Abstract
Most strains of Candida albicans are capable of switching frequently and reversibly between a number of phenotypes distinguishable by colony morphology. A number of different switching systems have been defined according to the limited set of phenotypes in each switching repertoire, and each strain appears to possess a single system. Switching can affect many aspects of cellular physiology and morphology and appears to be a second level of phenotypic variability superimposed upon the bud-hypha transition. The most dramatic switching system so far identified is the "white-opaque transition." This system dramatizes the extraordinary effects switching can have on the budding cell phenotype, including the synthesis of opaque-specific antigens, the expression of white-specific and opaque-specific genes, and the genesis of unique cell wall structures. Switching has been demonstrated to occur at sites of infection and between episodes of recurrent vaginitis, and it may function to generate variability in commensal and infecting populations for adaptive reasons. Although the molecular mechanisms involved in the switch event are not understood, recent approaches to its elucidation are discussed and an epigenetic mechanism is proposed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Soll D. R. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J Gen Microbiol. 1986 Jul;132(7):2035–2047. doi: 10.1099/00221287-132-7-2035. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Cundiff L., Schnars B., Gao M. X., Mackenzie I., Soll D. R. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989 Feb;57(2):458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Mihalik R., Soll D. R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990 Jan;172(1):224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio O. M., Billington B. L., Gottschling D. E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991 Sep 20;66(6):1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- Athar M. A., Winner H. I. The development of resistance by candida species to polyene antibiotics in vitro. J Med Microbiol. 1971 Nov;4(4):505–517. doi: 10.1099/00222615-4-4-505. [DOI] [PubMed] [Google Scholar]
- Auger P., Joly J. Factors influencing germ tube production in Candida albicans. Mycopathologia. 1977 Oct 28;61(3):183–186. doi: 10.1007/BF00468014. [DOI] [PubMed] [Google Scholar]
- Barlow A. J., Aldersley T., Chattaway F. W. Factors present in serum and seminal plasma which promote germ-tube formation and mycelial growth of Candida albicans. J Gen Microbiol. 1974 Jun;82(2):261–272. doi: 10.1099/00221287-82-2-261. [DOI] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Werth A., Soll D. R. The regulation of nuclear migration and division during synchronous bud formation in released stationary phase cultures of the yeast Candida albicans. Exp Cell Res. 1980 May;127(1):103–113. doi: 10.1016/0014-4827(80)90418-8. [DOI] [PubMed] [Google Scholar]
- Bergen M. S., Voss E., Soll D. R. Switching at the cellular level in the white-opaque transition of Candida albicans. J Gen Microbiol. 1990 Oct;136(10):1925–1936. doi: 10.1099/00221287-136-10-1925. [DOI] [PubMed] [Google Scholar]
- Borgers M., De Nollin S. The preservation of subcellular organelles of Candida albicans with conventional fixatives. J Cell Biol. 1974 Aug;62(2):574–581. doi: 10.1083/jcb.62.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Chaffin W. L. Differential expression of cytoplasmic proteins during yeast bud and germ tube formation in Candida albicans. Can J Microbiol. 1981 Jun;27(6):580–585. doi: 10.1139/m81-088. [DOI] [PubMed] [Google Scholar]
- Buffo J., Herman M. A., Soll D. R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984 Mar 15;85(1-2):21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Cihlar R. L., Lee D. D., Hoberg K., Scheld W. M. Yeast adhesion in the pathogenesis of endocarditis due to Candida albicans: studies with adherence-negative mutants. J Infect Dis. 1985 Oct;152(4):710–715. doi: 10.1093/infdis/152.4.710. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A., Rotman M., Kraft B., Soll D. R. Developmental mechanisms regulating the rapid decrease in a cohesion glycoprotein mRNA in Dictyostelium function primarily at the level of mRNA degradation. Dev Biol. 1990 Oct;141(2):262–269. doi: 10.1016/0012-1606(90)90382-s. [DOI] [PubMed] [Google Scholar]
