Abstract
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease predominantly affecting the axial skeleton (sacroiliac joints and spine). Nonradiographic axSpA (axSpA without radiographic sacroiliitis) and ankylosing spondylitis (AS; radiographic form of axSpA) are considered nowadays as two consecutive stages of one disease. Nonsteroidal anti-inflammatory drugs (NSAIDs) are highly effective against the major symptoms of axSpA (pain and stiffness) and may have disease-modifying properties including retarding progression of structural damage in the spine. Therefore, NSAIDs, unless contraindicated, are the treatment of choice for the majority of patients with axSpA. Beyond NSAIDs, only tumour necrosis factor (TNF) α blockers are effective and approved for the treatment of active axSpA. Several novel drugs (i.e. monoclonal antibodies targeting interleukin-17, interleukin-12/23, inhibitors of phosphodiesterase-4 and kinases), which might be effective in axSpA, are currently under investigation. Pharmacological therapy of axSpA should always be combined with nonpharmacological treatment including education and regular exercise/physiotherapy.
Keywords: ankylosing spondylitis, axial spondyloarthritis, nonsteroidal anti-inflammatory drugs, treatment, tumour necrosis factor α blocker
Introduction
Spondyloarthritis (SpA) is a group of chronic inflammatory diseases of autoimmune nature sharing common clinical and genetic features, such as involvement of the axial skeleton (sacroiliac joints and spine), a certain pattern of peripheral joint involvement (usually asymmetric monoarthritis or oligoarthritis predominantly affecting the joints of the lower extremities), the presence of enthesitis, dactylitis, typical extra-articular manifestations such as acute anterior uveitis, psoriasis and inflammatory bowel disease (i.e. Crohn’s disease and ulcerative colitis), and association with HLA-B27 antigen. Current classification of SpA relies on the predominant clinical manifestation: either axial or peripheral [Rudwaleit et al. 2011]. Axial SpA (axSpA) is characterized by predominant involvement of the spine and/or sacroiliac joints: ankylosing spondylitis (AS), nonradiographic axial Spa (nr-axSpa, without definite sacroiliitis on X-ray), certain forms of psoriatic arthritis and reactive arthritis with axial involvement, and arthritis associated with inflammatory bowel disease. Importantly, nr-axSpA and AS are considered nowadays as two stages of one disease (axSpA) [Rudwaleit et al. 2005], although there are patients with an abortive course of the disease who remain at the nonradiographic stage without progression to established AS.
In peripheral SpA, peripheral arthritis, enthesitis and/or dactylitis dominate in the clinical presentation setting [Rudwaleit et al. 2011]. Reactive arthritis, psoriatic arthritis, arthritis associated with inflammatory bowel disease and certain forms of undifferentiated (oligo)arthritis could be generally classified as a peripheral SpA.
In this review, currently available and possible future treatment options for axSpA are discussed.
Current treatments for axial SpA
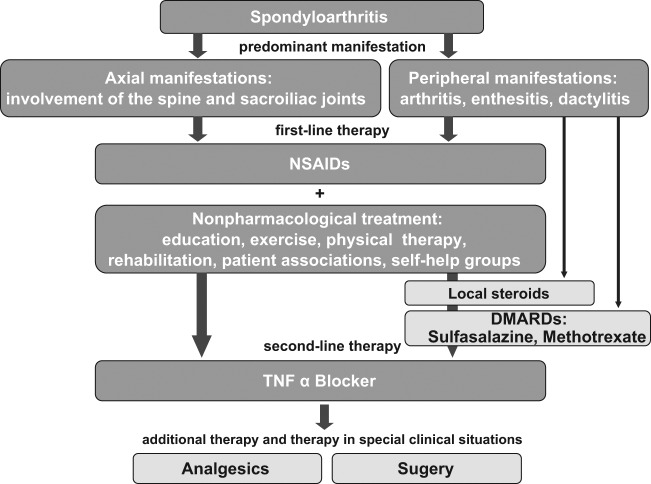
According to the actual evidence-based Assessment of Spondyloarthritis International Society (ASAS) and European League Against Rheumatism (EULAR) recommendations for the treatment of AS, the prototype disease of axSpA, the first-line therapy of this disease consist of nonsteroidal anti-inflammatory drugs (NSAIDs) and nonpharmacological treatment (such as education and regular exercise/physiotherapy) irrespectively of the of the predominant involvement (axial or peripheral) [Braun et al. 2011] (Figure 1).
