Abstract
Mucinous neoplasms of the appendix are a heterogeneous group of neoplasms ranging from simple mucoceles to complex pseudomyxoma peritonei. Considerable controversy exists on their pathologic classification and nomenclature. Clear understanding of the histopathologic diversity of these neoplasms helps in establishing proper communication between the radiologist, the pathologist and the surgeon. In this article, we present a brief discussion of the current taxonomy and nomenclature of mucinous neoplasms of the appendix followed by a review of their imaging features. Important points including the significance of identifying extra-appendiceal mucin at imaging, the new classification of pseudomyxoma peritonei into low- and high-grade varieties and the significance of simultaneous ovarian and appendiceal neoplasms are highlighted.
Keywords: Mucinous neoplasms, appendix, WHO classification, pseudomyxoma peritonei, ultrasonography, multidetector computed tomography
Introduction
Primary neoplasms of the appendix are present in less than 2% of surgical appendectomy specimens[1]. The major categories of primary neoplasms include epithelial tumors, mesenchymal tumors and lymphomas. Mucinous neoplasms of the appendix are a complex, diverse group of epithelial neoplasms often causing cystic dilation of the appendix due to accumulation of gelatinous material, morphologically referred to as mucoceles. First described by Rokitansky in 1842[2], clinically and radiologically, mucoceles are often incidentally detected in asymptomatic patients. However, it is important to recognize that the underlying cause of the mucocele can range from simple retention cyst to malignant adenocarcinoma and that rupture of the mucocele can result in the dreaded complication of pseudomyxoma peritonei (PMP)[2]. Accordingly, the goal of this article is to provide an up-to-date review of the pathology and imaging features of the mucinous neoplasms of the appendix including PMP.
Mucinous neoplasms of the appendix: pathology, taxonomy and nomenclature
Cystic dilatation of the appendix results from luminal obstruction caused by either non-neoplastic or neoplastic conditions. Simple mucoceles are retention cysts that can result from an obstructing appendicolith, endometriosis, extrinsic compression or inflammatory conditions. They are uncommon and rarely exceed 2 cm[3–5]. Mucosal hyperplasia and hyperplastic polyps of the appendix can uncommonly be associated with appendiceal dilatation[5]. An appendiceal mucinous neoplasm refers to a tumor associated with neoplastic adenomatous growth (adenoma or adenocarcinoma). Considerable controversy exits regarding the nomenclature of mucinous neoplasms of the appendix. Various classification schemes by Pai and Longacre, Misdraji et al., Carr and Sobin and the 2010 WHO classification have been proposed[6–9]. These are similar in that they all define benign neoplastic adenoma as confined to the mucosa, without mucin or cells penetrating the muscularis mucosa or evidence of perforation. Invasive adenocarcinoma is a frankly invasive neoplastic lesion with cellular invasion beyond the muscularis mucosa. The schemes differ in their classification of adenomatous growths with mucin dissection beyond the muscularis mucosa (termed broad front invasion) or mural perforation, where peritoneal dissemination can lead to PMP[10]. These are variably referred to as low-grade appendiceal mucinous neoplasms (LAMNs) or appendiceal neoplasms of uncertain malignant potential. The 2010 WHO classification recognizes 3 main categories of mucinous neoplasms: mucinous adenoma, LAMN and appendiceal adenocarcinoma[8]. It avoids the use of the terms cystadenomas and mucinous cystadenocarcinomas. Salient features of these 3 neoplasms are listed in Table 1.
Table 1.
Salient features of appendiceal mucinous neoplasms[5–9]
| Mucinous adenoma | Low-grade appendiceal mucinous neoplasm (LAMN) | Mucinous adenocarcinoma |
| Confined to appendiceal mucosa | Non-invasive glands with mucin dissecting beyond the appendix | Invasive glands extending beyond the appendix |
| No extra-appendiceal mucin | Acellular or cellular extra-appendiceal mucin | Invasive epithelium in the extra-appendiceal mucin |
| Not associated with PMP | Associated with low-grade PMP | Associated with high-grade PMP |
| Benign, no recurrences | Frequent recurrences | <10% 10-year survival |
Adenomas are usually low grade, confined to the mucosa of the appendix with no evidence of invasion beyond the muscularis mucosa. Adenomas are classified into tubular, tubulovillous and villous types (Fig. 1)[9]. Appendiceal adenomas, like colonic neoplasms, follow an adenoma-carcinoma sequence and act as premalignant lesions, requiring excision[11]. LAMNs are morphologically well-differentiated adenomas that can proliferate outside the appendix in a malignant fashion and are low-grade tumors[9]. The previous category of mucinous tumors of uncertain malignant potential is now included in the LAMN group. LAMNs are classified by a few authors into mucinous neoplasms with low- and high-risk of recurrence[5]. Mucinous adenocarcinomas are characterized by invasive glands containing high-grade cytologic atypia and extracellular mucin in more than 50% of the lesion[9].
Figure 1.
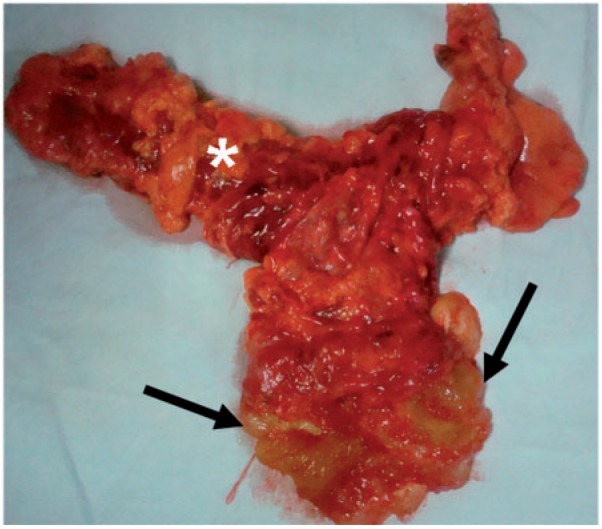
Mucinous adenoma of the appendix. Surgical specimen demonstrating a mucinous neoplasm (arrows) of the appendix. Note the adjacent right colon (*). Histopathology revealed mucinous adenoma.
PMP or mucinous carcinoma peritonei refers to growth of neoplastic mucin-secreting cells in the peritoneal cavity resulting in gelatinous mucinous ascites[10]. Ronnett et al. classified PMP into diffuse peritoneal adenomucinosis (DPAM) and high-grade peritoneal mucinous carcinomatosis (PMCA) based on cytologic atypia and survival rates[12,13]. According to the 2010 WHO classification, PMP is classified into low-grade PMP, which is associated with LAMN, and high-grade PMP, associated with mucinous adenocarcinoma[9]. The terms DPAM and borderline tumors are no longer recommended by the WHO classification.
Clinical features
Mucoceles are rare entities, seen in 0.2–0.3% of appendectomy specimens[14]. They affect women 4 times as often as men. Peak age of incidence is usually after 50 years[14]. However, adenocarcinomas of the appendix in general are more often seen in men in the sixth or seventh decades and have an increased association with other colonic neoplasia and chronic ulcerative colitis[9]. Prospective clinical diagnosis of mucinous neoplasms is often difficult. Half of the cases are asymptomatic, detected incidentally and are often stable for many years[15]. Other cases can present with abdominal pain, weight loss, nausea and vomiting, palpable mass, acute appendicitis, intussusception and localized rupture or peritoneal spread[15,16]. PMP can present with gradual abdominal distention or abdominal hernias.
