Abstract
Biological processes occur on a wide range of spatial and temporal scales: from femtoseconds to hours and from angstroms to meters. Many new biological insights can be expected from a better understanding of the processes that occur on these very fast and very small scales. In this regard, new instruments that use fast X-ray or electron pulses are expected to reveal novel mechanistic details for macromolecular protein dynamics. To ensure that any observed conformational change is physiologically relevant and not constrained by 3D crystal packing, it would be preferable for experiments to utilize small protein samples such as single particles or 2D crystals that mimic the target protein's native environment. These samples are not typically amenable to X-ray analysis, but transmission electron microscopy has imaged such sample geometries for over 40 years using both direct imaging and diffraction modes. While conventional transmission electron microscopes (TEM) have visualized biological samples with atomic resolution in an arrested or frozen state, the recent development of the dynamic TEM (DTEM) extends electron microscopy into a dynamic regime using pump-probe imaging. A new second-generation DTEM, which is currently being constructed, has the potential to observe live biological processes with unprecedented spatiotemporal resolution by using pulsed electron packets to probe the sample on micro- and nanosecond timescales. This article reviews the experimental parameters necessary for coupling DTEM with in situ liquid microscopy to enable direct imaging of protein conformational dynamics in a fully hydrated environment and visualize reactions propagating in real time.
Keywords: in situ microscopy, liquid TEM, dynamic TEM, time-resolved imaging
Introduction
Biological cells are dynamic systems comprised of a myriad of multiscale components that perform predefined tasks. Owing to the extraordinary complexity of these systems, scientists have relied on a bottom-up approach for piecing together structural and functional information derived from individual elements to understand the global activity. Ever since the first 3D protein structure was solved in 1958, determining the structure of every protein within a cell has been a central focus of biochemistry [1,2] and this emphasis has yielded more than 84 400 protein structures, using X-ray crystallography, nuclear magnetic resonance and electron microscopy techniques [3]. While conventional X-ray crystallography and nuclear magnetic resonance suffer from limitations of sample size or geometry, fourth-generation X-ray light sources such as the Linac Coherent Light Source (LCLS) have begun to expand the types of samples compatible with analysis by X-rays [4,5]. Whether individual proteins of average size (4 nm diameter [6]) will be amenable to structural analysis with LCLS remains to be seen; however, electron microscopy has already established its versatility for solving the structure of proteins arranged as single particles or 2D and 3D crystals.
Despite knowing over 80 000 protein structures, countless details regarding how changes in amino acid sequence correlate with variations in structure and function still remain obscure since most structures are typically solved under static conditions. While molecular dynamics simulations can be performed on known structures, the computational results are only models that ultimately need to be verified with additional structural data. Until such validation, the models remain speculative. Of course the optimal temporal resolution needed to identify variations in structure will depend on the type of conformational change that is expected. Intermolecular protein interactions generally occur on temporal scales longer than one ms, while intramolecular conformational changes such as protein domain, hinge-bending and subunit motion occur on the ms to μs timescale [7]. Furthermore, loop or rigid-body motions that can lead to activation or inactivation of a protein's functional state require access to the microsecond to nanosecond timescale [7]. Similarly, the magnitude of structural changes can range from sub-angstrom atomic fluctuations to several-nanometer protein domain motions or larger.
While no single instrument currently exists that can address all relevant temporal and spatial scales for biological processes, the second-generation Dynamic Transmission Electron Microscope being installed at Pacific Northwest National Laboratory has the potential to enhance the temporal resolution possible with electron microscopy and produce direct images of propagating biological reactions. In this paper, we review the recent advances in electron microscopy that will permit dynamic observations of proteins in realistic environments with atomic resolution and micro- to nanosecond temporal resolution. In particular, critical recent developments in the use of liquid stages inside conventional microscopes are described in detail that enable transferring live biological systems into the beam. We also review the expected contrast for imaging such systems and compare it with the state-of-the-art in cryo-EM. Finally, we discuss the modifications in the dynamic TEM (DTEM) (the use of aberration correctors, phase plates and energy filter) that will permit the experiments to be performed with the required spatiotemporal resolution.
Methods
In situ liquid scanning transmission electron microscope (STEM)
Purified ferritin molecules (Sigma Aldrich, USA) were diluted 100-fold in ddH2O and 1 μl was loaded into the continuous flow in situ holder as previously described [8]. The in situ liquid stage was subsequently inserted into a JEM-2100F/Cs with a spherical aberration corrector for scanning transmission electron microscopes (STEM) imaging mode. Prior to sample insertion, the microscope was aligned to a 1 Å resolution using the standard Platinum/Iridium calibration sample for the CEOS corrector system. STEM images were collected simultaneously on Bright Field and Dark Field detectors with dwell times of 2 µs per pixel giving rise to single-frame acquisition times of 524 ms for a 512 × 512 pixel image. The STEM probe had an average current density of 30 pA, yielding a dose of 60e– per Å2 (5 Å per pixel and 2 µs dwell time). Digital Micrograph (Gatan, Inc., USA) was utilized for all data acquisition.
