Abstract
We have previously reported an increase in interleukin (IL)-1β and IL-17 levels, and a continuous activation of caspase-1 in early rheumatoid arthritis (RA) patients. These results suggest that drugs targeting IL-1β regulatory pathways, in addition to tumor necrosis factor (TNF), may constitute promising therapeutic agents in early RA. We have recently used a THP-1 macrophage-like cell line to screen 2320 compounds for those that down-regulate both IL-1β and TNF secretion. Celastrol was one of the most promising therapeutic candidates identified in that study. Our main goal in the present work was to investigate whether administration of celastrol is able to attenuate inflammation in a rat model of adjuvant-induced arthritis (AIA). Moreover, since IL-1β is known to play a role in the polarization of Th17 cells, we also investigate whether administration of digoxin, a specific inhibitor of Th17 cells polarization, is able to attenuate inflammation in the same rat model. We found that celastrol administration significantly suppressed joint inflammation. The histological and immunohistochemical evaluation revealed that celastrol-treated rats had a normal joint structure with complete abrogation of the inflammatory infiltrate and cellular proliferation. In contrast, we observed that digoxin administration significantly ameliorated inflammation but only if administrated in the early phase of disease course (after 4 days of disease induction), and it was not efficient at inhibiting the infiltration of immune cells within the joint and in preventing damage. Thus, our results suggest that celastrol has significant anti-inflammatory and anti-proliferative properties and can constitute a potential anti-inflammatory drug with therapeutic efficacy in the treatment of immune-mediated inflammatory diseases such as RA. Furthermore, we find that early inhibition of Th17 cells polarization ameliorates arthritis but it is not as effective as celastrol.
Keywords: Celastrol, Digoxin, Wistar AIA rat model, IL-1β, Caspase-1, Th17 cells
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic immune-mediated inflammatory disease characterized by synovial hyperplasia caused by a large proliferative cellular infiltrate of immune cells, high expression of proinflammatory cytokines and consequent erosion and remodelling of joint cartilage and bone. RA management strategy has been revolutionized in the last decade with the discovery of specific treatments targeted against cytokines, such as tumor necrosis factor (TNF), and immune (B and T) cells. The current treatment goal for RA is to achieve a state of disease remission but, despite all available therapeutic approaches, RA remains an incurable, progressive, debilitating and destructive disease with only 20% of patients reaching remission [1]. Moreover, these novel treatment strategies are only effective in around 70% of the patients, with many of them eventually loosing response to the drugs or being forced to interrupt drug administration due to adverse effects. Anti-TNF treatment has been shown to be more effective when introduced early in the disease course [2,3]. However, still a relatively large proportion of patients fail to respond in these optimal conditions.
We have previously reported increased levels of interleukin (IL)-1β in very recent onset arthritis and in the synovial fluid of established RA patients [4]. This observation may be explained by activation of caspase-1, the pro-inflammatory enzyme which is activated by the inflammasome and which is responsible for the processing of pro-IL-1β, which we have also observed to be increased both in early and established RA patients [4]. Interestingly, the role of the inflammasomes has been recently addressed in the context of RA. In fact, it has been reported that polymorphisms in NLRP3 and CARD8 inflammasome genes are associated with anti-citrullinated protein antibodies (ACPA)-positive RA, an increased susceptibility for the disease and a worse prognosis for these patients [5–7]. Inflammasomes are activated by several different foreign and self-antigens and recent evidences suggest that these multiprotein complexes may participate in the development of the new syndrome termed ASIA, ‘Autoimmune (Auto-inflammatory) Syndrome [8], which is induced by adjuvants and assembles a spectrum of immune-mediated diseases triggered by an adjuvant stimulus [9,10].