Mucinous neoplasms may be occasionally detected at endoscopy where they demonstrate the pathognomonic volcano sign, which refers to an erythematous soft cecal mass with central crater from which mucin is discharged[17]. Endoscopy is recommended in patients diagnosed with mucinous neoplasms of the appendix due to increased incidence of synchronous or metachronous colonic polyps and masses[3,8]. Patients with mucinous neoplasms may have increased carcinoembryonic antigen (CEA) levels, which is of diagnostic and prognostic value[18]. Fine-needle biopsy of mucoceles is generally avoided as this can precipitate peritoneal spread and the differential diagnosis at cytology is usually difficult[19]. However, percutaneous biopsy of disseminated peritoneal lesions has high diagnostic yield [20–22].
Imaging of mucinous neoplasms of the appendix
Imaging plays an important role in the diagnosis and management of mucinous appendiceal neoplasms as it may bring attention to asymptomatic neoplasms, allows detection of local invasion, impending or actual rupture, and metastatic spread of these tumors. It also allows evaluation of synchronous or metachronous colonic and ovarian neoplasms.
Conventional radiography
Plain abdominal radiographs generally yield little information, but may occasionally show curvilinear right iliac fossa calcifications, and a mass effect on cecum, bowel or bladder. Barium enema is increasingly an imaging study of the past, and now replaced by more sensitive and specific cross-sectional imaging investigations. Barium enema signs include non-filling of the appendix, well-circumscribed submucosal lesion at the cecal pole, extraluminal compression of the cecum, and concentric ring appearance of cecal mucosal folds directed towards the appendiceal orifice (Fig. 2)[2].
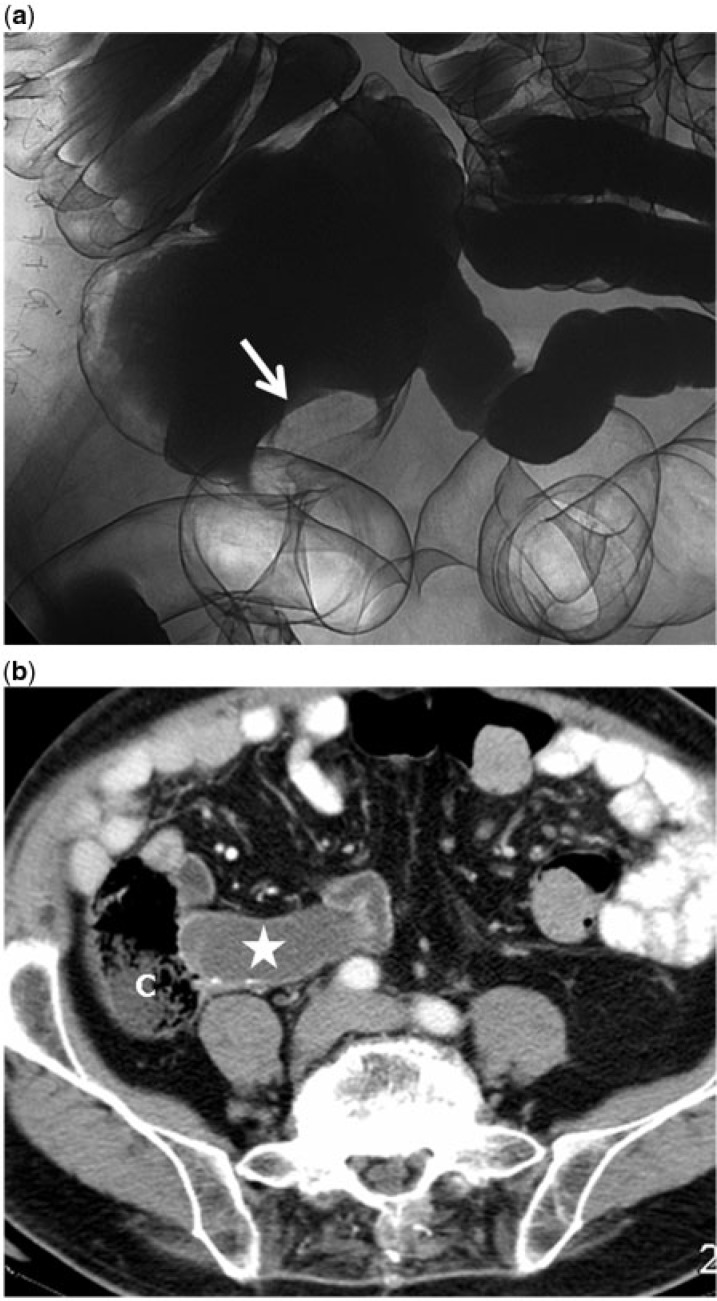
Figure 2.
A 78-year-old asymptomatic man with mucinous adenoma of the appendix confirmed at histopathology. (a) Double-contrast barium enema image showing a smooth oval filling defect at the tip of the cecum (arrow). (b) Contrast-enhanced axial CT image demonstrates the mucocele (star) with peripheral calcification indenting the cecal pole (C).
Ultrasonography
Ultrasonography shows a mucinous neoplasm as an encapsulated, elongated or ovoid cystic lesion in the expected position of the appendix, attached to the cecum. An internal onion-skin appearance, which represents lamellated mucin, is considered pathognomonic (Fig. 3)[23]. A porcelain appendix represents calcification in the appendiceal wall, creating an echogenic near wall and distal acoustical shadowing[2]. Discontinuity of the appendiceal wall, with leakage of internal contents into surrounding tissues indicates rupture of the mucinous neoplasm (Fig. 4).
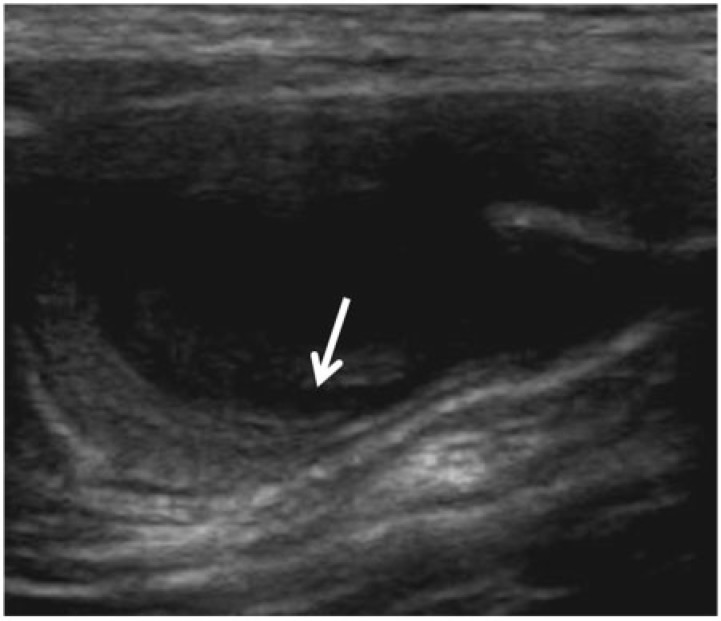
Figure 3.
A 78-year-old man with appendiceal mucinous adenoma manifesting as right lower quadrant pain. Longitudinal sonogram of the right lower quadrant reveals a cystic lesion with onion-skin (arrow) appearance of the intraluminal mucin.
