 1–8 [DOI] [PubMed] [Google Scholar]
- Vladar E.K., Antic D., Axelrod J.D. (2009) Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harbor Perspectives in Biology 1(3): a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalraj S., Selvamurugan N. (2012) MicroRNAs: synthesis, gene regulation and osteoblast differentiation. Curr Issues Mol Biol 15: 7–18 [PubMed] [Google Scholar]
- Wagner E., Zhu G., Zhang B., Luo Q., Shi Q., Huang E., et al. (2011) The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr Mol Pharmacol 4: 14–25 [PubMed] [Google Scholar]
- Wang K., Zhang Y., Li X., Chen L., Wang H., Wu J., et al. (2008) Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem 283: 23371–23375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Xu Z. (2010) miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun 402: 186–189 [DOI] [PubMed] [Google Scholar]
- Wexler E., Rosen E., Lu D., Osborn G., Martin E., Raybould H., et al. (2011) Genome-wide analysis of a Wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci Signal 4: ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting I., de Vries F., Thio B., Cooper C., Heerdink E., Leufkens H., et al. (2007) Lithium use and the risk of fractures. Bone 40: 1252–1258 [DOI] [PubMed] [Google Scholar]
- Winkler D., Sutherland M., Geoghegan J., Yu C., Hayes T., Skonier J., et al. (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zang W., Li X., Sun H. (2011) Proepithelin stimulates growth plate chondrogenesis via nuclear factor-kappaB-p65-dependent mechanisms. J Biol Chem 286: 24057–24067 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yaccoby S., Ling W., Zhan F., Walker R., Barlogie B., Shaughnessy J., Jr.(2007) Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109: 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Tang D., Chen M., Huang J., Xie R., Jonason J., et al. (2009) Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J Cell Sci 122: 3566–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Jerchow B., Sheu T., Liu B., Costantini F., Puzas J., et al. (2005) The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132: 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani A., Omrani G., Nasab M. (2009) Lithium’s effect on bone mineral density. Bone 44: 331–334 [DOI] [PubMed] [Google Scholar]
- Zanotti S., Smerdel-Ramoya A., Stadmeyer L., Durant D., Radtke F., Canalis E. (2008) Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology 149: 3890–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tu Q., Bonewald L., He X., Stein G., Lian J., et al. (2011) Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res 26: 1953–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Yan Y., Lim Y., Tang D., Xie R., Chen A., et al. (2009) BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. J Cell Biochem 108: 896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Khan D., Delling J., Tobiasch E. (2012) Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. TheScientificWorldJournal 2012: 793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., et al. (2009) Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res 24: 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]