Abstract
The contribution of vitamin D insufficiency to the pathogenesis of multiple sclerosis (MS) is reviewed. Among the multiple recently discovered actions of vitamin D, an immunomodulatory role has been documented in experimental autoimmune encephalomyelitis and in humans. This action in the peripheral immune system is currently the main known mechanism through which vitamin D might influence MS, but other types of actions could be involved within the central nervous system. Furthermore, vitamin D insufficiency is widespread in temperate countries and in patients with MS at the earliest stages of the disease, suggesting that the deleterious effects related to vitamin D insufficiency may be exerted in these patients. In fact, many genetic and environmental risk factors appear to interact and contribute to MS. In genetics, several human leukocyte antigen (HLA) alleles (more particularly HLA-DRB1*1501) could favour the disease whereas some others could be protective. Some of the genes involved in vitamin D metabolism (e.g. CYP27B1) also play a significant role. Furthermore, three environmental risk factors have been identified: past Epstein–Barr virus infection, vitamin D insufficiency and cigarette smoking. Interactions between genetic and environmental risk or protective factors may occur during the mother’s pregnancy and could continue during childhood and adolescence and until the disease is triggered in adulthood, therefore possibly modulating the MS risk throughout the first decades of life. Furthermore, some clinical findings already strongly suggest that vitamin D status influences the relapse rate and radiological lesions in patients with MS, although the results of adequately powered randomized clinical trials using vitamin D supplementation have not yet been reported. While awaiting these incontrovertible results, which might be long in coming, patients with MS who are currently in vitamin D insufficiency should be supplemented, at least for their general health status, using moderate doses of the vitamin.
Keywords: Epstein–Barr virus, genetics, multiple sclerosis, smoking, vitamin D
Introduction
Our knowledge of the multiple actions of vitamin D in the body and of the pathogenesis of multiple sclerosis (MS) has developed considerably during the past 12 years and it now appears highly likely that this vitamin is involved in MS [Hayes, 2000; Van Amerogen et al. 2004; Ascherio and Munger, 2007b; Holick, 2007; Ebers, 2008; Niino et al. 2008; Ascherio et al. 2010, 2012a; Pierrot-Deseilligny and Souberbielle, 2010; Hanwell and Banwell, 2011; Mowry, 2011; Simon et al. 2012a; Hølmoy et al. 2012; van der Mei et al. 2012b], a connection that was already suggested a long time ago [Goldberg, 1974]. In the first part of this article, dealing with the rationale for an involvement of vitamin D in MS, we will mainly review findings suggesting that this vitamin has a general immunomodulatory effect, including in patients with MS, and data showing that a widespread insufficiency in vitamin D exists in temperate and Nordic countries, including in patients with MS. In the second part of this review, we will see how vitamin D insufficiency is likely one of the risk factors for MS, among multiple other environmental and genetic risk factors, and that numerous interactions appear to exist between all these risk and protective factors, resulting in a likely continuous modulation of MS risk from conception to the beginning of the disease, years later. Lastly, in the third part of this paper, we will review data showing that vitamin D status may also influence the main clinical and radiological variables of patients with MS once the disease has started and conclude by recommending simple clinical measures that should be applied without further delay to take into account these new findings.
Rationale for the involvement of vitamin D in multiple sclerosis
Role of vitamin D
Vitamin D metabolism
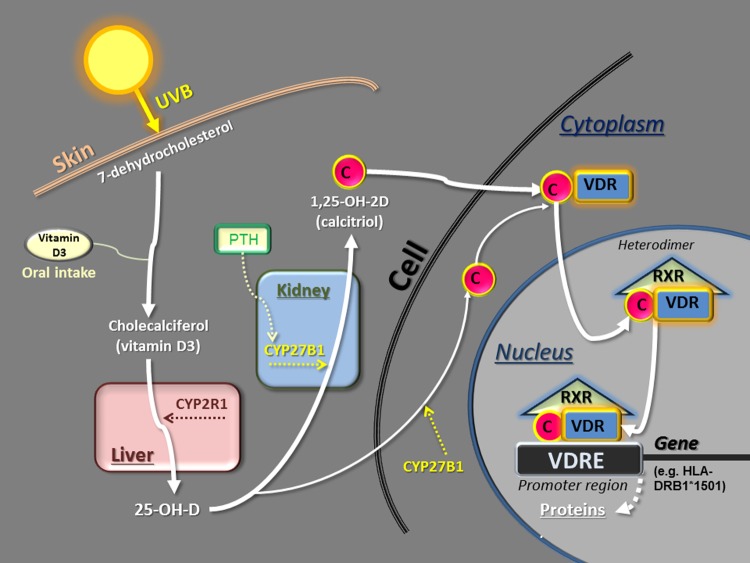
The main steps of vitamin D metabolism are well known and will not be detailed here [Lips, 2006; Holick et al. 2007; Norman and Bouillon, 2010] (Figure 1). After transformation of 7-dehydrocholesterol into cholecalciferol (vitamin D3) in the skin through the action of ultraviolet B radiation (UVB) or after direct oral intake of vitamin D3 (or D2), there is a first hydroxylation in the liver catalyzed by several vitamin D-25-hydroxylase enzymes, the most important being CYP2R1 [Prosser and Jones, 2004]: this results in 25-OH-D, which is the metabolite measured in the blood to evaluate the vitamin D status (see below). Then, a second hydroxylation takes place in the proximal tubule of the kidney, catalysed by the enzyme 1α-hydroxylase (CYP27B1) [Prosser and Jones, 2004], resulting in 1,25-OH-2D (calcitriol), which is the active metabolite of vitamin D. Low calcium intake and the parathyroid hormone (PTH) stimulate this renal hydroxylation and increase the calcitriol level in the blood, whereas the phosphaturic hormone fibroblast growth factor 23 (FGF23) and a high level of calcitriol have the opposite effect. Furthermore, the vitamin D 24-hydroxylase, another enzyme located in the renal tubule and encoded by the CYP24A1 gene, is also able to induce an inactivating pathway for vitamin D metabolites. This enzyme is tightly regulated by FGF23 and the level of calcitriol. The importance of this inactivating pathway has recently been highlighted in the literature with the demonstration that inactivating mutations of the CYP24A1 gene induced severe neonatal hypercalcaemia [Schlingman et al. 2011]. Vitamin D and its diverse metabolites, including calcitriol, are transported in the blood by the vitamin D-binding protein (DBP) (which is a serum globulin mainly produced in the liver) and to a lesser extent by albumin. The calcitriol dissociates from DBP when entering a target cell and first binds to a specific receptor of vitamin D (VDR) within the cytoplasm (Figure 1). Then, this complex enters the nucleus and forms a heterodimer by connecting to a nuclear receptor, that is, the retinoid X receptor (RXR). The heterodimer calcitriol–VDR–RXR finally binds to vitamin D-responsive elements (VDREs), which constitute a specific sequence of DNA within the promoter region of the target genes, the whole regulating (by activation or suppression) gene transcription and expression and finally protein synthesis (e.g. cytokines, etc.) in approximately 5–10% of the genome [Wang et al. 2005; Niino et al. 2008; Norman and Bouillon, 2010; Pike and Meyer, 2010] (Figure 1). Thus, calcitriol, secreted into the bloodstream by the kidney and exerting its actions in various other tissues by binding to a specific receptor, can be considered as a hormone.
Figure 1.
Schematic representation of vitamin D metabolism.
Note that, at the gene level, the heterodimer comprising calcitriol may stimulate or repress protein synthesis, depending on the cell. C, calcitriol; CYP2R1, vitamin D-25-hydroxylase; CYP27B1, 1α-hydroxylase; PTH, parathyroid hormone; RXR, retinoid X receptor; UVB, ultraviolet B radiation; VDR, vitamin D receptor; VDRE, vitamin D-responsive element.
Multiplicity of vitamin D actions
VDRs are widespread in almost all cells of the organism [Walters, 1992], including immunity cells: macrophages, monocytes, dentritic cells (DCs) and lymphocytes T and B [Bahlla et al. 1983; Provvedini et al, 1983, Morgan et al. 1996; Vedman et al. 2000; Chen et al. 2007]. VDRs are also present in all types of central nervous system (CNS) cells, that is, neurons, oligodendrocytes, astrocytes and glial cells [Walters, 1992; Baas et al. 2000; Garcion et al. 2002; Eyles et al. 2005]. These different immune and nervous cells express the CYP27B1 enzyme and are able to transform in situ the circulating 25-OH-D into calcitriol [Zehdner et al. 2001; van Etten et al. 2008] (Figure 1), resulting in intracrine and paracrine actions in these cells and neighbouring cells [Morris and Anderson, 2010]. Besides its role in calcium physiology and bone health, vitamin D also has numerous potential extra-bone actions: protective for the cardiovascular system, antiproliferative (in certain cancers), anti-infectious (innate immunity) and anti-inflammatory and immunomodulatory (adaptive immunity), an effect which could be involved in autoimmune diseases such as type 1 diabetes, Crohn’s disease, rheumatoid arthritis and MS [Holick, 2004, 2007; Vieth, 2007; Vieth et al. 2007; Borradale and Kimlin, 2009; Hewison, 2012]. The specific role of calcitriol within CNS cells remains to be clarified [Smolders et al. 2011b]: it may have potential actions in neuronal functioning, neuroprotection and myelination [Wergeland et al. 2011], but also in innate and adaptive immunity of the CNS, through the invading lymphocytes. Accordingly, the presence of VDRs and CYP27B1 in the different immune and nervous cells constitutes a first indication for potential actions of vitamin D in MS. The immunodulatory action of vitamin D through the general immune system, likely important for MS pathogenesis, is specifically reviewed in the following sections.
Immunodulatory effect of vitamin D in experimental autoimmune encephalomyelitis
Since experimental autoimmune encephalomyelitis (EAE) is the best animal model of MS, it is of interest to briefly review the effect of vitamin D in this disease, which has been studied for more than 20 years. Calcitriol has both a preventive and a curative effect in EAE [Lemire and Archer, 1991; Cantorna et al. 1996] but requires the presence of calcium [Cantorna et al. 1999] and VDRs [Mehan and DeLuca, 2002] for these actions. However, there are contradictory reports concerning the beneficial effect of vitamin D sufficiency [Fernandes de Abreu et al. 2011] or deficiency [DeLuca and Plum, 2011] on the severity and delay of onset of EAE. If vitamin D3 is used, the beneficial effect predominates in females, likely via a potentiation by oestrogens [Spach and Hayes, 2005; Nashold et al. 2009; Subramanian et al. 2012]. Various immunological mechanisms have been reported to explain the vitamin D and calcitriol effects: an anti-inflammatory effect [Spach et al., 2004], actions on macrophages [Nashold et al. 2000], on different types of cytokines [Cantorna et al. 1998; Spach et al. 2006; Pedersen et al. 2007], on regulatory T lymphocyte cells (Tregs), lymphocyte T helper 1 (Th1), Th17 and Th2 [Mattner et al. 2000; Muthian et al. 2006; Chang et al. 2010; Mayne et al. 2011] and invariant natural killer T-cells (iNKTs) [Cantorna et al. 2012]. Interestingly, with low VDR gene expression, EAE is facilitated, with an increase in Th1 and Th17, suggesting that, in a similar genetic situation in humans, the MS risk may be increased despite high ambient UV radiation (e.g. in Sardinia) [Spanier et al. 2012]. These diverse beneficial actions on T lymphocytes are favoured by some authors, who suggest that vitamin D positively influences Treg activity, restoring a better ratio between the Th2 (protective) and Th1 (aggressive) cells, the overall effect being a decrease in inflammation [Cantorna, 2006, 2008; Smolders et al. 2008a; Cantorna et al. 2012] (Figure 2). It should be noted that this mechanism is analogous to the mechanism of interferon β (IFNβ) [Axtell et al. 2010; Bushnell et al. 2012], used as an immunomodulatory treatment in MS, and that a potentiation exists between the beneficial effects of IFNβ and calcitriol analogue used together in EAE [Van Etten et al. 2007]. Despite all these findings observed with vitamin D or calcitriol in EAE, one research group recently reported results that led them to suggest that UVB plays the actual protective role in EAE instead of vitamin D [Becklund et al. 2010] and to question the role of VDR and vitamin D in EAE and MS [DeLuca and Plum, 2011; Wang et al. 2012]. However, even if it were the case that UVB exerts a specific immunosuppressive effect independent of vitamin D synthesis [Hart et al. 2011], such an effect has not yet been studied in humans and does not in fact rule out parallel, now well documented immunomodulatory mechanisms induced by vitamin D. Furthermore, whatever the current controversial points of view concerning the role of vitamin D in EAE, it should not be forgotten that this disease is not exactly comparable to human MS.
Figure 2.
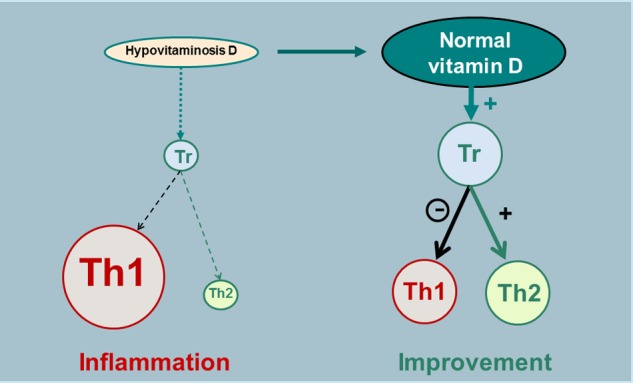
Schematic representation of one of the hypothetical immunomodulatory effects of vitamin D (through calcitriol).
Tr, regulatory T lymphocyte; Th1, lymphocyte T helper 1 (‘aggressive’); Th2, lymphocyte T helper 2 (‘protective’).
General immunodulatory effect of vitamin D in humans
The general immunodulatory effect of vitamin D in humans (for a review, see Hewison [2012]), that is, outside the CNS, is currently the best known mechanism through which vitamin D appears to influence MS risk and course. Furthermore, vitamin D could enhance innate immunity via its actions on macrophages and monocytes and regulate adaptive immunity in multiple ways [Adorini and Penna, 2008]. The presence of VDRs in human T lymphocytes [Provvedini et al. 1983; Baeke et al. 2010], in greater number in CD8 than in CD4 lymphocytes [Vedman et al. 2000], as well as in B lymphocytes [Provvedini et al. 1983; Chen et al. 2007], and the expression of CYP27B1 in lymph nodes [Zehdner et al. 2001] and T lymphocytes [Sigmundsdottir et al. 2007] constitute important indications of a potential role of vitamin D in adaptive immunity. Furthermore, a number of mechanisms by which vitamin D and calcitriol could favourably influence immunity have been reported in the past 30 years: it has been shown that vitamin D (through calcitriol) reduces differentiation of monocytes to DCs and differentiation and proliferation of DCs, thus decreasing T-cell stimulation [Griffin et al. 2001]; controls T-cell activation [von Essen et al. 2010] and inhibits T-cell proliferation [Rigby et al. 1990; Lemire et al. 1984]; reduces the production of interleukin (IL)-2 (growth factor for T cells) [Müller et al. 1993]; suppresses in vitro and in vivo production of proinflammatory Th1 cell-derived IFNγ and tumour necrosis factor α [Reichel et al. 1987; Lemire et al. 1995; Baeke et al. 2010; Zhang et al. 2012]; reduces proinflammatory Th17 activity and IL-17 production [Tang et al. 2009; Ikeda et al. 2010; Bruce et al. 2011; Joshi et al. 2011; Allen et al. 2012]; enhances the production of the anti-inflammatory cytokine IL-10 [Heine et al. 2008; Baeke et al. 2010; Allen et al. 2012]; promotes in vitro and in vivo the development of Tregs expressing cytotoxic T lymphocyte antigen 4 and forkhead box P3, resulting in an anti-inflammatory effect [Jeffery et al. 2009; Prietl et al. 2010; Khoo et al. 2012; Urry et al. 2012]; enhances the transformation of CD4 T lymphocytes into a Th2 phenotype (with a protective role) [Boonstra et al. 2001; van Etten and Mathieu, 2005; Sloka et al. 2011b]; and furthermore, inhibits B-cell differentiation [Chen et al. 2007]. Accordingly, vitamin D has general immunomodulatory and anti-inflammatory effects not only by reducing DCs, Th1, Th17, B-cell proliferation and proinflammatory cytokines but also by promoting Th2 phenotype, Treg activity and anti-inflammatory cytokines. The vitamin D action on Tregs, as mentioned above in the context of EAE, could itself reduce Th1 activity and re-equilibrate the balance between Th1 and Th2 cells, resulting in a reduction of inflammation [Cantorna, 2006; Smolders et al. 2008a] (Figure 2). Such an action profile of vitamin D (through calcitriol) strongly suggests that an insufficiency of this vitamin could play a role in the pathophysiology of autoimmune diseases, including MS, and may constitute one of the risk factors involved in these diseases.
Immunomodulatory effect of vitamin D in patients with multiple sclerosis
The multiple immunological studies on patients with MS reported recently have shown that the general immunomodulatory actions of vitamin D on T and B cells already described in animals and normal humans likely also exist in this disease. One of the first studies dealing with the immunological action of vitamin D in patients with MS was a controlled trial in which it was observed that vitamin D supplementation (1000 IU/day for 6 months) significantly increased tumour growth factor β1, a cytokine inhibiting T cells and secreted by different types of cells, including Tregs [Mahon et al. 2003]. More recently, it has been shown in patients with MS that calcitriol inhibits in vitro T-cell proliferation, inhibits the development of IL-6- and IL-17-producing cells, enhances IL-10 production and the number of Tregs [Correale et al. 2009] and stimulates CD 46 and IL-10 [Kickler et al. 2012], all these mechanisms contributing to an anti-inflammatory action. Furthermore, a correlation was found between the vitamin D and calcitriol serum levels and the Treg number [Royal et al. 2009], or only between the vitamin D serum level and the inhibitory action of Tregs on Th1 cells, with a beneficial effect in IFNβ users [Smolders et al. 2009] and without correlation with calcitriol, PTH and calcium [Smolders et al. 2010a]. In a small controlled trial, MS-associated abnormal T reactivities were suppressed in vivo by vitamin D supplementation at serum 25-OH-D concentrations higher than 100 nmol/liter [Kimball et al. 2011b]. In another small study, in which patients with relapsing–remitting MS (RRMS) were supplemented with high doses of vitamin D (20,000 IU/day) for 3 months, Tregs were unchanged but the proportion of IL-10+ CD4+ T cells was increased [Smolders et al. 2010b]. Furthermore, in patients with MS, a low vitamin D serum level was associated with T-cell proliferation [Grau-Lopez et al. 2012], vitamin D inhibited in vitro the differentiation and maturation of DCs [Bartosik-Psujek et al. 2010] and enhanced in vivo anti-inflammatory cytokines [Moysayebi et al. 2011], and calcitriol reduced in vitro proinflammatory cytokines and enhanced anti-inflammatory cytokines [Lysandropoulos et al. 2011]. However, there was no substantial effect on phenotypic markers of B-cell differentiation in circulating B cells in a study using supplementation with high doses of vitamin D3 [Knippenberg et al. 2011]. Lastly, the immunomodulatory and anti-inflammatory effects of vitamin D appear to be more marked in women than in men in patients with MS as well as in healthy subjects, maybe due to synergic effects between calcitriol and 17-β estradiol [Correale et al. 2010]. Altogether, these different studies show that vitamin D has potentially beneficial immunomodulatory and anti-inflammatory effects in patients with MS, though their actual impact on the course of the disease remains to be accurately evaluated by randomized, controlled trials (RCTs) using vitamin D supplementation.