Figure 1.
Summary of the ASAS/EULAR recommendations for treatment of AS.
AS, ankylosing spondylitis; DMARD, disease-modifying antirheumatic drug; NSAIDs, nonsteroidal anti-inflammatory drug; TNF, tumour necrosis factor.
NSAIDs are especially effective in patients with axial involvement, reducing pain and stiffness substantially in a majority of patients, as shown in a number of clinical trials with nonselective cyclooxygenase (COX) inhibitors as well as with selective COX-2 antagonists [Barkhuizen et al. 2006; Dougados et al. 1999; Sieper et al. 2008; van der Heijde et al. 2005a]. Clinically significant improvement of back pain in AS is usually reported by more than 60% of the patients treated with NSAIDs [Amor et al. 1995; Sieper et al. 2008; van der Heijde et al. 2005a], as compared with only about 15% of patients with chronic low back pain of noninflammatory causes [Amor et al. 1995].
So far, clinical trials demonstrating clinical efficacy of NSAIDs in axSpA were performed in patients with AS only. However, it can be expected that NSAIDs are also effective in patients with nr-axSpA, who did not develop radiographic sacroiliitis yet. That is also being confirmed by daily practice. Considering nr-axSpA and AS as two stages of axSpA, it is reasonable to extrapolate data on treatment efficacy from AS to the early stage of the disease. Therefore, nr-axSpA patients should generally be treated in the same way as patients with AS [Braun et al. 2011].
In general, all NSAIDs, regardless of their COX selectivity, have similar clinical efficacy in their therapeutic doses (usually equal to maximal recommended) [Dougados et al. 2011] in axSpA. In the majority of cases, NSAIDs reduce pain and stiffness rapidly and the full effect can normally be observed after 48–72 hours. In some cases, however, a longer treatment period (up to 2 weeks) is necessary in order to reach the complete anti-inflammatory and analgesic effect of a NSAID [van der Heijde et al. 2005a]. Moreover, considering the high individual variation in response to NSAIDs on the patients’ level, it is worthwhile to try at least another NSAID if the first NSAID has failed.
NSAID therapy in axSpA, if effective and tolerable, should usually be continued for a long-term period. Dose and intake frequency could be adjusted according to the intensity of the patient’s symptoms (back pain, stiffness). In general, on-demand use of an effective dose should be recommended. In some AS patients a moderate dose might be sufficient for long-term treatment, while in others the highest tolerated dose taken continuously might be necessary in order to achieve an optimal therapeutic effect. Safety aspects, especially gastrointestinal and cardiovascular risks, should always be taken into account when starting NSAID therapy.
Gastrointestinal toxicity is a well-known adverse effect of NSAIDs. Serious gastrointestinal adverse events include gastroduodenal ulceration and ulcer complications such as gastrointestinal bleeding, perforation and gastric outlet obstruction. The risk of gastrointestinal adverse events of NSAIDs is strongly dependent on the presence of risk factors: previous gastrointestinal events (especially if complicated), age, concomitant use of anticoagulants, corticosteroids, other NSAIDs including low-dose aspirin, high-dose NSAID therapy, chronic debilitating disorders, especially cardiovascular disease, and Helicobacter pylori infection [Lanza et al. 2009].
Currently available evidence suggests that both selective COX-2 inhibitors (coxibs) and nonselective NSAIDs, with the possible exception of full-dose naproxen, increase cardiovascular risk to nearly the same extent [Kearney et al. 2006; Trelle et al. 2011]. The individual cardiovascular risk depends on numerous well-known traditional cardiovascular risk factors such as age, smoking, diabetes and history of previous cardiovascular events. Large clinical trials demonstrated that rates of cardiovascular events were especially low in younger patients and patients with low baseline cardiovascular risk: less than 1 event per 100 patient-years [Cannon et al. 2006; Solomon et al. 2005]. It is especially relevant in axSpA, because this is a disease of young people starting normally in the third decade of life without cardiovascular risk factors and comorbidities.
