Abstract
The treatment of familial amyloid polyneuropathy (FAP) requires a multidisciplinary approach, mainly neurological and cardiological. It includes specific treatments to stop the progression of systemic amyloidogenesis, the symptomatic treatment of the peripheral and autonomic neuropathy and the treatment of organs severely involved by amyloidosis (heart, eyes, kidneys). First-line specific treatment of met30 transthyretin (TTR) FAP is liver transplantation, which allows suppression of the main source of mutant TTR, to stop the progression of the neuropathy in 70% of cases in the long term and to double the median survival. In cases of severe renal or cardiac insufficiency, a combined kidney–liver or heart–liver transplantation can be discussed. Tafamidis (Vyndaqel) is a novel specific stabilizer of TTR which, in the very early stages of met30 TTR FAP, slows the progress of peripheral neuropathy. This drug should be proposed in cases of stage 1 symptomatic polyneuropathy. Other innovative medicines have been developed by biopharmaceutical companies to block the hepatic production of mutant and wild type TTR which are harmful in late-onset FAP (> 50 years old), including RNA interference therapeutics and antisense oligonucleotides, and to remove the amyloid deposits (monoclonal antibody antiserum amyloid P). Clinical trials should first assess patients with late onset FAP or non-met30 TTR FAP who are less responsive to liver transplantation or in case of significant progression of the neuropathy with Vyndaqel. Initial cardiac assessment and periodic cardiac investigations are important for patients with FAP because of the frequency of cardiac impairment, which is responsible for the high rate of mortality. Prophylactic pacemaker treatment should be discussed. Symptomatic treatments are required to improve patients’ quality of life. Familial screening of people with TTR mutation and regular follow up are essential. Appropriate clinical examination and complementary investigations are vital for the early detection of disease onset and to start specific therapy as soon as possible.
Keywords: familial amyloidosis with polyneuropathy, gene therapy, liver transplantation, tafamidis, treatment
Introduction
Familial amyloid polyneuropathy (FAP) is a progressive, disabling and life-threatening polyneuropathy affecting the peripheral and autonomic nervous system. FAP is an autosomal transmission disorder which is usually due to a point mutation of the transthyretin (TTR) gene [Benson and Kincaid, 2007]. The natural history of the disease was described 30 years ago in patients of Portuguese origin with early onset (<50 years) [Coutinho et al. 1980] and more recently in patients with late onset in Japan (≥50 years) [Koike et al. 2012] (Table 1). Study and follow up of a cohort of 483 Portuguese patients with FAP in Porto enabled the disease course to be described in three stages [Coutinho et al. 1980]. Another staging system concerned the progression of walking disability in FAP [Steen and Ek, 1983] (Table 2). The course of late onset met30 TTR FAP has recently shown major functional severity, faster progression of sensorimotor polyneuropathy and reduced survival [Koike et al. 2012].
Table 1.
Particularities of early and late onset familial amyloid polyneuropathy and its course.
| Early onset met30 TTR FAP [Coutinho et al. 1980; Koike et al. 2002] | Late onset met30 TTR FAP [Koike et al. 2002 ; Koike et al. 2012] | |
|---|---|---|
| Age of onset | <50 years | >50 years |
| Country | Portugal, Japan, Brazil | Sweden, France, UK, Italy, Japan |
| Positive family history | 94% | 48% |
| Initial clinical presentation | ||
| Peripheral neuropathy | 57% | 81% |
| Autonomic neuropathy | 48% | 10% |
| Weight loss | 5% | 0 |
| Mean delay for aid for walking | >5.6 years | 3 years |
| Cardiac | Progressive conduction disorders | Restrictive cardiomyopathy Cardiac insufficiency |
| Median survival | 11 years | 7.3 years |
| Causes of death | Cachexia, infections | Cardiac insufficiency Sudden death Cachexia or secondary infection |
FAP, familial amyloid polyneuropathy; TTR, transthyretin.
Table 2.
Stages of familial amyloid polyneuropathy for locomotion.
| Coutinho et al. [1980] | Duration of stage | Yamamoto et al. [2007] | ||
|---|---|---|---|---|
| Stage 1 | The disease is limited to the lower limbs Walking without any help. Slight weakness of the extensors of the big toes. | 5.6 ± 2.8 years | PND I | Sensory disturbances in extremities Preserved walking capacity |
| Stage 2 | Motor signs progress in lower limbs with steppage and distal amyotrophies, the muscles of the hands begin to be wasted and weak. The patient is by then obviously handicapped but can still move around, although needing help. | 4.8 ± 3.6 years | PND II PND IIIa PND IIIb | Difficulties walking but without the need for a walking stick One stick or one crutch required for walking. Two sticks or two crutches required for walking |
| Stage 3 | The patient is bedridden or confined to a wheelchair, generalized weakness and areflexia. | 2.3 ± 3.1 years | PND IV | Patient confined to a wheelchair or a bed |
PND, a modified polyneuropathy disability score.