Image simulations
Bright field electron microscope images of individual apo-ferritin molecules were simulated using the TF SIM operation of the SPIDER software suite [9] using complex atomic scattering amplitudes from the known structure (PDB: 1 aew). Standard values were used for all simulations with Cs = 2 mm and defocus = −500 nm for conventional cryo-EM and Cs = 0.005 mm and defocus = −50 nm for aberration corrected TEM. Electron diffraction pattern simulations were calculated using modified Crystfel scripts [10] wherein the X-ray-relevant parameters and scattering factors were replaced with electron relevant parameters. Individual bacteriorhodopsin trimers of known structure (PDB: 1 fbb & 1 fbk) were assembled into a model 2D crystal using unit cell parameters of a = b = 63 Å, α = β = 90° and γ = 120° with P3 symmetry. Owing to the high intensity of low-order reflections, the diffraction patterns were high-pass filtered at a resolution of 3.0 nm to more easily detect differences in the intensities of the high-resolution Bragg spots.
Results
In situ imaging in liquids
Cryogenic electron microscopy (cryo-EM) was developed over 30 years ago to address issues related to biological sample preservation, since it was observed that samples at room temperature suffered severe radiation damage effects that hampered high-resolution imaging. In this method, a sample is rapidly frozen by plunging it into a liquid ethane or propane slurry that is separately cooled by liquid nitrogen [11]. Since the slurry is maintained well below its boiling point, the Leidenfrost effect is avoided and rapid thermal transfer occurs allowing the protein and buffer or water solution to be vitrified. The resulting low-density vitrified ice is non-crystalline and immobilizes the samples in a frozen hydrated state [11]. This method preserves high-resolution components of the protein sample and also provides better tolerance against beam-induced damage during imaging. However, the process of freezing completely negates the possibility of observing dynamics in real time.
In an effort to overcome this limitation, time-lapse cryo-EM was developed using laser flash photolysis, microfluidic mixing, or directed spraying of an aerosol onto a sample-coated grid to trigger a reaction just prior to plunge freezing the grid [12–15]. Unfortunately, time-lapse cryo-EM solely provides ‘snapshots’ of processes frozen in time and remains restricted to millisecond temporal resolution. Thus, to permit real-time TEM observations of dynamics that occur on a microsecond or faster timescale such as loop and domain motion, biological samples need to be imaged in a fully hydrated and non-frozen state. This can be achieved using windowed environmental cells where two thin electron transparent membranes create a sample chamber that is isolated from the high vacuum of the TEM [16].
The concept of a windowed environmental cell for studying biological samples in a wet environment using TEM has been around for more than 75 years [17,18]. The earliest wet cell used two 500 nm thick aluminum foils [18] to encase the sample and permit wet imaging conditions at a resolution of several hundred nanometers. However, recent applications of windowed environmental cells generally use thin (<50 nm) silicon-based membranes to enclose the sample within a continuous flow capable [16] or hermetically sealed chamber [19,20] and achieve a several-nanometer or better resolution. Gas environmental chambers for TEM have imaged muscle thick filaments and myosin filaments in a hydrated state by maintaining an equilibrium with circulating water vapor [21,22]. Furthermore, applications using liquid environmental chambers for in situ liquid TEM and STEM of biological materials under fully hydrated conditions have visualized whole cells [23,24], acrosomal filaments [25] and individual ferritin molecules and nanolipoprotein discs [8].
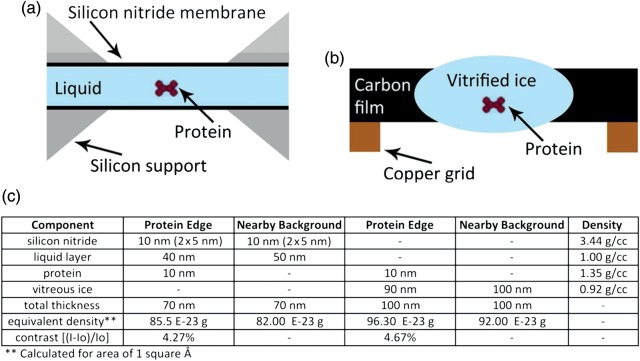
Fortunately, the experimental design of in situ stages mimics the overall thickness and equivalent density used for cryo-EM and similar contrast levels are achievable between the two techniques. Figure 1 compares the typical sample geometry for cryo-EM and in situ liquid microscopy and details the analogous effective density and resulting contrast that occur at the edge of a 10 nm diameter globular protein. Although the overall thickness of the in situ chamber is less than the cryo-EM example, the similar contrast is due to the higher density and scattering cross-section for the silicon nitride membranes. This means that any sample that can be currently visualized with cryo-EM should be amenable to in situ experiments with the caveat each experiment will need to be modified to match the optimal fluid path length or membrane thickness to the expected sample size or desired contrast or resolution.
Fig. 1.
Sample geometry similarities. (a) Schematic of in situ liquid S(TEM) chamber using two thin membranes to enclose the sample. (b) Cryo-EM layout showing the vitrified ice layer with trapped sample. (c) Simplified comparison of edge contrast from in situ and cryo-EM experiments from a generic 10 nm diameter protein.
