SUMMARY
The 22 γ-protocadherins (γ-Pcdhs) potentially specify thousands of distinct homophilic adhesive interactions in the brain. Neonatal lethality of mice lacking the Pcdh-γ gene cluster has, however, precluded analysis of many brain regions. Here, we use a conditional Pcdh-γ allele to restrict mutation to the cerebral cortex and find that, in contrast to other central nervous system phenotypes, loss of γ-Pcdhs in cortical neurons does not affect their survival or result in reduced synaptic density. Instead, mutant cortical neurons exhibit severely reduced dendritic arborization. Mutant cortices have aberrantly high levels of protein kinase C (PKC) activity and of phosphorylated (inactive) myristoylated alanine-rich C-kinase substrate, a PKC target that promotes arborization. Dendrite complexity can be rescued in Pcdh-γ mutant neurons by inhibiting PKC, its upstream activator phospholipase C, or the γ-Pcdh binding partner focal adhesion kinase. Our results reveal a distinct role for the γ-Pcdhs in cortical development and identify a signaling pathway through which they play this role.
INTRODUCTION
Aberrant dendrite development is associated with the synaptic dysfunction that characterizes autism spectrum disorders and mental retardation. In patient samples and in the brains of transgenic mice engineered to model these disorders, a reduction in dendrite complexity accompanied by disruptions in spine morphology and synaptic density is observed (Dierssen and Ramakers, 2006; Kishi and Macklis, 2010; Kwon et al., 2006). Given that adhesion molecules are major contributors to the progression of synaptogenesis (Sanes and Yamagata, 2009), it follows that they should also be implicated in dendrite arborization, as has increasingly been found to be the case. The ~19,000 distinct ectodomain splice variants of the Drosophila immunoglobulin superfamily molecule Dscam1 mediate homophilic self-recognition of a neuron’s dendrites and are necessary for repulsive signaling that leads to arbor spread (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007; Wojtowicz et al., 2007). Intriguingly, mammalian DSCAM and DSCAML1, despite lacking the splicing diversity of Drosophila Dscam, appear to serve a similar function in the developing mouse retina (Fuerst et al., 2009). Interactions between alternatively spliced neurexins and neuroligins, which are important for synapse function (Varoqueaux et al., 2006), have also been shown to promote growth of dendritic arbors in Xenopus (Chen et al., 2010).
Several members of the cadherin superfamily, which encompasses ~20 classical cadherins and ~80 protocadherins (Pcdhs), have been implicated in dendrite arborization in addition to synapse development. Drosophila CadN (Zhu and Luo, 2004) and mammalian N-cadherin (Marrs et al., 2006; Tanabe et al., 2006) are critical for the normal formation of dendrite arbors in several neuronal types, as are the catenins, cadherin signaling partners (Yu and Malenka, 2003; Elia et al., 2006). Functions of the diverse Pcdhs, which contain varying numbers of cadherin repeats in their ectodomains but do not signal through catenins, are less understood. However, roles in dendrite arborization have been established for Drosophila Flamingo, a seven-transmembrane Pcdh, and for its mammalian Celsr homologs (Gao et al., 2000; Grueber et al., 2002; Shima et al., 2007). Perhaps the most intriguing neuronal Pcdhs are those belonging to three gene clusters, Pcdh-α, Pcdh-β, and Pcdh-γ, encompassing ~1 megabase on human chromosome 5q31, with similar arrangements in other vertebrates (Wu and Maniatis, 1999; Wu, 2005). The Pcdh-γ gene cluster contains 22 “variable” (V) exons expressed from their own promoters and spliced to three short, invariant “constant” (C) exons (Tasic et al., 2002; Wang et al., 2002a; Wu and Maniatis, 1999). Each V exon encodes six cadherin repeats, a transmembrane domain, and a cytoplasmic domain of a single isoform, with a shared carboxyl (C) terminus encoded by the C exons. Pcdh-γ genes are expressed throughout the developing central nervous system, including the cortex, with each neuron expressing a subset (Wang et al., 2002b; Kaneko et al., 2006; Zou et al., 2007). The γ-Pcdhs promiscuously form cis-tetramers that interact in a strictly homophilic manner in trans, which indicates that this family could specify at least 104 distinct adhesive interfaces (Schreiner and Weiner, 2010).
Mice in which all 22 Pcdh-γ genes have been deleted (Pcdh-γdel/del) die within hours of birth (Wang et al., 2002b). Here we use a conditional Pcdh-γ mouse mutant and a fore-brain-restricted Cre line to circumvent this neonatal lethality. We show that loss of the γ-Pcdhs results in a severe reduction in cortical neuron dendrite arborization during the third and fourth postnatal weeks. Through both biochemical analyses of developing cortical tissues and pharmacological manipulation of cortical neuron cultures, we provide evidence that the γ-Pcdhs promote dendrite development by negatively regulating PKC activity via focal adhesion kinase (FAK) and phospholipase C (PLC), maintaining myristoylated alanine-rich C-kinase substrate (MARCKS) in its dephosphorylated, active state.