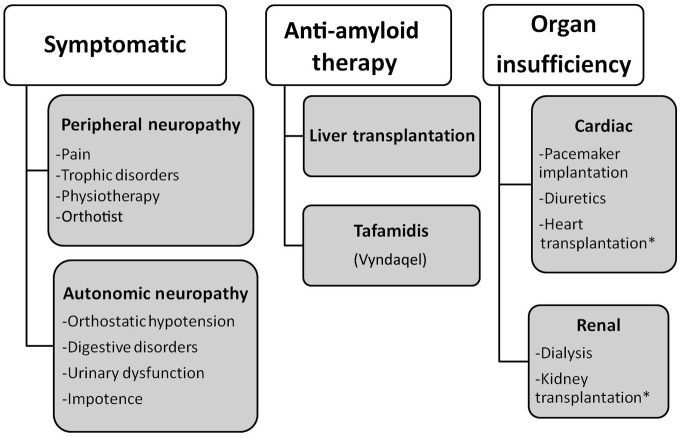
FAP is a multivisceral and life-threatening disease resulting in multiple disabilities for which a national referral center was set up in France in 2005 [Adams et al. 2012b]. Therapy for FAP is complex and requires a multidisciplinary approach: first, specific therapy to prevent production of amyloids; second, symptomatic therapy of sensorimotor and autonomic polyneuropathy, and cardiac and renal injury; finally, treatment of end-stage cardiac or renal failure (Figure 1). During the past 20 years treatment of FAP has benefited from advances in drugs that have enabled management of the disease and modification of its natural history.
Figure 1.
Principles of therapy for familial amyloid polyneuropathy.
*: in association with liver transplantation.
Symptomatic treatments
Symptomatic treatments are important in patients with FAP to improve their quality of life. These include treatment of manifestations of sensorimotor polyneuropathy, prevention and cure of trophic disorders, physiotherapy, help of an orthesist (Orthotist), control of autonomic dysfunction (digestive – diarrhea, gastroparesis; urinary and genital dysfunction; orthostatic hypotension), treatment of associated ophthalmologic manifestations (glaucoma, vitreous opacities) and cardiac disorders.
Treatment of organ complications
Cardiac involvement
Treatment of cardiac complications depends on the type and the severity of the cardiopathy. Assessment of the cardiac function must be systematic in patients with FAP as it is usually subclinical and requires a cardiac checkup. Conduction disorders, which are common and evolutive, must be tested using an electrocardiogram (ECG), Holter recording and an intracardiac ECG if necessary [Eriksson et al. 1984]. Detection of severe conduction disorders often requires prophylactic pacemaker implantation [Eriksson and Olofsson, 1984]. Evaluation of cardiac function and impact of myocardial amyloid infiltration on ventricular function is extremely important. Severe cardiac injury may constitute a contraindication for liver transplantation (LT), while a combined heart and liver transplantation can be considered.
In severe renal injury due to amyloidosis, hemodialysis and later a renal transplantation may be required.
Anti-amyloid therapy
It is important to search systematically for familial amyloidosis in cases of suspected amyloid light-chain amyloidosis because anti-amyloid therapy differs radically and requires chemotherapy.
Twenty years’ experience of liver transplantation
LT was proposed 20 years ago to suppress the main source of mutant TTR, which is amyloidogenic, to prevent formation of amyloidosis and to stop progression of the disease [Holmgren et al. 1991, 1993]. More than 2000 patients with FAP underwent LT worldwide [Wilczek et al. 2011], half of them in Portugal and 200 in France. The results of the LT depend on the TTR gene mutation, the age of the patient and the stage of the disease.
The initial results concerned the biological effect, with 98% reduction of mutated TTR in the days following LT [Adams et al. 2000; Holmgren et al. 1991]. Afterwards, the effects of LT on neuropathy were evident since its progression was stopped in 70% of patients at mid term. This has been established for met30 TTR FAP according to the following objective parameters: clinical, functional, neurophysiological and histopathological, with a major reduction in axon loss [Adams et al. 2000]. These findings were confirmed in the long term. LT does not allow either clinical or functional recovery [Adams et al. 2000; Yamamoto et al. 2007]; autonomic dysfunctions are unchanged.
Worsening of walking ability is possible after LT, seen in one-third of our cohort of patients after LT (personal data). Twenty percent required an aid for walking after LT (median delay of 11 years) (unpublished data). Independent risk factors for requiring aid for walking were major weight loss, previous walking difficulties and a late onset at LT. Worsening of walking ability may be explained by accumulation of wild type TTR in the nerve after LT [Liepnieks et al. 2010]. For non-met30 TTR FAP, success of LT is less common. The benefit of LT on neuropathy is better when LT is performed at an early stage, before the onset of walking difficulties that require aid.