Recent recommendations of the American College of Gastroenterology suggest a careful assessment of both gastrointestinal and cardiovascular risks in order to choose an optimal regimen of NSAIDs treatment. In patients with low gastrointestinal risk (no risk factors) and low cardiovascular risk (no intake of low-dose aspirin), nonselective NSAIDs can be administered safely. In case of moderate gastrointestinal risk (age >65 years or high-dose NSAID therapy or a previous history of uncomplicated ulcer, or concurrent use of aspirin including low dose, corticosteroids or anticoagulants) a selective COX-2 inhibitor or a combination of a nonselective NSAID with a gastroprotector (proton-pump inhibitor or misoprostol) should be used. In patients with high gastrointestinal risk [history of a previously complicated ulcer, especially recent, or multiple (>2) risk factors] an alternative therapy should be considered or, if not possible, a combination of a COX-2 inhibitor with a gastroprotective agent [Lanza et al. 2009]. In patients with high cardiovascular risk (defined as the need of low-dose aspirin intake) and low or moderate gastrointestinal risk, a combination of naproxen with gastroprotective agents is recommended. If both cardiovascular and gastrointestinal risks are high, NSAIDs should be generally avoided and an alternative therapy should be considered [Lanza et al. 2009].
Interestingly, in a recent large observational study from Norway, frequent use of NSAIDs in AS was clearly and statistically significant associated with decreased overall mortality [Bakland et al. 2011]. This data require confirmation, but it could be speculated that an anti-inflammatory effect of NSAIDs, might counterbalance increase of the cardiovascular risk in AS related to systemic inflammation and to the NSAID treatment itself [Song et al. 2008].
There is increasing evidence that NSAIDs might possess not only symptomatic efficacy but also disease-modifying properties in axSpA retarding progression of structural damage in the spine. In 1976, Boersma showed that a continuous use of phenylbutazone was associated with retardation of spinal ossification in AS [Boersma, 1976]. In a more recent study by Wanders and colleagues continuous (daily) use of NSAIDs (all starting with celecoxib but changing to other NSAIDs in the case of clinical inefficacy or intolerance) was also associated with an inhibition of radiographic progression in the spine over 2 years [Wanders et al. 2005]. The most recent data from the German Spondyloarthritis Inception Cohort (GESPIC) provided support for these findings: high NSAIDs intake (more than 50% of the maximal recommended dose) over 2 years was associated with lower rate of radiographic spinal progression in AS in comparison with patients with low NSAIDs intake. In nr-axSpA, no significant differences regarding radiographic progression between patients with high and low NSAIDs intake was found that was most likely related to the low level of spinal damage in general in this group [Poddubnyy et al. 2012].
Importantly, retardation of radiographic spinal progression with NSAIDs therapy was nearly exclusively seen in patients with risk factors for such a progression (presence of syndesmophytes at baseline and elevated C-reactive protein [CRP]) [Poddubnyy et al. 2012]. These data were also confirmed in a resent post hoc analysis of the study by Wanders and colleagues: an inhibitory effect of continuous NSAIDs use was observed only in the group of patients with elevated acute phase reactants (CRP or erythrocyte sedimentation rate [ESR]) [Kroon et al. 2012].
Nonpharmacological treatment (first of all, education and regular exercises) is considered to be of nearly the same importance as NSAIDs in the first-line therapy of axSpA [Braun et al. 2011]. It is generally accepted that regular exercise/physiotherapy is effective in reducing symptoms and increasing function and spinal mobility in axSpA in a short-term perspective that is also supported by evidence [Dagfinrud et al. 2008]. However, the influence of nonpharmacological treatment on the long-term outcomes and radiographic spinal progression is less clear.
Classic disease-modifying antirheumatic drugs (DMARDs; such as methotrexate, sulfasalazine and, to a lesser extent, leflunomide) are usually not effective in axial disease, but might be beneficial in the case of peripheral joint involvement [Braun et al. 2006; Haibel et al. 2005a, 2007]. Therefore, DMARDs are currently reserved for patients with predominant peripheral manifestation.
Local steroids are also recommended mainly for treatment of peripheral manifestation (arthritis, enthesitis, dactylitis) but can be also effective in the treatment of active sacroiliitis (CT-guided injections) in pure axial disease [Braun et al. 1996].
Systemic steroids are generally not recommended in axSpA, but short-term treatment (intravenous pulse therapy with methylprednisolone or oral prednisolone for 2 weeks) might be beneficial if rapid reduction of disease activity is required [Haibel et al. 2012; Peters and Ejstrup, 1992].