RESULTS
Reduction of Cortical Layer I without Lamination Defects in Pcdh-γ Mutants
To identify functional roles for the γ-Pcdhs in cortical development, we crossed Pcdh-γfcon3 conditional mutant mice (Prasad et al., 2008) with a line expressing Cre from the Emx1 locus (see Figures S1A–S1G available online). The Emx1-Cre line has been used extensively to excise floxed alleles in progenitors that give rise to primary glutamatergic neurons as well as astrocytes in the cortex, while sparing ganglionic eminence-derived GABAergic cortical interneurons (Gorski et al., 2002). We confirmed that Emx1-Cre efficiently recombined the Pcdhγfcon3 allele by immunostaining in neonatal Emx1-Cre; Pcdh-γfcon3/+ brains (Figures S1A–S1G).
Emx1-Cre; Pcdh-γfcon3/fcon3 mutants were born in Mendelian ratios and were viable and fertile. Gross examination of the brain revealed no obvious abnormalities or changes in overall size. Comparison of sections through the primary somatosensory cortex (S1), however, revealed that the mutant cortex was thinner than that of controls. Close examination showed that this was due entirely to a reduction of the superficial, cell-sparse layer I: layers II–VI were remarkably similar in side-by-side micrographs of controls and mutants (Figures 1A and 1B), and quantitative analysis of a variety of cortical layer markers indicated no difference in cell number or in lamination (Figures S1H–S1M). Layer I thinning occurred between postnatal day 18 (P18) and P28, with the distance between layer II and the pia reduced by 42% (n = 48 total measurements from three animals per genotype; Figure 1C). Apoptosis was similarly low in control and mutant cortex throughout the postnatal period (Figures S1N and S1O), and loss of the γ-Pcdhs in the primary neurons of the cortex also did not affect the numbers of cortical interneurons (Figures S1P and S1Q; data not shown).
Figure 1. Loss of Layer I Apical Branches in Pcdh-γ Mutant Cortex.
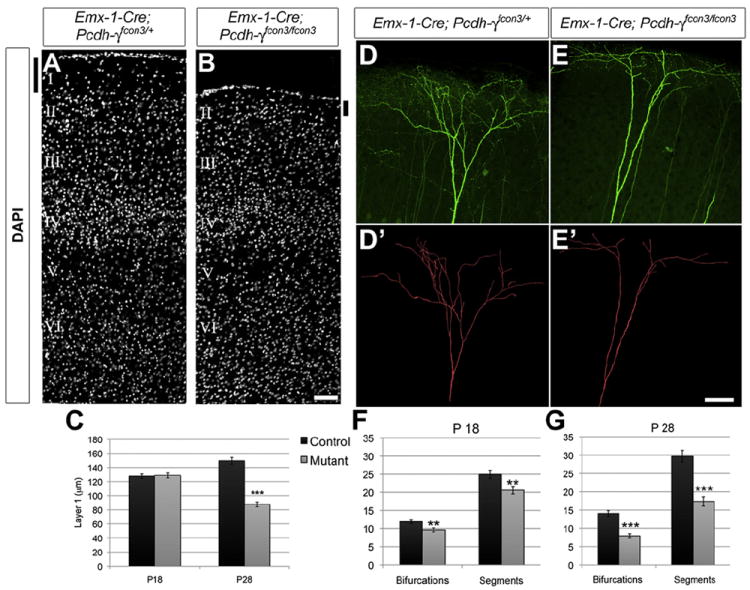
(A and B) Sections through P28 Emx1-Cre; Pcdh-γfcon3/fcon3 cortex demonstrate a reduction in thickness of layer I (black bars) despite normal lamination in layers II–VI. (C) This reduction occurs between P18 and P28. (D–E′) Apical tufts of layer V neurons in Thy1-YFPH-expressing controls (D, YFP; D′, reconstruction) and Emx1-Cre; Pcdh-γfcon3/fcon3 mutants (E and E′) were analyzed for complexity. (F and G) Dendrite segments (between two branch points) and bifurcations (branch points) were reduced at P18 (F), but this phenotype worsened significantly by P28 (G). Images are from P28 mice; scale bar represents 100 μm in (A) and (B) and 75 μm in (D)–(E′). **p < 0.01; ***p < 0.001.
Reduced Dendrite Arborization in the Absence of γ-Pcdhs
Because cortical layer I is composed mainly of apical dendritic tufts of deep-layer pyramidal neurons, loss of layer I in the mutants could be due to defects in dendrite arborization. To investigate this, we crossed Emx1-Cre; Pcdh-γfcon3 mice with the Thy1-YFPH transgenic line (Feng et al., 2000), in which a population of layer V neurons throughout the cortex strongly expresses yellow fluorescent protein (YFP). We analyzed confocal stacks from 100 μm vibratome sections of Emx1-Cre; Pcdh-γfcon3/fcon3; Thy1-YFPH mutants and littermate controls between P18 and 3 months of age. As in controls, mutant layer V pyramidal neurons extended apical dendrites into layer I (Figures 1D and 1E), and their axons correctly exited the cortex through the internal capsule to form the corticospinal tract (data not shown).
