Abstract
With its unique structure of two compartments, Janus particles inadequate, including to be used as a drug delivery system to deliver multiple payloads with widely different solubility. Here we report on a fluidic nanoprecipitation system (FNPS), capable of fabricating biocompatible Janus polymeric nanoparticles comprised of the FDA-approved polymer poly(lactic-co-glycolic acid) (PLGA). The FNPS contains dual inlets, one for each half of the particle, that insert into the precipitation stream. The system provides a one-step approach for production of Janus polymeric particles with submicrometer diameters and is likely amenable to substantial scale-up. To the best of our knowledge, this is the first demonstration of biocompatible Janus nanoparticles that encapsulate a hydrophobic drug (paclitaxel) on one side and a hydrophilic drug (doxorubicin hydrochloride) on the other.
Interest in delivering multiple drugs, or drugs linked with diagnostic agents (theranostics), is expanding. Occasionally this can be accomplished with a uniform polymeric particle.1 However, such particles are not suitable when drugs or diagnostic agents have widely disparate solubility because it is difficult to encapsulate both payloads into a single polymer formulation. There are also concerns that mixing payloads with different physiochemical properties may cause the complexity of release profiles. This problem can be overcome by passively absorbing or chemically cross-linking the second payload to the particle, a method often used for imaging purposes.2–8 Unfortunately, cross-linking and absorption are difficult to control and suffer from low efficiency.9
Particles with two compartments are named after the mythological Roman god of gates, Janus. Most Janus particles are spherical with two discernible hemispheres, but Janus cylinders and disks have also been developed (for review, see refs 10–12). Janus particles can be used for many applications for which monomorphic particles are inadequate because of their dimorphic nature; i.e., each side of the particle could be fine-tuned to encapsulate different payloads. Although Lahann et al. showed that polymeric Janus nanoparticles can be fabricated by using electrified jetting,13 these particles were not capable of carrying payloads with disparate solubility. Here we report on a fluidic nanoprecipitation system (FNPS), capable of one-step fabricating biocompatible Janus polymeric nanoparticles comprised of the FDA-approved polymer poly-(lactic-co-glycolic acid) (PLGA). To the best of our knowledge, this is the first report of Janus polymeric nanoparticles capable of carrying a hydrophobic drug (paclitaxel) and a hydrophilic drug (doxorubicin hydrochloride) in a single particle.
RESULTS AND DISCUSSION
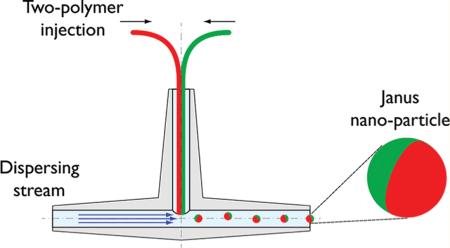
We previously reported on a fluidic nanoprecipitation system (FNPS) capable of fabricating highly uniform polymeric nanoparticles14—particularly particles comprised of poly-(lactic-co-glycolic acid) (PLGA), which is biodegradable15 and biocompatible16–18 and is one of the only nonbiologic polymers approved for use in humans. The FNPS we designed is comprised of an inlet stream that feeds a solution of polymer into a dispersing channel, where it precipitates to a highly uniform particle. Here, we extend this system to fabricate polymeric Janus particles. The FNPS was configured to contain a pair of inlet channels that abut each other just prior to the entry of the dispersing stream. Thus, each inlet channel can feed a different polymer and payload into the dispersing stream (Figure 1a). The two separate polymers make contact at the exit of the inlet streams, which are arranged in side-by-side geometry to guarantee equal exposure to the sheer force of the dispersing phase. This perpendicular arrangement of inlet and dispersing channels ensures an equal volume of each polymer is contained in each droplet. Turbulence at the junction between inlet and dispersing channels facilitates formation and release of Janus droplets (Figure 1b), which solidified through the “nanoprecipitation” process19 to submicrometer particles at a yield of ~80%.
Figure 1.