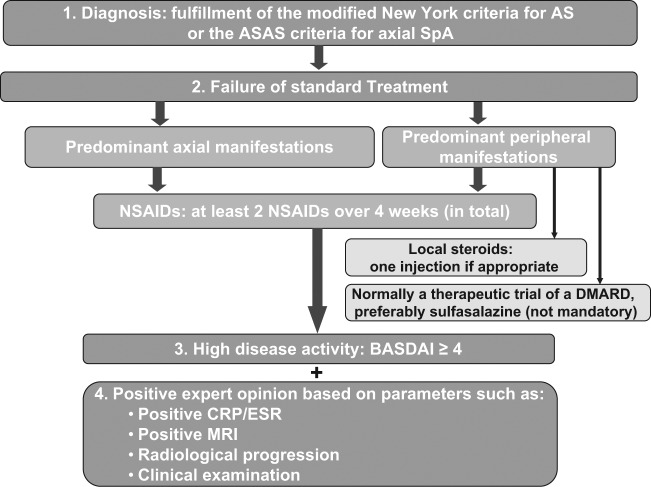
In patients who do not respond to first-line therapy, a tumour necrosis factor (TNF) α blocker represents the only reliable treatment option available at the moment (Figure 1). Although, similarly to NSAIDs, the vast majority of evidence of TNF blockers efficacy in axSpA was obtained in clinical trials conducted in established AS, it is reasonable to expect the same (or even higher) clinical response in patients at the earlier disease stage, nr-axSpA. This idea was implemented in the recent update of the ASAS recommendation for treatment of axial SpA with anti-TNF α agents (Figure 2) [van der Heijde et al. 2011]. According to these recommendations, patients with definite axSpA (fulfilling either the ASAS classification criteria for axial SpA [Rudwaleit et al. 2009b] or the modified New York criteria for AS [van der Linden et al. 1984]) having high disease activity (defined as Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] ≥4) despite adequate NSAIDs treatment (defined as no response to at least two NSAIDs for at least 4 weeks in total unless contraindicated; local steroids and DMARDs might be used in patients with peripheral disease if appropriate) are considered as candidates for anti-TNF α therapy [van der Heijde et al. 2011]. A positive opinion of a rheumatologist based on assessment of acute phase reactants, MRI, radiographic data and radiographic progression of AS is also required. Efficacy of anti-TNF α therapy should be assessed after at least 12 weeks of treatment and should first consider clinical improvement (BASDAI improvement by ≥50% or by ≥2 absolute points, 0–10 scale) [van der Heijde et al. 2011].
Figure 2.
ASAS recommendations for the use of an anti-TNF agent in patients with axial SpA.
AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis International Society; BASDAI, the Bath Ankylosing Spondylitis Disease Activity Index; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAID, nonsteroidal anti-inflammatory drug; SpA, spondyloarthritis; TNF, tumour necrosis factor.
All four TNF α blockers currently available for the treatment of AS (adalimumab, etanercept, golimumab and infliximab) demonstrated similar high efficacy (with major reduction of symptoms as measured by the percentage of patients achieved an ASAS40 response in about 40–50% of the cases) in patients who did not respond to previous NSAIDs therapy [Davis et al. 2005; Inman et al. 2008; van der Heijde et al. 2005b, 2006]. Despite high clinical efficacy, TNF α blockers were not able to retard radiographic spinal progression in AS over a period of 2 years in recent clinical trials [van der Heijde et al. 2008a, 2008b, 2009]. From this point of view, a combination of a TNF α blocker with a NSAID (i.e. in patients with risk factors for radiographic progression and clinical indications for NSAID therapy) might provide additional therapeutic benefits. It is important to stress, however, that according to the current recommendations NSAIDs should be administered to symptomatic patients only [Braun et al. 2011], because only in these patients do the benefits of NSAID treatment (symptomatic and disease modifying) overweigh the potential risks related to NSAID intake (gastrointestinal and cardiovascular side effects). Whether the possible structure-modifying effect of NSAIDs alone can justify continuous NSAID treatment in asymptomatic patients (e.g. in those who are treated with a TNF α blocker) cannot be answered positively today. More data on the long-term relevance of the structure-modifying effect of NSAIDs especially in combination with TNF α blockers are needed.