- Davies D. R. The structure and function of the aspartic proteinases. Annu Rev Biophys Biophys Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- Defever K. S., Whelan W. L., Rogers A. L., Beneke E. S., Veselenak J. M., Soll D. R. Candida albicans resistance to 5-fluorocytosine: frequency of partially resistant strains among clinical isolates. Antimicrob Agents Chemother. 1982 Nov;22(5):810–815. doi: 10.1128/aac.22.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. D., Merz W. G., Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980 Jul;18(1):158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton S., Penn C. W. Biological attributes of colony-type variants of Candida albicans. J Gen Microbiol. 1989 Dec;135(12):3363–3372. doi: 10.1099/00221287-135-12-3363. [DOI] [PubMed] [Google Scholar]
- Evans E. G., Odds F. C., Richardson M. D., Holland K. T. The effect of growth medium of filament production in Candida albicans. Sabouraudia. 1974 Mar;12(1):112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D. Bud position in Saccharomyces cerevisiae. J Bacteriol. 1960 Oct;80:567–568. doi: 10.1128/jb.80.4.567-568.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney R., Langtimm C. J., Soll D. R. The programs of protein synthesis accompanying the establishment of alternative phenotypes in Candida albicans. Mycopathologia. 1985 Jul;91(1):3–15. doi: 10.1007/BF00437280. [DOI] [PubMed] [Google Scholar]
- Fruit J., Cailliez J. C., Odds F. C., Poulain D. Expression of an epitope by surface glycoproteins of Candida albicans. Variability among species, strains and yeast cells of the genus Candida. J Med Vet Mycol. 1990;28(3):241–252. doi: 10.1080/02681219080000301. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Effect of pH and human saliva on protease production by Candida albicans. Infect Immun. 1981 Jan;31(1):323–326. doi: 10.1128/iai.31.1.323-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M., Abu Elteen K. Correlative relationship between proteinase production, adherence and pathogenicity of various strains of Candida albicans. J Med Vet Mycol. 1986 Oct;24(5):407–413. doi: 10.1080/02681218680000621. [DOI] [PubMed] [Google Scholar]
- Gil C., Pomés R., Nombela C. A complementation analysis by parasexual recombination of Candida albicans morphological mutants. J Gen Microbiol. 1988 Jun;134(6):1587–1595. doi: 10.1099/00221287-134-6-1587. [DOI] [PubMed] [Google Scholar]
- Goshorn A. K., Scherer S. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics. 1989 Dec;123(4):667–673. doi: 10.1093/genetics/123.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S., Esposito R. E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989 Mar 10;56(5):771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Gow N. A., Henderson G., Gooday G. W. Cytological interrelationships between the cell cycle and duplication cycle of Candida albicans. Microbios. 1986;47(191):97–105. [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961 Oct;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Physiological properties of mutagen-induced variants of Candida albicans resistant to polyene antibiotics. J Med Microbiol. 1972 Nov;5(4):425–440. doi: 10.1099/00222615-5-4-425. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J Gen Microbiol. 1986 Sep;132(9):2421–2431. doi: 10.1099/00221287-132-9-2421. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974 Oct 18;251(5476):656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- Hube B., Turver C. J., Odds F. C., Eiffert H., Boulnois G. J., Köchel H., Rüchel R. Sequence of the Candida albicans gene encoding the secretory aspartate proteinase. J Med Vet Mycol. 1991;29(2):129–132. [PubMed] [Google Scholar]
- Ito-Kuwa S. [Ultrastructural changes in the cell wall during germ tube and bud formation in the dimorphic fungus Candida albicans]. Shigaku. 1986 Feb;73(6):1586–1602. [PubMed] [Google Scholar]