Individual neurons were reconstructed through confocal stacks by using a program (Neuromantic) to disambiguate processes from those of any neighboring YFP+ cells. We found that loss of dendrite complexity in mutant neurons could account for the reduction in layer I that we observed (Figures 1D and 1E). We quantified two measures of apical tuft complexity: number of dendritic segments (between branch points) and number of bifurcations (branch points). By both measures, arborization of apical tufts was significantly reduced in the absence of γ-Pcdhs (Figures 1F and 1G; n = 20 tufts per genotype, per time point). Immunostaining for markers of axons, astrocytes, and Cajal-Retzius cells did not reveal any other abnormalities within the mutant layer I (Figure S2A).
We also measured dendrite arborization in confocal reconstructions of whole neurons (Figures 2A and 2B) by using Sholl analysis with crossings measured at 10 μm increments from the soma. Arborization of mutant layer V neurons was not reduced at P18 but was severely disrupted by P28 (Figures 2C, 2D, and 2G). Similar analyses of 3-month-old mice showed that the reduced complexity of mutant dendritic arbors persisted into adulthood (Figures 2E and 2G; n = 20 neurons per genotype, per time point). This was observed throughout the entire dendritic arbor: when basal and oblique dendritic compartments were quantified separately, the results were essentially identical (Figures S2B–S2D). By 3 months, a population of layer II/III pyramidal neurons in Thy1-YFPH mice accumulates sufficient YFP signal to image their dendritic arbors. This allowed us to ask whether the defects we observed were limited to layer V neurons or represented a broader effect of γ-Pcdh on cortical neurons. Sholl analysis demonstrated that dendritic arbor complexity was also significantly reduced in mutant layer II/III neurons (Figures 2F and 2G).
Figure 2. Reduced Dendrite Arborization in Pcdh-γ Mutant Cortical Neurons.
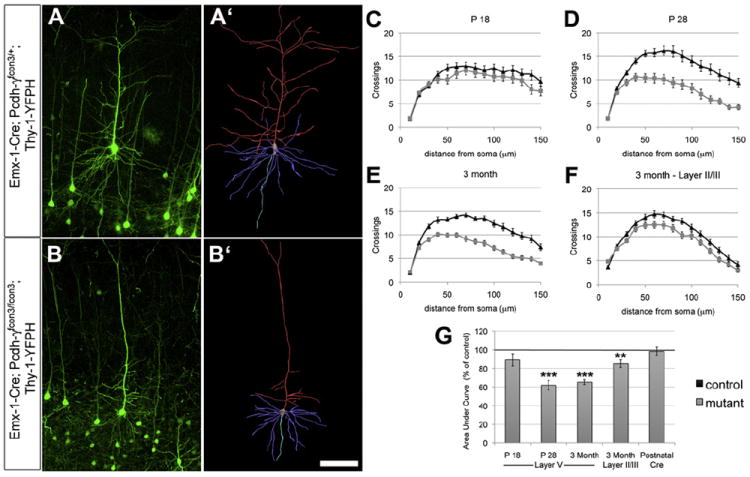
(A–B′) Sholl analyses were performed on reconstructed layer V neurons in S1 of Thy1-YFPH-expressing controls (A and A′) and Emx1-Cre; Pcdh-γfcon3/fcon3 mutants (B and B′). (C, D, and G) Arborization was similar in mutants and controls at P18 (C and G) but was significantly reduced in mutants by P28 (D and G). (E, F, and G) This disruption persisted at 3 months (E and G). Dendrite arborization was also significantly decreased in layer II/III neurons at 3 months (F and G). If the Pcdh-γ locus is disrupted only after P28 by tamoxifen injection in Cre-ER; Pcdh-γfcon3/fcon3 mice and analyzed 4 weeks later, arborization is similar to controls (G). Graphs in (C)–(F) plot the means ± SEM of Sholl crossings at indicated distances from soma; graph in (G) plots area under the curve of these Sholl graphs (as percentage of matched control). Scale bar represents 150 μm. ***p < 0.001; **p < 0.01.
We next asked whether deleting the Pcdh-γ genes after dendritic arbors had already formed would cause their existing complexity to be lost. We addressed this question by crossing Pcdh-γfcon3/fcon3 mice with a tamoxifen-inducible Cre-ER transgenic line (Guo et al., 2002). We injected Cre-ER; Pcdh-γfcon3/fcon3; Thy1-YFPH conditional mutants and controls with tamoxifen at P28 and performed Sholl analysis on layer V neurons 4 weeks later. In parallel littermates injected with the same tamoxifen dose, we confirmed that the Pcdh-γfcon3 allele was excised in all or nearly all neurons, as observed previously (Garrett and Weiner, 2009). Loss of the γ-Pcdhs after P28 did not lead to a reduction in dendrite complexity at 2 months (Figure 2G; n = 20–26 neurons per genotype). Together, these data suggest that the γ-Pcdhs are critical for the elaboration of dendritic arbors between P18 and P28, but not for their maintenance.