Vitamin D requirements and insufficiency
Optimal vitamin D serum level
25-OH-D is the vitamin D metabolite usually measured in the blood since it is representative of the vitamin D store in the organism [Heaney, 2000; Zerwekh, 2008]. The limits usually recommended are between 75 and 200 nmol/liter (i.e. 30 and 80 ng/mL) [Dawson-Hughes et al. 2005; Binkley and Krueger, 2008; Souberbielle et al. 2010]. The question of the lower cut-off (75 nmol/liter) is a key point to understand the whole vitamin D problem. This limit has not been determined from classical control groups of ‘normal’ adults (i.e. with the 2.5th or 5th percentile found in an apparently healthy population) since vitamin D insufficiency is widespread in general populations (see below), but it has not been empirically fixed either. Defining vitamin D insufficiency corresponds to determining the 25-OH-D serum level below which adverse outcomes may occur or above which beneficial effects of vitamin D may be observed. Ideally, this supposes that RCTs demonstrating positive effects of vitamin D compared with placebo on clinical (‘hard’) outcomes are available and that the 25-OH-D concentrations in the ‘vitamin D groups’ of theses RCTs have been evaluated. It must be emphasized that, with the exception of the effect on the risk of falls, the many lines of evidence concerning the various potential extra-skeletal effects of vitamin D are mostly based on observational and mechanistic studies. Although numerous prospective studies have shown that subjects in the highest quantile of 25-OH-D serum concentrations (usually >70–80 nmol/liter) have a lower relative risk for many diseases than those in the lowest quantile (usually <30–40 nmol/liter) [Munger et al. 2006; Bodnar et al. 2007; Leu and Giovannucci, 2011; Ma et al. 2011], the observational nature of these studies precludes any conclusion regarding a causal relationship between low vitamin D status and these diseases, and there are consequently no clear clinical cutoff(s) to optimize the potential vitamin D effects. It must be acknowledged that the 75 nmol/liter cutoff is only ‘reasonably’ evidence based (i.e. based on RCTs) for the musculoskeletal effects of vitamin D: in the RCTs that have shown positive effects of vitamin D on nonvertebral fractures [Bischoff-Ferrari et al. 2009b] and falls [Bischoff-Ferrari et al. 2009a], subjects in the ‘vitamin D groups’ generally had 25-OH-D levels of more than 75 nmol/liter, whereas those in the ‘placebo groups’ had levels mostly in the 30–60 nmol/liter range. Consistent with these RCTs, bone biopsy data showed that histomorphometric signs of defect in the mineralization of bone were not detected in subjects with a 25-OH-D serum level of more than 75 nmol/liter whereas they were present, as defined by the most conservative threshold of the osteoid volume/bone volume ratio of 2%, in approximately 20% of subjects with a 25-OH-D serum level between 50 and 75 nmol/liter [Bischoff-Ferrari et al. 2004; Priemel et al. 2010]. Furthermore, patients with a basal 25-OH-D serum level of up to 70 nmo/liter decreased their PTH serum concentration when they were given vitamin D (without calcium) [Okazaki et al. 2011], whereas it has been reported that the PTH serum concentration may increase when the 25-OH-D serum level is below 75–80 nmol/liter [Chapuy et al. 1996; Holick, 2007; Durazo-Arvizu et al. 2010]. It has also been shown that calcium absorption was improved in menopausal women when the 25-OH-D serum level increased to approximately 80 nmol/liter [Heaney et al. 2003b] and calcium excretion was no longer directly dependent on 25-OH-D serum concentrations below the level of 75 nmol/liter [Kimball et al. 2011a]. Lastly, recent data indicate that a 25-OH-D serum level of at least 82 nmol/liter is required to optimize the antifracture efficacy of bisphosphonates [Carmel et al. 2012].
Due to the convergence of the findings provided by all of these different approaches on the 75 nmol/liter level, most medical laboratories in the world have now adopted this level as the lower normal limit, even if this point is not yet consensual [Ross et al. 2011; Heaney and Holick, 2011; Holick et al. 2011]. Furthermore, it should be noted that the ‘physiological’ zone between the 75 and 200 nmol/liter 25-OH-D serum levels grossly corresponds to the serum levels observed in outdoor workers [Haddad and Chyu, 1971; Haddock et al., 1982; Barger-Lux and Heaney, 2002; Azizi et al. 2012], as well as in traditionally living populations in East Africa [Luxwolda et al. 2012]. This zone is far below the toxic zone, which appears to be located above the 400 nmol/liter serum level [Hathcock et al. 2007; Burton et al. 2010].
Vitamin D requirements
On the basis of these new metabolic and pathological findings, the daily requirement of vitamin D has recently been reassessed and is now thought to be far higher than the 200–400 IU/day dose that, until a few years ago, was generally estimated to be sufficient. The previously held belief regarding the optimal requirement was principally based on the results of experiments in the rat almost one century ago in the context of studies on rickets prevention. Nowadays, it is more readily accepted that humans are different from rats, as a species as well as in terms of weight for determining treatment doses, and that rickets prevention is not the only vitamin D action to be taken into account. The daily requirement does of course depend on what the optimal target 25-OH-D serum level is considered to be: for a 25-OH-D serum level of 50 nmol/liter, 800-1000 IU/day of vitamin D appears sufficient, but to bring most people above the 75 nmol/liter level, a dosage of between 1000 and 4000 IU/day (depending on the individual, but on average 2000 IU/day) is required [Heaney et al. 2003a, 2009; Grant and Holick, 2005; Hollis, 2005; Bischoff-Ferrari et al. 2006, 2009b, 2012; Vieth, 2006; Hall et al. 2010; Schwalfenberg et al. 2010; Whiting and Calvo, 2010; Cashman et al. 2011; Garrett-Mayer et al. 2012; Holick, 2011, 2012]. However, vitamin D intake via (unfortified) food is very marginal in normal Western diets, even in those considered well balanced, and generally provides less than 100–200 IU/day, rarely reaching little more than 400 IU/day with fortified food [Calvo et al. 2004; Moore et al. 2005; Välimäki et al. 2007; O’Donnell et al. 2008; Vatanparast et al. 2010; von Geldern and Mowry, 2012]. Sunshine therefore remains the principal natural source of vitamin D, providing 80–90% of the requirement in the absence of fortified food. However, in temperate and Nordic countries, vitamin D may be synthesized in the skin via UVB only a few months per year (around summer), that is, when the sun is seasonally sufficiently high in the sky for UVB to penetrate all the layers of the atmosphere, and vitamin D stocks disappear in a few weeks after exposure to the sun (or oral intake) if they are not regularly replenished [Holick, 2007]. It should also be noted that modern lifestyles have tended to reduce most people’s outdoor activities and exposure to the sun. Moreover, exposure to the sun is often avoided due to dermatological concerns and people with dark skins and older people synthesize vitamin D less easily than people with light skins and the young [Vieth, 1999; Armas et al. 2007; Binkley et al. 2007]. Lastly, in people who are overweight, (liposoluble) vitamin D is partly sequestered in adipocytes, which may contribute to a worsening of insufficiency [Earthman et al. 2012].
Widespread vitamin D insufficiency
The characteristics of vitamin D physiology, the effects of latitude and climate and multiple societal factors related to vitamin D synthesis result in an insufficiency in this vitamin in most people living beyond the 40th parallels, that is, in Europe, the northen half of the United States, Canada and the former Soviet Union for the northen hemisphere, and New Zealand and Tasmania for the southern hemisphere [Holick, 2007; Pierrot-Deseilligny and Souberbielle, 2011; van der Mei et al. 2012b]. In these countries (for a review, see Pierrot-Deseilligny and Souberbielle [Pierrot-Deseilligny and Souberbielle, 2010]), 25-OH-D serum levels in ‘normal’ adults are between 40 and 70 nmol/liter on average, with generally only slight differences depending upon the season and consequently, at least for a large part of the year, 75% of people are in a state of insufficiency with a 25-OH-D serum level cut-off of 75 nmol/liter and still almost half of the population is in a state of insufficiency if one considers that the cutoff should be 50 nmol/liter. In tropical or subtropical countries, vitamin D serum levels are generally higher, at least for people not systematically avoiding sun exposure, and a correlation exists between latitude and vitamin D serum levels in white people at the world scale [Hagenau et al. 2009]. Such a correlation has also been observed in France, a relatively small country [Chapuy et al. 1996].
Vitamin D insufficiency in patients with multiple sclerosis
In patients with MS living in temperate and Nordic countries, as in the general populations of these countries, vitamin D insufficiency is widespread, whatever the cutoff (50 or 75 nmol/liter) for the lower limit of the 25-OH-D serum level (Figure 3): indeed, as early as the earliest stages of the disease, that is, in patients with clinically isolated syndrome (CIS) or with RRMS, average serum levels are between 42 and 74 nmol/liter, depending on the studies and the seasons, with a general mean close to 60 nmol/liter [Soilu-Hänninen et al. 2005, 2012; Smolders et al. 2008b; Hiremath et al. 2009; Kragt et al. 2009; Mowry et al. 2010; Pierrot-Deseilligny and Souberbielle, 2010, 2012; Simpson et al. 2010; Banwell et al. 2011; Dabbaghmanesh and Yousefipour, 2011; Lonergan et al. 2011; Neau et al. 2011; Steffensen et al. 2011; Yildiz et al. 2011; Bäärnhielm et al. 2012; Kampman et al. 2012; Kirbas et al. 2012; Løken-Amsrud et al. 2012; Moen et al. 2012; Runia et al. 2012; Soilu- Hänninen et al. 2012; Šaltyte. Benth et al. 2012; Triantafyllou et al. 2012] (Table 1). In some of these studies, there was a control group in addition to the patient group and there was not always a significant difference in vitamin D serum levels between the two groups. However, this point may be considered secondary since we now know that most control ‘normal’ subjects are also in a state of more or less marked vitamin D insufficiency. Thus, the possible role of vitamin D status in any disease, including in MS, must always be interpreted in conjunction with the actions of multiple other environmental and genetic risk factors interacting with this status (see below). In this context, vitamin D insufficiency appears to be only one risk factor favouring the disease, interacting in concert with multiple other risk factors, which may explain a significant deleterious effect on MS risk of this widespread vitamin insufficiency at a population scale but does not account for the totality of individual situations of patients with MS, in whom the cumulative effects of several other risk factors may have at times a crucial role, independently of vitamin D status. In particular, in the relatively rare cases of patients with MS in whom normal spontaneous vitamin D serum levels are observed throughout the year, it may be hypothesized that, in these patients, either other environmental (infectious, toxic, etc.) and genetic risk factors play a determinant role or (genetic) errors in the metabolism and actions of vitamin D exist downstream to the 25-OH-D serum level determination. Conversely, the fact that the great majority of ‘normal’ subjects who are in a state of vitamin D insufficiency (on the same basis as patients with MS) do not eventually develop MS may be explained by the existence, in these subjects, of other protective environmental or genetic factors.
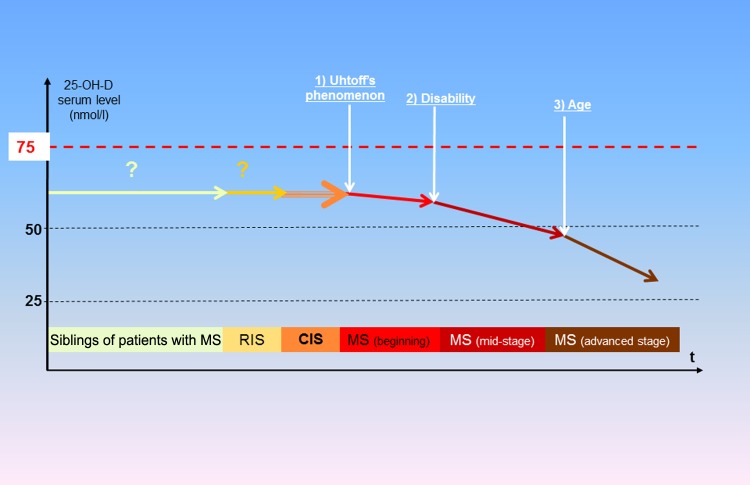
Figure 3.
Schematic representation of the evolution of 25-OH-D serum level according to multiple sclerosis stage.
Note that there are so far no data in patients with RIS and siblings of patients with MS but it may be inferred that their 25-OH-D serum concentrations are not very different from those of ‘normal’ populations. CIS, clinically isolated syndrome; MS, multiple sclerosis; RIS, radiologically isolated syndrome.
Table 1.
25-OH-D serum levels in different cohorts of patients with multiple sclerosis, mainly at the earliest stages of the disease.
| Reference | Cohort provenance: country (region or city) | Age (years) Mean ± SD (range) | Sample size, N | 25-OH-D serum level (nmol/liter): mean ± SD (range) | MS form |
|---|---|---|---|---|---|
| Soilu-Hänninen et al. [2005] | Finland (Turku) | 36 ± 1.4 | 40 | 41 ± 5 (W), 58 ± 3 (S) | RRMS |
| Smolders et al. [2008b] | The Netherlands (Maastricht) | NA | 126 | 72 ± 31 | RRMS |
| Hiremath et al. [2009] | USA (Baltimore) | 42 ± 13 | 199 | 71 ± 39 | CIS, RRMS, SPMS |
| Kragt et al. [2009] | The Netherlands (Amsterdam) | 45 ± NA | 103 | 59 ± 25 (W), 97 ± 34 (S) | RRMS, SPMS |
| Mowry et al. [2010] | USA (San Francisco and New York) | 15 ± 3 | 110 | 55 ± 22 | CIS, RRMS |
| Pierrot-Deseilligny and Souberbielle [2010] | France (Paris region) | 36 ± 12 | 32 | 45 ± 16 | CIS |
| Shaygannejad et al. [2010] | Iran (Isfahan) | 36 (15–55) | 50 | 48 ± NA | RRMS |
| Simpson et al. [2010] | Australia (Tasmania) | 44 ± 10 | 145 | 42 ± NA (W), 74 ± NA (S) | RRMS |
| Banwell et al. [2011] | Canada (multiple centres) | 9.5 ± 4.5 | 302 | 63 ± 28 | CIS |
| Dabbaghmanesh and Yousefipour [2011] | Iran (Shiraz) | 35 ± 8 | 82 | 55 ± 54 | RRMS, SPMS |
| Lonergan et al. [2011] | Ireland (3 centres) | 46 (21–80) | 329 | 38 (13–161) | RRMS, SPMS |
| Neau et al. [2011] | France (Poitiers region) | 46 ± 23 | 170 | 46 ± 23 | RRMS, SPMS |
| Steffensen et al. [2011] | Norway (Tromsø) | 39 (21–50) | 35 | 55 ± 29 | RRMS |
| Yildiz et al. [2011] | Switzerland (St Gallen) | 38 ± 10 | 80 | 57 ± 29 | RRMS |
| Bäärnhielm et al. [2012] | Sweden (Stockholm) | 39 ± 10 | 1013 | 63 ± NA | RRMS |
| Kampmann et al. [2012] | Norway (Tromsø) | 40 (21–50) | 35 | 48 (20–120) | RRMS |
| Kirbas et al. [2012] | Turkey (Rize) | NA (18–40) | 30 | 67 ± 35 | RRMS |
| Løken-Amsrud et al. [2012] | Norway (multiple centres) | 39 (19–58) | 88 | 67 (26–121) | RRMS |
| Moen et al. [2012] | Norway (Oslo) | NA | 99 | 68 ± 24 | CIS, RRMS |
| Pierrot-Deseilligny et al. [2012] | France (Paris region) | 39 ± 10 | 156 | 49 ± 22* | RRMS |
| Runia et al. [2012] | The Netherlands (Rotterdam) | 39 (19–55) | 73 | 69 ± NA | RRMS |
| Šaltyte. Benth et al. [2012] | Norway (multiple centres) | 39 (19–48) | 92 | 68 ± 26 | RRMS |
| Soilu-Hänninen et al. [2012] | Finland (Turku) | 39 (22–53) | 66 | 54 (19–82)* | RRMS |
| Triantafyllou et al. [2012] | Greece (Athens) | 39 ± 10 | 119 | 62 ± 25 | RRMS |
Before vitamin D supplementation.
CIS, clinically isolated syndrome; MS, multiple sclerosis; NA, not available; RRMS, relapsing–remitting multiple sclerosis; S, in summer; SD, standard deviation; SPMS, secondary progressive multiple sclerosis; W, in winter.
Furthermore, it should be noted that the vitamin D serum level usually tends to decrease throughout the course of MS because of the conjugate actions of three worsening factors for vitamin D insufficiency, successively intervening and accumulating during this course (Figure 3): as early as the beginning of MS, Uhtoff’s phenomenon (heat sensitivity) may lead some patients to spontaneously avoid sun exposure and the associated heat, an attitude that until recently was often encouraged by neurologists, leading to an accelerated decline in vitamin D synthesis; in the mid course of the disease, disability reduces outdoor activities and, consequently, sun exposure; in older patients, vitamin D synthesis is physiologically reduced by age. These different factors contributing to the deterioration of vitamin D status likely partly explain why vitamin D serum levels are lower in secondary progressive MS (SPMS) than at the earliest stages of the disease, with concentrations usually close to 40 nmol/liter [Nieves et al. 1994; Ozgocmen et al. 2005; Smolders et al. 2008b; Pierrot-Deseilligny and Souberbielle, 2010; Neau et al. 2011]. These associated factors might also contribute to ‘reverse causality’ (i.e. with the disease worsening the initial insufficiency in vitamin D), at least in mid and advanced stages of MS. However, it should not be ignored that a marked hypovitaminosis D is observed as early as the earliest stages of MS (i.e. before these associated factors can be exerted) and consequently may contribute to triggering the disease (see below). From a preventive point of view, it would also be of particular interest to study the vitamin D status of subjects with radiologically isolated syndromes and in siblings of patients with MS (Figure 3), since they all have an increased risk for MS.
Vitamin D insufficiency is likely one of the risk factors for multiple sclerosis
It is nowadays commonly accepted that MS is a multifactorial disease that appears in subjects who are genetically predisposed and who encounter one or more deleterious environmental factors [Goodin, 2009].
Genetic risk factors for multiple sclerosis possibly involving vitamin D
It has long been known that siblings of patients with MS have about a 1–5% risk of developing the disease and this risk reaches 20–30% for a homozygote twin, which shows that genetics does indeed partly influence MS risk [Dyment et al. 2006]. A recent genome-wide association study (GWAS) has suggested that about 50 genes, comprising approximately 20% of the heritability of MS, are involved in the risk of this disease, half of them being implicated in immune processes, that is, T-cell differentiation, B-cell regulation and cytokine pathways [Sawcer et al. 2011]. The genetic contribution to MS risk has recently been reviewed [Lin et al. 2012] and here we will mainly deal with the genetic aspects that may be related to vitamin D.