Spatial resolution limits for in situ liquid microscopy
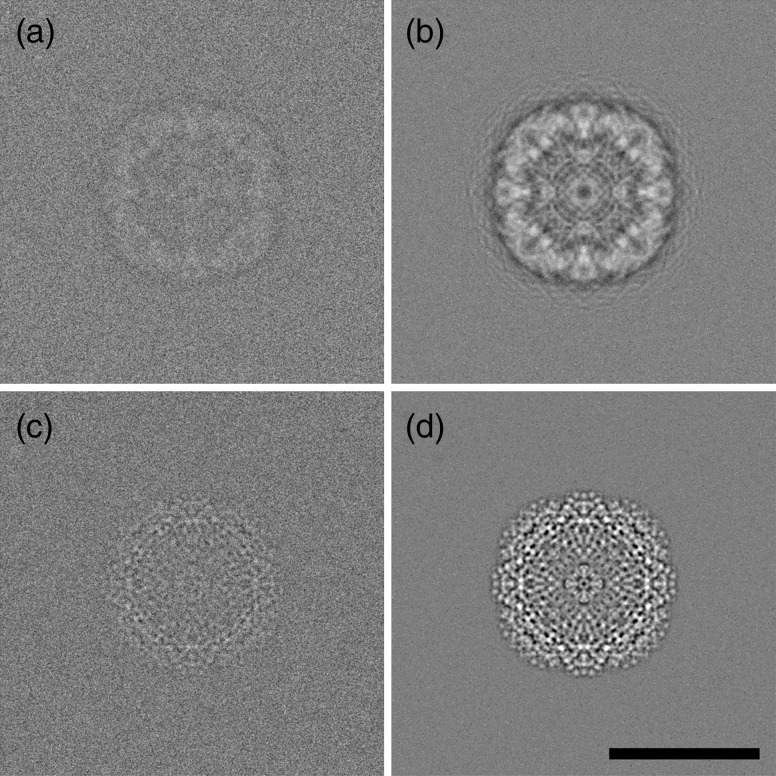
All the previous publications for in situ liquid (S)TEM of biological macromolecules have realized spatial resolutions of only a few nanometers at best. However, even higher resolution will be required to fully monitor and understand protein conformational dynamics. It is clear from in situ liquid experiments studying nanoparticle growth that resolutions better than 2 Å can be achieved with current technology [16]. Other studies have revealed experimental conditions that permit atomic resolution while also permitting quantitative analysis of the liquid environment with electron energy loss spectroscopy [26]. The two main considerations for enhancing contrast and resolution are the membrane thickness used to enclose the sample and the fluid path length. Thinner versions for both parameters provide better contrast and enable higher resolution. The incorporation of aberration correction also facilitates ideal imaging conditions for obtaining high-resolution images even when the size of the sample or the thickness of the environmental cell would limit the contrast in a conventional microscope. Figure 2 shows an ideal simulated image of apo-ferritin for conventional cryo-EM image conditions as well as the simulated image for in situ liquid TEM of apo-ferritin using an aberration-corrected microscope. These simulations used optimized defocus conditions that maximized the intensity for the first Thon ring at a 0.5 nm spatial resolution with a point resolution of 0.35 nm according to the contrast transfer function.
Fig. 2.
Simulations of single particles. (a and b) Individual and averaged (n = 100) bright field cryo-EM simulations of apo-ferritin within an ice layer of a 100 nm thickness and using a defocus of −500 nm with Cs = 2.0 mm. (c and d) Individual and averaged (n = 100) bright field in situ liquid DTEM simulation of apo-ferritin between two 5 nm thick silicon nitride membranes and within a 50 nm fluid path length and with Cs = 0.005 mm. Both images assumed a total dose of 10 electrons per Å2 and a signal-to-noise ratio of 4%. Note the lack of the strong black halo artifact in c and d due to the closer to focus imaging conditions made possible with aberration correction. Scale bar is equivalent for all panels and represents 10 nm.
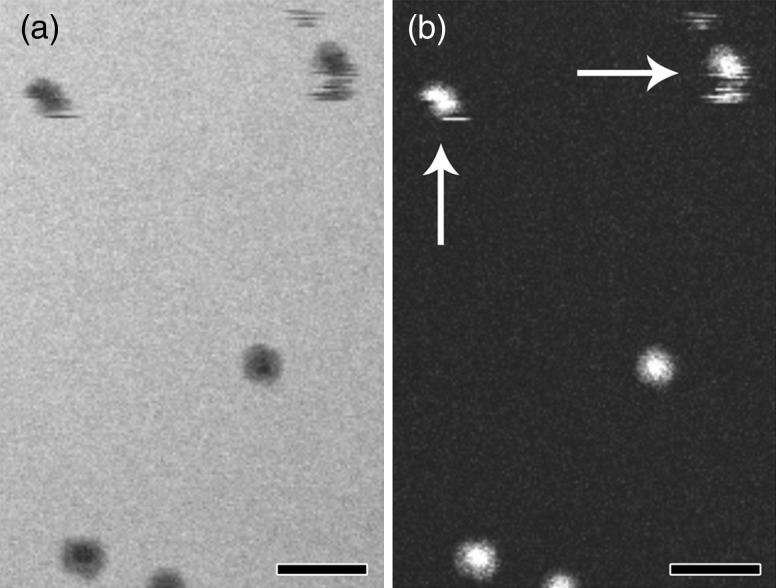
Interestingly, the simulations and results from in situ liquid imaging inorganic materials indicate that a much better resolution should be achieved for biological samples than actually reported. However, that assumption fails to account for the flexibility and mobility of biological samples which appear to limit the attainable spatial resolution with conventional in situ liquid microscopy. Figure 3 illustrates this effect where particles that moved during the scanning process are seen as intermittent streaks rather than circular particles with a denser core. Whether these particles move because of Brownian motion, local fluid flow or beam-induced charging is a topic for discussion and further research. For example, if the observed movement of ∼2–5 nm during the 50 ms image acquisition was solely due to Brownian motion, a displacement of 2000 nm would be expected [27]. The three orders of magnitude difference in displacement suggests that the ferritin molecules in Fig. 3 may have experienced inhibited Brownian motion possibly due to the wall effect caused by the close proximity of two (possibly charged) silicon nitride membranes. Alternatively, the electron beam may have created a localized electrophoretic field that dominated or impeded Brownian motion. Recent work focused on nanoparticle nucleation and growth [28] and nanoparticle motion [29] has begun laying the foundation for experiments aimed at quantifying and understanding electron beam effects for in situ liquid microscopy. Interestingly, the research involving nanoparticle motion, as a function of electron dose, determined that the observed motion of platinum nanoparticles was significantly slower than that expected from Brownian motion and that electrophoretic charging was the dominant force [29]. While the similarities in results are intriguing, further experiments will be required to fully understand the mechanism of displacement for biological samples. Nevertheless, if the movement follows a linear trend, then faster imaging conditions could mitigate the effective image blur by interacting with the sample on a short enough timeframe that the particle appears to be static.