There are several predictors of positive treatment response to TNF α blockers in axSpA. The most important of these are young age, short disease duration, low level of functional disability, elevated acute phase reactants and signs of active inflammation on MRI [Rudwaleit et al. 2004, 2009a; Vastesaeger et al. 2011]. Thus, patients with early and active disease respond generally better to anti-TNF α treatment in comparison with patients with more advanced disease. This was also confirmed in several clinical trials performed in patients with early axSpA (nonradiographic in the majority of cases) [Barkham et al. 2009; Haibel et al. 2008; Song et al. 2010a]. Recently, results of the first phase III trial investigating efficacy of a TNF α blocker (adalimumab) in nr-axSpA were presented [Sieper et al. 2011]. Although the treatment response (ASAS40) to adalimumab at week 12 in the whole group was not impressive (36% as compared with 15% in the placebo group, p < 0.001), this was clearly higher in patients with short disease duration (less than 5 years: 49% of the adalimumab-treated patients achieved an ASAS40 response), elevated CRP (55%) and presence of active inflammation on MRI of the sacroiliac joints (49%) [Sieper et al. 2012c]. As a result, in June 2012 adalimumab became the first TNF α blocker to receive a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for the treatment of adults with severe axSpA without radiographic evidence of AS but with objective signs of inflammation by elevated CRP and/or MRI, who have had an inadequate response to or are intolerant to NSAIDs [EMA, 2012]. This positive opinion is currently being followed by the approval of adalimumab for nr-axSpA in EU countries. Similar phase III clinical trials in patients with nr-axSpA with etanercept, golimumab and certolizumab pegol are ongoing. It can be expected that all currently available TNF α blockers will extend their official labels to nr-axSpA in the next 2 years.
The use of analgesics can be recommended for patients in whom pain cannot be effectively reduced with the other treatment methods described above [Braun et al. 2011].
Surgery might be of benefit in patients with axial disease and severe spinal deformities (i.e. ankylosis with hyperkyphosis) with a serious impact on patient’s functional status and quality of life (spinal corrective osteotomy) [Braun et al. 2011].
New treatment targets and future treatment modalities in axial SpA
As already mentioned above, only TNF α blockers are currently available as a second-line treatment in patients with AS/axSpA who do not respond to NSAIDs. Therefore, treatment options for axSpA patients with a lack of response to a TNF α blocker are limited. Unfortunately, several non-anti-TNF biologics that have being successfully used for the treatment of active rheumatoid arthritis failed to show efficacy in axSpA. Interleukin (IL)-1 blockade with anakinra, B-cell depleting therapy with rituximab and modulation of T-cell costimulation with abatacept did not show convincing results in patients with active AS in pilot trials [Haibel et al. 2005b; Song et al. 2010b, 2011], although there was a positive signal for a possible rituximab effect in anti-TNF α-naïve patients, but not in anti-TNF α failures. Most recently, monoclonal antibodies against IL-6 receptor tocilizumab and sarilumab also failed to demonstrate clinical efficacy in AS in two large placebo-controlled trials [Sieper et al. 2012a, 2012b].
Much more promising are data related to the blockade of IL-17 in axSpA. It has being suggested that IL-17 might be a key mediator of inflammation in AS [Shen et al. 2009]. In AS, an elevated level of serum IL-17 and increased number of circulating polyfunctional Th17 cells were reported [Jandus et al. 2008; Mei et al. 2011; Wendling et al. 2007]. Moreover, an immunohistological analysis of IL-17-secreting cells in facet joints from AS patients showed that the frequency of IL-17- producing cells was significantly higher compared with spine samples obtained from patients with osteoarthritis [Appel et al. 2011]. A fully human antibody to IL-17A secukinumab (formerly AIN457) is under investigation now in a number of chronic inflammatory disorders including AS. In a small phase II study in AS, the primary study endpoint, ASAS20-response at week 6, was achieved in 61% (14 out of 23) of AS patients who received secukinumab as compared with 17% of the patients receiving placebo [Baeten et al. 2010]. A larger phase III trial is ongoing.
Blockade of IL-23 represents also an attractive target in axSpA. IL-23 together with transforming growth factor (TGF)-β and IL-6 stimulates naïve precursor cells to differentiate into Th17 T cells. Even more important is the role of IL-23 in maintaining the Th17 phenotype and in acquiring the full effector function [Matsui, 2007]. Recent immunohistological study demonstrated that a number of IL-23-positive cells in bone marrow of facet joints of AS patients was significantly higher in comparison with samples obtained from patients with osteoarthritis and individuals without spinal disease [Appel et al. 2010]. A monoclonal antibody against IL-12/IL-23 (ustekinumab) has been approved for the treatment of psoriasis and is currently under investigation in a pilot trial for active AS.