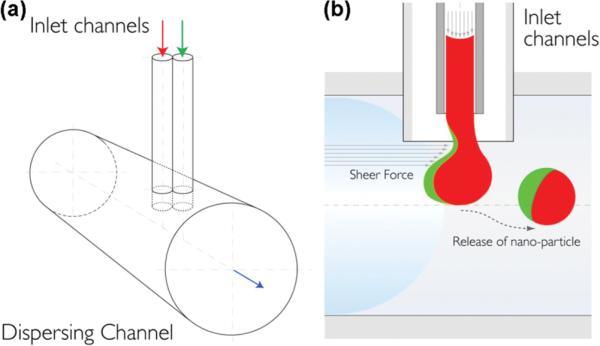
Schematic of the modified fluidic nanoprecipitation system (FNPS). (a) Cartoon of FNPS. Sample inlets are inserted into the dispersing channel via a “T” connector. The inlet channels contain two PLGA polymers that make contact at the exit of the inlet streams and precipitate upon contact with the surfactant in the dispersing channel, solidifying the particles. (b) Side view of the channels. PLGA droplets are exposed to the hydrodynamic force of the continuous flow.
To determine whether the modified FNPS could produce Janus particles, we included different fluorescent dyes with polymer in each inlet stream. In this example, one inlet fed a solution containing 25 mg/mL of PLGA PURESORB7502 (PLA/PGA 75:25, inherent viscosity 0.19 g/mL) in dimethylformamide (DMF) and Nile red. A second inlet fed a solution containing 25 mg/mL PLGA Resomer RG504H (PLA/PGA 50:50, inherent viscosity 0.45–0.6 g/mL) in acetone and rhodamine 6G. The dispersing channel contained a solution of 1% poly(vinyl alcohol) (PVA) in water. The flow rate of the inlets was set at 100 μL/h and the dispersing channel at 10 mL/min. The resulting nanoparticles were analyzed with fluorescence microscopy, and images were obtained at emission wavelengths of 568 and 488 nm. The Janus morphology is confirmed by the fact that the two fluorophores are evident on separate hemispheres of the particles (Figure 2a). While some particles appear to fluoresce with one color more than another, these are likely to be particle in which one phase of the Janus is turned toward the microscope lens and the other phase turned away. This type of effect is also apparent in the AFM images in Figure 3a.
Figure 2.
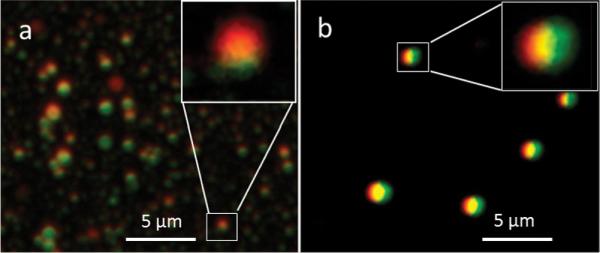
Polymeric Janus particles with dyes partitioned to separate hemispheres. The images are overlays of confocal laser scanning microscopy images obtained at 568 and 488 nm. (a) Janus particles loaded with two hydrophobic dyes, Nile red (red) and Rh-6G (green). (b) Janus particles loaded with Nile red and the hydrophilic dye FITC-dextran (green).
Figure 3.
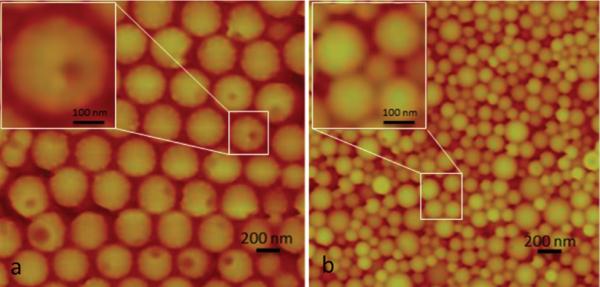
Morphology of Janus nanoparticles made is polymer-dependent. Atomic force microscope images of (a) Janus nanoparticles prepared by flowing thiol-terminated PLGA solution and PLGA Resomer RG502 solution and (b) Janus nanoparticles prepared by flowing PLGA Resomer RG504H solution and PLGA Resomer RG502 solution.