Effects on survival
Although LT doubles the median survival in early onset met30 TTR FAP, the impact of LT on survival for non-met30 TTR FAP is less obvious. The rate of survival at 5 years for patients with FAP after LT is 82% in met30 TTR FAP versus 59% in non-met30 TTR FAP according to the world register of LT in FAP [Wilczek et al. 2011]. Independent risk factors of mortality in our center are severe autonomic dysfunction, late onset and non-met30 TTR mutation (unpublished data). Long-term follow up of early onset met30 TTR FAP shows a rate of survival of 100% in patients after LT versus 56.1% in those who did not have a transplant after 10 years [Yamashita et al. 2012].
Effects on organ and ocular involvement
The effects of LT on cardiac involvement depend on the type of initial cardiopathy and the genotype, early or late onset, or TTR variant (met30 or non-met30). Progress of cardiopathy is possible after LT, marked by the following:
Progressive thickening of the myocardium on echocardiogram after LT in late onset met30 TTR FAP in Sweden, explaining the possible development of congestive heart failure after LT [Olofsson et al. 2002].
Development of conduction disorders requiring pacemaker implantation in 8% [Yamamoto et al. 2007] to 20% of cases after LT (personal communication, unpublished data).
Worsening of cardiopathy after LT can be explained by accumulation of wild type TTR in the myocardium [Yazaki et al. 2000]. Periodic assessment of patients after LT is necessary due to the ongoing risk of progress of cardiopathy after LT, both on conduction disorders and restrictive cardiomyopathy.
Renal function is stable in most patients after LT, but cases of deterioration during the first quarter after LT requiring hemodialysis have been reported [Rocha et al. 2011]. Chronic renal failure (34%), including 5% terminal end-stage disease after LT, is also possible [Ferreira et al. 2012].
Combined heart and liver transplantation [Raichlin et al. 2011] and kidney and liver transplantation must be discussed in cases of severe life-threatening cardiomyopathy or end-stage renal disease requiring dialysis.
Ocular manifestations are not influenced by LT, with a persisting risk of developing glaucoma or vitreous opacities in 8% and 12%, respectively due to the retinal source of mutated TTR [Sandgren et al. 2008].
Morbidity and mortality
In our series of 200 patients who have had LT since 1993, 38% died, including 14% in the first year until 15 years after LT. Mortality during the first year is higher in patients with non-met30 TTR FAP versus met30 TTR FAP. Deaths during the first year are due to cardiac causes (sudden death, cardiac insufficiency), infections or hepatic failure, as a direct result of surgery in 3.5% [Yamamoto et al. 2007]. After the first year, patients died from progress of the amyloid disease (50%), affecting the heart or peripheral nervous system, or varied causes, including infection, stroke, suicide, hepatic causes.
Delay for LT varies from 6 to 12 months according to the rules fixed by the National Biomedical Agency. The waiting list for LT may vary from 6 to 18 months due to the shortage of donor organs (cadaveric donor) and a need to match donor and patient blood and body type. A patient with a very common blood type has less chance of quickly finding a suitable liver. Avoiding a long wait is possible if a patient has a living donor who is willing to donate part of their liver (living donor transplantation) via a partial hepatic graft. In such cases, the delay may be as short as 4 months. If the donor is a family member, they must not carry the TTR gene mutation.
Side effects of liver transplantation
LT is associated with the risks of surgery and acute liver rejection, which is usually controlled by pulses of methylprednisolone or modification of immunosuppressive drugs. LT is associated with an increased risk of infections, mainly during the first year after surgery, including septicemia which are usually favored by urinary incontinence [Adams et al. 2000; Yamamoto et al. 2007], and pulmonary infections which may be opportunistic in half of the cases (pneumocystosis, cytomegalovirus). Patients may develop acute renal failure (26%) after orthotopic LT [Ferreira et al. 2012] or chronic renal failure (34%) which may lead to end-stage renal disease (5.1%). The risk of chronic renal insufficiency is associated with renal insufficiency before LT and onset of acute renal failure after LT (Ferreira et al. 2012). pre-OLT is an independent risk factor [Ferreira et al. 2012; Lobato and Rocha, 2012]. Orthotopic LT may also be associated with cancer or lymphoproliferative syndrome (1% of cases) both favored by immunosuppressive drugs.
