Abstract
Objective
To examine whether dietary fish oil supplementation reduces development of spontaneous endometriosis-associated adhesions using an established model.
Design
Laboratory-based study.
Setting
Medical center research laboratory.
Patient(s)/Animal(s)
Disease-free women of reproductive age and nude mice.
Intervention(s)
Women were not provided any intervention. Mice were randomized to receive fish oil supplementation or control diet.
Main Outcome Measure(s)
Experimental endometriosis was established in mice via injection of human endometrial tissue within 16 hours of ovariectomy. Mice were provided standard or menhaden fish oil–supplemented diets for ≥2 weeks before initiation of experimental endometriosis and until killing them 1 week later. At necropsy, mice were examined for the presence and extent of adhesions and endometriotic-like lesions. Tissues were excised and morphologically characterized.
Result(s)
Adhesions/lesions were reduced in mice provided with dietary fish oil compared with control animals. Leukocytes were more numerous within the adhesions/lesions of the mice maintained on the standard diet compared with animals provided with fish oil. As indicated by staining intensity, collagen deposition was greater at adhesion sites within control mice compared with fish oil–supplemented animals.
Conclusion(s)
Wound-healing associated with surgery created an inflammatory peritoneal microenvironment that promoted the development of both experimental endometriosis and adhesions in a murine model. Targeting excessive inflammation with fish oil may be an effective adjuvant therapy to reduce the development of postsurgical adhesions related to endometriosis.
Keywords: Omega-3 fatty acids, adhesions, endometriosis, mice, inflammation
Endometriosis, the growth of endometrial glands and stroma outside the uterus, is thought to occur most frequently following retrograde menstruation into the peritoneal cavity of reproductive-age women (1, 2). Significantly, retrograde menstruation normally induces a peritoneal inflammatory response by the innate immune system which aids in the clearance of displaced endometrial tissue; however, excessive production of proinflammatory cytokines and chemokines may trigger immune cell behaviors that serve to promote the development or progression of endometriosis (3). Equally important, altered inflammatory processes within the peritoneal cavity associated with a woman’s risk for developing endometriosis may contribute to the development of adhesive disease (4, 5), a common comorbidity among these patients. Abdominal adhesions can subsequently lead to bowel obstruction, chronic pelvic pain, and/or infertility (6), potentially leading to additional surgeries and increasing the likelihood of further damage to organs within the peritoneal cavity. Therefore, preventing adhesion formation in response to various stimuli, including surgical procedures related to endometriosis, would represent a significant clinical advance.
Using an experimental endometriosis model, we previously demonstrated that the proinflammatory microenvironment associated with a recent surgical injury combined with the presence of human endometrial tissue fragments synergistically promoted development of adhesive disease at sites distal to the surgical injury (4). Taken with the observations of others (5, 6), our studies suggested that inflammatory processes associated with normal wound healing represent major contributors to development of endometriosis-associated adhesive disease (4–6). For example, immune cells, including macrophages and neutrophils that migrate to sites of injury, promote inflammation-related wound healing by releasing proinflammatory cytokines and proangiogenic factors. Whereas immune cells promote the clearance of menstrual debris under normal physiologic conditions, the inflammatory microenvironment of acute injury may disrupt this activity. Additionally, the pathophysiology of endometriosis is associated with both reduced immunosurveillance and heightened inflammatory responses within the peritoneal cavity (7–9). Ultimately, this skewed inflammatory response can lead to the development of chronic inflammation (10), thereby stimulating the progression of endometriosis and the associated development of adhesive disease (4, 6, 11).
Multiple experimental studies using a variety of model systems have examined the efficacy of limiting inflammatory processes in an effort to reduce adhesion formation (12–15). For example, using a traditional animal model of adhesions, in which the peritoneal mesothelium is directly traumatized, investigators have demonstrated the effectiveness of sildenafil, resveratrol, and rosiglitazone in the inhibition of adhesion formation (16–19). Significantly, these agents each exhibit antiinflammatory properties, which likely contributed to their success in reducing adhesive disease. Relevant to the present study, Pierce et al. (20) reported a reduction in development of adhesions in rabbits after placement of surgical mesh embedded with fish oil compared with animals receiving the same mesh without fatty acids. Using an in vitro approach, Victory et al. (21) demonstrated that docosahexaenoic acid (one of the fish oil fatty acids) significantly reduced the expression of adhesion-related markers in cultured peritoneal cells. Taken together, these studies support the further examination of the antiinflammatory omega-3 fatty acids, found in fish oil, to prevent endometriosis-associated adhesive disease. Therefore, in the present study, we explored the impact of nutritional intervention with the use of fish oil–supplemented diets, directed at reducing systemic/peritoneal inflammation, to prevent endometriosis-related adhesions in our chimeric human/murine model.