Nanoprecipitation is governed by the Marangoni effect,19 wherein movement in an interface is caused by longitudinal variations of interfacial tension.20 Since the physiochemical properties of polymer influences interfacial tension,21 we tested the effect of feeding inlet streams with distinct polymer types on the morphology of particles. One inlet fed a solution of 25 mg/mL Resomer RG502 (PLA/PGA 50:50, inherent viscosity 0.16–0.24 g/mL) in DMF. A second inlet contributed a solution of 25 mg/mL thiol-terminated PLGA solution (PLGA RG502SH, 25 mg/mL acetone solution). The dispersing channel contained a solution of 1% PVA in water. The flow rate of the inlets was set at 100 μL/h and the dispersing channel at 75 mL/min. The resulting particles (305 ± 8 nm) were examined by atomic force microscopy and had “dents”, making them appear like pitted olives (Figure 3a). We suspected the “dents” were caused by the way AFM senses the thiol-terminated alkane chain on the PLGA fed from the second inlet. Therefore, we replaced thiol-terminated PLGA with RG504H, which has a carboxylate end group. The resulting particles are spherical and lack dents (Figure 3b), suggesting the addition of the bulky thiol-terminated chain is the reason for the unique particle morphology; however, the precise mechanism underlying the formation of Janus “dents” is not entirely clear. The ability to reproducibly fabricate particles where the size of the dent is uniform illustrates the potential for making particles other than those with an almost perfectly hemispherical separation of the Janus phases.
An important application of Janus particles is codelivery of a hydrophobic and hydrophilic molecule in the same particle. In this regard, a major issue is whether a Janus particle can be fabricated where one side is derived from an emulsion, which increases the encapsulation efficiency of hydrophilic molecules.22 To test this possibility, we prepared a solution of FITC-dextran (green) in 1% PVA. This solution was combined with methylene chloride containing PLGA (Rosemer RG502H) and sonicated to form an emulsion. For the other phase a solution containing 25 mg/mL PLGA/hydrophobic Nile red in acetone was made. The two solutions were fed through separate inlet channels (200 μL/h) into the dispersing stream (1% PVA in water) flowing at 75 mL/min. Particle morphology was assessed with fluorescence microscopy; the particles were spherical, with each dye localized to an almost perfect hemisphere (Figure 2b). The ability to fabricate particles where one side comes from an emulsion makes it feasible to fabricate Janus nanoparticles containing two agents with widely different solubility. Since these particles have diameters far less than 1 μm, they are likely suitable for delivering drugs to tumors, where the leaky vasculature allows for entry of nanoparticles.
Given this opportunity, we attempted to make Janus particles containing hydrophobic and hydrophilic drugs. For this test we chose two widely used anticancer drugs, paclitaxel (PTX) and doxorubicin hydrochloride (DOX), which are frequently used in combination.23,24 The two drugs also have very different solubility (logP PTX = 3.96; logP DOX = 0.17, logP is the logarithm of the ratio of the concentrations of the un-ionized solute in the solvents), making them ideally suited for the test. In addition, systemic delivery of each of these drugs is problematic. PTX is insoluble and delivered in a solution of polyethoxylated castor oil and ethanol, which causes side effects and limits the dose.25 DOX elicits dose-limiting cardiotoxicity.26 Both limitations can be overcome by encapsulating the drugs into particles. Abraxane, approved by the FDA in 2005, is a nanoparticle in which PTX is combined with human serum albumin.27 Other work shows encapsulation of DOX into a PLGA particle eliminates cardiotoxicity.
Janus nanoparticles were prepared by feeding a solution of PTX/PLGA in acetonitrile through one inlet stream and an emulsion of DOX/PLGA in the other. Inlet streams were fed at 200 μL/h into a dispersing stream of 1% PVA in water with a flow rate of 75 mL/min. Monophasic control particles containing each drug individually were fabricated under essentially the same conditions. The degree of encapsulation of PTX in Janus particles was 1.15 wt %, equating to an encapsulation efficiency of 80%. DOX was present in Janus particles at 0.6%, equating to an encapsulation efficiency of 15%. Monophasic particles contained 3.44% PTX (encapsulation efficiency 86%) or 1.25% DOX (encapsulation efficiency 19%). Based on these values, drugs were encapsulated into each phase with essentially the same efficiency as for monophasic particles.
The drug release profiles of Janus particles were compared to monophasic particles. The in vitro release profile of drug-loaded nanoparticles was investigated by incubating the particles in 1 × PBS containing 0.1% tween80 on an orbital shaker at 37 °C. At specific time intervals, the dispersion was centrifuged at 16000g for 30 min; all the supernatant was removed and replaced by fresh release media. The supernatant were freeze-dried and then dissolved by acetonitrile for PTX measurements or by DMSO for DOX measurement. The PTX concentration was measured by HPLC and DOX concentration was measured by a fluorescence microplate reader. The cumulative percentage release is shown in Figure 4. In all cases, release of drug went through two phases: a burst release and a slower continuous release (Figure 4). PTX was released differently from Janus vs monophasic particles (Figure 4a). Compared to monophasic particles, more PTX was released from Janus particles in the burst phase. This implies PTX is encapsulated differently in Janus than in monophasic nanoparticles. This distinct release profile can also be taken as evidence the particles have Janus features. In contrast, water-soluble DOX was released from Janus particles in a manner almost identical to that of monophasic particles (Figure 4b). In all cases, greater than 60% of each drug was released during the first 24 h.