Immunosuppressants to use and their long-term risk/benefit ratio
The immunosuppressants we usually use are calcineurin inhibitors (CNI): ciclosporin or tacrolimus. The risk of acute rejection is about 20% with a loss of graft by chronic rejection below 5%. Liver transplant recipients are at high risk of developing acute and chronic renal failure as a consequence of the use of CNI. These two drugs have also a similar toxicity profile, which may compromise the long term benefits of liver transplantation (LT) and the quality of life after liver transplantation: at 10-year-post LT, the rate of chronic renal failure, arterial hypertension, and diabetes is 30%, 40% and 10-20% of cases, respectively. In addition, there is an overall increase of cardiovascular complications and of de novo tumors. Frequently, mycophenolate mofetil, a non-nephrotoxic immunosuppressor, is added to CNI to avoid overdose of CNI and to preserve post-transplant renal function.
Timing of surgery
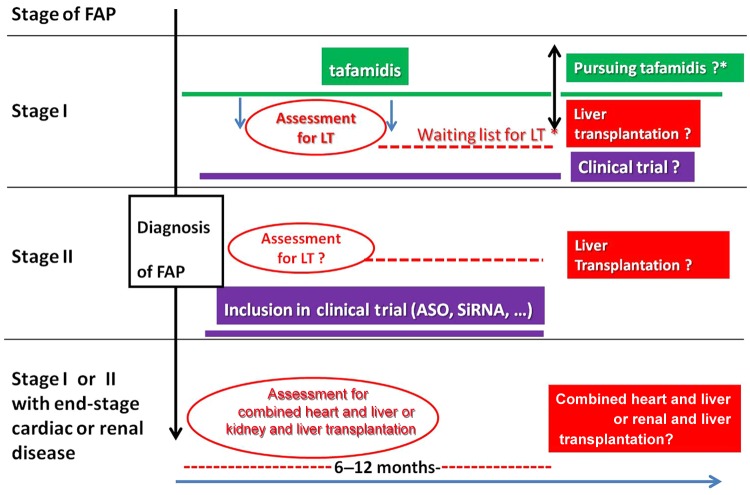
We must take into account the waiting list which varies between 6 and 18 months; during this time the disease may worsen (Figure 2). As LT is able to stop progress of the disease, LT should be performed as soon as possible, early in the course of the disease [Yamashita et al. 2012].
Figure 2.
Therapeutic strategy in patients with familial amyloid polyneuropathy (FAP) according to the stage of the disease [Coutinho et al. 1980]. Stage III (wheelchair bound or bedridden) is a contraindication for any transplantation, liver alone or combined liver plus kidney and liver plus heart.
ASO, antisense oligonucleotide; LT, liver transplantation; SiRNA small interfering RNA.
Domino liver transplantation
To compensate for the lack of donors for LT, it is proposed that livers from patients with FAP which are normal except for production of the mutant TTR are used as a graft for patients with severe liver diseases. This is called domino LT (DLT). These patients are at risk of developing acquired amyloid neuropathy [Adams et al. 2011; Stangou et al. 2005] that may justify retransplantation. In a prospective study to assess the risk–benefit ratio of DLT, systemic amyloidosis developed early with amyloid deposits on labial salivary gland biopsy (LSGB) 5 years after DLT in half of patients [Adams et al. 2011] or on rectal biopsy in one-third of patients [Llado et al. 2010]. In DLT recipients, nerve biopsy is crucial [Adams et al. 2011] to prove that the neuropathy is due to amyloidosis and not other causes [Adams et al. 2011; Llado et al. 2010]. The delay to declare polyneuropathy ranges from 3.5 [Adams et al. 2011] to 9 years [Conceicao et al. 2010]. De novo amyloid neuropathy mimics FAP of early onset [Adams et al. 2011].
Anti-amyloid medicine
During the past 5 years, several therapeutic strategies for FAP have been developed as an alternative to LT. This has allowed the first anti-amyloid medicine to benefit from a marketing authorization in Europe and France but not in the USA. Multicentric clinical trials for other medicines for FAP should start soon (Table 3).
Table 3.
State of progress of clinical trials with anti-amyloid drugs.
| Treatment | Preclinical experimental | Administration | Phase I | Phase II | Phase III | Marketing authorization |
|---|---|---|---|---|---|---|
| Tafamidis* | – | Oral | 2007 | −>2009 | EMA : November 2011 ANSM: September 2011 FDA: No | |
| Diflunisal$ | – | Oral | −>2010 | |||
| DOXY-TUDCA‡ | + | Oral | Current | |||
| ASOs§ | + | SCI | Current | 2013 | ||
| SiRNA¶ | + | IVI | 2011–2012 | 2012 | 2013? | |
| Anti-SAP Mab# | + | IVI | 2013? |
Coelho et al. [2012]; $ Berk et al. [2011]; ‡ Cardoso et al. [2010], Obici et al. [2012]; § Ackermann et al. [2012], Benson et al. [2006]; ¶ Coelho et al. [2011]; # Bodin et al. [2010].