The importance of IL-1β in the early phase of RA is further highlighted by reports of its ability to promote the differentiation of Th17 cells [11,12] through the induction of the transcription factors IFR4 and RORγt expression [11]. These cells are characterized by the production of IL-17, a cytokine that is also up-regulated in the early phase of RA [13]. Interestingly, IL-17 serum levels and Th17 frequency are decreased in Cryopyrin-associated periodic syndromes (CAPS) patients following in vivo IL-1β blockage [14]. Therefore, it is possible that IL-1β plays an important role in early rather than late stages of the disease and that pathways regulating this cytokine and TNF, such as the inflammasome/caspase-1 and NF-kB, can potentially constitute promising combined therapeutic targets. Based on this background and on the results of a recent drug screen performed in our laboratory for compounds that simultaneously inhibit IL-1β and TNF secretion (Figueiredo et al., unpublished), we have identified celastrol as a promising therapeutic candidate for arthritis. Celastrol, a pentacyclic-triterpene extract from Trypterigium wilfordii Hook, is used in traditional Chinese medicine and was recently shown to possess anti-tumor [15,16] and anti-inflammatory [17] effects. Our aim in this study was to investigate whether celastrol administration is able to attenuate inflammation in a rat model of adjuvant-induced arthritis (AIA) and which mechanisms might be important for its protective effect. Moreover, since IL-1β is known to play a role in the polarization of Th17 cells, we have also analyzed the anti-inflammatory and anti-proliferative properties of digoxin, a specific inhibitor of RORγt transcriptional activity and consequently inhibitor of Th17 cells polarization [18], in the same rat model. We found that celastrol, in contrast to digoxin, has significant anti-inflammatory and anti-proliferative properties and can putatively constitute an anti-inflammatory drug with therapeutic efficacy in the treatment of immune-mediated inflammatory diseases such as RA.
2. Methods
2.1. Compounds
Celastrol and digoxin were purchased from Sigma (Missouri, USA).
2.2. IL-1β and TNF secretion assay
THP-1 cells were stimulated with 4% PFA-fixed DH5 Escherichia coli (E. coli) at a Multiplicity of Infection (MOI) of 20 bacterial cells per THP-1 cell, 1 hour after incubation with celastrol. Cell supernatants were collected and IL-1β and TNF cytokines quantified by enzyme linked immunosorbent assay (ELISA) technique (R&D systems, Minnesota, USA) according to the provider’s instructions.
2.3. Cell culture
THP-1 (ATCC TIB-202) macrophage-like cell line and THP-1/NF-kB reporter cell line were cultured in R10 - RPMI media 1640 supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) penicillin-streptomycin, 1% (v/v) pyruvate, 1% (v/v) L-glutamine, 1% (v/v) non-essential aminoacids, 1% (v/v) hepes buffer and 2-mercaptoethanol to a final concentration of 0.05 M, as recommended by the American Tissue Culture Collection (ATCC). Cells were cultured at 250.000 cells/mL, incubated with 10 μM of celastrol for 1 h at 37 °C 5% CO2, and then stimulated with PFA-fixed E. coli (20 E. coli per cell) for 8 h and 24 h at 37 °C 5% CO2. Simultaneously, non-stimulated negative control cells were also cultured at the same density as the stimulated population for comparison. Caspase-1 activity was measured in THP-1 macrophage-like cell line using the Carboxyfluorescein FLICA Detection kit for Caspase Assay (Immunochemistry Technologies, LLC, Minnesota, USA) following the reagent instructions. Briefly, cells from the different assays were protected from light exposure while incubated for 1 hour at 37 °C with 30X FLICA solution at a 1:30 ratio. NF-kB activity was measured in THP-1/NF-kB reporter cell line. Lentiviral particles carrying a NF-kB-responsive GFP-expressing reporter gene (Cignal Lenti Reporters, SABiosciences, Maryland, USA) were used to infect THP-1 cells and to establish a stable cell line. All samples were analyzed by flow cytometry using a FACS Calibur (BD biosciences, New Jersey, USA). The data collected were further analyzed using FlowJo software (Tree Star Inc, Oregon, USA).
2.4. Animal experimental design
Wistar AIA rats were purchased from Charles River Laboratories International (Massachusetts, USA). Female Wistar AIA rats weighing 125–150 g were maintained under specific pathogen free (SPF) conditions and all experiments were approved by the Animal User and Ethical Committees at the Instituto de Medicina Molecular, according to the Portuguese law and the European recommendations. Celastrol and digoxin were administrated at a dose of 1 μg/g and 2 μg/g body weight every day, respectively [18,19]. Drugs and vehicle control were dissolved in normal saline solution and injected intraperitoneally to AIA rats (N=5–10 animals per group) after 4 days (early treatment group) and after 11 days (late treatment group) of disease induction, when arthritis was already present. The inflammatory score, ankle perimeter and body weight were measured during the period of treatment. Inflammatory signs were evaluated by counting the score of each joint in a scale of 0–3 (0 — absence; 1 — erythema; 2 — erythema and swelling; 3 — deformities and functional impairment) [20]. The total score of each animal was defined as the sum of the partial scores of each affected joint. Rats were sacrificed after 19 days of disease evolution and paw samples were collected for histological and immunohistochemical evaluation.