Figure 4.
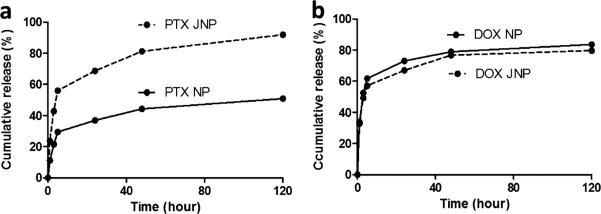
Cumulative release of drugs from nanoparticles. Release profiles of (a) paclitaxel (PTX) and (b) doxorubicin hydrochloride (DOX) from Janus particles (JNP) and monophasic particles (NP) into PBS at 37 °C. For each experiment, 2 mg/mL Janus particles and 1 mg/mL monophasic particles were used. Each experiment was repeated three times.
There are several advantages of using Janus particles as drug carriers. First, it can avoid the interference of drugs when mix them in the same carrier. Second, from production standpoint of view, there is no one-for-all method to encapsulate drugs with best encapsulation efficiency. Nanoprecipitation is best for some hydrophobic drugs, and water-soluble drugs have a better encapsulation using emulsion method. Our fluidic system provides a one-step process to encapsulate two different drugs using the methods best for them: nanoprecipitation for PTX and emulsion for DOX. Last, Janus particles can also provide two surfaces (using polymers with different end-groups) for modification such as targeting, imaging purpose.
CONCLUSION
In summary, the FNPS with dual inlets provides a one-step approach for production of Janus polymeric particles with submicrometer diameters. The system is simple to assemble, and the process is likely amenable to substantial scale-up. For example, the yield for dual drug-loaded Janus nanoparticles described here is 6 mg/h by one pair of inlets; higher yield can be easily achieved by using more inlets. The ability to encapsulate payloads with widely disparate solubility is likely to broaden substantially the application of polymeric Janus particles.
EXPERIMENTAL SECTION
Device Fabrication and Experimental Setup
A fluidic nanoprecipitation system (FNPS) device was fabricated by using two stainless steel capillaries with an i.d. of 0.127 mm as the sample inlet channels. These were confined by a transparent plastic tube to ensure a side-by-side orientation. Tygon R-3603 tubing was used as the dispersing channel. It was connected to the sample inlets via a “T” connector (Spectrum Cat. # 961-11135).
Sample solutions were fed into the inlet channels as follows: two 3 mL syringes were attached using the dual-syringe applicator assembly (FibriJet SA-0100, Micromedics, St. Paul, MN). Each syringe was loaded with a specific sample solution and connected to a sample inlet. Flow was controlled by a single syringe pump (KDS100, KD Scientific, Holliston, MA). A stream of surfactant solution passing through the dispersing channel was fed by a Fisher peristaltic mini-pump.
Synthesis of Thiol-Termiated PLGA
Thiol-terminated PLGA was synthesized by dissolving 6-(tritylthio)hexanoic acid in DMF along with HBTU/HOBt and triethylamine at room temperature (molar ratio 1:1.2:1.2:2). After 1 h, 1,4-bezenediamine was added, and the mixture was stirred for 2 h. The resulting mixture was partitioned between water and ethyl acetate. The organic layers were pooled, evaporated, and purified on a silica gel column eluted with ethyl acetate–hexane (10–50%) to provide compound I. Compound I was covalently attached to PLGA-COOH (RG 502H) in DMF with HBTU/HOBt and triethylamine at room temperature for 2 h (same molar ratio mentioned above). The resulting polymer was precipitated with ethyl ether and repeatedly washed in an ice-cold mixture of ethyl ether and methanol to remove impurities. After drying under vacuum, the -Trt protecting group was removed by dissolving the polymer in 1% trifluoroacetic acid (TFA) methylene chloride solution at room temperature for 2 h. The thiol-terminated PLGA (RG502SH) was precipitated with cold methanol, washed with the same solvent, and then dried under vacuum and used for particle preparation without further treatment.