ANSM, Agence nationale de sécurité du médicmanet et des produits de santé; ASO, antisense oligonucleotide; DOXY-TUDCA, doxycycline taurodesoxycholic acid; EMA, European Medicines Agency; FDA, US Food and Drug Administration; IVI, intravenous infusion; SCI, subcutaneous injection.
Kinetic stabilizer of transthyretin
The mechanism of action of this class of medicine is stabilization of mutant TTR by binding to the T4 binding sites and stabilizing the TTR tetramer, allowing prevention of dissociation in monomers and amyloidogenic and toxic intermediaries [Johnson et al. 2012]. Efficiency of this treatment has been shown in vitro in the serum of treated patients and later in a multicenter international clinical phase II/III trial in 128 patients (Fx-005). In this double-blind study 20 mg/day tafamidis was compared with placebo over 18 months. The primary criteria were percentage of patients without an increase by two points of Neuropathy Impairment Score in the Lower Limbs (NIS-LL), and modification of total quality of life (TQOL). The mean age of patients was 39 years; all had a met30 TTR variant with a recent neuropathy and a median NIS-LL score in the treated group of 4/88. Most of the patients were of Portuguese origin, and were enrolled during the first 2 years of the disease. The results of the study were nonsignificant in the intention to treat group but were significant in the group of 87 patients. This was evaluable by the absence of progression of neuropathy in 60% of patients in the tafamidis group versus 38% in the placebo group, and a better preserved TQOL in the tafamidis group versus the placebo group (p = 0.045) [Coelho et al. 2012].
The French marketing authorization committee gave a favorable opinion for tafamidis (Vyndaqel, Pfizer, New York, USA) in September 2011. Tafamidis is indicated for the treatment of TTR amyloidosis in adult patients with symptomatic polyneuropathy of stage I to slow progression of the neuropathy. Many questions about tafamidis remain unanswered:
What are the longer-term outcomes, including ambulation, survival?
What is the efficacy in moderate and advanced stages of the disease or in non-met30 TTR FAP?
What is the efficacy in autonomic dysfunction?
Is tafamidis able to prevent impotence or postural hypotension?
What is the effect on cardiomyopathy?
This treatment is a disease-modifying-drug. Its most frequent side effects (very common, at least one in ten patients) are urinary tract infections and diarrhea. We do not know the long-term side effects, or its potential drug–drug interactions [Coelho et al. 2012].
France is the first country to obtain marketing authorization for tafamidis. The availability of tafamidis has allowed modification of the care of patients with FAP with stage I disease, enabling them to benefit from an anti-amyloid treatment as soon as the diagnosis is made. Until now, it was necessary to wait 6–12 months for LT. This alternative treatment also enables patients with FAP and contraindications to LT [age > 70 years (a third of patients), evolutive cancer] to benefit from anti-amyloid therapy [Adams et al. 2012b] (Figure 2).
Indications for available anti-amyloid therapies (LT, tafamidis) depend on the stage of the disease, the age of the patient, associated diseases, severity of cardiopathy and contraindications for LT [Adams et al. 2012a].
Tafamidis is preferential for treating early stage symptomatic FAP. Neuropathy must be symptomatic, with amyloidogenic mutant TTR (according to molecular biology study), and amyloidosis proven by biopsy, and stage I of the disease (walking without a cane). Treatment should be initiated and monitored by a physician knowledgeable in the management of patients with TTR amyloid polyneuropathy. Physicians should continue to assess the need for other therapy, including LT, as part of the standard of care. Patients should benefit from complete neurological assessment (clinical, neurophysiological, nerve conduction study in the four limbs), cardiac assessment and periodic assessment (at least every 6 months).
The conditions for renewal of prescription every 6 months are a good safety profile and absence of significant progress of the disease. Women of childbearing potential should use appropriate contraception when taking tafamidis. Significant criteria for progress of the neuropathy are worsening of ambulation (increase of a modified polyneuropathy disability score by one point) (Table 2), onset of orthostatic hypotension or impotence, progress of cardiopathy with worsening of stage of cardiac insufficiency (one point on the New York Heart Association classification system) or worsening of conduction disorders [Adams et al. 2012a]. If neuropathy or cardiopathy progresses significantly, LT or another therapeutic protocol [short interfering RNA (siRNA), antisense oligonucleotide (ASO), etc.] should be proposed when available (Table 3).
Other therapeutic perspectives
Transthyretin kinetic stabilizer: diflunisal
Diflunisal, a nonsteroidal anti-inflammatory drug (NSAID) which binds to T4 binding sites of tetrameric TTR, stabilizes TTR and suppresses formation of amyloid fibrils [Miller et al. 2004]. A randomized, controlled study against placebo is ongoing to determine if diflunisal is able to modify progression of neurological disease in FAP. A total of 130 patients of varied age and TTR mutations were enrolled. Until now, very few complications in the NSAID group have occurred. Data collection will be realized by the end of 2012 [Berk et al. 2011].