MATERIALS AND METHODS
Acquisition and Culture of Human Endometrial Tissue
Approval for human tissue use was obtained from the Vanderbilt University Institutional Review Board and Committee for the Protection of Human Subjects. After written informed consent, endometrial samples (n = 9) were obtained by Pipelle biopsy (Unimar) during the proliferative phase (days 9–12) from a donor population (age 21–45 y) at Vanderbilt University Medical Center. Donors exhibited normal menstrual cycles with no known history of adhesions or endometrial disease, including endometriosis. A serum P level of <1.5 ng/mL at the time of biopsy was required for inclusion. Individuals with a history (<3 months) of hormonal therapy (i.e., oral contraceptives) or other medications that could affect study results (e.g., Omacor, Celebrex) were excluded.
Endometrial specimens were immediately rinsed in Dulbecco Modified Eagle Medium/Nutrient Mixture F-12 (Gibco) to remove residual blood and mucous. Tissues were minced into 1–2-mm3 cubes and maintained overnight under serum-free conditions in the same medium supplemented with 1 nmol/L E2 (Sigma), 1% insulin-transferrin-selenium (Collaborative Biomedical), and 0.1% Excyte (Miles Scientific) and incubated in a 5% CO2 humidified chamber at 37°C.
Mice and Dietary Information
Virgin, female (5 weeks) Athymic Nude-Foxn1nu mice were purchased from Harlan Laboratories (Indianapolis) and housed in Vanderbilt University’s mouse vivarium under standard conditions (22°C and on a 12-h light-dark cycle) and provided food and water ad libitum. After 1 week of acclimatization, mice were randomized to receive low-phytoestrogen rodent chow (V502; Purina) or the same diet supplemented with 5% or 10% menhaden fish oil. The supplemented diets were produced on our behalf by Purina Test Diets and provided in pellet form similar to the control diet. Menhaden fish oil, donated by Omega Protein, has an established fatty acid profile (~40% omega-3 fatty acids) and was subjected to molecular distillation by that company to remove dioxins/PCBs. The fish oil diets were maintained in vacuum-sealed bags at −20°C until use and once provided to mice were replaced every 3 days. Mice were maintained on the fish oil–supplemented diet for ≥2 weeks before surgery and until we killed them (5 days after human tissue injection). All mice were weighed at the initiation of these studies (when mice were randomized to the various diets), at time of surgery, and when we killed them. At each time, weights were similar between groups, suggesting minimal differences in food consumption.
An ideal diet has been suggested to contain a 3:1 ratio of omega-6 to omega-3; however, among Western countries the typical diet contains 15:1 omega-6 to omega-3 with ratios in the U.S. often reaching 40:1 (22, 23). The diets used herein contained omega-6/omega-3 ratios of 10:1 (standard diet), 3:1 (5% fish oil diet), and 1.5:1 (10% fish oil diet). Protein, total fat, and energy content were similar among all of the diets.
Ovariectomy
Mice were anesthetized with isoflurane and subjected to standard surgical ovariectomy via a single 5 mm dorsal/ventral incision between the rib cage and hind limb as previously described (4). At the time of surgery, all mice were implanted subcutaneously with a slow-release E2 capsule assembled in our laboratory (24). Experiments described herein were approved by Vanderbilt University’s Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act.
Experimental Endometriosis/Adhesion Model
We recently reported that endometriosis-associated adhesive disease develops in mice as a consequence of introduction of human endometrial tissue fragments near the time of peritoneal surgery (4). Specifically, at necropsy 5 days after tissue injection, we found that mice that received endometrial tissues 16 hours after ovariectomy exhibited extensive adhesive disease and robust experimental endometriosis (4). Therefore, in the present study, ovariectomy was performed 16 hours before intraperitoneal injection of human endometrial tissues (Supplemental Fig. 1, available online at www.fertstert.org). After overnight culture, endometrial fragments were washed in sterile prewarmed phosphate-buffered saline solution (PBS) and injected intraperitoneally along the ventral midline with the use of an 18-gauge needle as previously described (25). Each biopsy was typically adequate for induction of experimental disease in 6–8 mice. Each experiment used one biopsy from a single donor and included both the control (standard diet) group of mice and either the 5% or 10% fish oil–supplemented group or both experimental groups. Within each separate experiment (using a single biopsy), 2–3 mice per treatment group were established with experimental disease. Each group was replicated with at least four human endometrial biopsies.