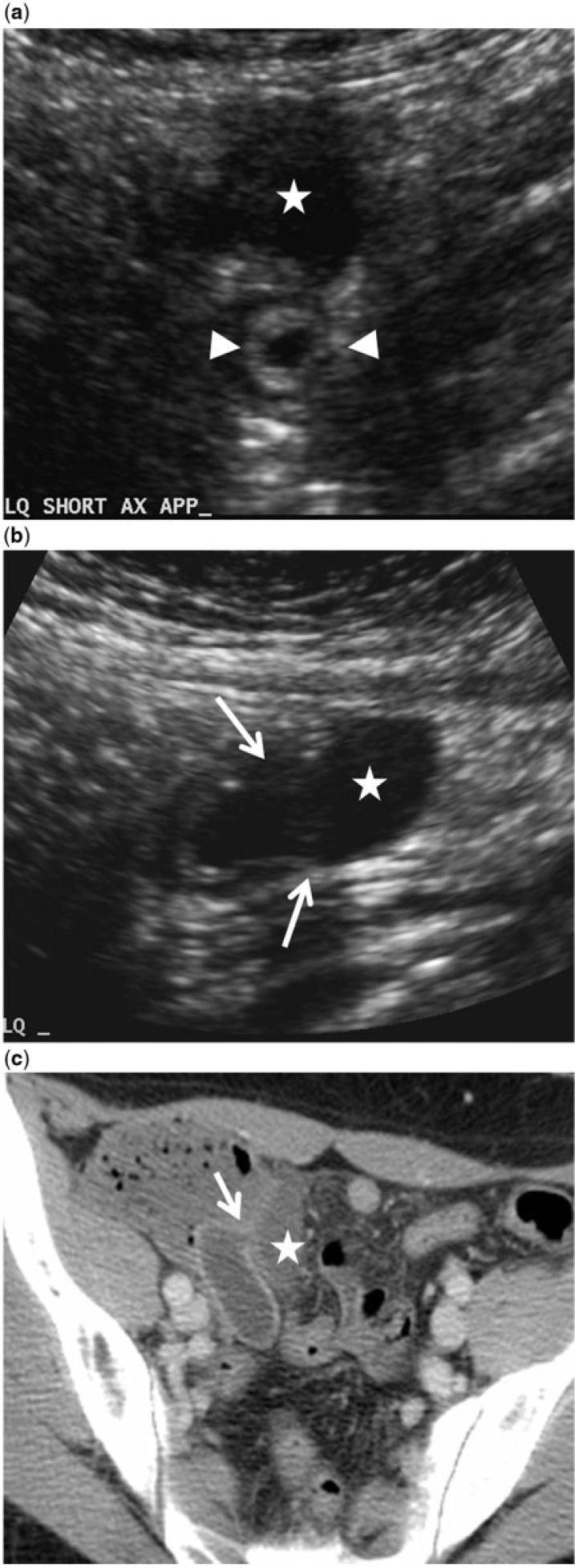
Figure 4.
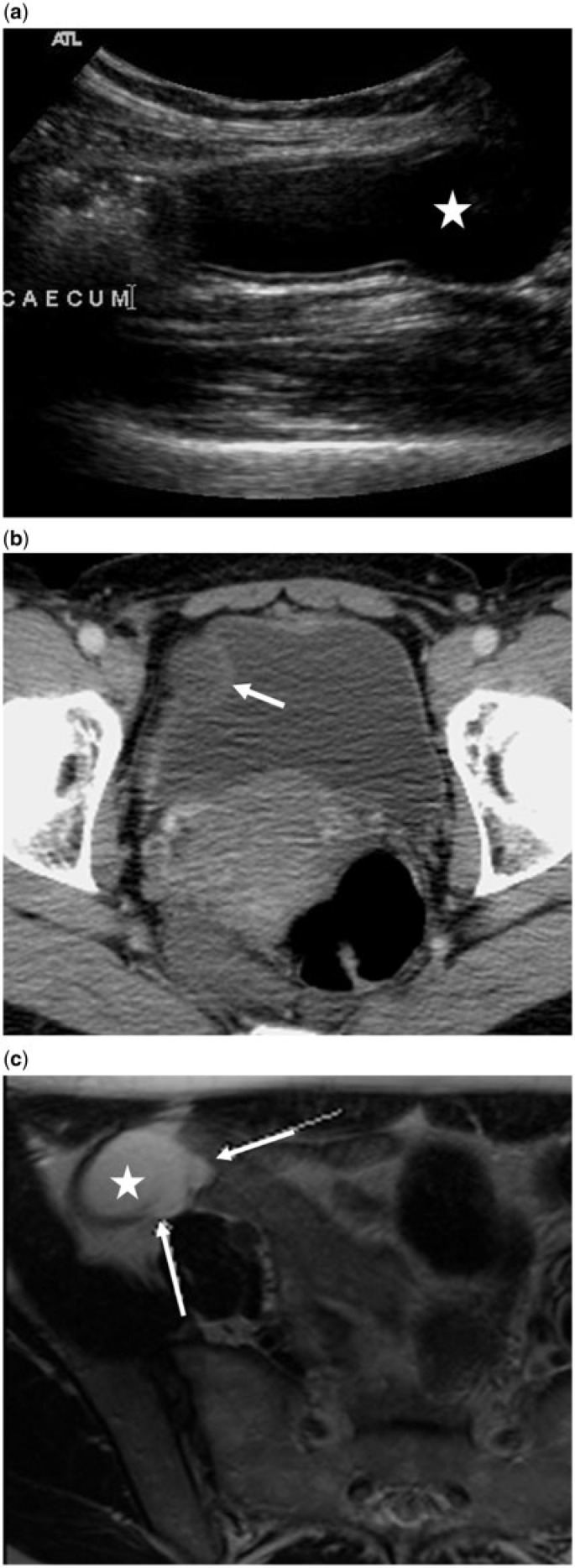
A 50-year-old woman with mucinous neoplasm of the appendix and peri-appendiceal leakage of mucin confirmed at surgery and histology. The presence of extra-appendiceal mucin qualifies it as an LAMN. (a,b) Transverse (a) and longitudinal (b) sonographic images of the right lower quadrant demonstrate a distended proximal appendix (arrowheads), discontinuity of the appendiceal wall (arrows) with mucinous intraluminal contents spilling into the peritoneal space (star). (c) Axial contrast-enhanced CT images demonstrate an abnormally distended appendix, measuring over 20 mm, with peri-appendiceal mucin (star) entering the peritoneal space through a defect in the appendiceal wall (arrow).
Multidetector computed tomography
Multidetector computed tomography (MDCT) offers multiplanar, high-definition anatomic depiction of appendiceal lesions[24]. Simple mucoceles are seen in the expected position of the appendix, as well-encapsulated, peripherally enhancing, low attenuation cystic structures with a smooth wall of variable thickness. Mural curvilinear or punctate calcification occurs in less than 50% of patients[14,25]. Computed tomography (CT) colonography can show a cecal contour abnormality or intraluminal smooth lesion at the expected position of the appendiceal orifice (Fig. 5). An appendix with a diameter of more than 15 mm, soft tissue mass, wall thickening or irregularity should raise the suspicion of mucinous neoplasm[3,9].
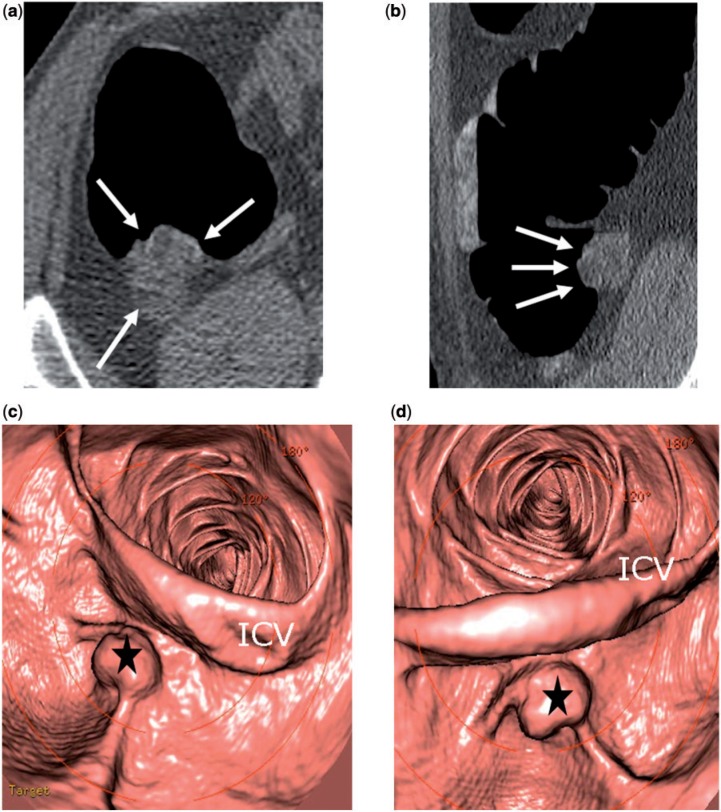
Figure 5.