Identification of a PKC Signaling Pathway Dysregulated in Pcdh-γ Mutant Cortex
Little is known about signaling events downstream of γ-Pcdhs. Thus, we screened cortical tissues from Pcdh-γ mutants for dysregulation of signaling pathways implicated in dendrite arborization (Jan and Jan, 2010; Urbanska et al., 2008) by assessing the phosphorylation state of component proteins. For the majority of the proteins examined, including PDK-1, ERK, AKT, PTEN, and mTOR, no differences in phosphorylation could be detected (Figure S3A). In contrast, we found that levels of phosphorylated MARCKS, an actin-binding membrane-associated protein (Hartwig et al., 1992; Li et al., 2008; Swierczynski and Blackshear, 1995), were significantly higher in Pcdh-γ mutant cortex samples as dendrite branching defects emerged (Figure 3A). MARCKS phosphorylation was also elevated in Pcdh-γdel/del neuronal cultures (Figure S3B). This is consistent with the observed dendritic phenotype, because phosphorylation of MARCKS leads to its dissociation from actin and the plasma membrane and results in reduced dendrite complexity in cultured hippocampal neurons (Hartwig et al., 1992; Li et al., 2008; Swierczynski and Blackshear, 1995).
Figure 3. Aberrant Upregulation of a FAK/PKC/MARCKS Signaling Pathway in Pcdh-γ Mutant Cortex.
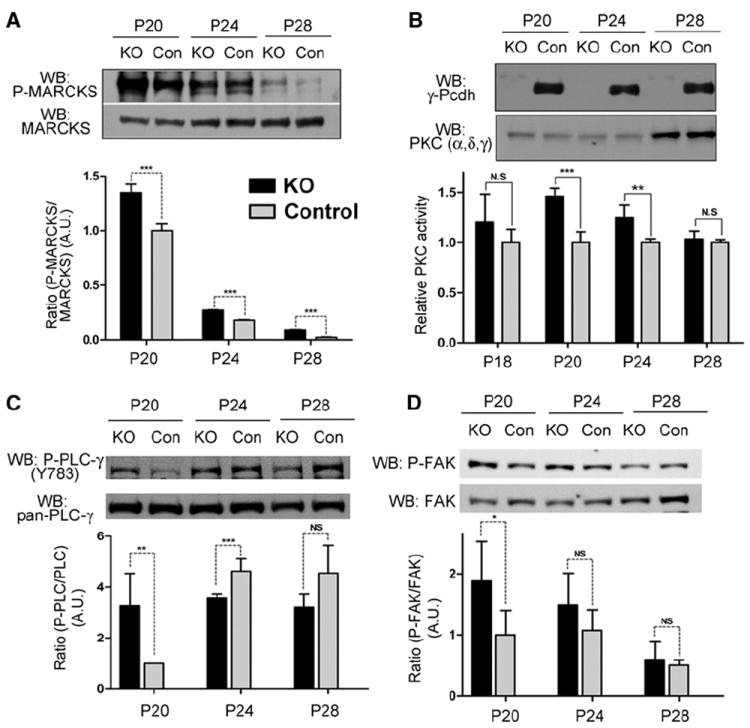
(A) Western blots were performed on cortical lysates from Emx1-Cre; Pcdh-γfcon3/fcon3 mutants (KO) and littermate controls by using antibody specific for the S152/S156 phosphorylated form of MARCKS or all MARCKS. Levels of phospho-MARCKS (normalized to total MARCKS to control for equal loading; shown as arbitrary ratio units with P20 control set at 1) were significantly higher in the mutant cortex. (B) Levels of PKC activity were measured from membrane preparations of mutant and control cortex. PKC activity (graphed as relative to control values at each age) was significantly higher at P20 and P24, despite similar levels of three PKC proteins (α, δ, and γ). (C) Levels of phospho-PLCγ1 (Y783; normalized to total PLCγ1 levels) were ~3-fold higher in the mutant cortex at P20. (D) Levels of phospho-FAK (Y397; normalized to total FAK) were significantly higher in the mutant cortex at P20. Graphs show means ± SEM of three experiments; **p < 0.05; ***p < 0.01; ns, not significant.
MARCKS is a classic substrate for PKC, which phosphorylates it on serine residues 152, 156, and 163 (Heemskerk et al., 1993). PKC activity itself can be a negative regulator of dendrite complexity (Metzger and Kapfhammer, 2000), suggesting a possible upregulation of PKC activity in Pcdh-γ mutant cortex. Direct biochemical measurement of PKC activity in cortical membrane preparations showed that it was, indeed, significantly higher between P20 and P24 in mutants compared to controls (Figures 3B and S3D). We also immunoprecipitated specific PKC isoforms and measured activity from the isolated material. Activities of PKC-α, PKC-δ, and PKC-γ (Figures S3E–S3H) were all similarly increased in the mutant cortex, suggesting a common mechanism leading to their dysregulation. Classical PKC isoforms, such as PKC-α and PKC-γ, require both intracellular Ca2+ and diacylglycerol (DAG) to become activated, whereas novel isoforms, such as PKC-δ, require only DAG (Rosse et al., 2010). The fact that all three of these isoforms are hyperactive in Pcdh-γ mutant cortex thus suggested that PLC activation, which leads to production of DAG, might also be elevated. A major brain isoform, PLCγ1, is activated by phosphorylation at tyrosine 783; in western blots of cortical lysates, Y783-phospho-PLCγ1 levels were indeed significantly higher in mutants at P20 (Figure 3C).