- Johnson E. M., Richardson M. D., Warnock D. W. In-vitro resistance to imidazole antifungals in Candida albicans. J Antimicrob Chemother. 1984 Jun;13(6):547–558. doi: 10.1093/jac/13.6.547. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Rogers A. L., Hanselmen L. R., Soll D. R., Yancey R. J., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988 Jun;102(3):149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Abraham J. A. Involvement of double-strand chromosomal breaks for mating-type switching in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1984;49:77–88. doi: 10.1101/sqb.1984.049.01.011. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotila M. P., Diamond R. D. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990 May;58(5):1174–1179. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Land G. A., McDonald W. C., Stjernholm R. L., Friedman L. Factors affecting filamentation in Candida albicans: changes in respiratory activity of Candida albicans during filamentation. Infect Immun. 1975 Jul;12(1):119–127. doi: 10.1128/iai.12.1.119-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose D. S., Schurman D. J., Feldman D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature. 1981 Oct 8;293(5832):477–479. doi: 10.1038/293477a0. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- Manning M., Mitchell T. G. Analysis of cytoplasmic antigens of the yeast and mycelial phases of Candida albicans by two-dimensional electrophoresis. Infect Immun. 1980 Nov;30(2):484–495. doi: 10.1128/iai.30.2.484-495.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon D., Balish E., Phillips A. W. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969 Nov;100(2):701–707. doi: 10.1128/jb.100.2.701-707.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies L. A., Odds F. C. A morphology index for characterization of cell shape in Candida albicans. J Gen Microbiol. 1989 Nov;135(11):3143–3152. doi: 10.1099/00221287-135-11-3143. [DOI] [PubMed] [Google Scholar]
- Merz W. G. Candida albicans strain delineation. Clin Microbiol Rev. 1990 Oct;3(4):321–334. doi: 10.1128/cmr.3.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Connelly C., Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988 May;26(5):842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B., Anderson J., Wilson J., Soll D. R. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J Gen Microbiol. 1989 May;135(5):1201–1208. doi: 10.1099/00221287-135-5-1201. [DOI] [PubMed] [Google Scholar]
- Niczyporuk W., Małyszko E., Szarmach H., Roszkiewicz J. In vitro-Untersuchungen zur proteolytischen und lipolytischen Aktivität hefeähnlicher Pilze sowie deren Virulenz gegenüber Mäusen. Mykosen. 1986 Feb;29(2):89–95. [PubMed] [Google Scholar]
- Odds F. C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Merson-Davies L. A. Colony variations in Candida species. Mycoses. 1989 Jun;32(6):275–282. doi: 10.1111/j.1439-0507.1989.tb02245.x. [DOI] [PubMed] [Google Scholar]
- Olaiya A. F., Sogin S. J. Ploidy determination of Canadida albicans. J Bacteriol. 1979 Dec;140(3):1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaiya A. F., Steed J. R., Sogin S. J. Deoxyribonucleic acid-deficient strains of Candida albicans. J Bacteriol. 1980 Mar;141(3):1284–1290. doi: 10.1128/jb.141.3.1284-1290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillus L., Rine J. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell. 1989 Nov 17;59(4):637–647. doi: 10.1016/0092-8674(89)90009-3. [DOI] [PubMed] [Google Scholar]
- Pomés R., Gil C., Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985 Aug;131(8):2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- Pugh D., Cawson R. A. The cytochemical localization of phospholipase in Candida albicans infecting the chick chorio-allantoic membrane. Sabouraudia. 1977 Mar;15(1):29–35. [PubMed] [Google Scholar]
- Remold H., Fasold H., Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968 Oct 8;167(2):399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988 Feb;170(2):895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Strathern J. N., Hicks J. B., Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979 Dec;93(4):877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P., Sherman F., Hicks J. B. Chromosomal rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J Bacteriol. 1990 Mar;172(3):1276–1283. doi: 10.1128/jb.172.3.1276-1283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryley J. F., Wilson R. G., Barrett-Bee K. J. Azole resistance in Candida albicans. Sabouraudia. 1984;22(1):53–63. [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Sadhu C., McEachern M. J., Rustchenko-Bulgac E. P., Schmid J., Soll D. R., Hicks J. B. Telomeric and dispersed repeat sequences in Candida yeasts and their use in strain identification. J Bacteriol. 