Appendiceal mucinous adenoma. (a,b) Two-dimensional axial images of CT colonography show a small, heterogeneous solid-appearing mass in the expected position of the appendix (arrow). The appendix is not identified separately. (c,d) Three-dimensional endoluminal views of CT colonography show the mass (star) protruding into the cecal lumen, below the ileocecal valve (ICV).
Adenomas are seen as tumors confined to the appendix with no peri-appendiceal mucin (Fig. 6). With continued mucin production and neoplastic expansion, mucinous neoplasms can develop focal mural expansions, a blistered appearance or frank rupture due to mucin dissecting the wall (Fig. 7). The presence of any mucin outside the appendix excludes the diagnosis of adenoma according to the WHO classification[9]. Extra-appendiceal mucin is a feature of LAMN and mucinous adenocarcinoma (Fig. 8). MDCT is particularly useful in detecting mucin outside the appendix but still confined to the right lower quadrant, also referred to as localized PMP[7]. According to the TNM staging, this is staged as T4a disease, whereas intraperitoneal metastasis beyond the right lower quadrant, including PMP, is staged as distant metastasis (M1a)[9,26]. Localized PMP with cellular mucin at histopathology is associated with a significantly greater risk of recurrence compared with acellular mucin (23% vs 4%)[27]. Rarely, mucinous neoplasms may be complicated by intussusception[4]. MDCT helps in detecting local recurrence after surgery (Fig. 9).
Figure 6.
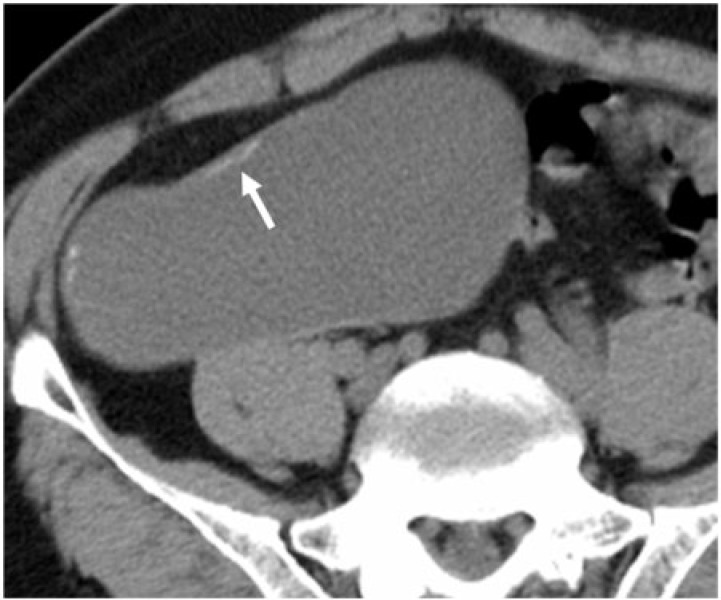
A 76-year-old man with appendiceal mucinous adenoma manifesting as right lower quadrant discomfort. Axial unenhanced CT image shows a well-defined appendiceal lesion with intact margins, curvilinear mural calcifications (white arrow) and uniform low-density internal contents.
Figure 7.
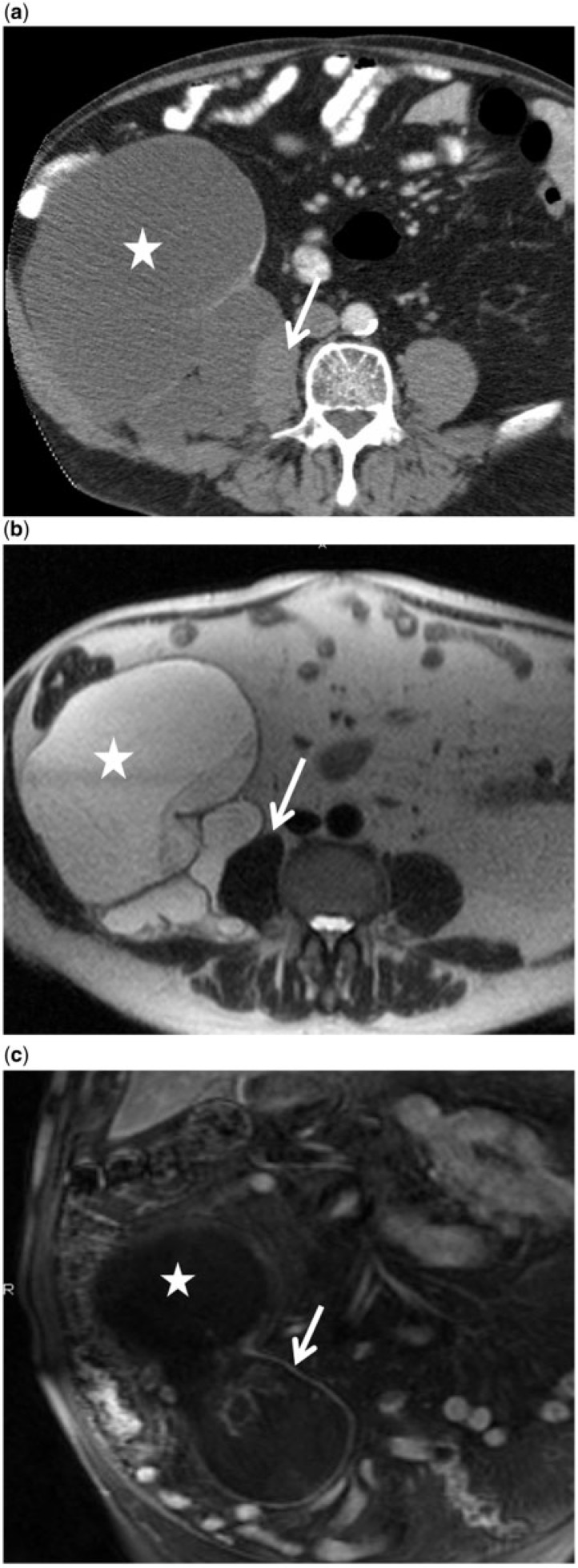
A 49-year-old woman with incidentally detected mucinous neoplasm of the appendix complicated by impending rupture. (a) Longitudinal ultrasonographic image demonstrates uncomplicated mucocele (star). (b) Axial contrast-enhanced CT image shows the mucocele adherent to the bladder wall (arrow). (c) Axial T2-weighted MR image shows the mucocele to be a hyperintense (star) with focal bulge and thinning of the wall (arrows). Histopathology revealed an LAMN with localized peritoneal adenomucinosis.
Figure 8.