Although aberrant upregulation of PLC and PKC leading to MARCKS hyperphosphorylation is a plausible mechanism for explaining the dendritic defects observed, it leaves open the question of how the γ-Pcdhs regulate such a pathway. Little is known about intracellular binding partners of the γ-Pcdhs; recently, however, it was shown that FAK binds to the γ-Pcdh constant domain, and this inhibits its autophosphorylation on tyrosine residue 397, a key step in its activation (Chen et al., 2009). Additionally, FAK’s Y397 autophosphorylation site interacts with the C-terminal SH2 domain of PLCγ1, and overexpression of FAK can increase PLCγ1 activity indirectly (Zhang et al., 1999; Tvorogov et al., 2005). We thus examined whether FAK phosphorylation might be aberrantly high in the cortex of postnatal Pcdh-γ mutants. Levels of phospho-FAK were indeed significantly higher in the mutant cortex compared to controls at P20 (Figure 3D). In confirmation, we also found increased levels of phosphorylated p130-CAS, a downstream substrate of FAK, in mutants (Figure S3C).
Rescue of Dendrite Arborization in Pcdh-γ Mutant Neurons
To establish a system in which we could manipulate neurons pharmacologically and genetically, we cultured dissociated neurons from Pcdh-γdel/del and control cortex. To label the morphology of individual neurons at random, we lipofected cultures (~1%–5% efficiency) with a construct encoding YFP and measured dendrite arborization by Sholl analysis at 2, 8, and 14 days in vitro (DIV). Consistent with in vivo analyses, we found that dendrite complexity was significantly reduced in mutant neurons (Figures 4A, 4B, and 4I; Figures S4A–S4F). As expected for homophilic adhesion molecules, loss of the γ-Pcdhs affected dendrite arborization in a cell-autonomous manner, as shown by Cre transfection of individual Pcdh-γfcon3/fcon3 neurons (Figures S4G and S4H).
Figure 4. Rescue of Dendrite Arborization in Pcdh-γ Mutant Cortical Neurons.
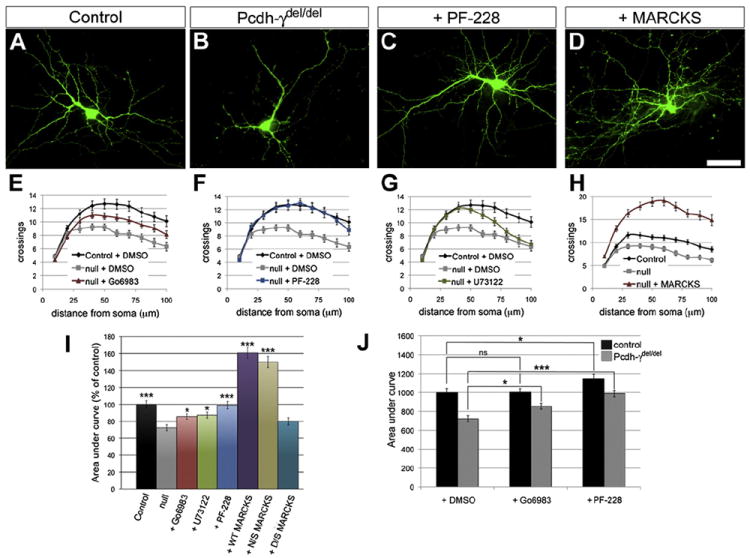
(A–D) Cortical neurons were cultured from neonatal control and Pcdh-γdel/del mice and transfected with a plasmid encoding YFP to reveal morphology. (C–I) Cultures were treated with vehicle (DMSO) only or with specific pharmacological inhibitors of PKC (Gö6983; E and I), PLC (U73122; G and I), or FAK (PF-228; C, F, and I). Inhibition of FAK completely rescued dendritic arborization in mutant neurons, whereas inhibition of PKC or PLC partially rescued arborization. In some cultures, neurons were transfected with constructs encoding GFP-tagged MARCKS (D, H, and I), an unphosphorylatable mutant (N/S-MARCKS; I), or a pseudophosphorylated mutant (D/S-MARCKS; I). Overexpression of wild-type or N/S-MARCKS (but not D/S-MARCKS) rescued dendritic arborization in mutant neurons. (J) Addition of pharmacological inhibitors had a significantly greater effect on mutant neurons than on control neurons, consistent with the γ-Pcdhs regulating a linear FAK-PKC pathway. Scale bar represents 50 μm. Graphs show means ± SEM from three experiments; *p < 0.05; ***p < 0.001; ns, not significant.
Next, we cultured cortical neurons in the presence of three pharmacological inhibitors. We broadly inhibited PKC isoforms with Gö6983 (Gschwendt et al., 1996), which significantly rescued dendrite arborization in mutant neurons toward control levels (Figures 4E and 4I), as did the addition of U73122, a potent inhibitor of PLC activity (Bleasdale et al., 1989) (Figures 4G and 4I). A recently characterized inhibitor of FAK, PF-573228 (referred to as PF-228; Slack-Davis et al., 2007), completely rescued the phenotype, increasing arborization in mutant neurons such that they were indistinguishable from controls (Figures 4A, 4C, 4F, and 4I).