Fig. 3.
In situ liquid STEM of soluble proteins. (a and b) Bright field and corresponding dark field STEM images of purified ferritin complexes with iron oxide core. Note the two particles at the top of each image that suffered from Brownian motion or beam-induced charging during image rastering (white arrows). Particles in the lower half of the image appear stable but may be tumbling end-over-end in solution. Scale bars represent 25 nm.
Temporal resolution and the second-generation dynamic TEM
Dynamic Transmission Electron Microscopy is a burgeoning technology that combines pulsed laser systems with the electron optics of a standard TEM to capture images of reactions on fast timescales. Researchers at Technische Universität Berlin were the first to begin to appreciate the benefits of such a capability and their system achieved a 100 nm spatial resolution at 10 ns [30]. The development of the first-generation DTEM at Lawrence Livermore National Laboratory improved the spatial resolution to ∼5 nm at 15 ns [31]. While both of these instruments have yielded novel insights into the salient dynamics of inorganic material microstructure, the spatial resolution performance is not well suited for biological samples. Case in point, a resolution of 5 nm would not visualize differentiating details between two protein particles that are 4 nm in diameter – the average size for eukaryotic proteins [6].
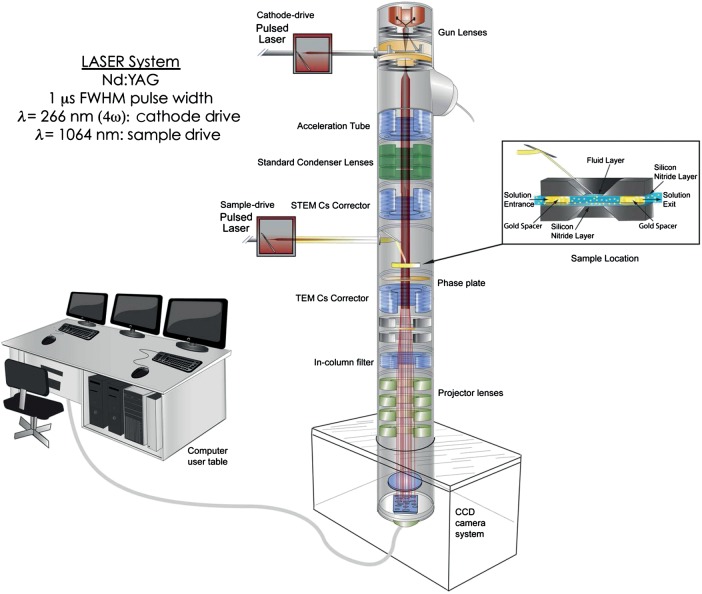
Therefore, a new second-generation DTEM is currently being installed at Pacific Northwest National Laboratory that is optimized for biological and organic materials. The four main technological advancements that improve the performance of the second-generation DTEM beyond the capabilities of its predecessor are the incorporation of spherical aberration correctors, an improved integration of the laser systems, a higher brightness gun and the use of phase plates to enhance image contrast. Fig. 4 shows a schematic illustration of the second-generation DTEM. In brief, the pulsed cathode drive laser irradiates a photocathode source (a hairpin of a low work function metal, e.g. tungsten, tantalum, zirconium) with a photon energy greater than the target work function. A flux of electrons is then produced via photoemission with approximately the same time duration as the stimulating laser pulse. After this photoemission process, the microscope processes the emitted electron ‘packet’ in the traditional way (acceleration, focusing, magnification, detection, etc.). This means that images can be obtained with the same time resolution as the pulse duration – which is currently set by the user at any time between 1 µs and 10 ns. If the photoemission pulse is synchronized with a second laser that stimulates the sample, in situ pump-probe reactions can also be initiated and studied with high time precision.
Fig. 4.