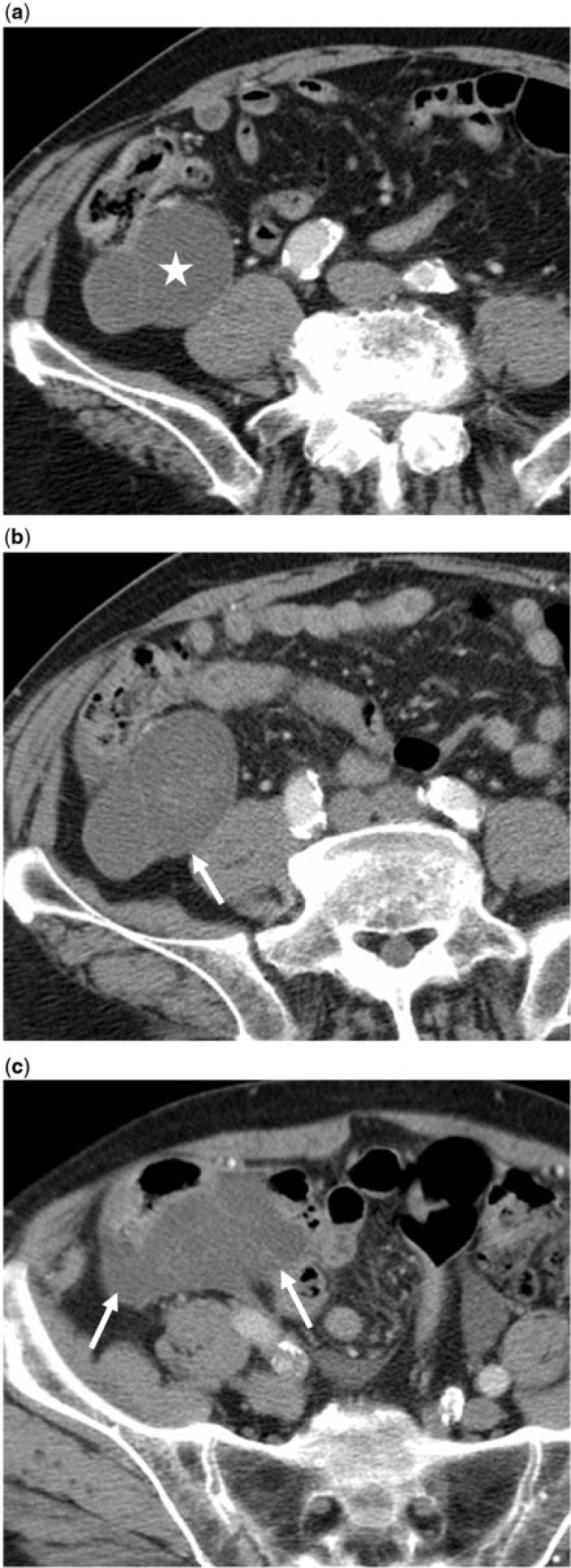
An 87-year-old man with esophageal cancer and incidentally detected mucinous neoplasm of the appendix, which was neglected and later complicated by rupture. (a) Baseline axial contrast-enhanced CT image reveals a lobulated mucocele (star) abutting the cecum. (b,c) Axial contrast-enhanced CT images 2 years later show local rupture of the mucocele with cecal adhesions (arrows). Histopathology revealed an LAMN and localized PMP.
Figure 9.
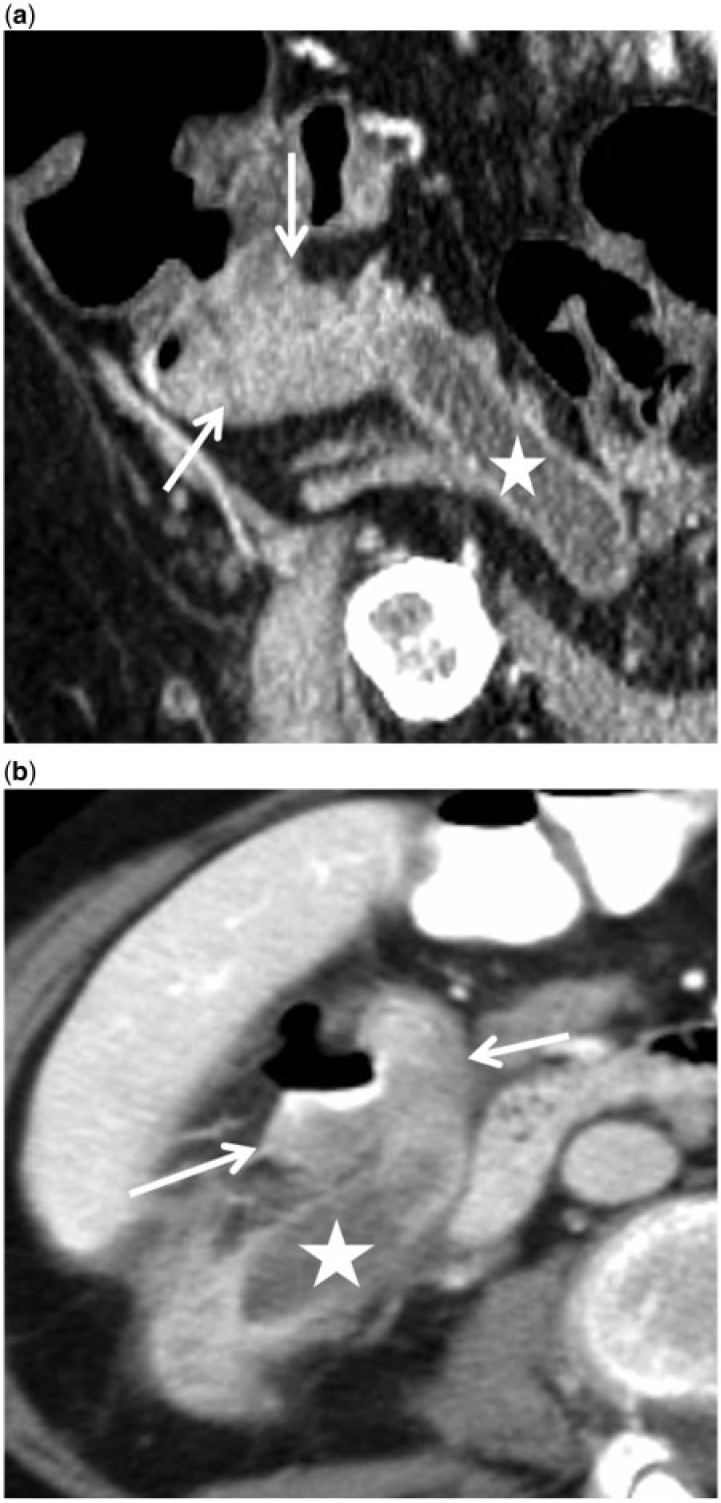
A 49-year-old woman with complicated appendiceal mucinous adenocarcinoma manifesting as a 2-year history of chronic right lower quadrant discomfort and increasing right flank mass. Incomplete resection at another hospital revealed appendiceal mucinous adenocarcinoma. A post-operative retrocecal collection was treated as an abscess and drained percutaneously through the right flank. (a,b) Axial (a) and sagittal reformatted (b) images of the follow-up contrast-enhanced CT scan revealed mucinous carcinoma appearing as a low-density, elongated mass (arrows) with internal calcifications, dorsal to the cecum, extending along the drainage tract through the abdominal wall aponeurosis and superficial fascia to the skin surface as a mucinous fistula. This was later completely resected. The patient is under follow-up.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is helpful to detect incidental appendiceal adenomas, rupture and extra-appendiceal mucin and to differentiate large mucinous neoplasms from other intraperitoneal and retroperitoneal pathologies. Mucinous neoplasms appear as hyperintense tubular distention of the appendix on T2-weighted images (Fig. 7)[25]. The signal intensity on T1-weighted images depends on the mucin concentration. Intraluminal and peri-appendiceal mucin appears bright on T2-weighted images. Contrast-enhanced MRI may show smooth mucosal enhancement in simple lesions, whereas nodules and solid components are seen in adenomas, LAMNs and adenocarcinomas (Fig. 10). Peri-appendiceal enhancement may be seen when there are adhesions or extraluminal mucin, which raises the possibility of underlying LAMN or adenocarcinoma.
Figure 10.
An 80 year-old man with large appendiceal mucinous adenoma with retroperitoneal growth pattern mistaken for sarcoma. (a) Axial contrast-enhanced CT image shows a large, lobulated retroperitoneal cystic lesion (star) with mural calcifications, displacing the right colon anteriorly and infiltrating the psoas (arrow). (b) Axial T2-weighted MR image reveals a complex tubular hyperintense retroperitoneal tumor (star) infiltrating the psoas muscle (arrow). (c) Coronal contrast-enhanced T1-weighted MR image shows enhancement of the wall with nodularity (arrow). It was successfully resected, with no recurrence at 5 years.