Human leukocyte antigen system
The HLA system drives many immune responses. In the analysis performed by Sawcer and colleagues, the allele HLA-DRB1*1501, which is present in 14–30% of populations from countries with high risk for MS [Schmidt et al. 2007], has the strongest association with this disease, representing 11% of the whole heritability, but other HLA-DRB1 alleles are also involved to a lesser degree [Sawcer et al. 2011]. By contrast, HLA-A*0201 could be protective. The risk for MS is also increased in children with one or more HLA-DRB1*15 alleles [Banwell et al. 2011; Disanto et al. 2011a]. It should be noted that a VDRE exists within the promoter region of HLA-DRB1 [Ramagopalan et al. 2009b; Handunnetthi et al. 2010], that is, the main risk variant for MS: this VDRE was highly conserved (no mutations on over 600 chromosomes) in the major MS-associated DR2 haplotype bearing the HLA-DRB1*15 allele and not conserved generally among non-MS associated haplotypes. In addition, this VDRE influenced gene expression and conferred calcitriol sensitivity to HLA-DRB1*15, whereas the variant VDRE present on other, non-MS-associated HLA-DRB1 haplotypes was not responsive to calcitriol. The authors hypothesized that a lack of vitamin D in early childhood can affect the expression of HLA-DRB1 in the thymus and result in an increase in the risk of autoimmunity later in life [Handunnetthi et al. 2010]. It should also be noted that the frequency of the MS-associated HLA-DRB1*1501 allele is much higher in white individuals than in other racial types, which could explain why MS prevalence is relatively low in people with dark skins living in temperate countries, independently of their vitamin D status [Handunnetthi et al. 2010]. Furthermore, the frequency of HLA-DRB1*15 is higher in women than in men with MS [Hensiek et al. 2002; Chao et al. 2010; Irizar et al. 2012] and the penetrance of HLA-DRB1*15 has increased over time in women [Chao et al. 2009]. This could contribute to the well known recent increase in female predominance of the disease and also, through the calcitriol effect on HLA-DRB1*15 expression, to a lower incidence of MS in women with higher vitamin D serum levels [Kragt et al. 2009]. Furthermore, it may be that decreasing vitamin D serum levels in the general population [Yetley, 2008] due to diverse changes in lifestyle (fewer outdoor activities, more protection from sunshine and higher obesity levels) has increased the frequency of HLA-DRB1*15 in MS over time [Handunnetthi et al. 2010]. In a recent study, it was confirmed that the majority of HLA-DRB1 alleles (including HLA-DRB1*1501) express the VDRE, but an independent contribution of VDRE motif variation to an increased MS risk was not discernible [Nolan et al. 2012]; however, HLA-DRB1*04, *07 and *09 alleles, which express the ‘nonresponsive’ VDRE motif, were associated with a significantly reduced risk of MS. It has also been suggested that VDR variants could modulate the risk of MS conferred by HLA-DRB1*1501 [Agliardi et al. 2011; Huang and Xie, 2012] and, in a cohort of Sardinian patients with MS (i.e. a population with a specific high genetic risk for MS), VDREs do not seem to play a significant role in the promoter region of DRB1 in susceptibility to MS [Cocco et al. 2012]. Accordingly, even if there is no doubt that some HLA alleles play a crucial role in MS risk or protection, further studies are required to understand better the different HLA-VDRE mechanisms involved in this disease.
Other genes
The study by Sawcer and colleagues has shown the involvement in MS risk of two genes involved in vitamin D metabolism: CYP27B1, controlling 1-α-hydroxylase and therefore calcitriol synthesis, and CYP24A1, controlling calcitriol catabolism [Sawcer et al. 2011]. The genetic involvement of CYP27B1 in MS risk was also found in another GWAS considering a specific pathway in which eight genes within a module of 13 genes influenced by vitamin D were associated with MS [ANZgene and the Australia and New Zealand Multiple Sclerosis Genetics Consortium, 2009] and is now confirmed thanks to several other types of genetic research methods used to study patients with MS with vitamin D-dependent rickets [Torkildsen et al. 2008], with rare variants [Ramagopalan et al. 2011a] or with single nucleotide polymorphisms [Sundqvist et al. 2010; Simon et al. 2011]. However, it should be mentioned that no association was found between genes involved in vitamin D metabolism and MS risk in two other studies [Orton et al. 2011a; Smolders et al. 2011c], but these studies were performed with a much smaller sample than the one used by Sawcer and colleagues [Sawcer et al. 2011]. Furthermore, the genes encoding CYP27B1 and CYP24A1 could be epigenetically regulated [Kim et al. 2009; Novakovic et al. 2009], which may influence the vitamin D serum level and MS risk [Burrell et al. 2011]. Moreover, interactions between CYP27B1 and HLA-DRB1*15 may exist and influence the MS risk [Simon et al. 2011] (see below). Polymorphisms in the VDR gene such as the TaqI variant could be weakly linked to MS [Cox et al. 2012], but some other variants and single-nucleotide polymorphisms, located for example near DHCR7 [Alloza et al. 2012], may be involved in vitamin D insufficiency and the MS risk, a point that does, however, require confirmation. Recent findings have also provided new insights into how vitamin D influences the genetic regulation of B cells in MS (for a review, see Disanto and colleagues) [Disanto et al. 2012c]. Lastly, a genetic regulation of DBP may also influence the MS risk [Disanto et al. 2011c]. Accordingly, these different genetic links reported between vitamin D and MS, in particular those related to CYP27B1, strongly suggest that vitamin D insufficiency is involved in the pathogenesis of this disease. However, in a few of the aforementioned studies, some results are not concordant, which may simply result from methodological questions and require further studies. Furthermore, the exact molecular mechanisms as to how VDREs exert control over numerous genes and cells are not yet understood and are currently being studied [Berlanga-Taylor et al. 2011 Disanto et al. 2012e].
Environmental risk factors for multiple sclerosis
Besides genetic risk factors, three main environmental risk factors for MS have been identified: past infection with Epstein-Barr virus (EBV), vitamin D insufficiency and smoking [Ascherio and Munger, 2007a, 2007b; Ascherio et al. 2012a].
Epstein–Barr virus infection
Almost all, if not all, patients with MS have previously been infected by EBV [Wagner et al. 2000; Wandinger et al. 2000; Ascherio and Munger, 2007a; Pakpoor et al. 2012]. Following primary infection, EBV remains latent in the memory B-cell population for life and EBV antibody titres are increased with potentially deleterious immunologic effects, for example by favouring certain autoimmune diseases. EBV infection is ubiquitous and 95% of the general population has been infected by this virus, but MS is extremely rare in EBV-negative adult individuals [Ascherio and Munger, 2007a; Levin et al. 2010]. Like MS, but independently of this disease, EBV infection is also positively correlated with latitude [Disanto et al. 2012d]. Individuals with a history of late infectious mononucleosis (after childhood) have a twofold to threefold increased risk of developing MS [Thacker et al. 2006; Nielsen et al. 2007b; Ramagopalan et al. 2009c; Handel et al. 2010b]. Furthermore, plasma antibody titres against the EBV nuclear antigen 1 (EBNA1) increase several years before the clinical onset of MS [Sundström et al. 2004; Levin et al. 2005; Lünemann et al. 2010; Sundqvist et al. 2012a], EBNA1 levels are correlated with MS risk [Lucas et al. 2011a; Munger et al. 2011b; Simon et al. 2012b] and antibody response to EBV antigens is generally higher in MS [Lindsey et al. 2012]. It should particularly be noted that MS risk appears to be 30-fold higher in subjects with the highest anti-EBNA1 level (> 300) [Munger et al. 2011b; Ascherio et al. 2012a]. Therefore, there no longer appears to be any doubt that EBV infection contributes to the pathogenesis of MS, but the exact mechanisms that lead to the disease have yet to be determined [Tselis, 2012]. EBV was found in 90% of meningeal B-cell follicles and in perivascular cuffs of patients with MS in one study [Serafini et al. 2007] but was either not found or not frequently observed in a number of subsequent studies [Willis et al. 2009; Aloisi et al. 2010; Lassmann et al. 2010; Peferoen et al. 2010; Sargsyan et al. 2010; Torskilden et al. 2010; Owens and Bennett, 2012; Tracy et al. 2012]. Nevertheless, latent EBV infection, with residual viral particles possibly remaining chronically in B lymphocytes, may contribute to the inflammatory milieu in active MS lesions by activating an innate and adaptive immune response, including B-cell activation and proinflammatory IFNα production [Serafini et al. 2010; Tzartos et al. 2012; Ascherio et al. 2012a]. As an alternative, or in addition to this still uncertain direct effect of chronic or latent EBV infection, it may be that an altered long-lasting immune response following primary EBV infection contributes to trigger or perpetuate demyelinating disease [Niller et al. 2008; Mameli et al. 2012; Perron et al. 2012]. Links with MS risks appear to exist between EBV immunological response and HLA-DRB1*1501 [De Jager et al. 2008; Sundström et al. 2009; Lucas et al. 2011a; Sundqvist et al. 2012a], HLA-B*0702 [Jilek et al. 2012], vitamin D insufficiency [Hayes and Donald Acheson, 2008; Holmøy, 2008; Lossius et al. 2011] and smoking [Simon et al. 2010]. However, the latter link was not found in another study [Sundqvist et al. 2012b] and the exact deleterious mechanisms involved in these diverse cumulative risk factors remain currently unclear and require further studies. From a practical point of view, even if a vaccine against EBV is developed [Sokal et al. 2007], its efficacy in preventing autoimmune diseases (including MS) or even its total inocuity could be difficult to evaluate. By contrast, given the extreme rarity of MS in EBV-seronegative patients, the serological test may be useful in certain difficult diagnostic circumstances since a negative test result, at least in adults, or low anti-EBNA titres could constitute an argument against MS [Lünemann et al. 2010]. Accordingly, even if the simple encounter with EBV remains in the great majority of cases a banal infection without apparent deleterious long-term consequences, it could also, in some cases, be the first event triggering a long-lasting deleterious immunological cascade, worsened both by a late primo infection occurrence with symptomatic infectious mononucleosis and by the persistence of a high anti-EBNA1 level, eventually leading to the disease onset. These worsening infectious circumstances for the MS risk might also be cumulated with other risk factors (e.g. HLA-DRB1*1501, vitamin D insufficiency, smoking), which could increase even more the disease risk, in particular if the protective counterparts (e.g. HLA-A*0203 and normal vitamin D status) are absent (see below).
Vitamin D insufficiency
Although only a few epidemiological studies have so far directly implicated vitamin D status as an influencing factor in MS risk, multiple different environmental findings indirectly relating to vitamin D suggest that this vitamin plays an important role. It has long been known that latitude influences MS risk, the prevalence of the disease being minimal at the equator and increasing with either North or South latitude. This effect is observed on a world scale [Gale and Martyn, 1995; Alonso and Hernan, 2008; Simpson et al. 2011; Sloka et al. 2011a], at a continental level [Kurtzke, 1995; Puggliatti et al., 2006], in large countries, such as the United States [Acheson et al. 1960; Kurtzke et al. 1985, Kurtzke, 2008], the former Soviet Union [Boiko et al. 1995] and Australia [van der Mei et al. 2001; Taylor et al. 2010] and even in comparatively smaller countries, such as New Zealand [Taylor et al. 2008] and France, at least in farmers [Vukusic et al. 2007]. MS prevalence may change after migrations occurred during the second decade of life, with, for example, a beneficial effect for people who have migrated from a high-latitude region (with a high MS prevalence) to a sunnier, lower-latitude region (with a low MS prevalence) [Kurtzke et al. 1985; Gale and Martyn, 1995; Hammond et al. 2000; Ascherio and Munger, 2007a, 2007b; Handel et al. 2010a; McDowell et al. 2010; McLeod et al. 2011]. It should be noted that a reverse migration, that is, from a low-latitude region to a higher-latitude region, has less effect on MS prevalence, as though the environmental protection acquired in infancy and childhood, maybe related to the sunny climate of these regions (see below), is long lasting at adult age for these migrants. By contrast, no such protection seems to exist for their children, who have an MS risk similar to that of the natives of the high-latitude regions [Elian et al. 1990; Dean and Elian, 1997], which constitutes a further argument suggesting that this part of the risk is indeed environmental and not genetic.
However, MS prevalence does not depend upon latitude per se but upon more specific elements linked to latitude. Among these elements, climate and sun exposure likely play a crucial role. Indeed, there were very strong correlations between MS prevalence in the different states of the United States or in nine large-scale areas of North America and the corresponding mean annual amounts of UV in these areas [Beretich and Beretich, 2009]. In a meta-analysis performed on 52 studies from various countries around the world, a highly significant link (p < 10-8) existed between MS prevalence and the annual amount of UVB in the different countries, this link being 20 times more significant than that existing between MS prevalence and simple latitude [Sloka et al. 2011a]. In France, sunshine maps show large climate areas analogous to those of the main zones of MS prevalence identified in farmers by Vukusic and colleagues [Vukusic et al. 2007; Ebers, 2008, 2009; Handel et al. 2010a]. Furthermore, in two successive independent studies, a strong correlation was observed between the regional MS prevalence in French farmers and the annual UV radiation of the 22 French regions [Pierrot-Deseilligny and Souberbielle, 2010; Orton et al. 2011b], with a much higher significance for the link between UV radiation and MS prevalence (p = 4 × 10–6) than for that between MS prevalence and the latitude of the regions (p = 4 × 10–4) [Pierrot-Deseilligny and Souberbielle, 2010], and more marked results in women than in men [Orton et al. 2011b]. Similar links have also been reported between MS prevalence and UV radiation in Canada [Acheson et al. 1960], the United States [Freedman et al. 2000] and in England and Wales [Ramagopalan et al. 2011b]. Furthermore, at identical latitudes, the risk of MS is lower in the sunniest regions [van Amerogen et al. 2004; van der Mei et al. 2007a, 2007b], in particular in high-altitude regions compared with lowland regions [Kurtzke, 1967]. Accordingly, there are no longer any doubts that UV radiation influences MS risk. However, it may be that a part of the protection provided by UV radiation results from its hypothetical direct immunosuppressive effect, that is, bypassing vitamin D synthesis by UVB and the immunomodulatory action of this vitamin [Hart et al. 2011], as mentioned above in the section on EAE. Nevertheless, it remains that vitamin D is also directly involved in MS, independently of UV radiation, as suggested by a number of experimental, epidemiological, genetic, immunological and clinical studies reviewed in the different chapters of this review. In fact, these two mechanisms involved in the MS risk, that is, a possible direct immunosuppressive action of UVB, not involving vitamin D, and the immunomodulatory effect of vitamin D, mainly synthesized thanks to UVB, may play parallel, independent roles, as suggested in a few recent studies [Lucas et al. 2011b; Bäärnhielm et al. 2012].
The timing for MS protection by UVB and vitamin D mechanisms is also a key point. Multiple types of studies suggest that protection/risk from these environmental factors may occur at different epochs during the first part of life until early adulthood (Figure 4). For the period of adulthood, we have already mentioned that living in a sunny country or migrating to such a country after the age of 15–20 years are favourable factors for avoiding MS, but other, nonclimatic factors may be involved in this protection. In some studies, oral intake of vitamin D in the form of diverse vitamin supplements [Munger et al. 2004] or oily fish [Kampmann et al. 2007; Kampmann and Brustad, 2008] was found to be linked with a lower MS risk, but other associated protective factors cannot be ruled out in these studies. Of greater significance, since it was based on the serum level of vitamin D itself, was a study performed in young American soldiers who had given at least two serum samples a few years before the onset of any neurological symptoms during their military service [Munger et al. 2006]. The group of white soldiers with levels of vitamin D in the highest quintile (i.e. between 99 and 152 nmol/liter) had a significantly lower risk of MS than those with the lowest levels of vitamin D (i.e. between 15 and 63 nmol/liter) (p < 0.01). Furthermore, in a recent Swedish nested case–control study, an association was found between relatively high 25-OH-D serum levels (≥75 nmol/liter) during the years preceding disease onset and a decreased risk of MS [Salzer et al. 2012a].
The protection afforded by UV radiation/vitamin D may also be acquired before adulthood, that is, during childhood and adolescence. The studies analysing these points are based on questionnaires assessing the amount of time spent outdoors during holidays and weekends during the first two decades of life in patients with MS and control subjects. The risk of MS was significantly lower in subjects who spent the most time outdoors during their youth [Acheson et al. 1960; van der Mei et al. 2003; Kampman et al. 2007; Dwyer et al. 2008; Sloka et al. 2008], including within pairs of monozygotic twins [Islam et al. 2007]. These results are also supported by studies of skin actinic activity, measured on the back of the hand and reflecting total accumulated exposure to sun; the subjects who had the highest level of actinic activity also had the lowest MS risk [van der Mei et al. 2003; Lucas et al. 2011b]. Furthermore, in patients with MS who resided in low-to-medium solar radiation areas, low sun exposure in the autumn/winter during the ages of 6–15 years was significantly associated with a 2.1 year earlier symptom onset [McDowell et al. 2011]. Therefore, relatively frequent outdoor activities in childhood and adolescence, resulting in significant sun exposure during these epochs and consequently the likelihood of more vitamin D synthesis than with a lifestyle consisting of almost exclusively indoor activities, could be protective in terms of MS risk. However, in a study evaluating vitamin D intake during adolescence, no association was found with MS risk [Munger et al. 2011a]. By contrast, childhood or adolescence obesity, which is a cause of vitamin D insufficiency, increases the MS risk [Munger et al. 2009; Hedström et al. 2012]. It may be that the relatively long-lasting protection from MS acquired before adolescence by sun exposure or vitamin D status corresponds to a critical step of maturation of the thymus and the immune system [Tulic et al. 2012], which could be favourably influenced by these environmental factors during the first part of life. To be more precise about this hypothetical mechanism, it has been suggested that vitamin D insufficiency in utero and during childhood may affect expression of HLA-DRB1 in the thymus, allowing autoreactive T cells to escape thymic deletion [Ramagopalan et al. 2009a].
The month of birth and the vitamin D status of the mother during pregnancy may also have an impact on the MS risk for offspring when they reach adulthood. The risk of MS is lower for people born in autumn (especially in November) and higher for those born in spring (especially in May) in the northern hemisphere [Templer et al. 1992; Willer et al. 2005; Sotgiu et al. 2006; Fernandes de Abreu et al. 2009; Ramagopalan et al. 2009a; Bayes et al. 2009; Disanto et al. 2012a], with a reverse pattern in the southern hemisphere [Staples et al. 2010]; see also a recent meta-analysis confirming these findings [Dobson et al. 2012]. This seasonal effect is correlated with the presence of a familial risk factor for MS [Sotgiu et al. 2006] or with the phenotype HLA-DRB1 in Canada [Ramagopalan et al. 2009a] but not in Finland [Saastoimanen et al. 2012]. These results may be related to the vitamin D status of pregnant women [Willer et al. 2005; Salzer et al. 2010], since 25-OH-D serum levels are at their highest in autumn and their lowest in spring in the general population as well as in pregnant women [Hypponen and Power, 2007; Holmes et al. 2009; Handel et al. 2010a; Lewis et al. 2010]. In line with this hypothesis, it may be consistent that in sunnier countries, that is, with less contrast between the seasons, no seasonal difference in the month of birth has been observed for MS risk [Givon et al. 2012; Fragoso et al. 2012]. Furthermore, in a cohort of 927 US army veterans with MS, those who were born in winter and whose birthplace was in low solar radiation areas had disease symptom onset on average 2.8 years earlier than those born in seasons other than winter and in medium- and high-solar radiation areas [McDowell et al. 2010]. The influence of month of birth could exist for different immune-related diseases, including MS [Disanto et al. 2012a]. Furthermore, the predicted 25-OH-D level in pregnant mothers was inversely associated with the risk of MS in their daughters [Mirzaei et al. 2011]. It has recently been suggested [Disanto et al. 2012b], based on experimental findings [Harvey et al. 2010; Yu and Cantorna, 2011], that the influence on MS risk of month of birth and vitamin D status during pregnancy may be related to a critical step of development of the immune system in utero requiring vitamin D, and that an insufficiency of vitamin D at this crucial time could result in a state that cannot be corrected later, after birth, by vitamin D intakes. Accordingly, besides the positive influences on the MS risk of outdoor activities in childhood and adolescence and the beneficial role of a normal vitamin D status in early adulthood, both the vitamin D status in the pregnant mother and the month of birth, which is likely related to this status or sun exposure of the mother during the last months of pregnancy, may also influence the MS risk for a long-lasting period.