There are some data suggesting that apremilast, an oral phosphodiesterase-4 inhibitor, might be effective in AS [Pathan et al. 2011], results that need to be confirmed in a larger trial. It would also be interesting to find out whether oral kinase inhibitors such as Janus-kinase (JAK) inhibitors (e.g. JAK3 inhibitor tofacitinib) and spleen tyrosine kinase inhibitors (i.e. fostamatinib), which have been shown to be effective in rheumatoid arthritis, could also be effective for treating axSpA.
Haematopoietic stem cell transplantation (HSCT) has been increasingly used over the last 15 years for the treatment of severe life-threatening treatment-resistant cases of autoimmune diseases, such as systemic lupus erythematosus and systemic sclerosis. Concerning axSpA, only three published cases of successful HSCT (two of them were performed because of a haematological disease) are available now [Britanova et al. 2012; Jantunen et al. 2000; Yang et al. 2012]. Nonetheless, this kind of therapy might be a treatment option for patients with a very severe axSpA who did not respond to any of the currently available conventional treatments.
Conclusion
NSAIDs, due to their high symptomatic efficacy and possible disease-modifying properties, can be considered as a treatment of choice for the majority of patients with axSpA. The only effective treatment option nowadays for axSpA in the case of the inefficacy or intolerability of NSAIDs is a TNF α blocker. A number of potentially effective drugs (i.e. IL-17, IL-12/23 antagonist, small molecules), which might become an alternative for TNF α blockers in the future, are being investigated in axSpA now.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Denis Poddubnyy received consulting and speaking fees from Abbott, MSD, Pfizer, and UCB.
References
- Amor B., Dougados M., Listrat V., Menkes C., Roux H., Benhamou C., et al. (1995) Are classification criteria for spondylarthropathy useful as diagnostic criteria? Rev Rhum Engl Ed 62(1): 10–15 [PubMed] [Google Scholar]
- Appel H., Maier R., Wu P., Scheer R., Hempfing A., Kayser R., et al. (2011) Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther 13(3): R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel H., Rose A., Maier R., Hempfing A., Loddenkemper C., Sieper J. (2010) In situ analysis of IL-23 secreting cells in the spine of patients with ankylosing spondylitis. Ann Rheum Dis 69(Suppl. 3): 104 [Google Scholar]
- Baeten D., Sieper J., Emery P., Braun J., van der Heijde D., McInnes I., et al. (2010) The anti-IL17A monoclonal antibody secukinumab (AIN457) showed good safety and efficacy in the treatment of active ankylosing spondylitis. Arthritis Rheum 62: 3840 [Google Scholar]
- Bakland G., Gran J., Nossent J. (2011) Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis 70: 1921–1925 [DOI] [PubMed] [Google Scholar]
- Barkham N., Keen H., Coates L., O’Connor P., Hensor E., Fraser A., et al. (2009) Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum 60: 946–954 [DOI] [PubMed] [Google Scholar]
- Barkhuizen A., Steinfeld S., Robbins J., West C., Coombs J., Zwillich S. (2006) Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 33(9): 1805–1812, http://www.ncbi.nlm.nih.gov/pubmed/16960941 [PubMed] [Google Scholar]
- Boersma J.W. (1976) Retardation of ossification of the lumbar vertebral column in ankylosing spondylitis by means of phenylbutazone. Scand J Rheumatol 5: 60–64 [PubMed] [Google Scholar]
- Braun J., Bollow M., Seyrekbasan F., Haberle H., Eggens U., Mertz A., et al. (1996) Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and followup by dynamic magnetic resonance imaging. J Rheumatol 23: 659–664 [PubMed] [Google Scholar]
- Braun J., van den Berg R., Baraliakos X., Boehm H., Burgos-Vargas R., Collantes-Estevez E., et al. (2011) 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 70: 896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Zochling J., Baraliakos X., Alten R., Burmester G., Grasedyck K., et al. (2006) Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Ann Rheum Dis 65: 1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O., Bochkova A., Staroverov D., Fedorenko D., Bolotin D., Mamedov I., et al. (2012) First autologous hematopoietic SCT for ankylosing spondylitis: a case report and clues to understanding the therapy. Bone Marrow Transplant, in press. [DOI] [PubMed] [Google Scholar]
- Cannon C., Curtis S., FitzGerald G., Krum H., Kaur A., Bolognese J., et al. (2006) Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 368: 1771–1781 [DOI] [PubMed] [Google Scholar]
- Dagfinrud H., Kvien T., Hagen K. (2008) Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev 1: CD002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., van der Heijde D., Braun J., Dougados M., Cush J., Clegg D., et al. (2005) Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis 64: 1557–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougados M., Gueguen A., Nakache J., Velicitat P., Veys E., Zeidler H., et al. (1999) Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford) 38: 235–244 [DOI] [PubMed] [Google Scholar]
- Dougados M., Simon P., Braun J., Burgos-Vargas R., Maksymowych W., Sieper J., et al. (2011) ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 70: 249–251 [DOI] [PubMed] [Google Scholar]
- EMA (2012) Positive recommendations of Committee for Medicinal Products for Human Use (CHMP) on extensions of therapeutic indications for Humira (21 June 2012). Available at: http://www.emea.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500129074 (last accessed 1 August 2012).