Imaging of PMP
On imaging, PMP typically has low or proteinaceous attenuation ascites with serosal implants. Sonography demonstrates ascites, irregular peritoneal and omental thickening with anechoic areas, septations and echogenic foci (Fig. 11)[21]. CT shows fixed bowel loops, scalloping of liver margin, abdominal wall, or bowel loops. Septations may also be seen on CT, which may represent either the interface of mucinous nodules or fibrous tissue between nodules (Fig. 11). Mucinous nodules may show rim-like calcification[28].
Figure 11.
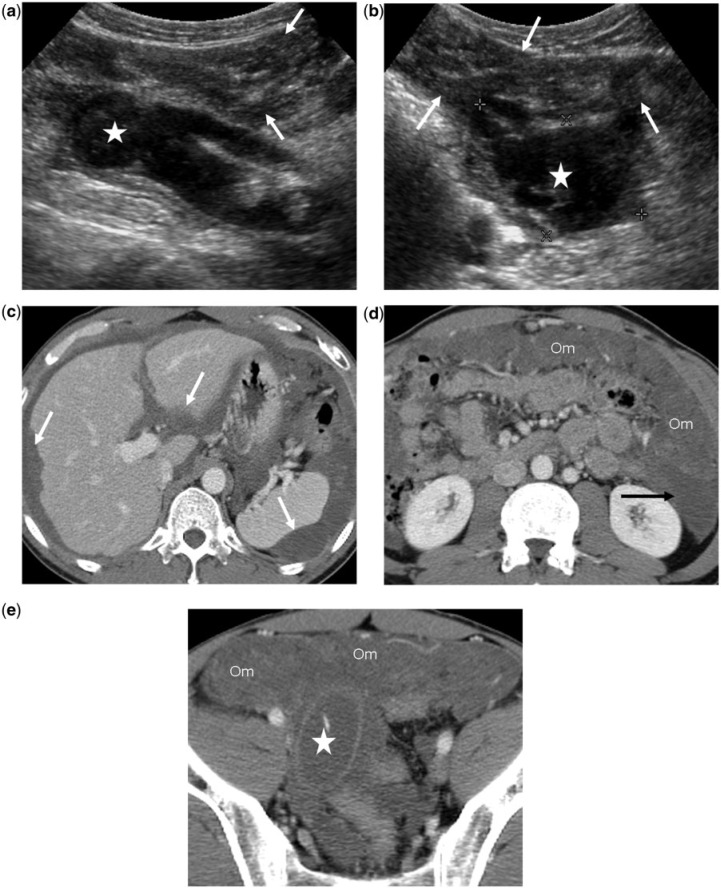
A 44-year-old man with low-grade PMP associated with an LAMN. Diagnosis was confirmed by percutaneous fine-needle aspiration of omental mucinous disease. (a,b) Transverse ultrasonographic images show diffuse complex peritoneal disease (arrows) and a dilated, ruptured appendiceal mucinous neoplasm (star). (c,d,e) Contrast-enhanced CT images demonstrate scalloped margins of the liver and spleen (arrows), fixed bowel loops, omental thickening (Om), high-density ascites (black arrow) and the appendiceal mucinous neoplasm (star).
The appendix is the most common site of the primary tumor in PMP; less common primary sites include colorectum, gallbladder, stomach, pancreas, fallopian tube, urachus, lung and breast[9]. The appendix is considered the primary site even in patients with simultaneous ovarian and appendiceal mucinous neoplasms based on molecular genetic studies[10,29]. The ovary is the primary site in the very rare condition of mucinous adenocarcinoma of intestinal type in mature cystic teratoma[9]. Low-grade PMP associated with LAMN has a characteristic distribution with large-volume disease in the greater omentum, beneath the right hemidiaphragm, in the right retrohepatic space, ligament of Trietz, left paracolic gutter and pelvis[9]. Peritoneal surfaces of the bowel are spared with no nodal metastasis or spread beyond the peritoneum. High-grade PMP arising from mucinous adenocarcinoma, on the other hand, invades the underlying organs and has hematogenous and nodal metastasis (Fig. 12)[9].
Figure 12.
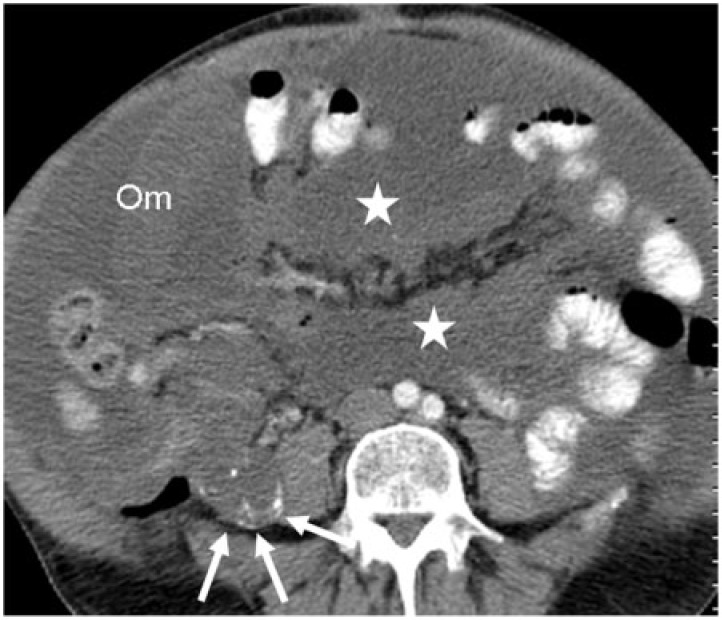
67-year-old man with appendiceal mucinous adenocarcinoma and high-grade pseudomyxoma peritonei. Axial contrast-enhanced CT image demonstrates mucinous carcinomatosis causing omental caking (Om), coating the small bowel loops and mesentery (star) and a lobulated, low-density right lower quadrant mass lesion (white arrows) with asymmetrical calcifications representing the appendiceal mucinous adenocarcinoma.
Differential diagnosis
Simple mucoceles may appear similar to cystic lymphangioma, mesenteric cyst, enteric duplication cyst, Meckel diverticulum, retroperitoneal tumor and ovarian cystic lesion[24]. Inflamed, perforated and leaking mucoceles may be indistinguishable from acute appendicitis, both clinically and radiologically (Fig. 13). An appendiceal diameter of more than 15 mm is a useful clue to the presence of chronic appendiceal obstruction raising the alarm for an underlying appendiceal or cecal neoplasm. Appendiceal obstruction from a cecal carcinoma, an important potential pitfall, is distinguished by the presence of a solid enhancing cecal mass (Fig. 14). PMP has the same imaging features as carcinomatosis from mucinous colorectal and other malignancies, but the appendix is the most common primary site for PMP. Percutaneous needle biopsy is helpful to confirm disseminated mucinous metastatic spread to the peritoneum[20].
Figure 13.