MARCKS associates with the plasma membrane in part through a basic effector domain (ED). PKC phosphorylation of four serines in the ED causes MARCKS to lose its associations with actin and the membrane (Swierczynski and Blackshear, 1995; Hartwig et al., 1992). In hippocampal neurons, transfection of MARCKS or a nonphosphorylatable version with all four ED serines mutated to asparagines (N/S-MARCKS), but not a pseudophosphorylated mutant with these serines replaced by aspartates (D/S-MARCKS) (Spizz and Blackshear, 2001), led to significant increases in dendrite arborization (Li et al., 2008). We tested these constructs for their ability to rescue arborization in Pcdh-γ mutant neurons. Cultures were transfected at 1 DIV and fixed for Sholl analysis at 8 DIV; all MARCKS constructs were efficiently expressed in transfected neurons (Figures S4I–S4K). Compared to controls, both MARCKS-GFP and N/S-MARCKS-GFP (but not D/S-MARCKS-GFP) greatly increased arborization in mutant neurons to levels even above those of untransfected control neurons (Figures 4D, 4H, and 4I).
Although these experiments alone cannot exclude the possibility that γ-Pcdhs affect dendrite branching through a distinct pathway parallel to the PKC pathway, this is unlikely. The activity of this pathway, but not other dendrite arborization-associated pathways, is significantly upregulated in acutely isolated mutant cortex; there would be little explanation for this if the γ-Pcdhs affected a parallel pathway. Second, if the γ-Pcdhs affected a parallel pathway, treatment with Gö6983 or PF-228 should increase branching in both control and mutant neurons without affecting the difference between them. However, we found that these inhibitors had a greater effect on mutant neurons than on control neurons (Figure 4J), with each treatment narrowing the difference in branching between the two genotypes. Finally, consistent with MARCKS being a major downstream component of signaling regulated by the γ-Pcdhs, overexpression of MARCKS brought branching in mutant and control neurons to nearly identically high levels (Figure S4L).
Together, our experiments provide strong support for a model in which the γ-Pcdhs bind to FAK and inhibit its activation, as shown by Chen et al. (2009). This inhibition leads to reductions in the activities of PLC and PKC, resulting in the maintenance of actin-associated, nonphosphorylated MARCKS at the membrane, thus promoting dendrite arborization. In the absence of the γ-Pcdhs, the activity of this FAK-PLC-PKC pathway is elevated, resulting in increased phosphorylation of MARCKS and the observed defects in arborization.
DISCUSSION
Here, we generated mice with forebrain-restricted loss of the γ-Pcdhs and used them in experiments that identify (1) an in vivo function for these diverse adhesion molecules in regulating dendrite arborization during cortical development and (2) a PKC/MARCKS signaling pathway through which the γ-Pcdhs exert this function. Previous analyses showed a role for the γ-Pcdhs in neuronal survival in the spinal cord (Wang et al., 2002b; Prasad et al., 2008), retina (Lefebvre et al., 2008), and hypothalamus (Su et al., 2010). Surprisingly, we found no evidence for increased apoptosis in Pcdh-γ mutant cortex. This could reflect greater genetic redundancy in the control of cortical neuron survival and/or a differential requirement for the γ-Pcdhs in distinct neuronal types. The latter possibility is supported by previous observations: distinct spinal cord (Prasad et al., 2008), retinal (Lefebvre et al., 2008), and hypothalamic (Su et al., 2010) populations exhibit increased apoptosis to different extents in the absence of γ-Pcdhs. Although it is tempting to suggest that interneurons require γ-Pcdhs, whereas “projection” neurons, such as layer V neurons, do not, this may be too simplistic: Lefebvre et al. (2008) documented significant apoptosis of Pcdh-γ mutant retinal ganglion cells, as well as of interneurons. Recently, Lin et al. (2010) identified the adaptor protein PDCD10/CCM3 as an interaction partner of the γ-Pcdh constant domain and downstream effector of the γ-Pcdhs’ regulation of neuronal survival. Though PDCD10 is ubiquitously expressed in the developing brain (Petit et al., 2006), there may be other unidentified γ-Pcdh/PDCD10 signaling partners whose restricted expression in particular neuronal types modulates dependence on the γ-Pcdhs for survival.
Our results raise the question of which cell types are involved in γ-Pcdh interactions regulating dendrite arborization. Are the defects we observe due to disrupted signaling between neurons, between neurons and glia, or among a neuron’s own dendrites? Given the cellular heterogeneity of the cortex, it is remarkable that our biochemical analyses were able to detect increased activity of the FAK and PKC in vivo. Because they are derived from the cortical ventricular zone in which the Emx1-Cre transgene is active, astrocytes, which express multiple γ-Pcdhs (Garrett and Weiner, 2009), are also mutant in Emx1-Cre; Pcdh-γfcon3/fcon3 cortex. Because much of the neuropil volume is taken up by astrocytes, the γ-Pcdhs may regulate this PKC pathway in glia as well as in neurons. That would be consistent with our prior demonstration that γ-Pcdh-mediated astrocyte-neuron interactions regulate spinal cord development (Garrett and Weiner, 2009). One intriguing question is whether the γ-Pcdhs could interact either directly or epistatically with DSCAM and DSCAML1. Although the mouse genes do not exhibit the splicing diversity of the fly gene, their mutation leads to defects similar to those in flies, suggesting that DSCAM proteins act as a general “nonstick coating” on dendrites (Fuerst et al., 2009). Neurons may thus require other diverse molecules (e.g., γ-Pcdhs) to mediate neuron-specific interactions that can locally overcome a repulsive effect of the DSCAMs. Indeed, there are indications that DSCAMs and Pcdhs are functionally antagonistic. In Dscam or DscamL1 mutants, there is a significant reduction in normal retinal cell death (Fuerst et al., 2009), in contrast to the increased cell death observed in Pcdh-γ mutant retinas (Lefebvre et al., 2008). Furthermore, overexpression of DSCAM in cultured neurons has been shown to decrease dendritic arborization (Alves-Sampaio et al., 2010).