Doxycycline–taurodesoxycholic acid
A combination of doxycycline and taurodesoxycholic acid (TUDCA) has been tested on transgenic mice for 15–30 days and it allowed suppression of TTR deposits in most of the old mice. The combination seems to have a synergistic action which acts during many steps of amyloidogenesis and tissular-induced damage [Cardoso et al. 2010]. Doxycycline acts primarily as a fibril disrupter in vitro. TUDCA, a biliary acid, acts as a potent antiapoptotic and antioxidant with the ability to reduce the toxic aggregates of TTR by 75% in young transgenic mice for human V30M TTR in a TTR null background.
Twenty patients have been enrolled in a phase II study [Obici et al. 2012]. Seven patients have completed 1 year of treatment. Safety is good and several patients are stable at 1 year.
Gene silencing
Gene silencing has been developed to block hepatic synthesis of both mutant and wild type TTR.
Antisense oligonucleotides
ASOs are short synthetic strings of nucleotides designed to prevent the expression of a targeted protein by selectively binding to the RNA that encodes the targeted protein, thereby preventing translation. ASOs have already been tested clinically for the treatment of viral diseases, cancer and metabolic diseases. Several recent clinical studies have documented that the antisense concept works in humans. ISIS-TTRRX is a second-generation antisense inhibitor of the molecular target TTR. ISIS-TTRRX is designed to bind within the nontranslated portion of the human TTR mRNA and results in the degradation of TTR mRNA, preventing production of both wild type and mutant TTR protein. When tested in a human TTR transgenic mouse model, ISIS-TTRRX showed a dose-dependent reduction of human TTR (up to 80%) at both the mRNA and protein levels in the serum after subcutaneous injection [Benson et al. 2006]. More recently, ISIS-TTRRX showed a dose-dependent reduction of human TTR in cerebrospinal fluid after intraventricular administration of ASO by suppression of choroid TTR expression [Benson et al. 2010]. Liver TTR mRNA and plasma TTR protein levels were also reduced by around 80% after 12 weeks of treatment in monkeys using subcutaneous injection. ISIS-TTRRX treatment was well tolerated in both rodents and monkeys and is currently under evaluation in a phase I clinical trial in normal healthy volunteers. ISIS-TTRRX will initially be developed for patients with FAP and a randomized, double-blind, placebo-controlled study to assess the long-term safety and efficacy of ISIS-TTRRx in patients with FAP will be initiated in 2013 [Ackermann et al. 2012].
Small interfering RNA
ALN-TTR01 is a systemically administered lipid nanoparticle formulation of a siRNA targeting wild type and all mutant forms of TTR. This formulation delivers the siRNA predominantly to the liver, thereby inhibiting TTR synthesis at the primary site of production. In transgenic mice expressing the human met30 transgene in a heat shock transcription factor 1 null background, ALN-TTR01 led to a robust reduction of hepatic TTR mRNA levels in the liver and TTR protein levels in the circulation, and significant regression of TTR protein in the peripheral nervous system and gut. These results demonstrate the potential therapeutic benefit of ALN-TTR01 for the treatment of TTR amyloidosis (ATTR). A phase 1 randomized, single-blind, placebo-controlled clinical trial of ALN-TTR01 was conducted in Portugal, Sweden, the United Kingdom and France. A phase II study with multiple injections of siRNA should begin soon [Coelho et al. 2011].
Antiserum amyloid P monoclonal antibodies
Another strategy consists of enhancing the clearance of amyloid deposits with monoclonal antibody against human serum amyloid P component (hu-SAPMab), a ubiquitous nonfibrillar plasma glycoprotein in amyloid deposits. Administration of hu-SAPMab to mice with amyloid deposits containing hu-SAP triggers a potent complement-dependent, macrophage-derived giant cell reaction that swiftly removes massive visceral amyloid deposits. This strategy has been efficient in an experimental model of human systemic amyloid A (AA) amyloidosis with splenic and hepatic AA protein, induced by chronic inflammation in C57BL/6 mice deficient in mouse SAP but transgenic for human SAP. This therapy could be applicable to TTR FAP [Bodin et al. 2010].
What are the future challenges in familial amyloid polyneuropathy?