2.5. Histological and immunohistochemical evaluation
For histopathological observation, paw, lung, liver, kidney and pancreas samples were collected at the time of sacrifice. Samples were fixed immediately in 10% neutral buffered formalin solution and then dehydrated with increasing ethanol concentrations (70%, 96% and 100%). Paw samples, after being fixed, were also decalcified in 10% formic acid. Samples were next embedded in paraffin, sectioned and stained with hematoxylin and eosin for morphological examination. Paws were also used for immunohistological staining with Ki67 antibody, a cellular proliferation marker. Tissue sections were incubated with primary antibody against rat polyclonal Ki67 (Abcam, Cambridge, UK) and with EnVision+ (Dako, Glostrup, Denmark). Colour was developed in solution containing diaminobenzadine-tetrahydrochloride (Sigma, Missouri, USA), 0.5% H2O2 in phosphate-buffered saline buffer (pH 7.6). Slides were counterstained with hematoxylin and mounted. All images were acquired using a Leica DM 2500 (Leica microsystems, Wetzlar, Germany) microscope equipped with a colour camera. Data regarding the degree of proliferation of synovial cells was scored from 0–3 (0 — fewer than three layers; 1 — three to four layers; 2 — five to six layers; 3 — more than six layers). Lymphoid cell infiltration was scored from 0–3 (0 — none to diffuse infiltration; 1 — lymphoid cell aggregate; 2 — lymphoid follicles; 3 — lymphoid follicles with germinal center formation) [21].
2.6. Intracellular IL-17 staining
Spleen cells were cultured in complete cell culture media at 37 °C 5% CO2. Cells were stimulated for 3 h with brefeldin A (0.01 mg/ml) (Epicenter Technologies, Nebraska, USA), phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) (Sigma, Missouri, USA) and ionomycin (500 ng/ml) (Calbiochem, Darmstadt, Germany). Next, cells were permeabilised using saponin (Sigma, Missouri, USA) and stained with anti-IL17 FITC (Biolegend, California, USA) to detect intracellular IL-17. Lastly, cells were acquired with a FACS LSR Fortessa (BD biosciences, New Jersey, USA) and data collected were further analyzed using FlowJo software (Tree Star Inc, Oregon, USA).
2.7. Statistical analysis
Statistical differences were determined with non-parametric Kruskal-Wallis and Mann–Whitney tests using GraphPad Prism (GraphPad, California, USA). Differences were considered statistically significant for p<0.05.
3. Results
3.1. Celastrol decreases IL-1β and TNF secretion
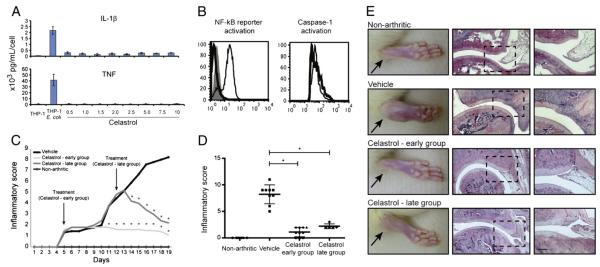
Based on the hypothesis that drugs which block the secretion of both IL-1β and TNF might be particularly effective at decreasing early disease activity in RA, we have recently used the human THP-1 macrophage-like cell line to screen for drugs that can simultaneously down-regulate the secretion of both cytokines. Among the 2320 tested drugs included in the Spectrum collection (Microsource Discovery Systems, Connecticut, USA), we found 45 that significantly decrease the levels of both IL-1β and TNF secretion (Figueiredo et al., unpublished). We further narrowed the selection by taking into account the possible human tolerability to chronic exposure and other biological properties that could be of interest in the context of RA. Celastrol was thus selected for testing in a rat model of adjuvant-induced arthritis (AIA), due to its prior human use in traditional Chinese medicine and due to its concomitant alleged anti-proliferative effects [15,16]. We started by validating the effect of celastrol in the inhibition of IL-1β and TNF secretion in a human THP-1 macrophage-like cell line, by using increasing concentrations of celastrol for 1 hour before challenging them with PFA-fixed E. coli for 6 hours. The conditioned media was then probed for the secretion of either IL-1β or TNF using ELISA technique. Celastrol was very effective at inhibiting the secretion of both cytokines over a wide range of tested concentrations (Fig. 1A).
Fig. 1.