Cigarette smoking
The role of smoking in MS has recently been reviewed [Wingerchuk, 2012] and this could be both a risk factor for MS and a deleterious element for the progression of the disease. In three large American and European cohort studies, it has been shown that cigarette smoking is a significant risk factor for MS, that this risk is dose dependent and perhaps due to cigarette smoking rather than nicotine [Hernan et al. 2001; Hedström et al. 2009; Carlens et al. 2010]. In smaller, less powered and, therefore, less important studies, a significant link also existed [Riise et al. 2003; Pekmezovic et al. 2006; Sundström et al. 2008] or was not found [Silva et al. 2009; Simon et al. 2010] between smoking and MS risk, but in a meta-analysis of 14 studies, smoking was indeed a significant risk for MS [Handel et al. 2011]. Passive smoking also constituted a risk factor for MS in a paediatric cohort for children exposed to parental smoking [Mikaeloff et al. 2007]. Furthermore, in a large Swedish cohort of adult patients with MS, the MS risk was increased among never smokers who had been exposed to passive smoking compared with never smokers who had never been exposed [Hedström et al. 2011a]. By contrast, no risk factor for MS was found for offspring of mothers who had smoked during their pregnancy [Montgomery et al. 2008]. As already emphasized in the preceding chapters, risk factors for MS are multiple and smoking may, therefore, interact with genetics and the main two other identified environmental risk factors. Smoking is associated with higher levels of EBNA1 [Nielsen et al. 2007a] and enhances the association between high EBNA1 titres and increased MS risk [Simon et al. 2010]. An interaction with HLA-DRB1*15 was also observed in smoker but not in nonsmoker patients with MS [Hedström et al. 2011b]. Smoking could also be a deleterious factor for the course of MS. Smoking significantly speeds conversion from CIS to confirmed MS [Di Pauli et al. 2008] and usually from RRMS to SPMS, also increasing the rate of accumulation of disability in established progressive forms of MS [Hernan et al. 2005; Sundström and Nyström, 2008; Healy et al. 2009; Pittas et al. 2009]. However, there was an exception to such worsening effects in one study [Koch et al. 2007] and only a trend for smoking to increase the risk of SPMS in a meta-analysis [Handel et al. 2011]. Accordingly, the findings already available strongly suggest that smoking increases the risk for MS and, in the course of the disease, is deleterious for disability, but further studies are required to confirm these points. A possible potentiation of MS risk by the association of smoking and vitamin D insufficiency also remains to be specifically studied.
Interactions between risk factors for multiple sclerosis
To summarize the previous sections, both genetic and environmental risk factors influence the MS risk; three main environmental factors – past EBV infection, vitamin D insufficiency and cigarette smoking – likely influence this risk; and the timing of significant interactions between genetics and these environmental risk factors appears to be variable throughout the first part of life, that is, from the mother’s pregnancy until the start of the disease in adolescence or adulthood [Handel et al. 2010a; Disanto et al. 2012b] (Figure 4). For the role of vitamin D insufficiency and lack of sun exposure, we have seen that the vitamin D status of a person’s mother during pregnancy, the month of birth, sun exposure during childhood and sun exposure and vitamin D status in early adulthood may influence the MS risk. Past infection with EBV could be crucial (compared with absence of EBV infection) for the MS risk, but the time of infection also appears to influence this risk, with a higher risk in the case of late infections (during adolescence and early adulthood). Lastly, passive smoking, during childhood, and active smoking during adolescence and in adulthood also determine two different exposure periods for the MS risk. Therefore, these three environmental risk factors may increase genetic susceptibility to MS at different epochs of life. These considerations lead to the concept that modulation of MS risk could be continuous from the mother’s pregnancy until adulthood, between genetic and environmental risk factors, and in genetics as well as in the environment, between deleterious and protective factors. Concerning the protective factors, we know that, in genetics, some HLA-DRB1 alleles are protective (see above) and that, in the environment, a normal vitamin D status is likely protective (versus vitamin D insufficiency) and a seronegative status for EBV could also be somewhat protective (versus a seropositive status, in particular, with high anti-EBV antibody titres). The mechanisms by which these multiple genetic and environmental risk interactions are exerted are starting to become clearer. In previous generations, as suggested by epigenetics, protective or deleterious environmental conditions may change the expression of important alleles for MS risk in future generations [Burell et al. 2011], for example, HLA-DRB1*1501 as a deleterious factor and HLA-A*0201 as a protective factor (Figure 4). Furthermore, it may be that the three currently known environmental risk factors for MS epigenetically influence the disease risk [Koch et al. 2012]. After conception, genetic susceptibility could be influenced, negatively or positively, by the existence or not of an infection with EBV, the vitamin D status, cigarette smoking and other, as yet undisclosed, environmental factors. All currently suspected risk factors, including genetic and environmental factors, share influences in the immune system, with possibly critical interventions at certain key periods of the maturation of this system, in particular in utero and before adolescence (see above). Theoretically, the combination of unfavourable genetics, more frequently encountered in siblings of patients with MS, past EBV infection, especially if it occurred late and with high residual anti-EBV antibody titres, chronic, long-lasting vitamin D insufficiency, in particular during the major part of infancy, childhood and early adulthood, and cigarette smoking, particularly in a case of a high level of tobacco intoxication, could maximize the MS risk, whereas the conjunction of converse conditions could confer maximal protection from this disease (Figure 4). Interestingly, it has recently been hypothesized and calculated that the cumulative effect of all currently known risk factors for MS could theoretically increase more than 400-fold the risk for this disease [Ascherio et al. 2012a]. In favour of the potential synergetic effects of the already identified multiple risk factors for MS, it should be noted that the risk for this disease is significantly increased in the case of the conjunction of HLA-DRB1*15 and either high anti-EBV antibody titres or clinically symptomatic infectious mononucleosis [De Jager et al. 2008; Nielsen et al. 2009; Sundström et al. 2009; Lucas et al. 2011a; Sundqvist et al. 2012a]; the existence of both HLA-DRB1*15 and cigarette smoking [Hedström et al. 2011b]; the addition of these three preceding risk factors [Simon et al. 2010]; with a probable regulation of the expression of HLA-DRB1*15 by vitamin D [Niino et al. 2000; Ramagopalan et al. 2009b]; and with a possible potentiation of the effects of EBV infection and vitamin D insufficiency in MS risk [Hayes and Donald Acheson, 2008; Holmøy, 2008; Disanto et al. 2011b, 2012b; Décard et al. 2012; Salzer et al. 2012b]. Interestingly, in a small cohort of patients with CIS (n = 25), who had donated their blood a few years prior to their first clinical event, vitamin D was insufficient (47 nmol/liter on average) and anti-EBNA high (186 IU/ml on average) [Décard et al. 2012]. Finally, vitamin D insufficiency and EBV infection may theoretically interact in different ways and at different periods: at the time of primo infection with EBV, the anti-infectious effect of vitamin D could be involved through its action on macrophages and an insufficiency in this vitamin could both facilitate this infection and increase its severity with deleterious consequenses years later; in the latent period between the EBV primo infection and MS triggering, the vitamin D status may influence favourably or unfavourably the long-lasting immunological cascade by the immunomodulatory action of this vitamin through peripheral T and B cells; and lastly, vitamin D could also influence the inflammation process within the CNS, in which this vitamin is present and may exert different effects, for example, anti-infectious on possible permanent residual viral particles, immunomodulatory on invading immune cells and neuroprotective on nerve cells. No definite conclusions can be drawn from the available but limited studies or simple hypotheses and only very large prospective randomized studies in normal populations lasting a few decades and taking into account all these potential risk factors could confirm their involvement in MS risk and their possible synergetic effects, but such studies will likely not be undertaken for practical and financial reasons. In the absence of such studies, but given the number of epidemiological findings already available and the fact that genetic and infectious risk factors are currently for the most part beyond our control, a reasonable, preventive approach to minimize MS risk would consist of improving the vitamin D status, if insufficient, at every epoch of life and avoiding cigarette smoking [Ascherio et al. 2012a].
Figure 4.
Modulation of multiple sclerosis risk from conception to the time of disease triggering.
Note that (a) risk factors for MS are multiple, genetic and environmental (lower part of the figure), (b) opposite conditions or other factors may be protective from MS occurrence (upper part of the figure), (c) interactions are numerous between all these risk and protective factors and (d) may occur throughout the first part of life, from conception until MS triggering. Note also that the period from conception to adolescence is crucial for the maturation of the immune system and thymus and could be particularly important for the interactions of the different protective and risk factors. In these successive events or situations, the likely risk factors are (see text): (1) unfavourable genetics, (1’) including HLA-DRB1*1501; (2) EBV infection, which may be a crucial event for subsequent MS (years later), with particularly an increase in MS risk if (2’) the primo-infection occurs late and (2”) is followed by a high anti-EBNA1 level; (3) vitamin D insufficiency, also increasing MS risk, (3’) including conditions likely related to this insufficiency or to insufficient exposure to sun; and (4) smoking, also contributing to this risk, even if (4’) it is only passive in childhood (however, with only one study having been reported so far). Reverse or other conditions could be protective: (1p) favourable genetics, (1p’) including HLA-A*0201; (2p) absence of EBV infection; (3p) vitamin D sufficiency, (3p’) including conditions likely related to a normal vitamin D status or sufficient exposure to sun; and (4p) numerous infections during childhood (hygienic hypothesis), possibly protective from subsequent auto-immune diseases. EBV, Epstein–Barr virus; HLA, human leukocyte antigen system; MS, multiple sclerosis.
Vitamin D status may influence the course of multiple sclerosis
This last section will be briefer since, in the clinical field of vitamin D in MS, once the disease has started, studies have so far been limited to observational, uncontrolled studies or insufficiently powered phase I/II trials using vitamin D supplementation. Several relatively large RCTs (phases II/III) have begun in Europe (including in France, i.e. the ‘CHOLINE’ study) and the United States [Munger and Ascherio, 2011; Smolders et al. 2011a; Dorr et al. 2012], but their first results will not been known for another 1–2 years.
Relapses
Since vitamin D has general immunomodulatory and anti-inflammatory actions, and furthermore, since some already documented immunological effects of this vitamin have been reported in patients with MS, most of whom are in a state of vitamin D insufficiency (see the first section), a potential influence of vitamin D status may be expected on the inflammatory component of MS, in particular on relapses during the initial stage of the disease. Vitamin D supplementation was associated with a decrease of about 50% in the number of relapses in a pioneering uncontrolled small study using 5000 IU/day of vitamin D for 2 years in 10 patients with RRMS [Goldberg et al. 1986]. There was also a decrease in relapses, albeit nonsignificant (–41% in the vitamin D arm versus –17% in controls), in a more recent controlled study using high doses of vitamin D (14,000 IU/day on average) for 1 year in patients with RRMS, with 25 treated versus 25 control patients [Burton et al. 2010], which is too small a sample and too short a follow up to draw definite conclusions about clinical outcomes. Furthermore, the very high vitamin D doses used in this study eventually resulted in high, supraphysiological vitamin D serum levels (i.e. close to 400 nmol/liter), which were well tolerated but may not be required to obtain a vitamin D effect (see below).
A few other small controlled studies using vitamin D supplementation in patients with RRMS have recently been reported and have found no effect on relapses. However, none of these studies were designed and adequately powered to address clinical outcomes, and in most of them, important methodological concerns existed. In the study by Stein and colleagues, only 23 patients with RRMS were randomized (i.e. into two groups of 11 and 12 patients, respectively) and were followed for only 6 months, with a vitamin D2 dose of 1000 IU/day (which already represents a notable supplementation dose) in one group and 7000 IU/day (i.e. a very high dose) in the other group [Stein et al. 2011]. There was no difference in terms of relapses or of magnetic resonance imaging (MRI) variables between the two groups, as could be expected with such a study design, but these two vitamin D doses were well tolerated for a 6-month period. In the study by Kampman and colleagues, 35 patients with RRMS received a vitamin D3 dose of 20,000 IU weekly and 33 other patients had a placebo, with a follow up of 96 weeks [Kampman et al. 2012]. No effect was observed on relapses or on other clinical or MRI variables. However, since the annualized relapse rate was 0.1 on average at baseline in both groups (which means one relapse every 10 years on average before the treatment intervention), no significant beneficial effect (of any substance) could be expected in such a benign form of the disease. Nevertheless the study showed that the relatively moderate vitamin D dose used was well tolerated for almost 2 years. In the study by Shaygannejad and colleagues, 50 patients with RRMS were randomized (into two groups of 25 patients), the first group receiving a daily dose of 0.5μg of calcitriol and the second group a placebo, with a follow up of 12 months [Shaygannejad et al. 2012]. No clinical effect was observed in the treated group, but it should be noted that all patients already had 25-OH-D serum levels higher than 100 nmol/liter at baseline, that is, were not in vitamin D insufficiency. Thus, in such a context of vitamin D sufficiency, the addition of moderate doses of calcitriol did not provide any apparent clinical beneficial effect. Lastly, in the study by Soilu-Hänninen and colleagues, 66 patients with RRMS under IFNβ-1b therapy were randomized to receive a vitamin D dose of 20,000 IU weekly or a placebo and were followed for 12 months [Soilu-Hänninen et al. 2012]. The primary outcomes of this study were MRI variables (see below), but no significant beneficial clinical effect was observed. Once again, this study was not adequately powered for analysing clinical outcomes and the follow up was also too short.
In contrast to these inconclusive small controlled studies, several recent association studies found a significant relationship between the vitamin D status and the relapse rate in patients with RRMS [Smolders et al. 2008b; Runia et al. 2012], with possible variable positive or negative interactions with IFNβ depending upon the vitamin D serum level, higher or lower than 50 nmol/liter respectively [Stewart et al. 2012]. In another recent association study, preliminary results provided evidence that low serum 25-OH-D levels are an important risk factor for conversion from CIS to MS, suggesting that vitamin D supplementation in combination with IFNβ-1b may improve outcomes in CIS [Ascherio et al. 2012b]. Interestingly, three other association studies, one in a paediatric cohort of patients with CIS or RRMS at the very beginning of the disease [Mowry et al. 2010] and the other two in adult cohorts of patients with RRMS [Simpson et al. 2010; Pierrot-Deseilligny et al. 2012], were comparable for sample size and follow up (Table 2). Furthermore, similar statistical models were used in these studies and predicted an analogous quantitative vitamin D effect on the relapse rate since an increase of 50 nmol/liter in the 25-OH-D serum level was associated with a marked decrease in the relapse rate (−50% to −68%), independently of the use of vitamin D supplementation or an association with a first-line immunomodulatory therapy (IMT) (Table 2). These quantitative predictions made by statistical models on the vitamin D effect may appear high, with a therapeutic action potentially similar to that of the best active treatments used in MS. However, such predictions are global and there are, of course, limits in the possibilities for a decrease in relapses (i.e. necessarily between 0% and 100%). It should also be noted that most of the patients in these three studies had relatively low vitamin D serum levels, close to 50 nmol/liter on average, including in our patients before supplementation [Pierrot-Deseilligny et al. 2012]. Furthermore, the patients in our study were systematically supplemented with moderate doses of vitamin D (3010 IU/day) for 2.5 years on average and their serum level passed from 49 ± 22 to 110 ± 26 nmol/liter with this supplementation. This resulted in a relatively wide range of vitamin D serum levels (before and under supplementation) and led us to observe a plateau effect of vitamin D action on the relapse rate beyond 110 nmol/liter (Figure 5), which might thus indicate an upper limit for vitamin D efficacy. Therefore, at least in our study, there appeared to be a marked vitamin D effect mainly within a relatively limited range of vitamin D serum levels, between 60 and 110 nmol/liter (Figure 5). It should be noted that this range extends from a lower limit approximately equivalent to the spontaneous level found in most patients in temperate countries to an upper limit reached by most of them if supplementation uses physiological doses of vitamin D, thus providing an almost maximal advantage of the potential vitamin D effect. Reverse causality, that is, the disease itself worsening the vitamin D insufficiency by limiting sunshine exposure, has been invoked to try to account for the correlations found between the spontaneous vitamin D serum level and the relapse rate of patients with RRMS in association studies. However, such an argument becomes very unlikely when patients are at the very beginning of the disease [Mowry et al. 2010] or are supplemented with vitamin D [Pierrot-Deseilligny et al. 2012]. Altogether, even if no definite conclusion can be drawn from simple association or observational studies, the fact that a similar marked vitamin D effect on the relapse rate has been found in three different cohorts of patients with RRMS of all ages, with or without associated IMT and with or without vitamin D supplementation, already strongly suggests that the vitamin D status significantly influence relapses. Of course, RCTs have to confirm this effect and accurately calibrate it.
Table 2.
Association studies analysing the 25-OH-D serum level and the relapse rate in patients with relapsing–remitting multiple sclerosis.
| Reference | Cohort rovenance | Age (years) mean ± SD | Sample size | Mean duration of follow up (years) | Association with IMT for most patients | Vitamin D supplementation for most patients | Relapse rate with an increase of 50 nmol/liter in 25-OH-D serum level |
|---|---|---|---|---|---|---|---|
| Mowry et al. [2010] | USA (San Francisco and New York) | 15 ± 3 | n = 110 | 1.7 | No | No | −68% |
| Simpson et al. [2010] | Australia (Tasmania) | 44 ± 10 | n = 145 | 3 | Yes | No | −50% |
| Pierrot-Deseilligny et al. [2012] | France (Paris region) | 39 ± 10 | n = 156 | 2.5 | Yes | Yes | −68% |
IMT, first-line immunomodulatory therapy; SD, standard deviation.
Figure 5.
Example of evolution of relapse incidence rate ratio according to the 25-OH-D serum level in patients with MS.
This study was performed before and under vitamin D supplementation (3010 IU/day on average) in a cohort of 156 consecutive patients with relapsing–remitting MS under first-line immunomodulatory therapy and followed for 2.5 years on average (for more details, see Pierrot-Deseilligny and colleagues [Pierrot-Deseilligny et al. 2012]. Note that in a multivariate model adjusted for the patient’s age, disease duration and previous use of an IMT prior to inclusion, a 13.7% decrease in the incidence rate of relapses (95% confidence interval 10.64%–16.64%) was associated with every 10 nmol/liter increase in the 25-OH-D serum level (p < 0.0001). Note also that most patients passed from a stage of vitamin D insufficiency before supplementation [left part of the figure, with for the whole population: mean (black arrow) = 49 nmol/liter; SD = 22] to a stage of relative vitamin D sufficiency under vitamin D supplementation [right part of the figure, with for the whole population: mean (black arrow) = 110 nmol/liter; SD = 26], with a potential vitamin D effect appearing particularly marked on relapses between 60 and 110 nmol/liter (green arrow). Furthermore, a plateau effect (orange arrow) was observed beyond 110 nmol/liter, suggesting that higher 25-OH-D serum concentrations are not required to obtain an optimal beneficial effect on the relapse rate. In black, whole population (n = 156); in blue, group 1 (n = 76, with IMT started prior to vitamin D supplementation); in red, group 2 (n = 80, with IMT started concomitantly with vitamin D supplementation). Adapted with permission from Pierrot-Deseilligny et al. [2012]. IMT, first-line immunomodulatory therapy; IRR, incidence rate ratio; SD, standard deviation.