Illustration of the second-generation Dynamic TEM being installed at PNNL that is optimized for biological imaging.
The single shot approach enabled by the DTEM means that the process being studied need not be perfectly reversible as all the information is obtained from a single specimen drive event; this type of imaging is required to observe events in liquids. However, the limitation to this method is that space-charge effects in the beam can lead to degradation of resolution, and even with an optimized microscope source, column and detector the high current will limit the overall temporal and spatial resolution of the instrument. The key to using this single-shot approach is therefore to optimize the components in the microscope to define the space-charge limited resolution of the instrument. The use of an arbitrary waveform generator to vary the temporal duration of the probe pulse between the nano- and microsecond timeframe permits flexibility for optimizing the temporal resolution for each experiment or minimizing space-charge effects that degrade spatial resolution [32]. Additionally, the combination of an aberration corrector and phase plate in the second-generation DTEM is intended to ensure that the maximum possible contrast and highest spatial resolution can be achieved. The increase in contrast for lower spatial frequencies afforded by a phase plate can significantly improve the ability to visualize biological samples within the liquid stage and this effect is exacerbated when combined with aberration correction [33]. In the case of a DTEM, the incorporation of a spherical aberration corrector has a two-fold effect on the spatial resolution. First, the spatial resolution (point resolution and information limit) of the microscope is increased by accurately correcting all first- to third-order aberrations. Second, aberration correction allows larger aperture angles to be used during imaging, which can significantly help reduce space-charge effects for nanosecond pulses by allowing the beam to be spread over a larger area and decrease the lateral distance between nearby electrons.
Outrunning beam-induced movement and Brownian motion
As mentioned earlier, the rate at which images can be acquired needs to be improved in order to observe conformational dynamics at high spatiotemporal resolution while avoiding blurring artifacts due to particle mobility. Conventional microscopes have only one electron present in the column at any given time and thus long exposure times are necessary to collect the requisite number of electrons to form a well-contrasted image. Most cryo-EM images use a dose of 5–20 electrons per Å2. Although faster readout speeds on a sub-ms timescale may become possible with new detector technologies [34], fast image acquisition will be limited by the electron doses emitted with a field emission source and tolerated by the sample. Based on the typical maximum probe current of 100 nA for Schottky field emission sources, acquisition times as short as 150 μs may be possible for an imaging dose of five electrons per Å2 with a beam diameter of 0.5 μm. However, more conventional microscope conditions use a probe current of 10 nA and in either case, accidental prior illumination of the area of interest would destroy the sample since the total dose per second is >31 500 electrons per Å2. Additionally, particle mobility due to Brownian motion and charging can still be significant on the 150 μs timescale.
Thus, to fully outrun these limiting artifacts, fast imaging with a pulsed electron source is required. Fortunately, the second-generation DTEM will operate in single-shot mode with temporal durations of 10–1000 ns and contain more than 109 electrons in each pulse. Each pulse contains all the electrons necessary for image formation. For example, a pulse of 109 electrons can be expanded to a beam diameter of 1.6 μm and still produce an image with a dose of five electrons per Å2. Future improvements in the gun brightness are expected to permit microsecond duration pulses containing 1010–1011 electrons that would provide even more flexibility for imaging conditions. Furthermore, compared with conventional microscopes where fast imaging would require high-dose conditions, the pulsed imaging mode allows expansion of the beam diameter to lower the corresponding dose per pulse. Broadening a pulsed beam containing 109 electrons to a diameter of 16 μm would cut the dose per pulse down to 0.05 electrons per Å2.
Improving spatial resolution for cryo-EM
While the temporal resolution of the DTEM offers options for outrunning particle mobility that adversely affect in situ liquid imaging, it also has the potential to improve the spatial resolution of cryo-EM. Two of the main limitations that currently inhibit routine atomic resolution imaging of biological samples using cryo-EM are due to the interaction of the specimen with the electron beam. Beam-induced movement and radiation sensitivity degrade the overall resolution for direct images and 3D reconstructions [35]. The degrading effects of these phenomenon increase at higher tilt angles and with increasing cumulative dose [36]. For electron crystallography of membrane proteins, the beam-induced movement at high tilt angles typically limits the resolution of a final 3D reconstruction to a lower value than the order of the 2D crystal would allow. However, these effects are currently observed for beam/specimen interaction times of several milliseconds or longer. Using DTEM, the time dependence of this beam/specimen interaction can be interrogated to determine whether a fast pulse of electrons could effectively outrun these image-degrading processes. This would be similar to the ‘diffract-and-destroy’ method developed with femtosecond duration X-ray free electron lasers [5]. Even if true ‘diffract-and-destroy’ conditions cannot be fully realized on micro- or nanosecond timescales to outrun radiolysis damage, the beam-induced movement for tilted samples should be mitigated with microsecond or faster pulses. Currently, movement of tilted crystals occurs on the scale of 1–5 angstroms for images collected over 100–500 ms [36]. The effect is exacerbated with longer exposures in a nearly linear manner. This means that pulses on a microsecond timescale should correspond to in-plane movements far below the sensitivity of the camera system unless pulsed imaging mode alters the interaction and causes a non-linear explosive movement on a microsecond or on a faster timescale. If diffract-and-destroy conditions are possible, the ability to probe the sample with many more electrons than currently tolerable would increase the attainable contrast and spatial resolution for any cryo-EM sample.