(A) IL-1β and TNF secretion are inhibited by celastrol treatment. Conditioned media samples from human THP-1 macrophage-like cell line cultured with growing concentrations of celastrol were analyzed by ELISA technique. Differences were considered statistically significant for p values<0.05. (B) The activation of NF-kB reporter and caspase-1 is decreased with celastrol treatment. NF-kB expression was measured by flow cytometry in a THP-1/NF-kB reporter cell line incubated with celastrol and then stimulated for 24 h with E. coli. Each thin line in the histogram corresponds to untreated but E. coli stimulated cells, the shaded area corresponds to drug-treated and E. coli stimulated cells and the thick line corresponds to untreated non-stimulated cells, used as a control. Caspase-1 activation was measured using flow cytometry in a THP-1 cell line incubated with celastrol and then stimulated for 8 h with E. coli. Each thin line in the histogram corresponds to untreated but E. coli stimulated cells used as control and the thick line corresponds to drug-treated and E. coli stimulated cells. (C) Celastrol is able to suppress inflammation throughout time. Notice that after 6 days of treatment the vehicle injected group increased the inflammatory manifestations sharply, whereas in celastrol-treated rats there was minimal inflammatory activity or even complete abrogation of arthritis manifestations. Arrows indicate the beginning of treatment after 4 and 11 days of disease induction. Differences were considered statistically significant for p values<0.05. (D) Celastrol possesses anti-inflammatory properties. Inflammation score in celastrol-treated AIA rats is maintained significantly diminished in comparison with vehicle-treated rats after treatment. Differences were considered statistically significant for p values<0.05. (E) Histological evaluation of joints after celastrol treatment. Notice that celastrol has completely prevented immune cellular infiltration and bone and cartilage invasion, allowing for a normal joint structure comparable to non-arthritic rats in both early and late treatment groups. Magnification 50× and 100×. Bars: 100 μm.
3.2. Celastrol inhibits the activation of NF-kB and caspase-1
The sequence of events culminating in IL-1β secretion is complex, but it can be summarized in two steps: induction of pro-IL-1β and its processing by activated caspase-1 (reviewed in [22]). Both pro-IL-1β and TNF depend on NF-kB activation for the transcription of their respective mRNAs. We therefore tested the effect of celastrol on these key pathways. To investigate its effect in the activation of NF-kB, we used an NF-kB reporter cell line made by stably infecting THP-1 cells with a commercial lentiviral GFP reporter under the control of a minimal CMV promoter and tandem repeats of the NF-kB transcriptional response element (TRE). We found that celastrol was able to suppress NF-kB reporter activation upon E. coli stimulation in comparison with cells that were also stimulated but did not receive treatment (Fig. 1B). To test the effect of this drug in caspase-1 processing and activation we used a caspase-1 fluorescent substrate and measured the relative active caspase-1 levels using FACS. Also in this case, celastrol administration decreased the activation of caspase-1 (Fig. 1B). We can thus conclude that celastrol inhibits NF-kB and caspase-1 activation.
3.3. Celastrol is able to suppress inflammation in Wistar rat adjuvant-induced arthritis
To study the anti-inflammatory properties of celastrol in vivo, AIA rats were treated daily with this drug after the disease had already become symptomatic. We started the treatment after 4 days of disease induction (early treatment group) and after 11 days of disease induction (late treatment group). The inflammatory score and ankle perimeter were evaluated during the period of treatment. As shown in Fig. 1C, all animals already presented arthritis by the fourth day of disease induction, which corresponds to the first day of treatment. After 6 days of treatment the vehicle-injected group increased the inflammatory manifestations sharply, while in early celastrol-treated rats there was minimal inflammatory activity or even complete abrogation of arthritis manifestations. In the late treatment group, drug administration was started after 11 days of disease evolution, when animals presented a mean inflammatory score of 5. Also in this group, by the second day of treatment with celastrol the inflammatory manifestations started to significantly decrease over time. This result shows that this drug has anti-inflammatory effects even when administrated in a later phase of arthritis. After 15 (early treatment group) and 8 (late treatment group) days of treatment, celastrol showed significant anti-inflammatory effects, as assessed by the evaluation of the inflammatory score shown in Fig. 1D and also by the evaluation of ankle perimeter (p=0.007 in early and late treatment groups vs. untreated animals).
3.4. Celastrol prevents joint immune cells infiltration and proliferation as well as cartilage and bone erosions
To evaluate the infiltration of immune cells within joints of AIA rats, joint tissue sections stained with hematoxylin and eosin were performed. The histological evaluation shown in Fig. 1E revealed that rats treated with celastrol had a normal joint structure with complete abrogation of the inflammatory infiltrate (p<0.0001 in early and p=0.006 in late treatment group vs. untreated animals). We also studied cell proliferation by staining joint tissue sections with Ki67. The immunohistochemical results revealed that rats treated with celastrol presented a reduced level of immune cells proliferation in the early treatment group (p=0.0009 vs. untreated animals). The late treatment group showed a less effective proliferation decrease (p=0.046 vs. untreated animals), even though the drug successfully diminished the inflammatory score. Early and late treatment with celastrol prevented cartilage and bone damage (Fig. 1E). We have not observed significant differences in body weight or any other side effects in treated rats, as revealed during autopsy and histological analysis made in lung, liver, kidney and pancreas (data not shown).