Other variables
The 25-OH-D serum level has also been inversely associated with the degree of disability in patients in various stages of MS [Van der Mei et al. 2007a; Kragt et al. 2009; Smolders et al. 2008b]. However, reverse causality (see above) cannot be ruled out for this clinical variable, which concerns patients with a more advanced disease and a permanent disability, consequently variably limiting their outdoor activities. Besides the main clinical variables, MRI findings are also important for evaluating the efficacy of a treatment or the association with a clinical variable. In a controlled therapeutic study comprising 66 patients with MS, 32 of whom were receiving placebo and 34 a weekly dose of 20,000 IU vitamin D for 1 year, there were fewer new T2 lesions (not significant) but a significantly lower number of T1 enhancing lesions in the treated patients [Soilu-Hänninen et al. 2012]. More importantly, in a 5-year longitudinal MS cohort study (n = 469) using multivariate analyses, each 10 ng/ml (25 nmol/liter) higher 25-OH-D serum level was associated with a 15% lower risk of a new T2 lesion and a 32% lower risk of a gadolinium-enhancing lesion, with an effect on the relapse rate (not significant) and a significant action on disability [Mowry et al. 2012]. There was also a significant association between the vitamin D serum level and the new gadolinium-enhancing lesions in another study on 88 patients during 6 months before the prescription of an IFNβ, this relation being no longer observed under this treatment for 18 months [Løken-Amsrud et al. 2012]. The absence of a significant effect of vitamin D supplementation on MRI findings in four other controlled studies may be explained, as in the case of clinical variables (see above), by the smallness of the sample size and the shortness of the follow up [Wingerchuk et al. 2005; Mosayebi et al. 2011; Stein et al. 2011], and the unusually benign profile of patients at baseline [Kampman et al. 2012].
Osteoporosis is frequent in patients with MS and of course both age and immobility due to disability contribute to this problem [Gibson and Summers, 2011]. However, it should also be noted that many relatively young ambulatory patients already have both marked osteoporosis and a chronic vitamin D insufficiency [Marrie et al. 2009; Sioka et al. 2009; Steffensen et al. 2010; Moen et al. 2011; Kirbas et al. 2012], which are even almost constant when patients have a marked level of disability [Dabbaghmanesh and Yousefipour, 2011]. However, there was no significant correlation between bone density and 25-OH-D serum level in 119 patients with MS [Triantafyllou et al. 2012] and 99 patients with CIS-MS [Moen et al. 2012], and no effect of vitamin D supplementation for 96 weeks on bone density in 35 patients with MS [Steffensen et al. 2011]. However, such studies were not adequately powered to reach definite conclusions. Lastly, in a small cohort of 59 patients with MS, there was a correlation between the vitamin D status and depressive symptoms but no relation with fatigue [Knippenberg et al. 2010]. These findings on frequent symptoms in MS are interesting but require larger and more powered studies for confirmation. Lastly, it has been reported that vitamin D has general beneficial muscular effects both by a genomic action on muscular fibre generation and a nongenomic action on calcium regulation and conduction at the muscular cell level, resulting in an improvement of muscle strength and walking speed [Ceglia and Harris, 2012]. Therefore, it would also be interesting to test a possible specific muscular vitamin D effect in patients with MS. Accordingly, these different clinical findings, including MRI results, suggest that vitamin D status and supplementation might influence the MS course, in particular at the early, inflammatory stage of the disease. RCTs are warranted for confirmation and accurate quantification of this vitamin D action.
Current practical clinical implications
Thus, there are now innumerable experimental, epidemiological, immunological, genetic and clinical arguments in support of the notion that vitamin D insufficiency is one of the risk factors for MS. Each of these five types of scientific approach has already provided strong suggestions of an involvement of vitamin D in this disease, but the most overwhelming argument in favour of this involvement is the very convergence of the conclusions of these different approaches, even if many questions remain to be studied, including the precise interaction mechanisms of this vitamin with the other genetic and environmental risk factors and an accurate quantification of its effects on MS course. The latest findings reviewed here have practical implications for the current clinical management of MS. Upstream to the disease, some categories of normal subjects (children or young adults) have been identified as having an increased genetic or environmental risk for MS. In the absence of RCTs in the field of MS prevention – an absence that is likely to be permanent – it may be a valuable option to check the vitamin D serum level in siblings of patients with MS, since the MS risk is noticeably increased in these subjects, and provide long-term supplementation for those with vitamin D insufficiency. The aim of this preventive supplementation is to raise a spontaneously low 25-OH-D serum level to the physiological range and then to maintain circulating vitamin D just above the 75–100 nmol/liter zone (see above) indefinitely. The same preventive measures may also be undertaken in subjects who have had infectious mononucleosis and continue to have a high anti-EBNA1 level since they have a theoretical 30-fold increase in relative MS risk (analogous to that of siblings of patients with MS). From a similar preventive perspective, subjects with a radiologically isolated syndrome who are found to be vitamin D insufficient could also be systematically supplemented with ‘physiological’ vitamin D doses, since, as far as we are aware, studies have not yet been undertaken in this context and the results of any future RCTs will not be available for a very long time. Furthermore, these different categories of subjects should also be encouraged not to smoke or to stop smoking.
In patients with MS, some limited RCTs using vitamin D supplementation have begun in different continents, but it will likely take a few more years before the results of several, adequately powered studies become known, a prerequisite for convincing the clinical scientific community of a potential beneficial effect of vitamin D supplementation on the disease course. However, since we already know that most patients with MS are in a state of vitamin D insufficiency, including as early as the earliest stages of the disease, a systematic vitamin D blood titration should be prescribed, and possibly repeated in winter if an initial result is normal in summer. In the case of insufficiency, a vitamin D supplementation should be undertaken in order to raise the vitamin D serum level at least up to the physiological range, that is, just above the 75–100 nmol/liter zone, and to maintain it within this range throughout the year. This point of view, which is shared by several research groups in the world working on vitamin D in MS [Vieth et al. 2007; Correale et al. 2009; Myrh, 2009; Pierrot-Deseilligny, 2009; Ascherio et al. 2012a; Holmøy et al. 2012; van der Mei et al. 2012a], is not a ‘recommendation’ (in the absence of RCT results) but may be considered as a wise provisional option for improving without further delay the general health status of patients with MS. It should be more generally accepted that a simple correction (using moderate vitamin doses) of a documented insufficiency in a ‘vitamin hormone’ which has multiple important extraneurological beneficial effects on health is not a premature intervention, even in patients with MS.
If one has the simple objective to raise the 25-OH-D serum level of most patients with MS currently in vitamin D insufficiency just beyond the 75–100 nmol/liter zone, that is, at the lower end of the physiological range, the following practical indications could be useful. First, concerning the possible risk of hypercalcaemia, calcaemia should be checked together with vitamin D before any vitamin D supplementation, to detect the rare cases of spontaneous hypercalcaemia. Controls of vitamin D and calcaemia titrations could be performed 6 and 12 months after the beginning of supplementation but are not required thereafter if vitamin D remains within the physiological range (75–200 nmo/liter) at two successive titrations. The risk of hypercalcaemia occurring under vitamin D supplementation is almost null if calcaemia is normal before the beginning of supplementation, classical contraindications are respected (granulomatous diseases and association with a few other specific well known medications), native vitamin D3 (cholecalciferol and not calcitriol) is used, and 25-OH-D serum levels are aimed to be within the physiological range (with vitamin D doses less than 10,000 IU/day, in fact generally between 1000 and 4000 IU/day) [Vieth, 2007]. Since calcium, in contrast to vitamin D, is normally delivered in equilibrated diets, it does not appear necessary to add calcium supplementation to vitamin D treatment, except after the age of 60 years, as in normal subjects in this age group. Second, concerning the dose of vitamin D supplementation required to reach a 25-OH-D serum level just beyond the 75–100 nmol/liter zone, a mean daily supplementation of 1500–3000 IU of vitamin D3 appears to be sufficient in most patients (see previous sections on ‘optimal vitamin D serum level’ and ‘vitamin D requirements’). From a neurological point of view, it may not be useful to obtain a vitamin D serum level higher than 110–120 nmol/liter since a plateau effect has been observed beyond this limit in a recent study, at least in terms of relapse prevention [Pierrot-Deseilligny et al. 2012]. These few clinical measures concerning vitamin D supplementation in MS are easy to manage in everyday medical practice, are safe, inexpensive, could already be considered mandatory for improving the general health status of patients and, even if definitive confirmation has not yet been provided, appear increasingly likely to have a beneficial neurological effect on the course of this disease.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Charles Pierrot-Deseilligny, Service de Neurologie 1, Hôpital de la Salpêtrière, Assistance Publique-Hôpitaux de Paris, Université Pierre et Marie Curie (Paris VI), Paris, France.
Jean-Claude Souberbielle, Service d’explorations fonctionnelles, Hôpital Necker-Enfants-Malades, Assistance Publique- Hôpitaux de Paris, Université René Descartes (Paris V), Paris, France.
References
- Acheson E., Bachrach C., Wright F. (1960) Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation and other variables. Acta Psychiatr Scand Suppl 35: 37–42 [DOI] [PubMed] [Google Scholar]
- Adorini L., Penna G. (2008) Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 4: 404–412 [DOI] [PubMed] [Google Scholar]
- Agliardi C., Guerini F., Saresella M., Caputo D., Leone M., Zanzottera M., et al. (2011) Vitamin D receptor (VDR) gene SNPs influence VDR expression and modulate protection from multiple sclerosis in HLA-DRB1*15 positive individuals. Brain Behav Immun 25: 1460–1467 [DOI] [PubMed] [Google Scholar]
- Allen A., Kelly S., Basdeo S., Kinsella K., Muready K., Mills K., et al. (2012) A pilot study of immunological effects of high-dose vitamin D in healthy volunteers. Mult Scler 18: 1797-1800 [DOI] [PubMed] [Google Scholar]
- Alloza I., Otaequi D., de Lapuente A., Antiqüedad A., Varadé G., Núnez C., et al. (2012) ANKRD55 and DHCR7 are novel multiple sclerosis risk loci. Genes Immun 13: 253–257 [DOI] [PubMed] [Google Scholar]
- Aloisi F., Serafini B., Magliozzi R., Howell O., Reynolds R. (2010) Detection of Epstein–Barr virus and B-cell follicles in the multiple sclerosis brain: what you find depends on how and where you look. Brain 133: 1–5 [DOI] [PubMed] [Google Scholar]
- Alonso A., Hernan M. (2008) Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 7: 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANZgene and the Australia and New Zealand Multiple Sclerosis Genetics Consortium (2009) Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 41: 824–828 [DOI] [PubMed] [Google Scholar]
- Armas L., Dowell S., Akhter M., Duthuluru S., Huerter C., Hollis B., et al. (2007) Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin colour. J Am Acad Dermatol 57: 588–593 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K. (2007a) Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 61: 288–299 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K. (2007b) Environmental risk factors for multiple sclerosis. Part II: non-infectious factors. Ann Neurol 61: 504–513 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K., Lünemann J. (2012a) The initiation and prevention of multiple sclerosis. Nat Rev Neurol 8: 602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A., Munger K., Simon K. (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9: 599–612 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K., Simon C., Kappos L., Polman C., Freedman M., et al. (2012b) Serum 25-hydroxyvitamin D concentrations among patients in BENEFIT predicts conversion to multiple sclerosis, MRI lesions, and brain volume loss. Mult Scler 18(Suppl. 4): 374–375 [Google Scholar]
- Axtell R., de Jong B., Boniface K., van der Voort L., Bhat R., De Samo P., et al. (2010) T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat Med 16: 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E., Pavlotski F., Kudish A., Flint P., Solomon A., Lerman Y., et al. (2012) Serum levels of 25-hydroxy-vitamin D3 among sun-protected outdoor workers in Israel. Photochem Photobiol 88: 1507–1512 [DOI] [PubMed] [Google Scholar]
- Bäärnhielm M., Hedström A., Kockum I., Sundqvist E., Gustafsson S., Hillert J., et al. (2012) Sunlight is associated with decreased MS risk: no interaction with HLA-DRB1*15. Eur J Neurol 19: 955–962 [DOI] [PubMed] [Google Scholar]
- Baas D., Prufer K., Ittel M., Kuchler-Boop S., Labourdette G., Sarlieve L., Brachet P. (2000) Rat oligodendrocytes express vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin (D)3. Glia 1: 59–68 [DOI] [PubMed] [Google Scholar]
- Baeke F., Korf H., Overbergh H., van Etten E., Verstuyf A., Gysemans C., Mathieu C. (2010) Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steriod Biochem Mol Biol 121: 221–227 [DOI] [PubMed] [Google Scholar]
- Banwell B., Bar-Or A., Arnold D., Sadovnick D., Narayanan S., McGowan S., et al. (2011) Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 10: 436–445 [DOI] [PubMed] [Google Scholar]
- Barger-Lux M., Heaney R. (2002) Effects of above average summer sun exposure on serum 25-hydroxyvitamin d and calcium absorption. J Clin Endocrinol Metab 87: 4952–4956 [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H., Tabarkiewicz J., Pocisnska K., Stelmasiak Z., Rolinski J. (2010) Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler 16: 1513–1516 [DOI] [PubMed] [Google Scholar]
- Bayes H., Weir C., O’Leary C. (2009) Timing of birth and risk of multiple sclerosis in the Scottish population. Eur Neurol 63: 36–40 [DOI] [PubMed] [Google Scholar]
- Becklund B., Severson K., Vang S., Deluca H.(2010) UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci U S A 107: 6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretich B., Beretich T. (2009) Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler 15: 891–898 [DOI] [PubMed] [Google Scholar]
- Berlanga-Taylor A., Disanto G., Ebers G., Ramagopalan S. (2011) Vitamin D-gene interactions in multiple sclerosis. J Neurol Sci 311: 32–36 [DOI] [PubMed] [Google Scholar]
- Bhalla A., Amento E., Clemens T., Holick M., Krane S. (1983) Specific high-affinity receptors for 1,25-dihydroxivitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab 57: 1308–1310 [DOI] [PubMed] [Google Scholar]
- Binkley N., Krueger D. (2008) Evaluation and correction of low vitamin D status. Curr Osteoporos Rep 6: 95–99 [DOI] [PubMed] [Google Scholar]
- Binkley N., Novotny R., Krueger D., Kawahara T., Daida Y., Lensmeyer G., et al. (2007) Low vitamin D status despite abundant sun exposure. J Endocrinol Metab 92: 2130–2135 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Dawson-Hughes B., Staehelin H., Orav J., Stuck A., Theiler R., et al. (2009a). Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339: b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Dietrich T., Orav E., Dawson-Hughes B. (2004) Positive association between 25-hydroxyvitamin D level and bone mineral density: a population-based study of younger and older adults. Am J Med 116: 634–639 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Giovannucci E., Willett W., Dietrich T., Dawson-Hughes B. (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84: 18–28 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Willett W., Orav E., Lips P., Meunie P., Lyons R., et al. (2012) A pooled analysis of vitamin D requirements for fracture prevention. New Engl J Med 367: 40–49 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H., Willett W., Wong J., Stuck A., Staehelin B., Orav E., et al. (2009b) Prevention of non-vertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 169: 551–561 [DOI] [PubMed] [Google Scholar]
- Bodnar L., Catov J., Simhan H., Holick M., Powers R., Roberts J. (2007) Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92: 3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko A., Deomina T., Favorova O., Gusev E., Sudomoina M., Turestkaya R. (1995) Epidemiology of multiple sclerosis in Russia and other countries of the former Soviet Union: investigations of environmental and genetic factors. Acta Neurol Scand Suppl 161: 71–76 [DOI] [PubMed] [Google Scholar]
- Boonstra A., Barrat F., Crain C., Heath V., Savekoul H., O’Garra A. (2001) 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naïve CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167: 4974–4980 [DOI] [PubMed] [Google Scholar]
- Borradale D., Kimlin M. (2009) Vitamin D in health and disease: an insight into traditional functions and new roles of ‘sunshine vitamin’. Nutr Res Rev 10: 1–19 [DOI] [PubMed] [Google Scholar]
- Bruce D., Yu S., Ooi J., Cantorna M. (2011) Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signalling. Int Immunol 23: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell A., Handel A., Ramagopalan S., Ebers G., Morahan J. (2011) Epigenetic mechanisms in multiple sclerosis and the major histocompatibility complex (MHC). Discov Med 11: 187–196 [PubMed] [Google Scholar]
- Burton J., Kimball S., Vieth R., Bar-Or A., Dosch H., Cheug R., et al. (2010) A phase I/II dose escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 74: 1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell S., Zhao Z., Stebbins C., Cadavid D., Buko A., Whalley E., et al. (2012) Serum IL-17F does not predict poor response to IM IFNβ-1a in relapsing-remitting MS. Neurology 79: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M., Whiting S., Barton C. (2004) Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr 80(Suppl.): 1710S–1716S [DOI] [PubMed] [Google Scholar]
- Cantorna M. (2006) Vitamin D and its role in immunology: multiple sclerosis and inflammatory bowel disease. Prog Biophys Mol Biol 92: 60–64 [DOI] [PubMed] [Google Scholar]
- Cantorna M. (2008) Vitamin D and multiple sclerosis: an update. Nutr Rev 66(10 Suppl. 2): S135–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M., Hayes C., DeLuca H. (1996) 1,25-Dihydoxyvitamin D3 reversibly blocks the progression of encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A 93: 7861–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M., Humpal-Winter J., DeLuca H. (1999) Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr 129: 1966–1971 [DOI] [PubMed] [Google Scholar]
- Cantorna M., Woodward W., Hayes C., DeLuca H. (1998) 1,25-Dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol 160: 5314–5319 [PubMed] [Google Scholar]
- Cantorna M., Zhao J., Zhang L. (2012) Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc 71: 62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlens C., Hergens M., Grunewald J., Ekbom A., Eklund A., Hoglund C., et al. (2010) Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med 181: 1217–1222 [DOI] [PubMed] [Google Scholar]