Preparation of Janus Particles
Janus nanoparticles were prepared by injecting 1 mL of each sample solution at a flow rate of 200 μL/h into 40 mL of dispersing phase (1% PVA solution). For dye-loaded particles, solution A was prepared by dissolving 1 mg of Nile red and 25 mg of PLGA (Rosemer RG502) in 1 mL of acetonitrile. Solution B, an emulsion, was prepared by first dissolving 1 mg of FITC-dextran in 1.5 mL of 1% PVA solution, which was added directly to a solution of 50 mg of PLGA (Rosemer RG502H) in 1.5 mL of methylene chloride, followed by sonication on ice for 60 s. For drug-loaded particles, solution A was prepared by dissolving 1 mg of paclitaxel and 25 mg of PLGA (Rosemer RG502) in 1 mL of acetonitrile. Solution B was prepared by first dissolving 1 mg of doxorubicin in 1.5 mL of 1% PVA solution, which was added directly to a 50 mg of PLGA (Rosemer RG502H) solution in 1.5 mL of methylene chloride/methanol (2:1). This solution was sonicated on ice for 60 s. Janus nanoparticles were collected into a beaker containing the dispersing solution. Janus particles were washed three times in Millipore water and lyophilized before use.
Preparation of Monophasic Nanoparticles
PLGA monophasic nanoparticles containing paclitaxel were prepared by injection of 1 mL of a solution of 1 mg of paclitaxel and 25 mg of PLGA (Rosemer RG502) in 1 mL of acetonitrile into one of the FNPS inlets. The other inlet was left empty. Monophasic nanoparticles containing doxorubicin hydrochloride were made by first dissolving 1 mg of doxorubicin in 1.5 mL of 1% PVA solution, which was added directly to a 50 mg of PLGA (Rosemer RG502H) solution in 1.5 mL of methylene chloride/methanol (2:1). This solution was sonicated on ice for 60 s. 1 mL of this solution was added into one of the inlets. Nanoparticles were collected into a beaker containing the dispersing solution. Particles were washed three times by Millipore water and lyophilized before use.
Characterization of the PLGA Nanoparticles
The size of the nanoparticles was determined using dynamic light scattering with a Beckman Coulter PCS submicrometer particle size analyzer. The morphology of the nanoparticles was examined using confocal microscopy (Radiance 2100/AGR-3Q BioRad multiphoton laser point scanning confocal microscope). Samples were dropped on glass slides and excited with an Ar ion laser at 488 nm for rhodamine 6G and a Kr laser at 568 nm for Nile red. Atomic force microscope images were obtained by dropping samples on fresh peeled mica and using the Digital Instruments Nanoscope IV.
The concentration of paclitaxel loaded in particles was measured by HPLC. The concentration of doxorubicin hydrochloride concentration was assayed by measuring the fluorescence at excitation 480 nm/emission 590 nm using a Molecular Devices SpectraMax GeminiEM. Encapsulation efficiency was calculated as the mass ratio of the entrapped drug in nanoparticles to the amount used in their preparation.
The rate of drug release was measured by incubating the particles in 1 × PBS containing 0.1% tween80 on an orbital shaker at 37 °C. At a predetermined time, an aliquot of the particle suspension was removed and centrifuged at 13.1K rpm for 30 min. The supernatant was lyophilized, and the drug was extracted using acetonitrile for paclitaxel or DMSO for doxorubicin. Concentrations were assayed by using the methods described above.