Finally, our results are consistent with several prior studies that show the following: (1) the γ-Pcdh constant domain binds to and inhibits FAK (Chen et al., 2009); (2) overexuberant dendrite arborization upon conditional deletion of FAK in cortical neurons in vivo (Beggs et al., 2003); (3) PKC activation suppresses arborization in cerebellar Purkinje cells (Metzger and Kapfhammer, 2000; Schrenk et al., 2002); and (4) dendrite arborization in hippocampal neurons depends on unphosphorylated MARCKS (Li et al., 2008). Here, we have linked these observations into a common pathway whose activity is normally suppressed by the γ-Pcdhs to allow dendrite arborization. A key remaining question is whether the γ-Pcdhs regulate this pathway constitutively or only upon the strictly homophilic trans-interactions that we have recently described as the mechanism of γ-Pcdh adhesion (Schreiner and Weiner, 2010).
EXPERIMENTAL PROCEDURES
Additional experimental details can be found in the Supplemental Experimental Procedures.
Cortical Cultures
Cortical cultures were prepared as described (Ghosh and Greenberg, 1995). Transfections were performed at 1 DIV. Each coverslip was incubated with 0.5 μg DNA and 0.5 μl Lipofectamine 2000 (Invitrogen) for 2 hr before being changed back to complete Neurobasal media. The MARCKS constructs were described in Swierczynski and Blackshear (1995). Pharmacological inhibitors were added at 6 DIV: 1 μM Gö6983 (Tocris), 100 nM U73122 (Tocris), and 1 μM PF-228 (Sigma-Aldrich).
Tissue Preparation and Immunofluorescence
Fixation, preparation, cutting, and staining of brain tissues was performed as described (Garrett and Weiner, 2009).
Western Blotting and Immunoprecipitation
Sample preparation, immunoprecipitation, western blotting, and band quantification was performed as described (Schreiner and Weiner, 2010).
PKC Activity Measurements
PKC activity was measured in crude membrane preparations with the PepTag nonradioactive protein kinase C assay (Promega) according to manufacturer’s instructions.
Image Analysis
Images were collected by using a Leica DM5000B epifluorescence microscope or a Leica SP2 AOBS laser-scanning confocal microscope.
Layer I Thickness Measurements
Images collected from the S1 region of control and mutant cortex were imported into NIH ImageJ. A line was drawn perpendicular to the surface of the brain from the top of layer II to the meninges and measured at multiple mediolateral points throughout the dorsal cortex.
Reconstructions
z stacks collected from 100 μm vibratome sections were imported into Neuromantic (http://www.reading.ac.uk/neuromantic/). Neurons were reconstructed by tracing dendrite branches in each confocal plane in the stack and then either analyzed for numbers of bifurcations and segments (for apical tufts) or exported to ImageJ for Sholl analysis. Images of cultured neurons were reconstructed using NeuronJ. Sholl analyses of reconstructions from Neuromantic or NeuronJ were performed with the Sholl Analysis plugin for ImageJ (Anirvan Ghosh Laboratory, UCSD). Area under the curve for each Sholl plot was calculated and data compared with two-way analysis of variance with Bonferroni posttests.
Supplementary Material
Acknowledgments
We are grateful to Joshua Sanes for sharing unpublished data; to Joshua Sanes and Robert Burgess for helpful comments; to Perry Blackshear for his gift of MARCKS constructs; to Alexandre Tiriac, Cassandra Coleman, Mark Blumberg, and Robert Burgess for help with experiments not included; and to Leah Fuller for expert assistance. Supported by R01 NS055272 from the NIH and by a Basil O’Connor Starter Scholar Award from the March of Dimes to J.A.W.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.neuron.2012.01.028.