Improving reliability of linking structure with function through pump-probe capabilities
In addition to improving the temporal resolution for imaging reactions in real time, the DTEM also enables a pump-probe experimental regime. This is an important parameter for observing fast reactions since reproducibility and interpretability depend on synchronization of all samples prior to initiating or triggering the reaction. This capability has already been demonstrated for material science samples such as reactive multilayer foils where the exothermic mixing of alternating nickel and aluminum layers is induced with a 10 ns laser pulse [37].
Although these previous DTEM experiments were performed under vacuum conditions, they showcase the ability to observe transient structures that are undetectable by other methods. This pump-probe imaging scheme has also been applied toward in situ liquid studies of lead sulfide (PbS) nanoparticle growth from a complex solution. Aberration-corrected in situ liquid microscopy proved that PbS nucleation and growth could be driven by the high-energy electrons used to form images. Unfortunately, using the imaging electrons to reduce the thioacetamide precursor removes the synchronization aspect for pump-probe experiments that will be critical to reliable interpretation of the underlying mechanisms. However, the first-generation DTEM was able to decouple the initiation of nanoparticle growth from the imaging electrons and instead rely on a single 532 nm laser pulse to drive the specific decomposition of thioacetamide [16].
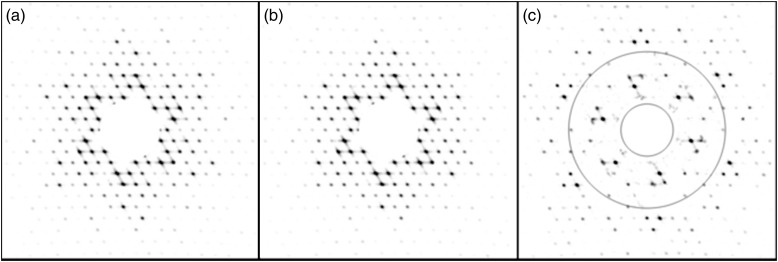
For biological purposes, the sample drive laser of the DTEM can be used to trigger localized temperature jumps, photoactivation of light stimulated proteins or photolysis of caged ligands or cofactors. The conformational changes can then be observed with either direct imaging or diffraction using micro- or nanosecond duration electron pulses. Figure 5 shows a simulation of the type of data expected from pulsed diffraction experiments. For this simulation, a 2D crystal of bacteriorhodopsin would be adhered to one of the silicon nitride membranes of the in situ liquid stage and kept hydrated for the entire experiment. After aligning the microscope and identifying an area of interest, an initial diffraction pattern of the bacteriorhodopsin ground state would be acquired. Subsequently, a 532 nm laser pulse would illuminate the crystal and initiate the photocycle. Approximately 50 μs after the sample drive laser pulse, the probing electrons would interact with the specimen and provide a diffraction pattern of the M-intermediate conformational state for bacteriorhodopsin. Additional experiments would be performed with varying delays between the pump and probe pulses to build a movie of conformational states as a function of time. While bacteriorhodopsin is a fairly well-known test case, the same experimental setup can be used for proteins of unknown structure or dynamics. For example, the conformational gating of energy transducing membrane proteins can be triggered by the sub-ns photorelease of caged calcium or zinc in the surrounding liquid environment to identify the structural changes and critical amino acids involved in enhancing the reduction of insoluble minerals or heavy metal contaminants.
Fig. 5.
DTEM pump-probe simulation of protein dynamics. Simulated electron diffraction patterns from 2D crystals of bacteriorhodopsin in the ground (a) and cytoplasmically open (b) M-intermediate conformational states. (c) Difference pattern between A and B with overlaid rings indicating 30 (inner) and 10 Å (outer) resolution.
Conclusions
The ability to visualize molecular details of live biological processes is close to becoming a reality. In situ liquid chambers have progressed to the point that atomic resolution is possible with real-time observations of nanoparticle dynamics. Although biological samples have not achieved this same resolution, the discrepancy is largely due to the flexibility of proteins, and therefore, faster temporal resolutions should effectively freeze-out this motion without requiring physical freezing. The integration of advanced technologies into the second-generation DTEM promises optimized conditions for imaging biological and organic samples on micro- and nanosecond timescales. The future coupling of in situ liquid stages with a biologically centric DTEM has the possibility of revolutionizing structural biology using electrons and providing direct synergy to the pump-probe capabilities of femtosecond X-rays.
Funding
National Institutes of Health (5RC1GM091755 to J.E.E.).
Acknowledgements
A portion of this work was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory. Pacific Northwest National Laboratory is operated by Battelle Memorial Institute for the U.S. Department of Energy under Contract No. DE-AC05-76RL01830.
References
- 1.Kendrew J C, Bodo G, Dintzis H M, Parrish R G, Wyckoff H, Phillips D C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–666. doi: 10.1038/181662a0. [DOI] [PubMed] [Google Scholar]
- 2.Watson J D, Crick F H. The structure of DNA. Cold Spring Harb. Symp. Quant. Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- 3. Available from: http://www.rcsb.org/pdb/statistics/holdings.do .