ACKNOWLEDGMENTS
The authors thank Dr. Christina Niemeyer, Dr. David T. Valenta, and Dr. Kosi Gramatikoff for insightful discussions. The work described in this manuscript was supported by a grant from the U.S. National Institutes of Health (HL080718) awarded to J.W.S.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Kolishett N, Dharc S, Valenciad PM, Lind LQ, Karnike R, Lippard SJ, Langer R, Farokhzad OC. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. U. S. A. 2010;107(42):17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Erdogan S, Torchilin VP. Gadolinium-loaded polychelating polymer-containing tumor-targeted liposomes. Methods Mol. Biol. 2010;605:321–334. doi: 10.1007/978-1-60327-360-2_22. [DOI] [PubMed] [Google Scholar]
- (3).Upadhyay KK, Bhatt AN, Castro E, Mishra AK, Chuttani K, Dwarakanath BS, Schatz C, Le Meins JF, Misra A, Lecommandoux S. In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol. Biosci. 2010;10:503–512. doi: 10.1002/mabi.200900415. [DOI] [PubMed] [Google Scholar]
- (4).Meng F, Engbers GH, Feijen J. Biodegradable polymersomes as a basis for artificial cells: encapsulation, release and targeting. J. Controlled Release. 2005;101:187–198. doi: 10.1016/j.jconrel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- (5).Nurunnabi M, Cho KJ, Choi JS, Huh KM, Lee YK. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials. 2010;31:5436–5444. doi: 10.1016/j.biomaterials.2010.03.057. [DOI] [PubMed] [Google Scholar]
- (6).Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69:1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- (7).Yildiz I, Deniz E, McCaughan B, Cruickshank SF, Callan JF, Raymo FM. Hydrophilic CdSe-ZnS core–shell quantum dots with reactive functional groups on their surface. Langmuir. 2010;26:11503–11511. doi: 10.1021/la1010488. [DOI] [PubMed] [Google Scholar]
- (8).Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007;99:1106–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- (9).Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31(11):3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Walther A, Muller AHE. Janus particles. Soft Matter. 2008;4:663–668. doi: 10.1039/b718131k. [DOI] [PubMed] [Google Scholar]
- (11).Jiang S, Chen Q, Tripathy M, Luijten E, Schweizer KS, Granick S. Janus particle synthesis and assembly. Adv. Mater. 2010;22:1060–1071. doi: 10.1002/adma.200904094. [DOI] [PubMed] [Google Scholar]
- (12).Wurm F, Kilbinger AFM. Polymeric Janus asymmetric particles. Angew. Chem., Int. Ed. 2009;48:8412–8421. doi: 10.1002/anie.200901735. [DOI] [PubMed] [Google Scholar]
- (13).Roh K-HMD, Lahann J. Biphasic Janus particles with nanoscale anisotropy. Nature Mater. 2005;4:759–763. doi: 10.1038/nmat1486. [DOI] [PubMed] [Google Scholar]
- (14).Xie H, Smith JW. Fabrication of PLGA nanoparticles with a fluidic nanoprecipitation system. J. Nanobiotechnol. 2010;8(18):xxx. doi: 10.1186/1477-3155-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Delivery Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- (16).Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24(19):3311–31. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- (17).Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- (18).Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(27):5821–5830. doi: 10.1016/j.biomaterials.2004.01.038. [DOI] [PubMed] [Google Scholar]
- (19).Fessi H, Puixeux F, Devissaguet J-P, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989;55:R1–R4. [Google Scholar]
- (20).Sternling CV, Scriven LE. Interfacial turbulence: Hydrodynamic instability and the marangoni effect. AIChE J. 1959;5(4):514–523. [Google Scholar]
- (21).Aubry J, Ganachaud F, Addad JC, Cabane B. Nano-precipitation of Polymethylmethacrylate by Solvent Shifting:1. Boundaries. Langmuir. 2009;1970:1979. doi: 10.1021/la803000e. [DOI] [PubMed] [Google Scholar]
- (22).Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- (23).Dombernowsky P, Gehl J, Boesgaard M, Jensen TP, Jensen BW, Ejlertsen B. Treatment of metastatic breast cancer with paclitaxel and doxorubicin. Semin. Oncol. 1995;23:13–17. [PubMed] [Google Scholar]
- (24).Gianni EM, Capri G, Fulfaro F, Tarenzi E, Villani F, Spreafico C, Laffranchi A, Caraceni A, Martini C, Stefanelli M, Valagussa P, Bonadonna G. Paclitaxel by 3-h infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequencefinding study. J. Clin. Oncol. 1995;13:2688–2699. doi: 10.1200/JCO.1995.13.11.2688. [DOI] [PubMed] [Google Scholar]
- (25).Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J. Natl. Cancer Inst. Monogr. 1993;15:141–147. [PubMed] [Google Scholar]
- (26).Saltiel E, McGuire W. Doxorubicin (Adriamycin) Cardiomyopathy—A Critical Review. West J. Med. 1983;139(3):332–341. [PMC free article] [PubMed] [Google Scholar]
- (27).Paclitaxel Albumin-stabilized Nanoparticle Formulation. National Cancer Institute Drug Information; [Google Scholar]