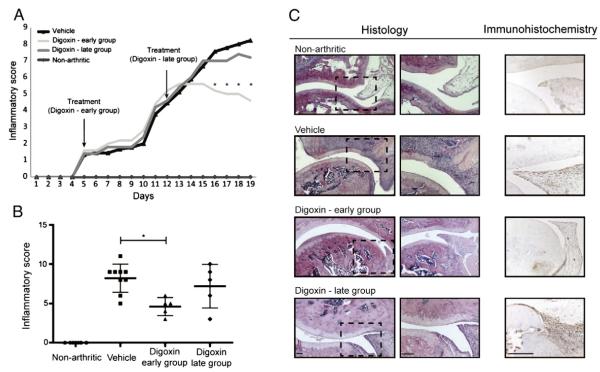
3.5. Digoxin delayed the course and reduced the severity of arthritis
To study the in vivo anti-inflammatory properties of digoxin, AIA rats were treated daily with this drug using the same experimental setup used for celastrol. As shown in Fig. 2A, after 11 days of treatment, we observed a delay in the course of arthritis in the early digoxin-treated rats, with a reduction in the severity of inflammatory signs in comparison with the vehicle-injected group. After 19 days of disease induction, digoxin showed significant anti-inflammatory effects, as assessed by the evaluation of the inflammatory score (Fig. 2B) and also by the evaluation of ankle perimeter (p=0.007 in early vs. untreated animals). In the late treatment group, there were no statistically significant differences in the inflammatory score when compared with the vehicle-treated rats (Fig. 2B). This result suggests that digoxin has anti-inflammatory effects only when administrated in the early phase of arthritis. Additionally, we observed that the percentage of IL-17-producing T cells in digoxin-treated rats was reduced as compared to vehicle-treated rats (p=0.0286 in early and p=0.0286 in late treatment group vs. untreated animals).
Fig. 2.
(A) Digoxin is able to reduce the severity of inflammation throughout time. After 11 days of treatment, the digoxin-injected group started to progressively reduce inflammatory manifestations. Arrow indicates the beginning of treatment after 4 and 11 days of disease induction. (B) Digoxin possesses anti-inflammatory properties. Inflammation score in early digoxin-treated AIA rats is significantly diminished in comparison with vehicle-treated rats after treatment. Of note, when treatment begins in the later phase of inflammation it has no effect in reducing the inflammatory process. Differences were considered statistically significant for p values<0.05. (C) Histological and immunohistochemical evaluation of joints after 15 days of treatment. Notice that digoxin reduced immune cellular proliferation only if treatment administration started in the early phase of arthritis but had no effect in immune cell infiltration within joints. Magnification 50× and 100× in histological images and a magnification 200× in immunohistochemical images. Bars: 100 μm.
3.6. Digoxin prevents proliferation but not infiltration of immune cells within joints
The histological evaluation shown in Fig. 2C revealed that digoxin was not able to suppress the infiltration of immune cells within joints (p=0.1201 in early and p=0.3475 in late treatment group vs. untreated animals). Furthermore, the immunohistochemical results revealed that rats treated with digoxin presented a reduced level of immune cell proliferation in the early treatment group (p=0.0042 vs. untreated animals), in contrast with the late treatment group which showed no effect in immune cell proliferation (p=0.4100 vs. untreated animals). Minimal cartilage and bone damage was present both in early and late digoxin treated animals (Fig. 2C). Also in the case of digoxin, we have not observed significant differences in body weight or any other side effects in treated rats, as revealed during autopsy and histological results (data not shown).
4. Discussion
In the present study, we demonstrated that AIA can be effectively treated through a possible inhibitory effect over IL-1β and TNF secretion induced by celastrol. The effect of this compound was profound as it induced a complete abrogation of joint immune cellular infiltration and proliferation, preventing cartilage and bone damage.