- Haibel H., Brandt H., Song I., Brandt A., Listing J., Rudwaleit M., et al. (2007) No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis 66: 419–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibel H., Fendler C., Listing J., Callhoff J., Braun J., Sieper J. (2012) Efficacy of oral prednisolone in active ankylosing spondylitis - results of a double blind placebo controlled trial. Ann Rheum Dis 71(Suppl. 3): 247. [DOI] [PubMed] [Google Scholar]
- Haibel H., Rudwaleit M., Braun J., Sieper J. (2005a) Six months open label trial of leflunomide in active ankylosing spondylitis. Ann Rheum Dis 64: 124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibel H., Rudwaleit M., Listing J., Heldmann F., Wong R., Kupper H., et al. (2008) Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 58: 1981–1991 [DOI] [PubMed] [Google Scholar]
- Haibel H., Rudwaleit M., Listing J., Sieper J. (2005b) Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann Rheum Dis 64: 296–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R., Davis J., Jr, Heijde D., Diekman L., Sieper J., Kim S., et al. (2008) Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 58: 3402–3412 [DOI] [PubMed] [Google Scholar]
- Jandus C., Bioley G., Rivals J., Dudler J., Speiser D., Romero P. (2008) Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 58: 2307–2317 [DOI] [PubMed] [Google Scholar]
- Jantunen E., Myllykangas-Luosujarvi R., Kaipiainen-Seppanen O., Nousiainen T. (2000) Autologous stem cell transplantation in a lymphoma patient with a long history of ankylosing spondylitis. Rheumatology (Oxford) 39: 563–564 [DOI] [PubMed] [Google Scholar]
- Kearney P., Baigent C., Godwin J., Halls H., Emberson J., Patrono C. (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon F., Landewe R., Dougados M., van der Heijde D. (2012) Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 71: 1623–1629 [DOI] [PubMed] [Google Scholar]
- Lanza F., Chan F., Quigley E. (2009) Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 104: 728–738 [DOI] [PubMed] [Google Scholar]
- Matsui M. (2007) Roles of the novel interleukin-12-associated cytokine, interleukin-23, in the regulation of T-cell-mediated immunity. Hepatol Res 37(Suppl. 3): S310–S318 [DOI] [PubMed] [Google Scholar]
- Mei Y., Pan F., Gao J., Ge R., Duan Z., Zeng Z., et al. (2011) Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol 30: 269–273 [DOI] [PubMed] [Google Scholar]
- Pathan E., Abraham S., Van-Rossen L., Withrington R., Keat A., Charles P., et al. (2011) Efficacy and safety of apremilast, an oral phosphodiesterase inhibitor, in ankylosing spondylitis. Arthritis Rheum 63(10 Suppl.): S646. [DOI] [PubMed] [Google Scholar]
- Peters N., Ejstrup L. (1992) Intravenous methylprednisolone pulse therapy in ankylosing spondylitis. Scand J Rheumatol 21: 134–138 [DOI] [PubMed] [Google Scholar]
- Poddubnyy D., Rudwaleit M., Haibel H., Listing J., Marker-Hermann E., Zeidler H., et al. (2012) Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 71: 1616–1622 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., Claudepierre P., Wordsworth P., Cortina E., Sieper J., Kron M., et al. (2009a) Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 36: 801–808 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., Khan M., Sieper J. (2005) The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 52: 1000–1008 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., Listing J., Brandt J., Braun J., Sieper J. (2004) Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 63: 665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudwaleit M., van der Heijde D., Landewe R., Akkoc N., Brandt J., Chou C., et al. (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70: 25–31 [DOI] [PubMed] [Google Scholar]
- Rudwaleit M., van der Heijde D., Landewe R., Listing J., Akkoc N., Brandt J., et al. (2009b) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68: 777–783 [DOI] [PubMed] [Google Scholar]
- Shen H., Goodall J., Hill Gaston J. (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum 60: 1647–1656 [DOI] [PubMed] [Google Scholar]
- Sieper J., Inman R., Badalamenti S., Radin A., Braun J. (2012a) Sarilumab for the treatment of ankylosing spondylitis: results of a phase 2, randomized, double-blind, placebo-controlled, international study (ALIGN). Ann Rheum Dis 71(Suppl. 3): 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., Klopsch T., Richter M., Kapelle A., Rudwaleit M., Schwank S., et al. (2008) Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 67: 323–329 [DOI] [PubMed] [Google Scholar]