1991 Jan;173(2):842–850. doi: 10.1128/jb.173.2.842-850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Voss E., Soll D. R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990 Jun;28(6):1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti N., Strippoli V., Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974 Jul 26;250(464):344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell R. G., Hermans I. F., Wilkins R. J., Corner B. E. Chromosomal variations in Candida albicans. Nucleic Acids Res. 1987 Apr 24;15(8):3625–3625. doi: 10.1093/nar/15.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Bedell G., Thiel J., Brummel M. The dependency of nuclear division on volume in the dimorphic yeast Candida albicans. Exp Cell Res. 1981 May;133(1):55–62. doi: 10.1016/0014-4827(81)90356-6. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
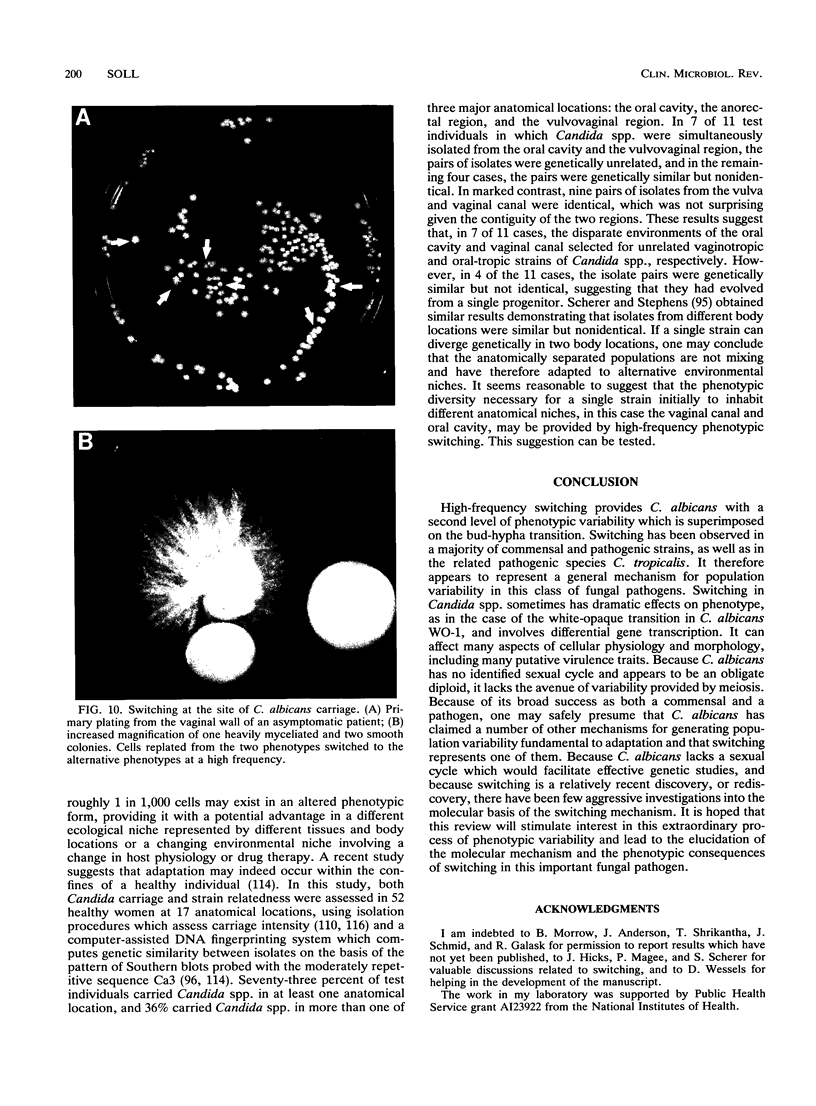
- Soll D. R., Galask R., Schmid J., Hanna C., Mac K., Morrow B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991 Aug;29(8):1702–1710. doi: 10.1128/jcm.29.8.1702-1710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Herman M. A., Staebell M. A. The involvement of cell wall expansion in the two modes of mycelium formation of Candida albicans. J Gen Microbiol. 1985 Sep;131(9):2367–2375. doi: 10.1099/00221287-131-9-2367. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Mitchell L. H. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983 Feb;96(2):486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. The regulation of cellular differentiation in the dimorphic yeast Candida albicans. Bioessays. 1986 Jul;5(1):5–11. doi: 10.1002/bies.950050103. [DOI] [PubMed] [Google Scholar]
- Soll D. R. The role of zinc in Candida dimorphism. Curr Top Med Mycol. 1985;1:258–285. doi: 10.1007/978-1-4613-9547-8_10. [DOI] [PubMed] [Google Scholar]
- Staebell M., Soll D. R. Temporal and spatial differences in cell wall expansion during bud and mycelium formation in Candida albicans. J Gen Microbiol. 1985 Jun;131(6):1467–1480. doi: 10.1099/00221287-131-6-1467. [DOI] [PubMed] [Google Scholar]
- Stewart E., Gow N. A., Bowen D. V. Cytoplasmic alkalinization during germ tube formation in Candida albicans. J Gen Microbiol. 1988 May;134(5):1079–1087. doi: 10.1099/00221287-134-5-1079. [DOI] [PubMed] [Google Scholar]
- Stiller R. L., Bennett J. E., Scholer H. J., Wall M., Polak A., Stevens D. A. Susceptibility to 5-fluorocytosine and prevalence of serotype in 402 Candida albicans isolates from the United States. Antimicrob Agents Chemother. 1982 Sep;22(3):482–487. doi: 10.1128/aac.22.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kobayashi I., Kanbe T., Tanaka K. High frequency variation of colony morphology and chromosome reorganization in the pathogenic yeast Candida albicans. J Gen Microbiol. 1989 Feb;135(Pt 2):425–434. doi: 10.1099/00221287-135-2-425. [DOI] [PubMed] [Google Scholar]
- TASCHDJIAN C. L., BURCHALL J. J., KOZINN P. J. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. AMA J Dis Child. 1960 Feb;99:212–215. doi: 10.1001/archpedi.1960.02070030214011. [DOI] [PubMed] [Google Scholar]
- Vogel R. A., Sponcler R. S. The study and significance of colony dissociation in Candida albicans. Sabouraudia. 1970 Feb;7(4):273–278. doi: 10.1080/00362177085190511. [DOI] [PubMed] [Google Scholar]
- Warnock D. W., Johnson E. M., Richardson M. D., Vickers C. F. Modified response to ketoconazole of Candida albicans from a treatment failure. Lancet. 1983 Mar 19;1(8325):642–643. doi: 10.1016/s0140-6736(83)91809-3. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Beneke E. S., Rogers A. L., Soll D. R. Segregation of 5-fluorocytosine-resistance variants by Candida albicans. Antimicrob Agents Chemother. 1981 Jun;19(6):1078–1081. doi: 10.1128/aac.19.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Magee P. T. Natural heterozygosity in Candida albicans. J Bacteriol. 1981 Feb;145(2):896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Soll D. R. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187(3):477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- Whelan W. L. The genetics of medically important fungi. Crit Rev Microbiol. 1987;14(2):99–170. doi: 10.3109/10408418709104437. [DOI] [PubMed] [Google Scholar]