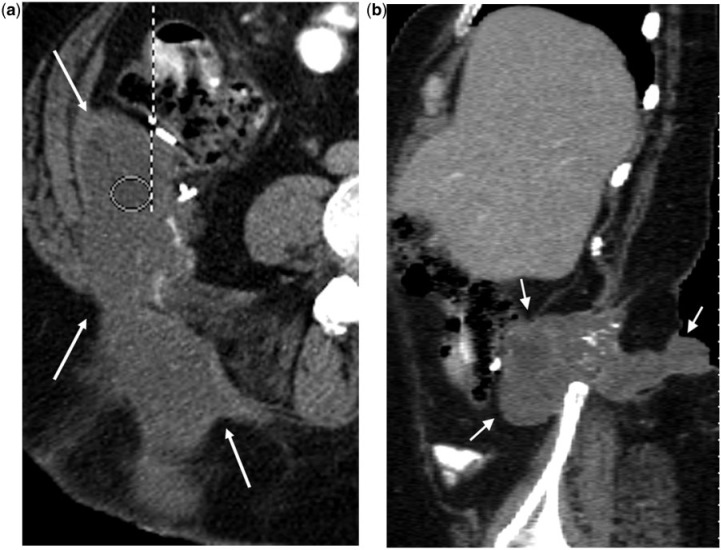
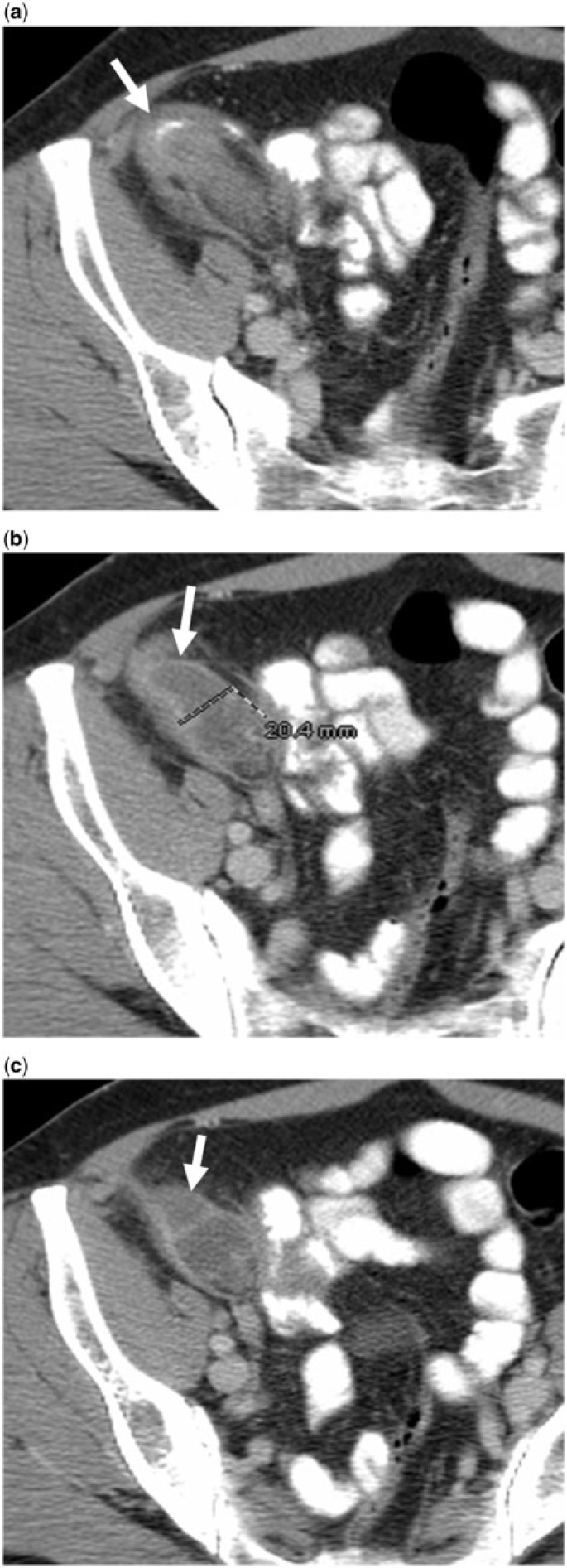
A 52-year-old woman with ruptured mucocele confused with acute appendicitis manifesting as right lower quadrant pain. (a,b,c) Axial contrast-enhanced CT images reveal a ruptured mucocele (arrows) with surrounding inflammation. Clues to the correct diagnosis include an appendiceal diameter of 20 mm or greater, (b) lobulated contour (arrow in (c)) and curvilinear mural calcification at the tip (arrow in (a)). Surgical pathology revealed an LAMN with rupture.
Figure 14.
Chronic appendiceal obstruction from cecal adenocarcinoma in 2 different patients. (a) Sagittal reformatted contrast-enhanced CT image reveal a solid cecal mass (arrows) and an obstructed appendix (star). (b) Axial contrast-enhanced CT image in another patient demonstrates locally invasive cecal cancer (arrows) obstructing the appendix (star).
Management and prognosis
Surgical treatment depends on the dimensions and histology of the mucinous neoplasm, as well as the clinical presentation. Retention cysts and small mucinous adenomas are treated with simple appendectomy, whereas bulky adenomas with large base may need cecal resection if there is a risk that the margin at the appendiceal stump would be positive [3]. All appendectomies should include a wide mesoappendix resection in order to assess for lymph node involvement[32]. Right hemicolectomy is reserved for invasive adenocarcinomas because of the potential for lymph node metastases, and, in these cases, only when peritoneal disease is absent[15]. It is important to recognize that all appendiceal mucoceles more than 2 cm need surgical excision because of the potential for a mucinous neoplasm.
In the setting of suspected appendicitis, when an appendiceal mucocele is encountered, the surgeon assumes extreme caution while handling the mucocele to avoid rupture and dispersion of mucus or epithelial cells into the peritoneal cavity as this is associated with a poorer prognosis[4]. The laparoscopic approach is usually not recommended for mucoceles, unless they are very benign in appearance, due to the risk of intra-operative rupture. When detected incidentally at laparoscopy, conversion to laparotomy is recommended[30,31]. If, however, the mucocele appears to be a homogeneous cyst, with no nodularity and no sign of perforation, then careful laparoscopic handling may be acceptable[32]. In general, laparotomy is advised to permit careful exploration of other viscera, in particular the colon and ovaries, because of the reported association between appendiceal mucocele and colonic and ovarian tumors in 11–20% of cases[3,8,11]. In addition, the entire abdomen should be inspected for signs of mucin and peritoneal dissemination. If mucin is present then it must be collected completely and sent for cytology, because the presence of cells in mucin localized to the right lower quadrant provides significant prognostic information[26].
For PMP, Sugarbaker[33,34] recommends aggressive peritonectomy with intra-operative hyperthermic chemotherapy and post-operative systemic chemotherapy. When significant peritoneal disease is detected at the time of exploration, then the goal must be to obtain a diagnosis. This includes an appendectomy with removal of the mesoappendix, generous sampling of the mucinous fluid and biopsy of peritoneal nodules. The extent of the disease, over the small intestine in particular, should be recorded to facilitate operative planning in the future. Closure of the abdomen should be followed by thorough irrigation to avoid tumor cell entrapment in the wound. Referral to a high-volume center for consideration of cytoreductive surgery and perioperative intraperitoneal chemotherapy should be pursued once the cytology and pathology results are available[32].
The prognosis for retention cysts and adenomas is excellent: 5-year survival is 91–100%[3]. Adenomas of the appendix, by definition, do not recur. Yantiss et al.[27], in their study of 65 patients with appendiceal mucinous neoplasms, found that 96% of patients with acellular extra-appendiceal mucin were disease free (mean 52 months), whereas 33% of patients with neoplastic proliferation outside the appendix developed widespread disease. Misdraji et al.[35], in their study of 107 mucinous appendiceal neoplasms, found that overtly malignant peritoneal disease had 3- and 5-year survival rates of 90% and 44%, respectively, whereas low-grade peritoneal disease was associated with 100% and 86% survival, respectively. Ten-year survival in patients with mucinous adenocarcinoma is usually less than 10%[6]. Features associated with poor prognosis include advanced stage and high grade. In PMP, spread of mucus beyond the right lower quadrant and cellular mucin are of prognostic significance[9]. Metastases to the ovaries are common.