References
- Alves-Sampaio A, Troca-Marín JA, Montesinos ML. NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down’s syndrome. J Neurosci. 2010;30:13537–13548. doi: 10.1523/JNEUROSCI.3457-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF, Pike JE. Inhibition of phospholipase C dependent processes by U-73, 122. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:590–593. [PubMed] [Google Scholar]
- Chen J, Lu Y, Meng S, Han MH, Lin C, Wang X. alpha- and gamma-Protocadherins negatively regulate PYK2. J Biol Chem. 2009;284:2880–2890. doi: 10.1074/jbc.M807417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Tari PK, She K, Haas K. Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo. Neuron. 2010;67:967–983. doi: 10.1016/j.neuron.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 2006;5(Suppl 2):48–60. doi: 10.1111/j.1601-183X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. Control of dendritic field formation in Drosophila: the roles of flamingo and competition between homologous neurons. Neuron. 2000;28:91–101. doi: 10.1016/s0896-6273(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Garrett AM, Weiner JA. Control of CNS synapse development by γ-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29:11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Heemskerk FM, Chen HC, Huang FL. Protein kinase C phosphorylates Ser152, Ser156 and Ser163 but not Ser160 of MARCKS in rat brain. Biochem Biophys Res Commun. 1993;190:236–241. doi: 10.1006/bbrc.1993.1036. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Bäumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-α and Pcdh-γ clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222:51–58. doi: 10.1016/j.expneurol.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. γ-protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen G, Zhou B, Duan S. Actin filament assembly by myristoylated alanine-rich C kinase substrate-phosphatidylinositol-4,5-diphosphate signaling is critical for dendrite branching. Mol Biol Cell. 2008;19:4804–4813. doi: 10.1091/mbc.E08-03-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Meng S, Zhu T, Wang X. PDCD10/CCM3 acts downstream of γ-protocadherins to regulate neuronal survival. J Biol Chem. 2010;285:41675–41685. doi: 10.1074/jbc.M110.179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs GS, Honda T, Fuller L, Thangavel R, Balsamo J, Lilien J, Dailey ME, Arregui C. Dendritic arbors of developing retinal ganglion cells are stabilized by beta 1-integrins. Mol Cell Neurosci. 2006;32:230–241. doi: 10.1016/j.mcn.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Metzger F, Kapfhammer JP. Protein kinase C activity modulates dendritic differentiation of rat Purkinje cells in cerebellar slice cultures. Eur J Neurosci. 2000;12:1993–2005. doi: 10.1046/j.1460-9568.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- Petit N, Blécon A, Denier C, Tournier-Lasserve E. Patterns of expression of the three cerebral cavernous malformation (CCM) genes during embryonic and postnatal brain development. Gene Expr Patterns. 2006;6:495–503. doi: 10.1016/j.modgep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-γ gene cluster. Development. 2008;135:4153–4164. doi: 10.1242/dev.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between γ-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci USA. 2010;107:14893–14898. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk K, Kapfhammer JP, Metzger F. Altered dendritic development of cerebellar Purkinje cells in slice cultures from protein kinase Cgamma-deficient mice. Neuroscience. 2002;110:675–689. doi: 10.1016/s0306-4522(01)00559-0. [DOI] [PubMed] [Google Scholar]
- Shima Y, Kawaguchi SY, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, Hirano T, Uemura T. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat Neurosci. 2007;10:963–969. doi: 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizz G, Blackshear PJ. Overexpression of the myristoylated alanine-rich C-kinase substrate inhibits cell adhesion to extracellular matrix components. J Biol Chem. 2001;276:32264–32273. doi: 10.1074/jbc.M103960200. [DOI] [PubMed] [Google Scholar]
- Su H, Marcheva B, Meng S, Liang FA, Kohsaka A, Kobayashi Y, Xu AW, Bass J, Wang X. γ-protocadherins regulate the functional integrity of hypothalamic feeding circuitry in mice. Dev Biol. 2010;339:38–50. doi: 10.1016/j.ydbio.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczynski SL, Blackshear PJ. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J Biol Chem. 1995;270:13436–13445. doi: 10.1074/jbc.270.22.13436. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Takahashi Y, Sato Y, Kawakami K, Takeichi M, Nakagawa S. Cadherin is required for dendritic morphogenesis and synaptic terminal organization of retinal horizontal cells. Development. 2006;133:4085–4096. doi: 10.1242/dev.02566. [DOI] [PubMed] [Google Scholar]
- Tasic B, Nabholz CE, Baldwin KK, Kim Y, Rueckert EH, Ribich SA, Cramer P, Wu Q, Axel R, Maniatis T. Promoter choice determines splice site selection in protocadherin α and γ pre-mRNA splicing. Mol Cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Tvorogov D, Wang XJ, Zent R, Carpenter G. Integrin-dependent PLC-gamma1 phosphorylation mediates fibronectin-dependent adhesion. J Cell Sci. 2005;118:601–610. doi: 10.1242/jcs.01643. [DOI] [PubMed] [Google Scholar]
- Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta Neurobiol Exp (Warsz) 2008;68:264–288. doi: 10.55782/ane-2008-1695. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Suüdhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-γ gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev. 2002a;16:1890–1905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002b;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. Comparative genomics and diversifying selection of the clustered vertebrate protocadherin genes. Genetics. 2005;169:2179–2188. doi: 10.1534/genetics.104.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. β-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G, Hanks SK. Focal adhesion kinase promotes phospholipase C-γ1 activity. Proc Natl Acad Sci USA. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
- Zou C, Huang W, Ying G, Wu Q. Sequence analysis and expression mapping of the rat clustered protocadherin gene repertoires. Neuroscience. 2007;144:579–603. doi: 10.1016/j.neuroscience.2006.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