Inclusion of patients with familial amyloid polyneuropathy in clinical trials
After the era of LT which has transformed the course and prognosis of neuropathy and survival in early onset Val30met TTR FAP, a new era has opened for patients with FAP: anti- amyloid medicines. Alternative therapies are required because FAP in nonendemic areas are usually late onset cases with a late diagnosis [Adams et al. 2012b], who usually have contraindications to LT. It is highly recommended that patients with FAP who cannot access LT or tafamidis are enrolled in multicenter clinical trials of phase I, II or III when available to assess both the safety and efficiency of medicines developed by biotechnology organizations.
Each country must pursue its efforts to identify new cases of FAP by training and raising awareness of physicians via national referral centers and eventually national networks for FAP [Adams et al. 2012b].
Familial screening
Genetic counseling is crucial when proposing presymptomatic screening. That concerns as a priority the sibship for this disease of autosomal transmission. Screening of carriers of genetic abnormalities can identify individuals who are at risk of developing a peripheral neuropathy but also a cardiomyopathy latent for a very long time. Only accurate cardiac screening will characterize a frequent indolent cardiomyopathy. For patients with early onset FAP, it is possible to propose prenatal screening.
Conclusion
FAP is an excellent example of the considerable progress which has been made during the last two decades in the development of innovative therapies, including LT, which is still the reference. Current and new medicines in development issued from biotechnology organizations have the main goal of stopping or slowing down the progression of this neurodegenerative polyneuropathy. Their efficacy in treating other organs, especially the heart, must also be carefully assessed. Care of patients with FAP must be multidisciplinary in a referral center. With the help of referral centers for FAP and eventually a national network, we hope that all newly diagnosed cases of FAP will benefit from these therapeutic advances.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author has acted as a consultant for Alnylam and ISIS and has participated in a symposium for Pfizer.
References
- Ackermann E., Guo S., Booten S., Alvarado L., Benson M., Hughes S., et al. (2012) Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid 19(Suppl. 1): 43–44 [DOI] [PubMed] [Google Scholar]
- Adams D., Lacroix C., Antonini T., Lozeron P., Denier C., Kreib A., et al. (2011) Symptomatic and proven de novo amyloid polyneuropathy in familial amyloid polyneuropathy domino liver recipients. Amyloid 18(Suppl. 1): 169–172 [DOI] [PubMed] [Google Scholar]
- Adams D., Lozeron P., Lacroix C. (2012a) Amyloid neuropathies. Curr Opin Neurol 25: 564–572 [DOI] [PubMed] [Google Scholar]
- Adams D., Lozeron P., Theaudin M., Mincheva Z., Cauquil C., Adam C., et al. (2012b) Regional difference and similarity of familial amyloidosis with polyneuropathy in France. Amyloid 19(Suppl. 1): 61–64 [DOI] [PubMed] [Google Scholar]
- Adams D., Samuel D., Goulon-Goeau C., Nakazato M., Costa P., Feray C., et al. (2000) The course and prognostic factors of familial amyloid polyneuropathy after liver transplantation. Brain 123: 1495–1504 [DOI] [PubMed] [Google Scholar]
- Benson M., Kincaid J. (2007) The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 36: 411–423 [DOI] [PubMed] [Google Scholar]
- Benson M., Kluve-Beckerman B., Zeldenrust S., Siesky A., Bodenmiller D., Showalter A., et al. (2006) Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve 33: 609–618 [DOI] [PubMed] [Google Scholar]
- Benson M., Smith R., Hung G., Kluve-Beckerman B., Showalter A., Sloop K., et al. (2010) Suppression of choroid plexus transthyretin levels by antisense oligonucleotide treatment. Amyloid 17: 43–49 [DOI] [PubMed] [Google Scholar]
- Berk J., Dyck P., Obici L., Zeldenrust S., Sekijima Y., Yamashita T., et al. (2011) The diflunisal trial: update on study drug tolerance and disease progression. Amyloid 18(Suppl. 1): 191–192 [DOI] [PubMed] [Google Scholar]
- Bodin K., Ellmerich S., Kahan M., Tennent G., Loesch A., Gilbertson J., et al. (2010) Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 468: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso I., Martins D., Ribeiro T., Merlini G., Saraiva M. (2010) Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T., Maia L., Martins da Silva A., Waddington Cruz M., Plante-Bordeneuve V., Lozeron P., et al. (2012) Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 79: 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T., Suhr O.B., Adams D., Lozeron P., Hawkins P., Mant T., et al. (2011) Interim clinical update for ALN-TTR01, a novel RNAI therapeutic for the treatment of transthyretin amyloidosis. J Peripher Nerv Syst 16: S25 [Google Scholar]