Conclusion
Classification and taxonomy of mucinous neoplasms is complex and controversial. Neoplasms confined to the mucosa of the appendix are adenomas, whereas neoplasms extending beyond the appendix can be LAMNs or adenocarcinomas. Concerning findings at imaging, especially MDCT, include the presence of a soft tissue mass, irregular wall thickening and complications such as rupture. Recognizing extra-appendiceal mucin is of utmost importance for proper staging and prognostication. A surgical consultation should be recommended for all appendiceal mucoceles more than 2 cm in diameter. Distribution of mucin and the presence of nodal and visceral metastases beyond the peritoneum help to differentiate low-grade and high-grade PMPs. PMP with simultaneous appendix and ovarian neoplasm should be treated as a primary appendiceal tumor. Clear communication between the radiologist, pathologist and surgeon is important for optimal patient management.
References
- 1.Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis colon and rectum. 1998;41:75–80. doi: 10.1007/BF02236899. [DOI] [PubMed] [Google Scholar]
- 2.Dachman A, Lichtenstein J, Friedman A. Mucocele of the appendix and pseudomyxoma peritonei. AJR Am J Roentgenol. 1985;144:923–929. doi: 10.2214/ajr.144.5.923. PMid:3885692. [DOI] [PubMed] [Google Scholar]
- 3.Persaud T, Swan N, Torreggiani WC. Giant mucinous cystadenoma of the appendix. Radiographics. 2007;27:553–557. doi: 10.1148/rg.272065134. PMid:17374868. [DOI] [PubMed] [Google Scholar]
- 4.Honnef I, Moschopulos M, Roeren T. Appendiceal mucinous cystadenoma. Radiographics. 2008;28:1524–1527. doi: 10.1148/rg.285075160. PMid:18794324. [DOI] [PubMed] [Google Scholar]
- 5.Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol. 2005;12:291–311. doi: 10.1097/01.pap.0000194625.05137.51. PMid:16330927. [DOI] [PubMed] [Google Scholar]
- 6.Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33:1425–1439. doi: 10.1097/PAS.0b013e3181af6067. PMid:19641451. [DOI] [PubMed] [Google Scholar]
- 7.Misdraji J. Appendiceal mucinous neoplasms: controversial issues. Arch Pathol Lab Med. 2010;134:864–870. doi: 10.5858/134.6.864. PMid:20524864. [DOI] [PubMed] [Google Scholar]
- 8.Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757–768. doi: 10.1002/1097-0142(19950201)75:3<757::aid-cncr2820750303>3.0.co;2-f. PMid:7828125. [DOI] [PubMed] [Google Scholar]
- 9.Carr N, Sobin L. Tumors of the appendix. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of tumours of the digestive system. World Health Organization classification of tumours vol 3. 4th. Lyon, France: IARC Press; 2010. pp. 122–125. [Google Scholar]
- 10.Panarelli NC, Yantiss RK. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med. 2011;135:1261–1268. doi: 10.5858/arpa.2011-0034-RA. PMid:21970481. [DOI] [PubMed] [Google Scholar]
- 11.Stocchi L, Wolff BG, Larson DR, Harrington JR. Surgical treatment of appendiceal mucocele. Arch Surg. 2003;138:585–590. doi: 10.1001/archsurg.138.6.585. PMid:12799327. [DOI] [PubMed] [Google Scholar]
- 12.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei". Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. PMid:7503361. [DOI] [PubMed] [Google Scholar]
- 13.Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. PMid:11443613. [DOI] [PubMed] [Google Scholar]
- 14.Madwed D, Mindelzun R, Jeffrey RB. Mucocele of the appendix: imaging findings. AJR Am J Roentgenol. 1992;159:69–72. doi: 10.2214/ajr.159.1.1609724. PMid:1609724. [DOI] [PubMed] [Google Scholar]
- 15.Dixit A, Robertson JH, Mudan SS, Akle C. Appendiceal mucoceles and pseudomyxoma peritonei. World J Gastroenterol. 2007;13:2381–2384. doi: 10.3748/wjg.v13.i16.2381. PMid:17511043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton DL, Stormont JM. The volcano sign of appendiceal mucocele. Gastrointest Endosc. 1989;35:453–456. doi: 10.1016/s0016-5107(89)72860-1. PMid:2792684. [DOI] [PubMed] [Google Scholar]
- 18.Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19–9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87:162–166. doi: 10.1002/jso.20107. PMid:15334630. [DOI] [PubMed] [Google Scholar]
- 19.Zuzarte JC, Liu YC, Cohen AM. Fine needle aspiration cytology of appendiceal mucinous cystadenoma. Acta Cytol. 1996;40:327–330. doi: 10.1159/000333762. PMid:8629421. [DOI] [PubMed] [Google Scholar]
- 20.Que Y, Wang X, Liu Y, Li P, Ou G, Zhao W. Ultrasound-guided biopsy of greater omentum: an effective method to trace the origin of unclear ascites. Eur J Radiol. 2009;70:331–335. doi: 10.1016/j.ejrad.2008.01.036. PMid:18328658. [DOI] [PubMed] [Google Scholar]
- 21.Que Y, Tao C, Wang X, Zhang Y, Chen B. Pseudomyxoma peritonei: some different sonographic findings. Abdom Imaging. 2012;37:843–848. doi: 10.1007/s00261-012-9843-0. PMid:22234650. [DOI] [PubMed] [Google Scholar]
- 22.Pfitzer P, Richartz G. Cytology of pseudomyxoma peritonei. Cytopathology. 1995;6:304–315. doi: 10.1111/j.1365-2303.1995.tb00576.x. PMid:8785368. [DOI] [PubMed] [Google Scholar]
- 23.Caspi B, Cassif E, Auslender R, Herman A, Hagay Z, Appelman Z. The onion skin sign. a specific sonographic marker of appendiceal mucocele. J Ultrasound Med. 2004;23:117–121. doi: 10.7863/jum.2004.23.1.117. PMid:14756359. [DOI] [PubMed] [Google Scholar]
- 24.Hoeffel C, Crema MD, Belkacem A, et al. Multi–detector row CT: spectrum of diseases involving the ileocecal area. Radiographics. 2006;26:1373–1390. doi: 10.1148/rg.265045191. PMid:16973770. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Levy AD, Rohrmann CA, Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003;23:645–662. doi: 10.1148/rg.233025134. PMid:12740466. [DOI] [PubMed] [Google Scholar]
- 26.Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. pp. 133–141. [Google Scholar]
- 27.Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33:248–255. doi: 10.1097/PAS.0b013e31817ec31e. PMid:18852679. [DOI] [PubMed] [Google Scholar]
- 28.Sulkin TVC, O'Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol. 2002;57:608–613. doi: 10.1053/crad.2002.0942. PMid:12096860. [DOI] [PubMed] [Google Scholar]
- 29.Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol. 1999;154:1849–1855. doi: 10.1016/S0002-9440(10)65442-9. PMid:10362811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González Moreno S, Shmookler BM, Sugarbaker PH. Appendiceal mucocele. Contraindication to laparoscopic appendectomy. Surg Endosc. 1998;12:1177–1179. doi: 10.1007/s004649900811. [DOI] [PubMed] [Google Scholar]
- 31.Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg. 2006;202:680–684. doi: 10.1016/j.jamcollsurg.2005.12.003. PMid:16571440. [DOI] [PubMed] [Google Scholar]
- 32.Miraliakbari R, Chapman 3rd WH. Laparoscopic treatment of an appendiceal mucocele. J Laparoendosc Adv Surg Tech A. 1999;9:159–163. doi: 10.1089/lap.1999.9.159. PMid:10235354. [DOI] [PubMed] [Google Scholar]
- 33.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. PMid:16389186. [DOI] [PubMed] [Google Scholar]
- 34.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. PMid:7826158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–1103. doi: 10.1097/00000478-200308000-00006. PMid:12883241. [DOI] [PubMed] [Google Scholar]