At necropsy, 5 days after intraperitoneal introduction of human tissues, the presence or absence of peritoneal adhesions and the presence and extent of ectopic sites of endometrial growth was determined. Scoring of adhesions was based on a system described by Lauder et al. (26), which considers the number, strength, and distribution of adhesions. Specifically: 0 = no adhesions; 1 = thin filmy adhesions; 2 = more than one thin adhesion; 3 = thick adhesion with focal point; 4 = thick adhesion with planar attachment; 5 = very thick vascularized adhesions or more than one planar adhesion. Endometriotic-like lesion burden was scored by number and size, with size measured in two dimensions, the larger denoted “a” and the smaller denoted “b.” The total volume was calculated by standard methodology (27) with the formula: V = a × b2 × 0.5.
Histochemical and Immunohistochemical Assessment of Ectopic Adhesions/Lesions
All tissues excised from mice at necropsy were formalin fixed, paraffin embedded, and subjected to standard hematoxylin and eosin staining. Additional sections were further subjected to Masson trichrome staining to assess the extent of collagen deposition and phosphotungstic acid hematoxylin staining (PTAH) to assess fibrin content. These stains were performed by the Vanderbilt Translational Pathology Shared Resource Core Laboratory with the use of standard methods.
Immunohistochemical localization of CD45, a panleukocyte marker, was used to assess the infiltration of leukocytes within adhesions/lesions. Briefly, formalin-fixed paraffin-embedded tissue sections were deparaffinized and subjected to antigen retrieval with the use of 1× Citrate Retrieval Buffer (Biogenex) in deionized water (dH20) at 70–75°C for 25 minutes. After cooling to room temperature, sections were rinsed twice with dH20 for 5 minutes each followed by blocking of endogenous peroxidase activity with the use of cold 3% hydrogen peroxide in methanol for 20 minutes. Sections were again rinsed twice with dH20 for 5 minutes each followed by incubation with 1× PowerBlock (Biogenex Laboratories) in dH20 for 15 minutes at room temperature. Sections were rinsed twice with 1× PBS for 5 minutes each then incubated at 4°C overnight with the primary antibody (1:150 rat antimouse CD45; BD Pharmagen) diluted in 1× PBS containing 0.5% Triton-X. Following two washes in 1× PBS, sections were incubated with an antirat secondary antibody (Biogenex Laboratories) at room temperature for 20 minutes, followed by two PBS washes for 5 minutes each. Sections were then incubated at room temperature using Strepavidin Peroxidase Label (Biogenex Laboratories) for 20 minutes, followed by two washes in 1× PBS and then chromagen detection with the use of Impact DAB (Vector Laboratories or Nova Red) according to the manufacturer’s instructions. Finally, sections were counterstained in Gill hematoxylin for 2 minutes followed by running tap water for 6 minutes, then dehydrated and coverslipped with the use of Cytoseal XYL (Thermofisher Scientific).
Leukocyte infiltrates within adhesions/lesions were enumerated by two authors (D.R.G., K.B.T.) in five random high-power fields (HPF) per group (at ×200 and at ×400 magnification) with the use of a method similar to that described by others for assessment of immune cell populations in tumors (28). The same five fields from each group and each magnification were enumerated by both observers, and the resulting ten numbers were averaged to semiquantitatively determine the number of CD45-positive cells present within control and 10% fish oil mice. Semiquantitative analysis of leukocyte infiltrates in mice receiving the 5% fish oil diet was limited to ×400 magnification owing to the limited number of adequately sized samples available. All slides were viewed with an Olympus BX51 microscope system and images captured with an Olympus DP71 digital camera.
Statistical Analysis
Analyses were performed with Graphpad Prism-5 software and presented as mean ± SEM. The statistical difference between samples was determined using one-way analysis of variance followed by Tukey post hoc test. P<.05 was considered to be significant.
RESULTS
Impact of Fish Oil Supplementation on Adhesion Formation and Endometriosis-Like Lesions
Using an established model of experimental endometriosis, we reported that adhesions rarely develop in mice that have been allowed to fully recover from ovariectomy (≥5 days) before intraperitoneal injection of human endometrial tissue, whereas adhesions are common among mice in which experimental endometriosis is established within 36 hours of surgery (4). Herein, we provided mice standard mouse chow or the same diet supplemented with 5% or 10% menhaden fish oil for 2 weeks before surgery and continuing until we killed them 5 days after establishment of experimental endometriosis. At necropsy, mice on the standard diet undergoing ovariectomy within 16 hours of receiving human endometrial tissues developed extensive adhesive disease associated with experimental endometriosis. Similar to our previous observations (4), all control mice developed lesions and adhesions at the site of surgical injury as well as at sites distal to the injury (Table 1; Fig. 1A and B). In contrast, both the adhesion score and experimental disease burden were significantly reduced in mice maintained on the 10% fish oil–supplemented diet (Table 1; Fig. 1C and D). Notably, the surgical site was the primary location for adhesion development in the supplemented mice, with distal occurrence rare.