Celastrol is a novel compound that has been shown to inhibit cancer progression and NF-kB activity [15,16,23]. Our results reveal that the anti-inflammatory properties of this drug might not only be related with its ability to inhibit the activation of NF-kB but also with its capacity to inhibit caspase-1 activation. Celastrol has also been reported to abrogate the release of IL-1β in LPS-stimulated human peripheral mononuclear cells [24] and to exert anti-inflammatory properties in animal models [17,25]. Interestingly, Pinna et al. described that celastrol inhibited pro-inflammatory cytokine secretion from mucosal inflammatory biopsies from Crohn’s patients, possibly due to the abrogation of cytokine gene transcription [26]. Of note, tripterine isolated from Tripterygium wilfordii Hook F has previously been shown to be effective on adjuvant [27] and collagen-induced arthritis [25] in rats, supporting the 2002 report showing that an ethanol/ethyl acetate extract of Tripterygium wilfordii Hook F shows therapeutic benefit in patients with refractory RA [28]. Furthermore, in an in vivo model of metastatic bone disease associated with breast cancer, celastrol inhibited bone resorption, consistent with the inhibitory effect on osteoclast formation and survival observed in in vitro experiments [19]. Of interest, these data support our findings that celastrol suppresses synovial immune cells infiltration and proliferation, preventing bone erosions. Importantly, celastrol treatment is effective when administrated both in the early and more established phase of arthritis which is relevant for the possible clinical implications of our findings. In RA the infiltration of immune cells and the proliferation of joint lining synovial fibroblasts lead to the formation of the tumor-like pannus tissue, which invades and destroys joint cartilage and bone. The cell proliferation inhibitory effect of celastrol may thus prove to be of interest to prevent and treat this complication of established RA.
Additionally, we also found that digoxin is able to ameliorate inflammatory signs in the same AIA rat model of arthritis. This is in agreement with the recent report from Huh et al. in which it was shown that digoxin was able to delay the onset and reduce disease severity in an experimental autoimmune encephalomyelitis (EAE) mice model, through the inhibition of RORγt transcriptional activity and, consequently, of Th17 cells differentiation [18]. However, despite our observation that digoxin was able to suppress the severity of inflammatory signs, we also found that it was not able to efficiently reduce the infiltration of immune cells within the joints. Importantly, we observed that digoxin treatment was only effective if the drug was administrated in the early phase of arthritis development, in contrast to what we found in the case of celastrol. In fact, blocking IL-1β and TNF simultaneously results in a significant inhibitory effect in arthritis progression and severity, even when administrated in a later phase of disease course, with a complete abrogation of the inflammatory score, infiltration and proliferation of immune cells within joints and prevention of structural damage. In contrast, we observed that digoxin had a slower and less efficient effect on disease progression. These data indicate that IL-1β, in the context of arthritis, might play a role independent from Th17/IL-17. Besides inducing Th17 cell polarization, IL-1β also directly stimulates the influx of neutrophils and macrophages into the damaged site. These cells in turn can destroy the tissue by the release of proteases and reactive oxygen species [29], and also by the formation of osteoclasts [30] leading to tissue damage and consequent functional disability characteristic of RA patients. IL-1β, together with IL-6 and TNF, also has a potent capacity to induce the receptor activator of nuclear factor kappa-B ligand (RANKL) expression on synovial fibroblasts/osteoblasts and to facilitate RANK signaling, thus directly contributing to the bone destruction process. In contrast, IL-17 seems to have a more limited effect on inflammatory cell influx and consequent inflammatory symptoms, as we observed in this study. Moreover, in agreement with this fact, a recent phase II study testing an anti-IL-17A drug in RA did not achieve its primary end point [31]. This does not preclude the important involvement of Th17 cells in driving the innate immune inflammation towards the adaptive (auto)immune chronic inflammation in RA, involving several other cytokines apart from IL-17A [32].
Despite this apparent crucial role of IL-1β signaling in RA, clinical benefits after IL-1β inhibition have been modest compared to anti-TNF drugs, at least in moderate to severe long established RA. Further, in 2004 a study which tested the efficacy of combination therapy using anakinra and etanercept in 244 long-standing and very active RA patients who have been treated unsuccessfully with MTX showed that concomitant IL-1β and TNF inhibition provides no added benefit and increased infections as compared to etanercept alone [33]. Possibly, in the context of RA inhibiting the IL-1β pathway at the receptor level is not an effective strategy but an upstream inhibition might work better, at least, in animal models. On top of that, downregulating TNF and IL-1β production might be safer than inhibiting completely TNF and IL-1β.