- Carmel A., Shieh A., Bang H., Bockman R. (2012). The 25(OH)D level needed to maintain a favourable bisphosphonate response is >33 ng/mL. Osteoporos Int 23: 2479–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman K., Fitzgerald A., Kiely M., Seamans K. (2011) A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr 106: 16171–627 [DOI] [PubMed] [Google Scholar]
- Ceglia L., Harris S. (2012) Vitamin D and its role in skeletal muscle. Calcif Tissue Int 12 September (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Chang J., Cha H., Lee D., Seo K., Kweon M. (2010) 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PlosOne 5: e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M., Herrera B., Ramagopalan S., Deluca G., Handunnetthi L., Orton S., et al. (2010) Parent-of-origin effects at the major histocompatility complex in multiple sclerosis. Hum Mol Genet 19: 3679–3689 [DOI] [PubMed] [Google Scholar]
- Chao M., Ramagopalan S., Herrera B., Lincoln M., Dyment D., Ebers G. (2009) Epigenetics in multiple sclerosis susceptibility: difference in transgenerational risk localizes to the major histocompatibility complex. Hum Mol Genet 18: 261–266 [DOI] [PubMed] [Google Scholar]
- Chapuy M., Preziosi P., Maamer M., Arnaud S., Galan P., Hercberg S., et al. (1996) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis Int 7: 439–443 [DOI] [PubMed] [Google Scholar]
- Chen S., Sims G., Chen X., Gu Y., Chen S., Lipsky P. (2007) Modulatory effect of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179: 1634–1647 [DOI] [PubMed] [Google Scholar]
- Cocco E., Meloni A., Murru M., Corongiu D., Tranquilli S., Fadda E., et al. (2012) Vitamin D responsive elements within the HLA-DRB1 promoter region in Sardinian multiple sclerosis alleles. PLoS One 7: e41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J., Ysrraelit M., Gaitan M. (2009) Immunomodulatory aspects of vitamin D in multiple sclerosis. Brain 132: 1146–1160 [DOI] [PubMed] [Google Scholar]
- Correale J., Ysrraelit M., Gaitan M. (2010) Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 185: 4948–4958 [DOI] [PubMed] [Google Scholar]
- Cox M., Ban M., Bowden N., Baker A., Scott R., Lechner-Scott J. (2012) Potential association of vitamin D receptor polymorphism Taq1 with multiple sclerosis. Mult Scler 18: 16–22 [DOI] [PubMed] [Google Scholar]
- Dabbaghmanesh M., Yousefipour G. (2011) Bone loss with multiple sclerosis: effect of glucocorticoid use and functional status. Iran Red Crescent Med J 13: 9–14 [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B., Heaney R., Holick M., Lips P., Meunier P., Vieth R. (2005) Estimates of optimal vitamin D status. Osteoporos Int 16: 713–716 [DOI] [PubMed] [Google Scholar]
- Dean G., Elian M. (1997) Age at immigration to England of Asian and Caribbean immigrants and the risk of developing multiple sclerosis. J Neurol Neurosurg Psychiatry 63: 565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décard B., von Ahsen N., Grunwald T., Streit F., Stoet A., Niggermier P., et al. (2012) Low vitamin D and elevated immunoreactivity against Epstein-Barr virus before first clinical manifestation of multiple sclerosis. J Neurol Neurosurg Psychiatry 83: 1170–1173 [DOI] [PubMed] [Google Scholar]
- De Jager P., Simon K., Munger K., Rioux J., Hafler D., Ascherio A. (2008) Integrating risk factors. HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology 70: 1113–1118 [DOI] [PubMed] [Google Scholar]
- DeLuca H., Plum L. (2011) Vitamin D deficiency diminishes the severity and delays onsets of experimental autoimmune encephalomyelitis. Arch Biochem Biophys 513: 140–143 [DOI] [PubMed] [Google Scholar]
- Di Pauli F., Reindl M., Ehling R., Schautzer F., Gneiss C., Lutterotti A., et al. (2008) Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler 14: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Disanto G., Chaplin G., Morahan J., Giovannoni G., Hyponnen E., Ramagopalan S. (2012a) Month of birth, vitamin D and risk of immune mediated disease: a case control study. BMC Med 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G., Magalhaes S., Handel A., Morrison K., Sadovnick A., Ebers G., et al. (2011a) HLA-DRB1 confers increased risk of pediatric-onset MS in children with acquired demyelination. Neurology 76: 781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G., Meier U., Giovannoni G., Ramagopalan S. (2011b) Vitamin D: a link between Epstein-Barr virus and multiple sclerosis development? Expert Rev Neurother 11: 1221–1224 [DOI] [PubMed] [Google Scholar]
- Disanto G., Morahan J., Ramagopalan S. (2012b) Multiple sclerosis: risk factors and their interactions. CNS Neurol Disord Drug Targets 11: 545–555 [DOI] [PubMed] [Google Scholar]
- Disanto G., Morahan J., Barnett M., Giovannoni G., Ramagopalan S. (2012c) The evidence or role of B cells in multiple sclerosis. Neurology 78: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G., Pakpoor J., Morahan J., Hall C., Meier U., Giovannoni G., Ramagopalan S. (2012d) Epstein–Barr virus, latitude and multiple sclerosis. Mult Scler 5 July (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Disanto G., Ramagopalan S., Para A., Handunnetthi L. (2011c) The emerging role of vitamin D binding protein in multiple sclerosis. J Neurol 258: 353–358 [DOI] [PubMed] [Google Scholar]
- Disanto G., Sandve G., Berlanga-Taylor A., Ragnedda G., Morahan J., Watson C., et al. (2012e) Vitamin D receptor binding, chromatin states and association with multiple sclerosis. Human Mol Genet 21: 3575–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R., Giovannoni G., Ramagopalan S. (2012) The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 18: 1522–1528 [DOI] [PubMed] [Google Scholar]
- Dorr J., Ohlraun S., Skarabis H., Paul F. (2012) Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized trial. Trials 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazo-Arvizu R., Dawson-Hughes B., Sempos C., Yetley E., Looker A., Cao G., et al. (2010) Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr 140: 595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T., van der Mei I., Ponsonby A., Taylo B., Stankovich J., McKay J., et al. (2008) Melanocortin 1 receptor genotype, past environmental sun exposure, and risk of multiple sclerosis. Neurology 71: 583–589 [DOI] [PubMed] [Google Scholar]
- Dyment D., Yee I., Ebers G., Sadovnick A. (2006) Multiple sclerosis in stepsiblings: recurrence risk and ascertainment. J Neurol Neurosurg Psychiatry 77: 258–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earthman C., Beckman L., Masodkar K., Sibley S. (2012) The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes 3: 387–396 [DOI] [PubMed] [Google Scholar]
- Ebers G. (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7: 268–277 [DOI] [PubMed] [Google Scholar]
- Ebers G. (2009) Editorial regarding ‘Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis’ by Beretich and Beretich. Mult Scler 15: 889–890 [DOI] [PubMed] [Google Scholar]
- Elian M., Nightingale S., Dean G. (1990) Multiple sclerosis among United Kingdom-born children of immigrants from the Indian subcontinent, Africa and the West Indies. J Neurol Neurosurg Psychiatry 53: 906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D., Smith H., Kinobe R., Hewison M., McGrath J. (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29: 21–30 [DOI] [PubMed] [Google Scholar]
- Fernandes de, Abreu D., Babron M., Rebeix I., Fontenille C., Yaouang J., Brassat D., et al. (2009) Season of birth and not vitamin D promoter polymorphisms is a risk factor for multiple sclerosis. Mult Scler 15: 1146–1152 [DOI] [PubMed] [Google Scholar]
- Fernandes de, Abreu D., Landel V., Féron F. (2011) Seasonal, gestational and postnatal influences of multiple sclerosis: the beneficial role of vitamin D supplementation in early life. J Neurol Sci 311: 64–68 [DOI] [PubMed] [Google Scholar]
- Fragoso Y., Shearer K., Adoni T., Alves-Leon S., Bidin Brooks J., Comini-Frota E., et al. (2012) Month of birth does not seem to interfere with the development of multiple sclerosis later in life in Brazilian patients. Neuroepidemiology 39: 70–71 [DOI] [PubMed] [Google Scholar]
- Freedman D., Dosemeci M., Alavanja M. (2000) Mortality from multiple sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med 57: 418–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C., Martyn C. (1995) Migrant studies in multiple sclerosis. Prog Neurobiol 47: 425–448 [PubMed] [Google Scholar]
- Garcion E., Wion-Barbot N., Monteiro-Menei C., Berger F., Wion D. (2002) New clues about vitamin d functions in the nervous system. Trends Endocrinol Metab 13: 100–105 [DOI] [PubMed] [Google Scholar]
- Garrett-Mayer E., Wagner C., Hollis B., Kindy M., Gattoni-Celli S. (2012) Vitamin D3 supplementation (4000 IU/d for 1 y) eliminates differences in circulating 25-hydroxyvitamin D between African American and white men. Am J Clin Nutr 96: 332–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., Summers G. (2011) Bone health in multiple sclerosis. Osteoporos Int 22: 2935–2949 [DOI] [PubMed] [Google Scholar]
- Givon U., Zeilig G., Dolev M., Achiron A. (2012) The month of birth and the incidence of multiple sclerosis in the Israeli population. Neuroepidemiology 38: 64–68 [DOI] [PubMed] [Google Scholar]
- Goldberg P. (1974) Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence (a view point). Part I: sunlight, dietary factors and epidemiology. Int J Envion Studies 6: 19–27 [Google Scholar]
- Goldberg P., Fleming M., Picard E. (1986) Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 21: 193–200 [DOI] [PubMed] [Google Scholar]
- Goodin D. (2009) The causal cascade to multiple sclerosis: a model for pathogenesis. PLoS OnE 4: e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W., Holick M. (2005) Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev 10: 94–111 [PubMed] [Google Scholar]
- Grau-Lopez L., Granada M., Raich D., Naranjo M., Borras F., Martinez-Caceres E., et al. (2012) Regulatory role of vitamin D in T-cell reactivity against myelin peptides in relapsing-remitting multiple sclerosis patients. BMC Neurol 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M., Lutz W., Phan V., Bachman L., McKean D., Kumar R. (2001) Dentritic cell modulation by 1 alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-independent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 98: 6800–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J., Chyu K. (1971) Competitive protein-binding radioassay for 25 hydroxycholecalciferol. J Clin Endocrinol Metab 33: 992–995 [DOI] [PubMed] [Google Scholar]
- Haddock L., Corcino J., Vasquez M. (1982) 25(OH)D serum levels in the normal Puerto Rican population and in subjects with tropical sprue and parathyroid disease. Puerto Rico Health Sci J 1: 85–91 [Google Scholar]
- Hagenau T., Vest R., Gissel T., Poulsen C., Erlandsem M., Mosekilde L., et al. (2009) Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 20: 133–140 [DOI] [PubMed] [Google Scholar]
- Hall L., Kimlin M., Aronov P., Hammock B., Slusser J., Woodhouse L. (2010) Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr 140: 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S., English D., McLeod J. (2000) The age-range risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain 123: 968–974 [DOI] [PubMed] [Google Scholar]
- Handel A., Giovannoni G., Ebers G., Ramagopalan S. (2010a) Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 6: 156–166 [DOI] [PubMed] [Google Scholar]
- Handel A., Williamson A., Disanto G., Dobson R., Giovannoni G., Ramagopalan S. (2011) Smoking and multiple sclerosis: an updated meta-analysis. PLoS One 6: e16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel A., Williamson A., Disanto G., Handunnetthi L., Giovannoni G., Ramagopalan S. (2010b) An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 5: e124986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handunnetthi L., Ramagopalan S., Ebers G., Handunnetthi L., Ramagopalan S., Ebers G. (2010) Multiple sclerosis, vitamin D, and HLA-DRB1*15. Neurology 74: 1905–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanwell H., Banwell B. (2011) Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta 1812: 202–212 [DOI] [PubMed] [Google Scholar]
- Hart P., Gorman S., Finley-Jones J. (2011) Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 11: 584–596 [DOI] [PubMed] [Google Scholar]
- Harvey L., Burne T., McGrath J., Eyles D. (2010) Developmental vitamin D3 deficiency induces alterations in immune organ morphology and function in adult offspring. J Steroid Biochem Mol Biol 121: 239–242 [DOI] [PubMed] [Google Scholar]
- Hathcock J., Shao A., Vieth R., Heaney R. (2007) Risk assessment for vitamin D. Am J Clin Nutr 85: 6–18 [DOI] [PubMed] [Google Scholar]
- Hayes C. (2000) Vitamin D: a natural inhibitor of multiple sclerosis. Proc Nutr Soc 59: 531–535 [DOI] [PubMed] [Google Scholar]
- Hayes C., Donald Acheson E. (2008) A unifying multiple sclerosis aetiology linking virus infection, sunlight, and vitamin D, through viral interleukin-10. Med Hypotheses 71: 85–90 [DOI] [PubMed] [Google Scholar]
- Healy B., Ali E., Guttmann C., Chitnis T., Glanz B., Buckle G., et al. (2009) Smoking and disease progression in multiple sclerosis. Arch Neurol 66: 858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney R. (2000) Vitamin D: how much do we need and how much is too much? Osteoporos Int 11: 553–555 [DOI] [PubMed] [Google Scholar]
- Heaney R., Davies K., Chen T., Holick M., Bager-Lux J. (2003a) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210 [DOI] [PubMed] [Google Scholar]
- Heaney R., Dowell M., Hale C., Bendich A.(2003b) Calcium absorption varies within the reference range for serum 25-hydroxy vitamin D. J Am Coll Nutr 22: 142–146 [DOI] [PubMed] [Google Scholar]
- Heaney R., Holick M. (2011) Why the IOM recommendations for vitamin D are deficient. J Bone Min Res 26: 455–457 [DOI] [PubMed] [Google Scholar]
- Heaney R., Horst R., Cullen D., Armas L. (2009) Vitamin D3 distribution and status in the body. J Am Coll Nutr 28: 252–256 [DOI] [PubMed] [Google Scholar]
- Hedström A., Bäärnhielm M., Olsson T., Alfredsson L. (2009) Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 73: 696–701 [DOI] [PubMed] [Google Scholar]
- Hedström A., Bäärnhielm M., Olsson T., Alfredsson L. (2011a) Exposure to environmental tobacco smoke is associated with increased risk for multiple sclerosis. Mult Scler 17: 788–793 [DOI] [PubMed] [Google Scholar]
- Hedström A., Olsson T., Alfredsson L. (2012) High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler 18: 1334–1336 [DOI] [PubMed] [Google Scholar]
- Hedström A., Sundqvist E., Bäärnhielm M., Nordin N., Hillert J., Kockum I., et al. (2011b) Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain 134: 653–664 [DOI] [PubMed] [Google Scholar]
- Heine G., Niesner U., Chang D., Steimeyer A., Zügel U, Zuberbier T., et al. (2008) 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol 38: 2210–2218 [DOI] [PubMed] [Google Scholar]
- Hensiek A., Sawcer S., Feakes R., Deans J., Mander A., Akesson E., et al. (2002) HLA-DR 15 is associated with female sex and younger age at diagnosis in multiple sclerosis. J Neurol Neurosurg Neuropsychiatry 72: 184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan M., Olek M., Ascherio A. (2001) Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol 154: 69–74 [DOI] [PubMed] [Google Scholar]
- Hernan M., Jick S., Logroscino G., Olek M., Ascherio A., Jick H. (2005) Cigarette smoking and the progression of multiple sclerosis. Brain 128: 1461–1465 [DOI] [PubMed] [Google Scholar]
- Hewison M. (2012) An update on vitamin D and human immunity. Clin Endocrinol 76: 315–325 [DOI] [PubMed] [Google Scholar]
- Hiremath G., Cettomai D., Baynes M., Ratchord J., Newsome S., Harrison D., et al. (2009) Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis. Mult Scler 15: 735–740 [DOI] [PubMed] [Google Scholar]
- Holick M. (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80(Suppl.): 1678S–1688S [DOI] [PubMed] [Google Scholar]
- Holick M. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Holick M. (2011) Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 12: 4–18 [DOI] [PubMed] [Google Scholar]
- Holick M. (2012) Vitamin D: extraskeletal health. Rheum Dis Clin North Am 38: 141–160 [DOI] [PubMed] [Google Scholar]
- Holick M., Binkley N., Bischoff-Ferrari H., Gordon C., Hanley D., Heaney R., et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930 [DOI] [PubMed] [Google Scholar]
- Holick M., Chen T., Lu Z., Sauter E. (2007) Vitamin D and skin physiology. J Bone Mineral Res 2(Suppl. 2): V28–V33 [DOI] [PubMed] [Google Scholar]
- Hollis B. (2005) Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 135: 317–322 [DOI] [PubMed] [Google Scholar]
- Holmes V., Barnes M., Alexander H., McFaul P., Wallace J. (2009) Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr 102: 876–881 [DOI] [PubMed] [Google Scholar]
- Holmøy T. (2008) Vitamin D status modulates the immune response to Epstein–Barr virus: synergistic effect of risk factors in multiple sclerosis. Med Hypotheses 70: 66–69 [DOI] [PubMed] [Google Scholar]
- Holmøy T., Kampman M., Smolders J. (2012) Vitamin D in multiple sclerosis: implications for assessment and treatment. Exp Rev Neurother 12: 1101–1112 [DOI] [PubMed] [Google Scholar]
- Huang J., Xie Z. (2012) Polymorphisms in the vitamin D receptor gene and multiple sclerosis risk. A meta-analysis of case control studies. J Neurol Sci 313: 79–85 [DOI] [PubMed] [Google Scholar]
- Hypponen E., Power C. (2007) Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 85: 860–868 [DOI] [PubMed] [Google Scholar]
- Ikeda U., Wakita D., Ohkuri T, Chamato K., Kitamura H., Iwakura Y., et al. (2010) 1alpha,25-Dihydroxyvitamin D(3) and all-transretinoic acid synergistically inhibit the differentiation and expression of Th17 cells. Immunol Lett 134: 7–16 [DOI] [PubMed] [Google Scholar]
- Irizar H., Munoz-Culla M., Zuriarran O., Goyenechea E., Castillo-Trivino T., Prada A., et al. (2012) HLA-DRB1*15:01 and multiple sclerosis: a female association? Mult Scler 18: 569–577 [DOI] [PubMed] [Google Scholar]
- Islam T., Gauderman W., Cozen W., Mack T. (2007) Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 69: 381–388 [DOI] [PubMed] [Google Scholar]
- Jeffery L., Burke F., Mura M., Zheng Y., Quershi O., Hewison M., et al. (2009) 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory cells expressing CTLA-4 and FoxP3. J Immunol 183: 5458–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek S., Schluep M., Harari A., Canales M., Lysandropoulos A., Zekeridou A., et al. (2012) HLA-B7-restricted EBV-specific CD8+ T cells are dysregulated in multiple sclerosis. J Immunol 188: 4671–4680 [DOI] [PubMed] [Google Scholar]
- Joshi S., Pantalena L., Liu X., Gaffen S., Liu H., Rohowsky-Cochan C., et al. (2011) 1,25-Dihydoxryvitamin B(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 31: 3653–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman M., Brustad M. (2008) Vitamin D: a candidate for the environmental effect in multiple sclerosis – observations from Norway. Neuroepidemiology 30: 140–146 [DOI] [PubMed] [Google Scholar]
- Kampman M., Steffensen L., Mellgren S., Jørgensen L. (2012) Effect of vitamin D3 supplementation on relapses, disease progression and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler 18: 1144–1151 [DOI] [PubMed] [Google Scholar]
- Kampman M., Wilsgaard T., Mellgren S. (2007) Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 254: 471–477 [DOI] [PubMed] [Google Scholar]
- Khoo A., Koenen H., Chai L., Sweep F., Netea M., van der Ven A., Joosten I. (2012) Seasonal variation in vitamin D3 levels is paralleled by changes in the peripheral human blood cell compartment. PlosOne 7: e29250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickler K., Ni Choileain S., Williams A., Richards A., Astier A. (2012) Calcitriol modulates the CD46 pathway in T cells. PLoS ONE 7: e48486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Kondo T., Takada I., Youn M., Yamamoto Y., Takahashi S., et al. (2009) DNA demethylation in hormone-induced transcriptional derepression. Nature 461: 1007–1012 [DOI] [PubMed] [Google Scholar]
- Kimball S., Burton J., O’Connor P., Vieth R. (2011a) Urinary calcium response to high dose vitamin D3 with calcium supplementation in patients with multiple sclerosis. Clin Biochem 44: 930–932 [DOI] [PubMed] [Google Scholar]