- Conceicao I., Evangelista T., Castro J., Pereira P., Silvestre A., Coutinho C., et al. (2010) Acquired amyloid neuropathy in a Portuguese patient after domino liver transplantation. Muscle Nerve 42: 836–839 [DOI] [PubMed] [Google Scholar]
- Coutinho P., Martins da Silva A., Lopes Lima J., Resende Barbosa A. (1980) Forty years of experience with type I amyloid neuropathy. Review of 483 cases. In: Glenner G., Costa P., de Freitas A. (eds), Amyloid and Amyloidosis. Amsterdam: Execerpta Medica, pp. 88–98 [Google Scholar]
- Eriksson P., Karp K., Bjerle P., Olofsson B. (1984) Disturbances of cardiac rhythm and conduction in familial amyloidosis with polyneuropathy. Br Heart J 51: 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P., Olofsson B. (1984) Pacemaker treatment in familial amyloidosis with polyneuropathy. Pacing Clin Electrophysiol 7: 702–706 [DOI] [PubMed] [Google Scholar]
- Ferreira A.C., Nolasco F., Sampaio S., Baptista A., Pessegueiro P., Monteiro E., et al. (2012) Orthotopic liver transplantation in familial amyloidotic polyneuropathy is associated with long-term progression of renal disease. Port J Nephrol Hypert 26(3): 199–205 [Google Scholar]
- Holmgren G., Ericzon B., Groth C., Steen L., Suhr O., Andersen O., et al. (1993) Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Holmgren G., Steen L., Ekstedt J., Groth C., Ericzon B., Eriksson S., et al. (1991) Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet 40: 242–246 [DOI] [PubMed] [Google Scholar]
- Johnson S., Connelly S., Fearns C., Powers E., Kelly J. (2012) The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol 421: 185–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Tanaka F., Hashimoto R., Tomita M., Kawagashira Y., Iijima M., et al. (2012) Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 83: 152–158 [DOI] [PubMed] [Google Scholar]
- Liepnieks J., Zhang L., Benson M. (2010) Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology 75: 324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado L., Baliellas C., Casasnovas C., Ferrer I., Fabregat J., Ramos E., et al. (2010) Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl 16: 1386–1392 [DOI] [PubMed] [Google Scholar]
- Lobato L., Rocha A. (2012) Transthyretin amyloidosis and the kidney. Clin J Am Soc Nephrol 7: 1337–1346 [DOI] [PubMed] [Google Scholar]
- Miller S., Sekijima Y., Kelly J. (2004) Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest 84: 545–552 [DOI] [PubMed] [Google Scholar]
- Obici L., Cortese A., Lozza A., Lucchetti J., Gobbi M., Palladini G., et al. (2012) Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid 19(Suppl. 1): 34–36 [DOI] [PubMed] [Google Scholar]
- Olofsson B., Backman C., Karp K., Suhr O. (2002) Progression of cardiomyopathy after liver transplantation in patients with familial amyloidotic polyneuropathy, Portuguese type. Transplantation 73: 745–751 [DOI] [PubMed] [Google Scholar]
- Raichlin E., Kushwaha S., Daly R., Kremers W., Frantz R., Clavell A., et al. (2011) Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc 43: 1871–1876 [DOI] [PubMed] [Google Scholar]
- Rocha A., Lobato L., Silva H., Beirao I., Santos J., Pessegueiro H., et al. (2011) Characterization of end-stage renal disease after liver transplantation in transthyretin amyloidosis (ATTR V30M). Transplant Proc 43: 189–193 [DOI] [PubMed] [Google Scholar]
- Sandgren O., Kjellgren D., Suhr O. (2008) Ocular manifestations in liver transplant recipients with familial amyloid polyneuropathy. Acta Ophthalmol 86: 520–524 [DOI] [PubMed] [Google Scholar]
- Stangou A., Heaton N., Hawkins P. (2005) Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med 352: 2356 [DOI] [PubMed] [Google Scholar]
- Steen L., Ek B. (1983) Familial amyloidosis with polyneuropathy. A long-term follow-up of 21 patients with special reference to gastrointestinal symptoms. Acta Med Scand 214: 387–397 [PubMed] [Google Scholar]
- Wilczek H., Larsson M., Ericzon B. (2011) Long-term data from the Familial Amyloidotic Polyneuropathy World Transplant Registry (FAPWTR). Amyloid 18(Suppl. 1): 188–190 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Wilczek H., Nowak G., Larsson M., Oksanen A., Iwata T., et al. (2007) Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 7: 2597–2604 [DOI] [PubMed] [Google Scholar]
- Yamashita T., Ando Y., Okamoto S., Misumi Y., Hirahara T., Ueda M., et al. (2012) Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology 78: 637–643 [DOI] [PubMed] [Google Scholar]
- Yazaki M., Tokuda T., Nakamura A., Higashikata T., Koyama J., Higuchi K., et al. (2000) Cardiac amyloid in patients with familial amyloid polyneuropathy consists of abundant wild-type transthyretin. Biochem Biophys Res Commun 274: 702–706 [DOI] [PubMed] [Google Scholar]