5. Conclusions
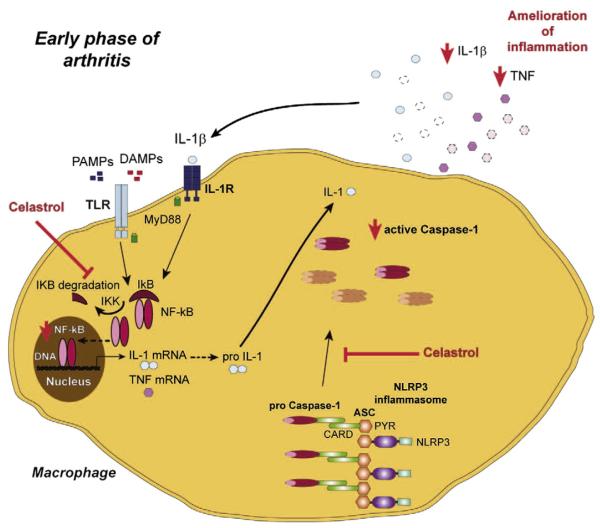
In conclusion, celastrol can putatively constitute an anti-inflammatory and anti-proliferative drug with therapeutic efficacy in the treatment of immune-mediated inflammatory diseases such as RA, possibly through the down-regulation of caspase-1 and inhibition of NF-kB activation (Fig. 3). Its anti-proliferative effects might be of additional value in RA to counteract the formation of the characteristic pannus leading to the destruction of cartilage and bone. In addition, we have shown that despite the ability to decrease the severity of early inflammatory signs in AIA, digoxin is not effective in reducing the infiltration of immune cells within the joints. We thus suggest that the isolated inhibition of Th17 polarization might be a strategy with limited efficacy, at least in established RA. Further animal experimentation is required to determine the real efficacy and safety of celastrol for arthritis treatment but these results suggest that it might be worth of entering into phase I clinical trials. Simultaneously, this study highlights the need for more research on the role of the inflammasome/caspase-1 and NF-kB pathways in the etiopathology of RA.
Fig. 3.
Proposed mechanism for celastrol anti-inflammatory effects. Celastrol showed anti-inflammatory and anti-proliferative properties in vivo, promoting a complete suppression of arthritis development and abrogation of joint immune cellular infiltration and proliferation, preventing cartilage and bone damage. This compound induced a down-regulation of caspase-1 and NF-kB activation and, consequently, lead to a decrease in IL-1β and TNF secretion. ASC — adaptor molecule apoptosis associated speck-like protein containing a caspase recruitment domain, CARD — caspase recruitment domain, DAMP — danger associated molecular pattern, IL — interleukin, IL-1R — IL-1 receptor, NF-kB — nuclear factor kappa-light-chain-enhancer of activated B cells, NLRP — NOD-like receptor protein, PAMP — pathogen associated molecular pattern, PYR — pyrin domain, TLR — toll-like receptor, TNF — tumor necrosis factor.
Take-home messages.
Celastrol has significant anti-inflammatory and anti-proliferative properties.
Digoxin is not as effective as celastrol treatment for AIA.
Blocking IL-1β and TNF (upstream to their receptors) is effective in arthritis.
Therapies targeting IL-1β pathway should be re-evaluated in RA patients.
Acknowledgements
The authors would like to acknowledge Ana Lopes for technical assistance and Ana Luisa Caetano for technical support with FACS.
Funding This work was supported by a grant (SFRH/BD/40513/2007) from Fundação para a Ciência e a Tecnologia (FCT). Work in Luis Moita’s laboratory is supported by FCT (PIC/IC/82991/2007 and PTDC/SAU-MII/100780/2008) and Fundação Luso-Americana para o Desenvolvimento (FLAD). The Gulbenkian Programme for Advanced Medical Education was sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde e FCT.
List of abbreviations
- RA
rheumatoid arthritis
- TNF
tumor necrosis factor
- IL
interleukin
- ACPA
anti-citrullinated protein antibodies
- AIA
adjuvant-induced arthritis
- CAPS
cryopyrin-associated periodic syndromes
- MOI
multiplicity of infection
- ELISA
enzyme linked immunosorbent assay
- SPF
specific pathogen free
- TRE
transcriptional response element
- EAE
experimental autoimmune encephalomyelitis
- RANKL
receptor activator of nuclear factor kappa-B ligand
Footnotes
Conflict of interests The authors declare that they have no competing interests.