- Kimball S., Vieth R., Dosch H., Ber-Or A., Cheung R., Gagne D., et al. (2011b) Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Met 96: 2826–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirbas A., Kirbas S., Anlar O., Turkyilmaz A., Cure M., Efe H. (2012) Investigation of the relationship between vitamin D and bone mineral density in newly diagnosed multiple sclerosis. Acta Neurol Belg 16 August (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Knippenberg S., Bol Y., Damoiseaux J., Hupperts R., Smolders J. (2010) Vitamin D status in patients with MS is negatively correlated with depression but not with fatigue. Acta Neurol Scand 124: 171–175 [DOI] [PubMed] [Google Scholar]
- Knippenberg S., Smolders J., Thewissen M., Peelen E., Terwaert J., Hupperts R., et al. (2011) Effect of vitamin D(3) supplementation on peripheral B cell differentiation and isotype switching in patients with multiple sclerosis. Mult Scler 17: 1418–1423 [DOI] [PubMed] [Google Scholar]
- Koch M., Metz L., Kovalchuk O. (2012) Epigenetic changes in patients with multiple sclerosis. Nat Rev Neurol 9: 35–43. [DOI] [PubMed] [Google Scholar]
- Koch M., van Harten A., Uyttenboogaart M., De Keyser J. (2007) Cigarette smoking and progression in multiple sclerosis. Neurology 69: 1515–1520 [DOI] [PubMed] [Google Scholar]
- Kragt J., van Amerongen B., Killestein J., Dijkstra C., Uitdehaag B., Polman C., et al. (2009) Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler 15: 9–15 [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (1967) On the fine structure of the distribution of multiple sclerosis. Acta Neurol Scan 43: 257–282 [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (1995) MS epidemiology world wide. One view of current status. Acta Neurol Scand 161(Suppl.): 23–33 [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (2008) Some contributions of the Department of Veterans Affairs to the epidemiology of multiple sclerosis. Mult Scler 14: 1007–1012 [DOI] [PubMed] [Google Scholar]
- Kurtzke J., Beebe J., Norman J. (1985) Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology 35: 672–678 [DOI] [PubMed] [Google Scholar]
- Lassmann H., Niedobitek G., Aloisi F., Middeldorp J. and the NeuroproMiSe EBV Working Group (2010) Epstein–Barr virus in the multiple sclerosis brain: a controversial issue-report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 134: 2772–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J., Adams J., Sakai R., Jordan S. (1984) 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. Clin Invest 74: 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J., Archer D. (1991) 1,25-Dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Ivest 87: 1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J., Archer D., Beck L., Spiegelberg H.(1995) Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr 125: 1704S–1708S [DOI] [PubMed] [Google Scholar]
- Leu M., Giovannucci E. (2011) Vitamin D: epidemiology of cardiovascular risks and events. Best Pract Res Clin Endocrinol Met 25: 633–646 [DOI] [PubMed] [Google Scholar]
- Levin L., Munger K., O’Reilly E., Falk K., Ascherio A. (2010) Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 67: 824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L., Munger K., Rubertone M., Peck C., Lenette E., Spiegeman D., et al. (2005) Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 293: 2496–2500 [DOI] [PubMed] [Google Scholar]
- Lewis S., Lucas R., Halliday J., Ponsonby A.(2010) Vitamin D deficiency and pregnancy: from preconception to birth. Mol Nutr Food Res 54: 1092–1102 [DOI] [PubMed] [Google Scholar]
- Lin R., Charlesworth J., van der Mei I., Taylor B.(2012) The genetics of multiple sclerosis. Pract Neurol 12: 279–288 [DOI] [PubMed] [Google Scholar]
- Lindsey J., Khan U., Ansari W., Powell T., Wang Y., Guirguis M. (2012) The antibody response to Epstein–Barr virions is altered in multiple sclerosis. J Neuroimmunol 254: 146–153. [DOI] [PubMed] [Google Scholar]
- Lips P. (2006) Vitamin D physiology. Prog Biophys Mol Biol 92: 4-8 [DOI] [PubMed] [Google Scholar]
- Løken-Amsrud K., Holmøy T., Bakke S., Beiske A., Bjerve K., Bjørnarå B., et al. (2012) Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology 79: 267–273 [DOI] [PubMed] [Google Scholar]
- Lonergan R., Kinsella K., Fitzpatrick P., Brady J., Murray B, Dunne C., et al. (2011) Multiple sclerosis prevalence in Ireland: relationship to vitamin D status and HLA genotype. J Neurol Neorosurg Psychiatry 82: 317–322 [DOI] [PubMed] [Google Scholar]
- Lossius A., Vartdal S., Holmøy T. (2011) Vitamin D sensitive EBNA-1 specific T cells in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol 240-241: 85–96 [DOI] [PubMed] [Google Scholar]
- Lucas R., Ponsonby A., Dear K., Valery P., Pender M., Burrows J., et al. (2011a) Current and past Epstein–Barr virus infection in risk of initial CNS demyelination. Neurology 77: 371–379 [DOI] [PubMed] [Google Scholar]
- Lucas R., Ponsonby A., Dear K., Valery P., Pender M., Taylor B., et al. (2011b) Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 76: 540–548 [DOI] [PubMed] [Google Scholar]
- Lünemann J., Tintoré M., Messmer B., Strowiq T., Rovira A., Perkal H., et al. (2010) Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol 57: 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxwolda M., Kuiperst R., Kema I., Dijck-Brouwer J., Muskiet F. (2012) Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/L. Br J Nutr 108: 1557-1561 [DOI] [PubMed] [Google Scholar]
- Lysandropoulos A., Jaquiéry E., Jilek S., Pantealo G., Schluep M., Du Pasquier R. (2011) Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and in healthy controls. J Neuroimmunol 233: 240–244 [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhang P., Wang F., Yang J., Liu Z., Qin H. (2011) Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 29: 3775–3782 [DOI] [PubMed] [Google Scholar]
- Mahon B., Gordon S., Cruz J., Cosman F., Cantorna M. (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 134: 128–132 [DOI] [PubMed] [Google Scholar]
- Mameli G., Poddighe L., Mei A., Uleri E., Sotgiu S., Serra C., et al. (2012) Expression and activation by Epstein Barr virus of human endogenous retroviruses in blood cells and astrocytes: inference for multiple sclerosis. PLoS One 7: e44991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie R., Cutter G., Tyry T., Vollmer T. (2009) A cross-sectional study of bone health in multiple sclerosis. Neurology 73: 1394–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F., Smiroldo S., Gabliati F., Muller M., Di Lucia P., Poliani P., et al. (2000) Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D3. Eur J Neuroimmunol 30: 498–508 [DOI] [PubMed] [Google Scholar]
- Mayne C., Spanier J., Relland L., Williams C., Hayes C. (2011) 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol 41: 822–832 [DOI] [PubMed] [Google Scholar]
- McDowell T., Amr S., Culpepper W., Langenberg P., Royal W., Bever C., et al. (2011) Sun exposure, vitamin D and age at disease onset in relapsing multiple sclerosis. Neuroepidemiology 36: 39–45 [DOI] [PubMed] [Google Scholar]
- McDowell T., Amr S., Langenberg B., Royal W., Bever C., Culpepper W., et al. (2010) Time of birth, residential solar radiation and age at onset of multiple sclerosis. Neuroepidemiology 34: 238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J., Hammond S., Kurtzke J. (2011) Migration and multiple sclerosis in immigrants to Australia from United Kingdom and Ireland: a reassessment. I. Risk of MS by age at immigration. J Neurol 258: 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan T., DeLuca H. (2002) The vitamin D receptor is essential for 1alpha,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys 408: 200–204 [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y., Caridade G., Tardieu M., Suissa S. and the KIDSEP Study Group (2007) Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain 130: 2589–2595 [DOI] [PubMed] [Google Scholar]
- Mirzaei F, Michels K., Munger K., O’Reilly E., Chitnis T., Forman M., et al. (2011) Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol 70: 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen S., Celius E., Sandvik L., Brustad M., Nordsletten L., Eriksen E., et al. (2012) Bone turnover and metabolism in patients with early multiple sclerosis and prevalent bone mass deficit. A population-based case-control study. PLoS One 7: e45703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen S., Celius E., Sandvik L., Nordsletten L., Eriksen E., Holmøy T. (2011) Low bone mass in newly diagnosed multiple sclerosis and clinically isolated syndrome. Neurology 77: 151–157 [DOI] [PubMed] [Google Scholar]
- Montgomery S., Bahmanyar S., Hillert J., Ekbom A., Olsson T. (2008) Maternal smoking during pregnancy and multiple sclerosis amongst offspring. Eur J Neurol 15: 1395–1399 [DOI] [PubMed] [Google Scholar]
- Moore C., Murphy M., Holick M. (2005) Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 135: 2478–2485 [DOI] [PubMed] [Google Scholar]
- Morgan J., Morgan D., Lasky S., Ford D., Kouttab N., Maizel A. (1996) Requirements for induction of vitamin D-mediated gene regulation in normal human B lymphocytes. J Immunol 157: 2900–2908 [PubMed] [Google Scholar]
- Morris H., Anderson P. (2010) Autocrine and paracrine actions of vitamin d. Clin Biochem Rev 31: 129–138 [PMC free article] [PubMed] [Google Scholar]
- Mosayebi G., Ghazavi A., Ghasami K., Kokhaei P. (2011) Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest 40: 627–639 [DOI] [PubMed] [Google Scholar]
- Mowry E. (2011) Vitamin D: evidence for its role as a prognostic factor in multiple sclerosis. J Neurol Sci 311: 19–22 [DOI] [PubMed] [Google Scholar]
- Mowry E., Krupp L., Milazzo M., Chabas D., Strober J., Belman A., et al. (2010) Vitamin D status is associated with relapse rate in pediatric-onset MS. Ann Neurol 67: 618–624 [DOI] [PubMed] [Google Scholar]
- Mowry E., Waubant E., McCulloch C., Okuda D., Evangelista A., Lincoln R., et al. (2012) Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol 72: 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Odum N., Bendizen K. (1993) 1,25-Dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett 35: 177–182 [DOI] [PubMed] [Google Scholar]
- Munger K., Ascherio A. (2011) Prevention and treatment of MS: studying the effects of vitamin D. Mult Scler 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K., Chitnis T., Ascherio A. (2009) Body size and risk of MS in two cohorts of US women. Neurology 73: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K., Chitnis T., Frazier A., Giovannucci E., Spiegelman D., Ascherio A. (2011a) Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol 258: 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K., Levin L., Hollis B., Howard N., Ascherio A. (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296: 2832–2838 [DOI] [PubMed] [Google Scholar]
- Munger K., Levin R., O’Reilly E., Falk K., Ascherio A. (2011b) Anti-Epstein Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personal. Mult Scler 17: 1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K., Zhang S., O’Reilly E., Hernan M., Olek M., Willet W., et al. (2004) Vitamin D intake and incidence of multiple sclerosis. Neurology 62: 60–65 [DOI] [PubMed] [Google Scholar]
- Muthian G., Raikvar H., Rajasingh J., Brigh J. (2006) 1,25-Dihydroxyvitamin D3 modulates JAK STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res 83: 1299–1309 [DOI] [PubMed] [Google Scholar]
- Myrh K. (2009) Vitamin D treatment in multiple sclerosis. J Neurol Sci 286: 104–108 [DOI] [PubMed] [Google Scholar]
- Nashold F., Miller D., Hayes D. (2000) 1,25-Dihydroxyvitamin D3 treatment decreases macrophage accumulation of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 103: 171–179 [DOI] [PubMed] [Google Scholar]
- Nashold F., Spach K., Spanier J., Hayes C. (2009) Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol 183: 3672–3681 [DOI] [PubMed] [Google Scholar]
- Neau J., Artaud-Uriot M., Lhomme V., Bounaud J., Lebras F., Boissonnot L., et al. (2011) Vitamin D and multiple sclerosis. A prospective survey of patients of Poitou-Charentes area. Rev Neurol 167: 317–323 [DOI] [PubMed] [Google Scholar]
- Nielsen T., Pedersen M., Rostgaard K., Frisch M., Hjalgrim H. (2007a) Correlations between Epstein–Barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Mult Scler 13: 420–423 [DOI] [PubMed] [Google Scholar]
- Nielsen T., Rostgaard K., Askling J., Steffensen R., Oturai A., Jersild C., et al. (2009) Effects of infectious mononucleosis and HLA-DRB1*15 in multiple sclerosis. Mult Scler 15: 431–436 [DOI] [PubMed] [Google Scholar]
- Nielsen T., Rostgaard K., Nielsen N., Koch-Henriksen N., Haarh S., Sørensen P., et al. (2007b) Multiple sclerosis after infectious mononucleosis. Arch Neurol 64: 72–75 [DOI] [PubMed] [Google Scholar]
- Nieves J., Cosman F., Herbert J., Shen V., Lindsay R. (1994) High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology 44: 1687–1694 [DOI] [PubMed] [Google Scholar]
- Niino M., Fukazawa T., Kikuchi S., Sasaki H. (2008) Therapeutic potential of vitamin D for multiple sclerosis. Cur Med Chem 15: 499–505 [DOI] [PubMed] [Google Scholar]
- Niino M., Fukazawa T., Yabe I., Kikuchi S., Sasaki H., Tashiro K. (2000) Vitamin D receptor gene polymorphism in multiple sclerosis and the association with HLA class II alleles. J Neurol Sci 177: 65–71 [DOI] [PubMed] [Google Scholar]
- Niller N., Wolf H., Minarovits J. (2008) Regulation and dysregulation of Epstein–Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity 41: 298–328 [DOI] [PubMed] [Google Scholar]
- Nolan D., Castley A., Tschoschner M., James I., Qiu W., Sayer D., et al. (2012) Contributions of vitamin D elements and HLA promoters to multiple sclerosis risk. Neurology 79: 538–546 [DOI] [PubMed] [Google Scholar]
- Norman A., Bouillon R. (2010) Vitamin D nutritional policy needs a vision for the future. Exp Biol Med 235: 1034–1045 [DOI] [PubMed] [Google Scholar]
- Novakovic B., Sibson N., Ng A., Manuelpillai U., Rakyan V., Down T., et al. (2009) Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of vitamin D levels at the fetomaternal interface. J Biol Chem 284: 14838–14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell S., Cranney A., Horsley T., Weiler H., Atkinson S., Hanley D., et al. (2008) Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am J Clin Nutr 88: 1528–1534 [DOI] [PubMed] [Google Scholar]
- Okazaki R., Sugimoto T., Kaji H., Fujii Y., Shiraki M., Inoue D., et al. (2011) Vitamin D insufficiency defined by serum 25-hydroxyvitamin D and parathyroid hormone before and after oral vitamin D3 supplementation load in Japanese patients. J Bone Miner Metab 29: 103–110 [DOI] [PubMed] [Google Scholar]
- Orton S., Ramagopalan S., Para A., Lincoln M., Handunnetthi L., Chao M., et al. (2011a) Vitamin D metabolic pathway genes and risk of multiple sclerosis in Canadians. J Neurol Sci 305: 116–120 [DOI] [PubMed] [Google Scholar]
- Orton S., Wald L., Confavreux C., Vukusic S., Krohn J., Ramagopalan S., et al. (2011b) Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 76: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G., Bennett J. (2012) Trigger, pathogen or bystander: the complex nexus linking Epstein-Barr virus and multiple sclerosis. Mult Scler 18: 1204–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgocmen S., Bulut S., IIhan N., Gulkesen A., Ardicoglu O., Ozkan Y. (2005) Vitamin D deficiency and reduced bone mineral density in multiple sclerosis: effect of ambulatory status and functional capacity. J Bone Miner Metab 23: 309–313 [DOI] [PubMed] [Google Scholar]
- Pakpoor J., Disanto G., Gerber J., Dobson R., Meier U., Giovannoni G., et al. (2012) The risk of developing multiple sclerosis in individuals negative for Epstein–Barr virus: a meta-analysis. Mult Scler 11 June (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Pedersen L., Nashold F., Spach K., Hayes C. (2007) 1,25 Dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokines synthesis and monocyte trafficking. J Neurosci Res 85: 2480–2490 [DOI] [PubMed] [Google Scholar]
- Peferoen L., Lamers F., Lodder L., Gerritsen W., Huitinga I., Melief J., et al. (2010) Epstein Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain 133: 1–4 [DOI] [PubMed] [Google Scholar]
- Pekmezovic T., Drulovic J., Milenkovic M., Jarebinski M., Stojsavljevic N., Mesaros S., et al. (2006) Lifestyle factors and multiple sclerosis: a case-control study in Belgrade. Neuroepidemiology 27: 212–216 [DOI] [PubMed] [Google Scholar]
- Perron H., Germi R., Bernard C., Garcia-Montojo M, Deluen C., Farinelli L., et al. (2012) Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler 18: 1721-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C. (2009) Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 256: 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Rivaud-Péchoux S., Clerson P., de Paz R., Souberbielle J. (2012) Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord 5: 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Souberbielle J. (2010) Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 133: 1869–1888 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Souberbielle J. (2011) Widespread vitamin D insufficiency: a new challenge for primary prevention with particular reference to multiple sclerosis. Presse Médicale 40: 349–356 [DOI] [PubMed] [Google Scholar]
- Pike J., Meyer M. (2010) The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am 39: 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas F., Ponsonby A., van der Mei I., Taylor B., Blizzard L., Groom P., et al. (2009) Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol 256: 577–585 [DOI] [PubMed] [Google Scholar]
- Priemel M., von Doramus C., Klatte T., Kessler S., Schlie J., Meier S., et al. (2010) Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 25: 305–312 [DOI] [PubMed] [Google Scholar]
- Prietl B., Pilz S., Wolf M., Tomaschitz A., Obermayer-Pietsch B., Graninger W., et al. (2010). Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? IMAJ 12: 136–139 [PubMed] [Google Scholar]
- Prosser D., Jones G. (2004) Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29: 664–673 [DOI] [PubMed] [Google Scholar]
- Provvedini D., Tsoukas C., Deftos L., Manolagas S. (1983) 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 221: 1181–1183 [DOI] [PubMed] [Google Scholar]
- Pugliatti M., Rosati G., Carton H., Riise T., Drulovic J., Vécsei L., et al. (2006) The epidemiology of multiple sclerosis in Europe. Eur J Neurol 13: 700–722 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S., Dyment D., Cader M., Morrison K., Disanto G., Morahan J., et al. (2011a) Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol 70: 881–886 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S., Handel A., Giovannoni G., Rutherford Siegel S., Ebers G., Chaplin G. (2011b) Relationship of UV exposure to prevalence of multiple sclerosis in England. Neurology 76: 1410–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan S., Link J., Byrnes J., Dyment D., Giovannoni G., Hintzen R., et al. (2009a) HLA-DRB1 and month of birth in multiple sclerosis. Neurology 73: 2107–2111 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S., Maugeri N., Handunnetthi I., Lincoln M., Orton S., Dyment D., et al. (2009b) Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 5: e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan S., Valdar W., Dyment D., DeLuca G., Yee I., Giovannoni G., et al. (2009c) Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology 32: 257–262 [DOI] [PubMed] [Google Scholar]
- Reichel H., Koeffler H., Tobler A., Norman A. (1987) 1 alpha,25-Dihyroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A 84: 3385–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby W., Waugh M., Graziano R. (1990) Regulation of human monocyte HLA-DR and CD4 antigen expression, and antigen presentation of 1,25-dihydroxyvitamin D3. Blood 76: 189–197 [PubMed] [Google Scholar]
- Riise T., Nortvedt M., Ascherio A. (2003) Smoking is a risk factor for multiple sclerosis. Neurology 61: 1122–1124 [DOI] [PubMed] [Google Scholar]
- Ross A., Manson E., Abrams S., Aloia J., Brannon P., Steven K., et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W., 3rd, Mia Y., Li H., Nauton K. (2009) Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol 213: 135–141 [DOI] [PubMed] [Google Scholar]
- Runia T., Hop W., de Rijke Y., Buljevac D., Hintzen R. (2012) Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 79: 261–266 [DOI] [PubMed] [Google Scholar]
- Saastamoinen K., Auvinen M., Tienari P. (2012) Month of birth is associated with multiple sclerosis but not with HLA-DR15 in Finland. Mult Scler 18: 563–568 [DOI] [PubMed] [Google Scholar]