- Sieper J., Porter-Brown B., Thompson L., Harari O., Dougados M. (2012b) Tocilizumab (TCZ) is not effective for the treatment of ankylosing spondylitis (AS): results of a phase 2, international, multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 71(Suppl. 3): 110 [Google Scholar]
- Sieper J., van der Heijde D., Dougados M., Mease P., Brown S.L., Pangan A.L. (2011) Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis – results from a phase 3 study. Arthritis Rheum 63(Suppl.): S970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., van der Heijde D., Dougados M., Mease P., Maksymowych W., Brown M., et al. (2012c) Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S., McMurray J., Pfeffer M., Wittes J., Fowler R., Finn P., et al. (2005) Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352: 1071–1080 [DOI] [PubMed] [Google Scholar]
- Song I., Hermann K., Haibel H., Althoff C., Listing J., Freundlich B., et al. (2010a) Effects of Etanercept vs. Sulfasalazine on acute inflammatory lesions as detected by whole body MRI in early axial spondyloarthritis - a 48 week randomized controlled trial. Ann Rheum Dis 69(Suppl. 3): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I., Heldmann F., Rudwaleit M., Haibel H., Weiss A., Braun J., et al. (2011) Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis 70: 1108–1110 [DOI] [PubMed] [Google Scholar]
- Song I., Heldmann F., Rudwaleit M., Listing J., Appel H., Braun J., et al. (2010b) Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum 62: 1290–1297 [DOI] [PubMed] [Google Scholar]
- Song I., Poddubnyy D., Rudwaleit M., Sieper J. (2008) Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum 58: 929–938 [DOI] [PubMed] [Google Scholar]
- Trelle S., Reichenbach S., Wandel S., Hildebrand P., Tschannen B., Villiger P., et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijde D., Baraf H., Ramos-Remus C., Calin A., Weaver A., Schiff M., et al. (2005a) Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheum 52: 1205–1215 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Dijkmans B., Geusens P., Sieper J., DeWoody K., Williamson P., et al. (2005b) Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 52: 582–591 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Kivitz A., Schiff M., Sieper J., Dijkmans B., Braun J., et al. (2006) Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 54: 2136–2146 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Landewe R., Baraliakos X., Houben H., van Tubergen A., Williamson P., et al. (2008a) Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 58: 3063–3070 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Landewe R., Einstein S., Ory P., Vosse D., Ni L., et al. (2008b) Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 58: 1324–1331 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Salonen D., Weissman B., Landewe R., Maksymowych W., Kupper H., et al. (2009) Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 11(4): R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijde D., Sieper J., Maksymowych W., Dougados M., Burgos-Vargas R., Landewe R., et al. (2011) 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis 70: 905–908 [DOI] [PubMed] [Google Scholar]
- van der Linden S., Valkenburg H., Cats A. (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27: 361–368 [DOI] [PubMed] [Google Scholar]
- Vastesaeger N., van der Heijde D., Inman R., Wang Y., Deodhar A., Hsu B., et al. (2011) Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis 70: 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders A., Heijde D., Landewe R., Behier J., Calin A., Olivieri I., et al. (2005) Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 52: 1756–1765 [DOI] [PubMed] [Google Scholar]
- Wendling D., Cedoz J., Racadot E., Dumoulin G. (2007) Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine 74: 304–305 [DOI] [PubMed] [Google Scholar]
- Yang H., Moon S., Shin J., Kwok S., Park K., Park S., et al. (2012) Regression of syndesmophyte after bone marrow transplantation for acute myeloid leukemia in a patient with ankylosing spondylitis: a case report. J Med Case Rep 6: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]