References
- [1].Lindqvist E, Saxne T, Geborek P, Eberhardt K. Ten year outcome in a cohort of patients with early rheumatoid arthritis: health status, disease process, and damage. Ann Rheum Dis. 2002;61:1055–9. doi: 10.1136/ard.61.12.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- [3].Klarenbeek NB, Guler-Yuksel M, van der Kooij SM, Han KH, Ronday HK, Kerstens PJ, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011;70:1039–46. doi: 10.1136/ard.2010.141234. [DOI] [PubMed] [Google Scholar]
- [4].Cascao R, Polido-Pereira J, Canhao H, Rodrigues AM, Navalho M, Raquel H, et al. Caspase-1 is active since the early phase of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(1):0144. [PubMed] [Google Scholar]
- [5].Kastbom A, Johansson M, Verma D, Soderkvist P, Rantapaa-Dahlqvist S. CARD8 p.C10X polymorphism is associated with inflammatory activity in early rheumatoid arthritis. Ann Rheum Dis. 2010;69:723–6. doi: 10.1136/ard.2008.106989. [DOI] [PubMed] [Google Scholar]
- [6].Fontalba A, Martinez-Taboada V, Gutierrez O, Pipaon C, Benito N, Balsa A, et al. Deficiency of the NF-kappaB inhibitor caspase activating and recruitment domain 8 in patients with rheumatoid arthritis is associated with disease severity. J Immunol. 2007;179:4867–73. doi: 10.4049/jimmunol.179.7.4867. [DOI] [PubMed] [Google Scholar]
- [7].Kastbom A, Verma D, Eriksson P, Skogh T, Wingren G, Soderkvist P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project) Rheumatology (Oxford) 2008;47:415–7. doi: 10.1093/rheumatology/kem372. [DOI] [PubMed] [Google Scholar]
- [8].Shoenfeld Y, Agmon-Levin N. ‘ASIA’ — autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- [9].Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5:648–52. doi: 10.1038/nrrheum.2009.196. [DOI] [PubMed] [Google Scholar]
- [10].Balofsky A, Agmon-Levin N, Shoenfeld Y. The new H1N1 and HPV vaccines and old fears. Curr Opin Rheumatol. 2010;22:431–6. doi: 10.1097/BOR.0b013e32833a43c3. [DOI] [PubMed] [Google Scholar]
- [11].Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–6. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- [13].Cascao R, Moura RA, Perpetuo I, Canhao H, Vieira-Sousa E, Mourao AF, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lasiglie D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He D, Xu Q, Yan M, Zhang P, Zhou X, Zhang Z, et al. The NF-kappa B inhibitor, celastrol, could enhance the anti-cancer effect of gambogic acid on oral squamous cell carcinoma. BMC Cancer. 2009;9:343. doi: 10.1186/1471-2407-9-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–21. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- [17].Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–57. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- [18].Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol Cancer Ther. 2009;8:2339–47. doi: 10.1158/1535-7163.MCT-09-0133. [DOI] [PubMed] [Google Scholar]
- [20].da Silva JA, Fonseca JE, Graca L, Moita L, Carmo-Fonseca M. Reinnervation of post-arthritic joints in the rat. Clin Exp Rheumatol. 1996;14:43–51. [PubMed] [Google Scholar]
- [21].Tsubaki T, Arita N, Kawakami T, Shiratsuchi T, Yamamoto H, Takubo N, et al. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthritis Res Ther. 2005;7:R825–36. doi: 10.1186/ar1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- [24].Ngassapa O, Soejarto DD, Pezzuto JM, Farnsworth NR. Quinone-methide triterpenes and salaspermic acid from Kokoona ochracea. J Nat Prod. 1994;57:1–8. doi: 10.1021/np50103a001. [DOI] [PubMed] [Google Scholar]
- [25].Li H, Jia YF, Pan Y, Pan DJ, Li D, Zhang LX. Effect of tripterine on collagen-induced arthritis in rats. Zhongguo Yao Li Xue Bao. 1997;18:270–3. [PubMed] [Google Scholar]
- [26].Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochem Biophys Res Commun. 2004;322:778–86. doi: 10.1016/j.bbrc.2004.07.186. [DOI] [PubMed] [Google Scholar]
- [27].Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- [28].Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–43. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- [29].Cascao R, Rosario HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev. 2010;9:531–5. doi: 10.1016/j.autrev.2009.12.013. [DOI] [PubMed] [Google Scholar]
- [30].Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- [31].Genovese M, Durez P, Richards H, Hugot S, Thangavelu K, Mpofu S. Secukinumab (AIN457), a novel monoclonal antibody targeting IL-17A demonstrates efficacy in active rheumatoid arthritis patients despite stable methotrexate treatment: results of a phase IIb study. ACR. 2010 abstr #L9:2010. [Google Scholar]
- [32].Ferraccioli G, Zizzo G. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov Med. 2011;11:413–24. [PubMed] [Google Scholar]
- [33].Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–9. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]