- Šaltytė Benth J., Myhr K., Løken-Amsrud K., Beiske A., Bjerve K., Hovdal H., et al. (2012) Modelling and prediction of 25-hydroxyvitamin D levels in Norwegian relapsing-remitting multiple sclerosis patients. Neuroepidemiology 39: 84–93 [DOI] [PubMed] [Google Scholar]
- Salzer J., Hallmans G., Nyström M., Stenlund H., Wadell G., Sundström P. (2012a) Vitamin D as a protective factor in multiple sclerosis. Neurology 79: 2140–2145 [DOI] [PubMed] [Google Scholar]
- Salzer J., Nyström M., Hallmans G., Stenlund H., Wadell G., Sundström P. (2012b) Epstein–Barr virus and vitamin D as risk factors for multiple sclerosis. Mult Scler 18(Suppl. 4): 373-374 [Google Scholar]
- Salzer J., Svenningsson A., Sundström P. (2010) Season of birth and multiple sclerosis in Sweden. Acta Neurol Scand 121: 20–23 [DOI] [PubMed] [Google Scholar]
- Sargsyan S., Shearer A., Ritchie A., Burgoon M., Anderson S., Hemmer B., et al. (2010) Absence of Epstein–Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 74: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S., Hellenthai G., Pirinen M., Spencer C., Patsopoulos N., Moutsanias L., et al. The International Multiple Sclerosis Genetics Consortium and Wellcome Trust Case Control Consortium 2 (2011) Genetic risk and primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann K., Kaufman M., Weber S., Inwin A., Goos C., John U., et al. (2011) Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410-421 [DOI] [PubMed] [Google Scholar]
- Schmidt H., Williamson D., Ashley-Koch A. (2007) HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am J Epidemiol 165: 1097–1109 [DOI] [PubMed] [Google Scholar]
- Schwalfenberg G., Genuis S., Hiltz M. (2010) Addressing vitamin D deficiency in Canada: a public health innovation whose time has come. Public Health 124: 350–359 [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Franciotta D., Magliozzi R., Reynolds R., Cinque P., et al. (2007) Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med 204: 2899–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B., Severa M., Columba-Cabezas S., Rosicarelli B., Veroni C., Chiappetta G., et al. (2010) Epstein–Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J Neuropathol Exp Neurol 69: 677–693 [DOI] [PubMed] [Google Scholar]
- Shaygannejad V., Janghorbani M., Ashtari F., Dehghan H. (2012) Effect of adjunct low-dose vitamin D on relapsing remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled study. Mult Scler Int 2012: 452541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H., Pan J., Debes J., Alt C., Habtezion A., Soler D., et al. (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8: 285–293 [DOI] [PubMed] [Google Scholar]
- Silva K., Alvarenga R., Fernandez Y., Alvarenga H., Thuler L. (2009) Potential risk factors for multiple sclerosis in Rio de Janeiro: a case-control study. Arq Neuropsiquiatr 67: 229–234 [DOI] [PubMed] [Google Scholar]
- Simon K., Munger K., Ascherio A. (2012a) Vitamin D and multiple sclerosis: epidemiology, immunology and genetics. Curr Opin Neurol 25: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Munger K., Kraft P., Hunter D., De Jager P., Ascherio A. (2011) Genetic predictors of 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Neurol 258: 1676–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., O’Reilly E., Munger K., Finerty S., Morgan A., Ascherio A. (2012b) Epstein–Barr virus neutralizing antibody levels and risk of multiple sclerosis. Mult Scler 18: 1185–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Van der Mei I., Munger K., Ponsonby A., Dickinson J., Dwyer T., et al. (2010) Combined effects of smoking, anti-EBNA antibodies and HLA-DBR1*1501 on multiple sclerosis risk. Neurology 74: 1365–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S., Jr, Blizzard L., Otahal P., Van der Mei I., Taylor B. (2011) Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 82: 1132–1141 [DOI] [PubMed] [Google Scholar]
- Simpson S., Taylor B., Blizzard L., Ponsonby A., Pittas F., Tremlett H., et al. (2010) Higher 25-hydroxyvitamin D is associated with lower relapse risk in MS. Ann Neurol 68: 193–203 [DOI] [PubMed] [Google Scholar]
- Sioka C., Kyritsis A., Fotopoulos A. (2009) Multiple sclerosis, osteoporosis, and vitamin D. J Neurol Sci 287: 1–6 [DOI] [PubMed] [Google Scholar]
- Sloka J., Pryse-Phillips W., Stefanelli M. (2008) The relation of ultraviolet radiation and multiple sclerosis in Newfoundland. Can J Neurol Sci 35: 69–74 [DOI] [PubMed] [Google Scholar]
- Sloka S., Silva C., Pryse-Phillips W., Patten S., Metz L, Yong V. (2011a) A quantitative analysis of suspected environmental causes of MS. Can J Neurol Sci 38: 98–105 [DOI] [PubMed] [Google Scholar]
- Sloka S., Silva C., Wang J., Yong V. (2011b) Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J., Damoiseaux J., Menheree P., Hupperts R. (2008a) Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 194: 7–17 [DOI] [PubMed] [Google Scholar]
- Smolders J., Hupperts R., Barkhof R., Grimaldi L., Holmoy T., Killestein J., et al. (2011a) Efficacy of vitamin D(3) as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta 1-a: a phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci 311: 44–49 [DOI] [PubMed] [Google Scholar]
- Smolders J., Menheere P., Kessels A., Damoiseaux J., Hupperts R. (2008b) Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 14: 1–5 [DOI] [PubMed] [Google Scholar]
- Smolders J., Menheere P., Thewissen M., Peelen E., Tervaert J., Hupperts R., et al. (2010a) Regulatory T cell function correlates with serum 25-hydroxyvitamin D, but not with 1,25 dihydroxyvitamin D, parathyroid hormone and calcium levels in patients with relapsing remitting multiple sclerosis. J Steroid Biochem Mol Biol 121: 243–246 [DOI] [PubMed] [Google Scholar]
- Smolders J., Moen S., Damoiseaux J., Huitinga I., Holmøy T. (2011b) Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci 311: 37–43 [DOI] [PubMed] [Google Scholar]
- Smolders J., Peelen E., Thewissen M., Cohen Tervaet J., Menheere P., Hupperts R., et al. (2010b) Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One 5(12): e15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J., Thewissen M., Peelen E., Menheere P., Tervaert J., Damoiseaux J., et al. (2009) Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 4: e6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J., Thewissen M., Theunissen R., Peelen E., Knippenberg S., Menheere P., et al. (2011c) Vitamin D-related gene expression profiles in immune cells of patients with relapsing remitting multiple sclerosis. J Neuroimmunol 235: 91–97 [DOI] [PubMed] [Google Scholar]
- Soilu-Hänninen M., Airas L., Monnonen I., Heikkilä A., Viljanen N., Hänninen A. (2005) 25 Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler 11: 266–271 [DOI] [PubMed] [Google Scholar]
- Soilu-Hänninen M., Aivo J., Lindström B., Elovaara I., Sumelhati M., Färkkilä M., et al. (2012) A randomised, double blind, placebo controlled trial with vitamin D3 as an add-on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 83: 565–571 [DOI] [PubMed] [Google Scholar]
- Sokal E., Hoppenbrouwers K., Vandermeulen C., Moutschen S., Léonard P., Moreels A., et al. (2007) Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein–Barr virus vaccine in healthy young adults. J Infect Dis 196: 1749–1753 [DOI] [PubMed] [Google Scholar]
- Sotgiu S., Pugliatti M., Sotgiu M., Fois M., Arru G., Sanna A., et al. (2006) Seasonal fluctuation of multiple sclerosis births in Sardinia. J Neurol 253: 38–44 [DOI] [PubMed] [Google Scholar]
- Souberbielle J., Body J., Lappe J., Plebani M., Shoenfeld Y., Wang T., et al. (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9: 709–715 [DOI] [PubMed] [Google Scholar]
- Spach K., Hayes C. (2005) Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175: 4119–4126 [DOI] [PubMed] [Google Scholar]
- Spach K., Nashold F., Dittel B., Hayes C. (2006) IL-10 signaling is essential for 1,25 dihydroxyvitamin d3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol 177: 6030–6037 [DOI] [PubMed] [Google Scholar]
- Spach K., Pedersen L., Nashold F., Kayo T., Yandell B., Prolla T., et al. (2004) Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics 18: 141–151 [DOI] [PubMed] [Google Scholar]
- Spanier J., Nashold F., Olson J., Hayes C. (2012) The Ifng gene is essential for VDR gene expression and vitamin D3-mediated reduction of pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis. J Immunol 189: 3188–3197 [DOI] [PubMed] [Google Scholar]
- Staples J., Ponsonby A., Lim L. (2010) Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis. BMJ 340: c 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen L., Jørgensen L., Straume B., Mellgren S., Kampman M. (2011) Can vitamin D supplementation prevent bone loss in persons with MS? A placebo-controlled trial. J Neurol 258: 1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen L., Mellgren S., Kampman M. (2010) Predictors and prevalence of low bone mineral density in fully ambulatory persons with multiple sclerosis. J Neurol 257: 410–418 [DOI] [PubMed] [Google Scholar]
- Stein M., Liu Y., Gray O., Baker J., Kolbe S., Ditchfield M., et al. (2011) A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 77: 1611–1618 [DOI] [PubMed] [Google Scholar]
- Stewart N., Simpson S., van der Mei I., Ponsonby A., Blizzard L., Dwyer T., et al. (2012) Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 79: 254–260 [DOI] [PubMed] [Google Scholar]
- Subramanian S., Miller L., Grafe M., Vanderbark A., Offner H. (2012) Contribution of GPR30 for 1,25-dihydroxyvitamin D3 protection in EAE. Metab Brain Dis 27: 29–35 [DOI] [PubMed] [Google Scholar]
- Sundqvist E., Bäärnhielm M., Alfredsson L., Hillert J., Olsson T., Kockum I. (2010) Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Gen 18: 1349–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist E., Sundström P., Lindén M., Hedström A., Aloisi F., Hillert J., et al. (2012a) Epstein–Barr virus and multiple sclerosis: interaction with HLA. Genes Immun 13: 14–20 [DOI] [PubMed] [Google Scholar]
- Sundqvist E., Sundström P., Lindén M., Hedström A., Aloisi F., Hillert J., et al. (2012b) Lack of replication of interaction between EBNA1 IgG and smoking in risk for multiple sclerosis. Neurology 79: 1363–1368 [DOI] [PubMed] [Google Scholar]
- Sundström P., Juto P., Wadell G., Hallmans G., Svenninggson A., Nyström R., et al. (2004) An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology 62: 2277–2282 [DOI] [PubMed] [Google Scholar]
- Sundström P., Nyström L. (2008) Smoking worsens the prognosis in multiple sclerosis. Mult Scler 14: 1031–1035 [DOI] [PubMed] [Google Scholar]
- Sundström P., Nyström L., Hallmans G. (2008) Smoke exposure increases the risk for multiple sclerosis. Eur J Neurol 15: 579–583 [DOI] [PubMed] [Google Scholar]
- Sundström P., Nyström R., Ruuth K., Lundgren E. (2009) Antibodies to specific EBNA-1 domains and HLA-DRB1*1501 interact as risk factors for multiple sclerosis. J Neuroimmunol 215: 102–107 [DOI] [PubMed] [Google Scholar]
- Tang J., Zhou R., Luger D., Zhu W., Silver P., Grajewski R., et al. (2009) Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunnol 182: 4624–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B., Lucas R., Dear K., Kilpatrick T., Pender M., van der Mei I., et al. (2010) Latitudinal variation in incidence and type of first central nervous system demyelinating events. Mult Scler 16: 398–405 [DOI] [PubMed] [Google Scholar]
- Taylor B., Richardson A., Mason D., Willoughby E., Abenethy D., Sabel C. (2008) Prevalence of multiple sclerosis in New Zealand. Mult Scler 14(Suppl. 1): S202 [Google Scholar]
- Templer D., Trent N., Spencer D., Trent A., Corgiat M., Mortensen P., et al. (1992) Season of birth in multiple sclerosis. Acta Neurol Scand 85: 107–109 [DOI] [PubMed] [Google Scholar]
- Thacker E., Mizraei F., Ascherio A. (2006) Infectious mononucleosis and risk of multiple sclerosis: a meta-analysis. Ann Neurol 59: 499–503 [DOI] [PubMed] [Google Scholar]
- Torkildsen Ø., Knappskog P., Nyland H., Myhr K. (2008) Vitamin D-dependent rickets as a possible risk factor for multiple sclerosis. Arch Neurol 65: 809–811 [DOI] [PubMed] [Google Scholar]
- Torskilden Ø., Stansberg C., Anglelskår S., Kooi E., Geurts J., van der Valk P., et al. (2010) Upregulation of immunoglobulin-related genes in cortical sections from multiple sclerosis patients. Brain Path 20: 720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Kakalacheva K., Lünemann J., Luzuriaga K., Middeldorp J., Thorley-Lawson D. (2012) Persistence of Epstein–Barr virus in self-reactive memory B cells. J Virol 86: 12330–12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou N., Lambrinoudaki I., Thoda P., Andreadou E., Kararizou E., Alexandrou A., et al. (2012) Lack of association between vitamin D levels and bone mineral density in patients with multiple sclerosis. J Neurol Sci 313: 137–141 [DOI] [PubMed] [Google Scholar]
- Tselis A. (2012) Epstein–Barr virus cause of multiple sclerosis. Curr Opin Rheumatol 24: 424–428 [DOI] [PubMed] [Google Scholar]
- Tulic M., Andrews D., Crook M., Charles A, Tourigny M., Moqbel R., et al. (2012) Changes in thymic regulatory T-cell maturation from birth to puberty: differences in atopic children. J Allergy Clin Immunol 129: 199–206 [DOI] [PubMed] [Google Scholar]
- Tzartos J., Khan G., Vossenkamper A., Cruz-Sadaba M., Lonardi S., Sefia E., et al. (2012) Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology 78: 15–23 [DOI] [PubMed] [Google Scholar]
- Urry Z., Chambers E., Xystrakis E., Dimeloe S., Richards D., Gabryšová L., et al. (2012) The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3(+) and IL-10(+) CD4(+) T cells. Eur J Immunol 42: 2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Välimäki V., Löyttyniemi E., Välimäki M. (2007) Vitamin D fortification of milk products does not resolve hypovitaminosis D in young Finnish men. Eur J Clin Nutr 61: 493–497 [DOI] [PubMed] [Google Scholar]
- Van Amerongen B., Dijkstra C., Lips P., Polman C. (2004) Multiple sclerosis and vitamin D: an update. Eur J Clin Nutr 58: 1095–1109 [DOI] [PubMed] [Google Scholar]
- Van der Mei I., Dore D., Winzenberg T., Blizzard L., Jones G. (2012a) Vitamin D deficiency in Tasmania: a whole life perspective. Intern Med J 42: 1137–1144 [DOI] [PubMed] [Google Scholar]
- Van der Mei I., Ponsonby A., Blizzard L., Dwyer T. (2001) Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology 20: 168–174 [DOI] [PubMed] [Google Scholar]
- Van der Mei I., Ponsonby A., Dwyer T., Blizzard L., Simmons R., Taylor B., et al. (2003) Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 327: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Mei I., Ponsonby A., Dwyer T., Blizzard L., Taylor B., Kilpatrick T., et al. (2007a) Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 254: 581–590 [DOI] [PubMed] [Google Scholar]
- Van der Mei I., Ponsonby A., Engelsen O., Pasco J., McGrath J., Eyles W., et al. (2007b) The high prevalence of vitamin D insufficiency across Australian population is only partly explained by season and latitude. Environ Health Perspect 115: 1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Mei I., Simpson S., Jr, Knippenberg S., Winzenberg T., Taylor B. (2012b) Role of vitamin D in multiple sclerosis: implications for disease management. Neuroden Dis Manage 1: 523–536 [Google Scholar]
- Van Etten E., Gysemans C., Branisteanu D., Verstuyf A., Bouillon R., Overbergh L., et al. (2007) Novel insights in the immune function of the vitamin D system: synergism with interferon-beta. J Steroid Biochem Mol Biol 103: 546–551 [DOI] [PubMed] [Google Scholar]
- Van Etten E., Mathieu C. (2005) Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97: 93–101 [DOI] [PubMed] [Google Scholar]
- Van Etten E., Stoffels K, Gysemans C., Mathieu C., Overbergh L. (2008) Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev 66(10 Suppl. 2): S125–S134 [DOI] [PubMed] [Google Scholar]
- Vatanparast H., Calvo M., Green T., Whiting S. (2010) Despite mandatory fortification of staple food, vitamin D intakes of Canadian children and adults are inadequate. J Steroid Biochem Mol Biol 121: 301–303 [DOI] [PubMed] [Google Scholar]
- Vedman C., Cantorma M., DeLuca H. (2000) Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374: 334–338 [DOI] [PubMed] [Google Scholar]
- Vieth R. (1999) Vitamin D supplementation, 25-hydoxyvitamin D concentrations, and safety. Am J Clin Nutr 69: 842–849 [DOI] [PubMed] [Google Scholar]
- Vieth R. (2006) What is the optimal vitamin D status for health? Prog Biophys Mol Biol 92: 26–32 [DOI] [PubMed] [Google Scholar]
- Vieth R. (2007) Vitamin D toxicity, policy and science. J Bone Mineral Res 22(Suppl. 2): V64–V68 [DOI] [PubMed] [Google Scholar]
- Vieth R., Bischoff-Ferrari H., Boucher B., Dawson-Hughes B., Garland C., Heaney R., et al. (2007) The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85: 649–650 [DOI] [PubMed] [Google Scholar]
- Von Essen M., Kongsbak M., Schjerling P., Olgaard K., Odum N., Geisler C. (2010) Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 4: 344–349 [DOI] [PubMed] [Google Scholar]
- Von Geldern G., Mowry E. (2012) The influence of nutritional factors on the prognosis of multiple sclerosis. Nat Rev Neurol 13: 678–689 [DOI] [PubMed] [Google Scholar]
- Vukusic S., Van Bokstael V., Gosselin S., Confavreux C. (2007) Regional variations of multiple sclerosis prevalence in French farmers. J Neurol Neurosurg Psychiatry 78: 707–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hennig H., Jabs W., Siekhaus A., Wessel K., Wandinger K. (2000) Altered prevalence and reactivity of anti-Epstein-Barr virus antibodies in patients with multiple sclerosis. Viral Immunol 13: 497–502 [DOI] [PubMed] [Google Scholar]
- Walters M. (1992) New identified actions of the vitamin D endocrine system. Endocr Rev 13: 719–764 [DOI] [PubMed] [Google Scholar]
- Wandinger K., Jabs W., Siekhaus A., Bubel S., Trillenberg P., Wagner H., et al. (2000) Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 55: 178–184 [DOI] [PubMed] [Google Scholar]
- Wang T., Tavera-Mendoza L., Laperriere D., Libby E., MacLeod N., Nagai Y. (2005) Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19: 2685–2695 [DOI] [PubMed] [Google Scholar]
- Wang Y., Marling S., Zhu J., Severson K., DeLuca H. (2012) Development of experimental autoimmune encephalomyelitis (EAE) in mice requires vitamin D and the vitamin D receptor. Proc Natl Acad Sci U S A 109: 8501–8504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergeland S., Torkildsen O, Myhr K., Aknes L., Mørk S., Bø L. (2011) Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One 6: e26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting S., Calvo M. (2010) Correcting poor vitamin D status: do older adults need higher repletion doses of vitamin D(3) than younger adults? Mol Nutr Food Res 54: 1077–1084 [DOI] [PubMed] [Google Scholar]
- Willer C., Dyment D., Sadovnick A., Rothwell P., Murray T., Ebers G., et al. (2005) Timing of birth and risk of multiple sclerosis: population based study. BMJ 330: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S., Stadelmann C., Rodig S., Caron T., Gattenloehner S., Mallozzi S., et al. (2009) Epstein–Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 132: 3318–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D. (2012) Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord 5: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D., Lesaux J., Rice J., Kremenchtzky M., Ebers G. (2005) A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76: 1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetley E. (2008) Assessing the vitamin D status of the US population. Am J Clin Nutr 88: 558S–564S [DOI] [PubMed] [Google Scholar]
- Yildiz M., Tettenborn B., Putzki N. (2011) Vitamin D levels in Swiss multiple sclerosis patients. Swiss Med Wkly 141: w13192. [DOI] [PubMed] [Google Scholar]
- Yu S., Cantorna M. (2011) Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol 186: 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehdner D., Bland R., Williams M., McNinch R., Howie A., Stewart P., et al. (2001) Extrarenal expression of 25-hydoxyvitamin D(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86: 888–894 [DOI] [PubMed] [Google Scholar]
- Zerwekh J. (2008) Blood biomarkers on vitamin D status. Am J Clin Nutr 87: 1087S–1091S [DOI] [PubMed] [Google Scholar]
- Zhang Y., Leung D., Richers B., Liu Y., Remiglio L., Riches D., et al. (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]