- 4.Boutet S, Lomb L, Williams G J, Barends T R M, Aquila A, Doak R B, Weierstall U, DePonte D P, Steinbrener J, Shoeman R L, Messerschmidt M, Barty A, White T A, Kassemeyer S, Kirian R A, Seibert M M, Montanez P A, Kenney C, Herbst R, Hart P, Pines J, Haller G, Gruner S M, Philipp H T, Tate M W, Hromalik M, Koerner L J, van Bakel N, Morse J, Ghonsalves W, Arnlund D, Bogan M J, Caleman C, Fromme R, Hampton C Y, Hunter M S, Johansson L, Katona G, Kupitz C, Liang M, Martin A V, Nass K, Redecke L, Stellato F, Timneanu N, Wang D, Zatsepin N A, Schafer D, Defever J, Neutze R, Fromme P, Spence J C H, Chapman H N, Schlichting I. High-resolution protein structure determination by serial femtosecond crystallography. Science. 2012;337:362–364. doi: 10.1126/science.1217737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman H N, Fromme P, Barty A, White T A, Kirian R A, Aquila A, Hunter M S, Schulz J, DePonte D P, Weierstall U, Doak R B, Maia F R, Martin A V, Schlichting I, Lomb L, Coppola N, Shoeman R L, Epp S W, Hartmann R, Rolles D, Rudenko A, Foucar L, Kimmel N, Weidenspointner G, Holl P, Liang M, Barthelmess M, Caleman C, Boutet S, Bogan M J, Krzywinski J, Bostedt C, Bajt S, Gumprecht L, Rudek B, Erk B, Schmidt C, Homke A, Reich C, Pietschner D, Struder L, Hauser G, Gorke H, Ullrich J, Herrmann S, Schaller G, Schopper F, Soltau H, Kuhnel K U, Messerschmidt M, Bozek J D, Hau-Riege S P, Frank M, Hampton C Y, Sierra R G, Starodub D, Williams G J, Hajdu J, Timneanu N, Seibert M M, Andreasson J, Rocker A, Jonsson O, Svenda M, Stern S, Nass K, Andritschke R, Schroter C D, Krasniqi F, Bott M, Schmidt K E, Wang X, Grotjohann I, Holton J M, Barends T R, Neutze R, Marchesini S, Fromme R, Schorb S, Rupp D, Adolph M, Gorkhover T, Andersson I, Hirsemann H, Potdevin G, Graafsma H, Nilsson B, Spence J C. Femtosecond X-ray protein nanocrystallography. Nature. 2011;470:73–77. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocchieri L, Karlin S. Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. 2005;33:3390–3400. doi: 10.1093/nar/gki615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPherson A, Eisenberg D. Structure and dynamics of membrane-cytoskeleton proteins. In: Advances in Protein Chemistry & Structural Biology: Protein Structure and Diseases. Donev R., editor. Oxford, UK: Academic Press; 2011. pp. 189–190. [Google Scholar]
- 8.Evans J E, Jungjohann K L, Wong P C, Chiu P L, Dutrow G H, Arslan I, Browning N D. Visualizing macromolecular complexes with in situ liquid scanning transmission electron microscopy. Micron. 2012;43:1085–1090. doi: 10.1016/j.micron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 10.White S. Membrane Proteins of Known Structure. [15 July 2012]; Available from: http://blanco.biomol.uci.edu/mpstruc/listAll/list .
- 11.Adrian M, Dubochet J, Lepault J, McDowall A W. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- 12.Berriman J, Unwin N. Analysis of transient structures by cryo-microscopy combined with rapid mixing of spray droplets. Ultramicroscopy. 1994;56:241–252. doi: 10.1016/0304-3991(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 13.Shaikh T R, Barnard D, Meng X, Wagenknecht T. Implementation of a flash-photolysis system for time-resolved cryo-electron microscopy. J. Struct. Biol. 2009;165:184–189. doi: 10.1016/j.jsb.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramaniam S, Henderson R. Electron crystallography of bacteriorhodopsin with millisecond time resolution. J. Struct. Biol. 1999;128:19–25. doi: 10.1006/jsbi.1999.4178. [DOI] [PubMed] [Google Scholar]
- 15.White H D, Thirumurugan K, Walker M L, Trinick J. A second generation apparatus for time-resolved electron cryo-microscopy using stepper motors and electrospray. J. Struct. Biol. 2003;144:246–252. doi: 10.1016/j.jsb.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Evans J E, Jungjohann K L, Browning N D, Arslan I. Controlled growth of nanoparticles from solution with in situ liquid transmission electron microscopy. Nano Lett. 2011;11:2809–2813. doi: 10.1021/nl201166k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams I M, McBain J W. A closed cell for electron microscopy. Science. 1944;100:273–274. doi: 10.1126/science.100.2595.273. [DOI] [PubMed] [Google Scholar]
- 18.Marton L. La Microscopie Electronique des Objets Biologiques. Bull. Acad. Roy. Med. Belg. 1935;21:600–617. [Google Scholar]
- 19.Williamson M J, Tromp R M, Vereecken P M, Hull R, Ross F M. Dynamic microscopy of nanoscale cluster growth at the solid-liquid interface. Nat. Mater. 2003;2:532–536. doi: 10.1038/nmat944. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H, Smith R K, Jun Y W, Kisielowski C, Dahmen U, Alivisatos A P. Observation of single colloidal platinum nanocrystal growth trajectories. Science. 2009;324:1309–1312. doi: 10.1126/science.1172104. [DOI] [PubMed] [Google Scholar]
- 21.Sugi H, Minoda H, Inayoshi Y, Yumoto F, Miyakawa T, Miyauchi Y, Tanokura M, Akimoto T, Kobayashi T, Chaen S, Sugiura S. Direct demonstration of the cross-bridge recovery stroke in muscle thick filaments in aqueous solution by using the hydration chamber. Proc. Natl. Acad. Sci. USA. 2008;105:17396–17401. doi: 10.1073/pnas.0809581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoda H, Okabe T, Inayoshi Y, Miyakawa T, Miyauchi Y, Tanokura M, Katayama E, Wakabayashi T, Akimoto T, Sugi H. Electron microscopic evidence for the myosin head lever arm mechanism in hydrated myosin filaments using the gas environmental chamber. Biochem. Biophys. Res. Commun. 2011;405:651–656. doi: 10.1016/j.bbrc.2011.01.087. [DOI] [PubMed] [Google Scholar]
- 23.Peckys D B, Veith G M, Joy D C, de Jonge N. Nanoscale imaging of whole cells using a liquid enclosure and a scanning transmission electron microscope. PLoS One. 2009;4:e8214. doi: 10.1371/journal.pone.0008214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jonge N, Poirier-Demers N, Demers H, Peckys D B, Drouin D. Nanometer-resolution electron microscopy through micrometers-thick water layers. Ultramicroscopy. 2010;110:1114–1149. doi: 10.1016/j.ultramic.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirsaidov U M, Zheng H, Casana Y, Matsudaira P. Imaging protein structure in water at 2.7 nm resolution by transmission electron microscopy. Biophys. J. 2012;102:L15–L17. doi: 10.1016/j.bpj.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jungjohann K L, Evans J E, Aguiar J A, Arslan I, Browning N D. Atomic-scale imaging and spectroscopy for in situ liquid scanning transmission electron microscopy. Microsc. Microanal. 2012;18:621–627. doi: 10.1017/S1431927612000104. [DOI] [PubMed] [Google Scholar]
- 27.Evans J E, Jungjohann K L, Browning N D. Dynamic transmission electron microscopy. In: Kaufmann E N, editor. Characterization of Materials. Hoboken, NJ: John Wiley & Sons, Inc.; 2012. pp. 1774–1787. [Google Scholar]
- 28.Woehl T J, Evans J E, Arslan I, Ristenpart W D, Browning N D. Direct in situ determination of the mechanisms controlling nanoparticle nucleation and growth. ACS Nano. 2012;6:8599–8610. doi: 10.1021/nn303371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White E R, Mecklenburg M, Shevitski B, Singer S B, Regan B C. Charged nanoparticle dynamics in water induced by scanning transmission electron microscopy. Langmuir. 2012;28:3695–3698. doi: 10.1021/la2048486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bostanjoglo O, Elschner R, Mao Z, Nink T, Weingartner M. Nanosecond electron microscopes. Ultramicroscopy. 2000;81:141–147. doi: 10.1016/s0304-3991(99)00180-1. [DOI] [PubMed] [Google Scholar]
- 31.LaGrange T, Campbell G H, Reed B W, Taheri M, Pesavento J B, Kim J S, Browning N D. Nanosecond time-resolved investigations using the in situ of dynamic transmission electron microscope (DTEM) Ultramicroscopy. 2008;108:1441–1449. doi: 10.1016/j.ultramic.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Reed B W, Armstrong M R, Browning N D, Campbell G H, Evans J E, LaGrange T, Masiel D J. The evolution of ultrafast electron microscope instrumentation. Microsc. Microanal. 2009;15:272–281. doi: 10.1017/S1431927609090394. [DOI] [PubMed] [Google Scholar]
- 33.Evans J E, Hetherington C, Kirkland A, Chang L Y, Stahlberg H, Browning N. Low-dose aberration corrected cryo-electron microscopy of organic specimens. Ultramicroscopy. 2008;108:1636–1644. doi: 10.1016/j.ultramic.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denes P, Bussat J-M, Lee Z, Radmillovic V. Active pixel sensors for electron microscopy. Nucl. Instrum. Meth. A. 2007;579:891–894. [Google Scholar]
- 35.Typke D, Gilpin C J, Downing K H, Glaeser R M. Stroboscopic image capture: reducing the dose per frame by a factor of 30 does not prevent beam-induced specimen movement in paraffin. Ultramicroscopy. 2007;107:106–115. doi: 10.1016/j.ultramic.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Gyobu N, Tani K, Hiroaki Y, Kamegawa A, Mitsuoka K, Fujiyoshi Y. Improved specimen preparation for cryo-electron microscopy using a symmetric carbon sandwich technique. J. Struct. Biol. 2004;146:325–333. doi: 10.1016/j.jsb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Kim J S, Lagrange T, Reed B W, Taheri M L, Armstrong M R, King W E, Browning N D, Campbell G H. Imaging of transient structures using nanosecond in situ TEM. Science. 2008;321:1472–1475. doi: 10.1126/science.1161517. [DOI] [PubMed] [Google Scholar]