TABLE 1.
Postsurgical adhesion and lesion scores (mean ± SEM) in mice.
| Treatment | n | Adhesion score | P value* | Lesion score | P value* |
|---|---|---|---|---|---|
| E + standard diet | 19 | 3.70 ± 0.159 | 3.03 ± 0.225 | ||
| E + 10% fish oil diet | 15 | 1.90 ± 0.150 | <.001 | 1.32 ± 0.163 | <.001 |
| E + 5% fish oil diet | 11 | 2.00 ± 0.135 | <.001 | 1.64 ± 0.279 | <.01 |
Note: E = estradiol.
Compared with mice on the standard diet.
Herington. Fish oil inhibition of adhesions. Fertil Steril 2013.
FIGURE 1.
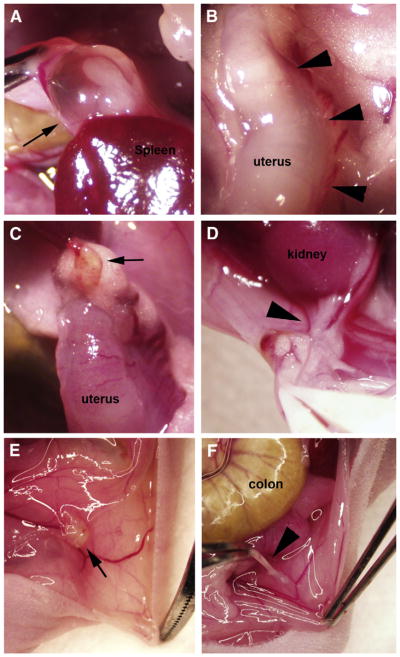
Gross morphology of lesions (arrows) and adhesions (arrowheads) in (A, B) control mice and mice maintained on the (C, D) 10% or (E, F) 5% fish oil diet. Lesions in control mice were significantly larger than those observed in supplemented mice, as illustrated by the large cyst attached to both the spleen and bowel (A) compared with the small nodular lesions observed in a 10% fish oil–supplemented mouse (C) or a 5% fish oil–supplemented mouse (E). Adhesions in control mice were frequently extensive, as shown in (B), in which the uterus is entirely adhered to the posterior peritoneum. In contrast, adhesions present in the 10% fish oil–supplemented group were less extensive, and typically involved the site of the surgical injury, as shown in (D), in which the tip of the left uterine horn is adhered to the kidney. In (F), a thin, filmy adhesion in a 5% fish oil–supplemented mouse is shown attaching the posterior peritoneum to the left uterine horn. Original magnification ×15.
Herington. Fish oil inhibition of adhesions. Fertil Steril 2013.
Recognizing that supplementation with 10% fish oil would be difficult to achieve in a patient population, we additionally examined the effectiveness of a 5% fish oil–supplemented diet. As shown in Table 1 and Figures 1E and 1F, we noted a reduction in both adhesion score and experimental disease burden similar to that observed in the mice provided 10% fish oil.
Collagen and Fibrin Deposition within Adhesions/Lesions
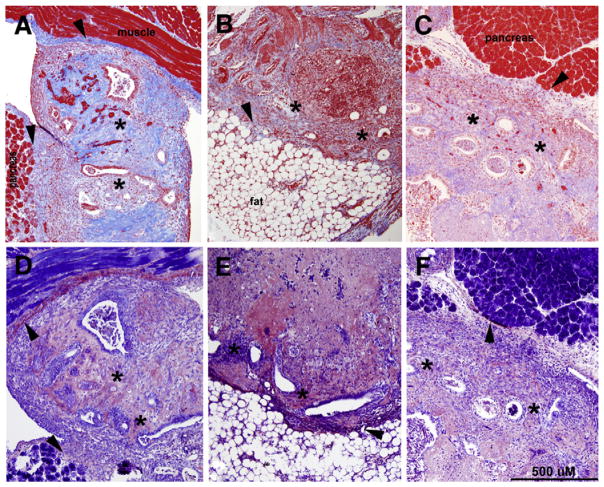
As reflected by the adhesion scores, gross observations at necropsy suggested the adhesions in mice maintained on either fish oil diet were physically weaker and had fewer attachment points compared with those developing in control animals. To more fully characterize the nature of the adhesions, formalin-fixed paraffin embedded tissues were subjected to Masson trichrome and PTAH staining. As shown in Figure 2, Masson trichrome staining of tissues from mice maintained on the standard diet revealed an intense bright blue stain (Fig. 2A) whereas adhesions/lesions obtained from the mice provided the fish oil–supplemented diets were only weakly stained (Fig. 2B and C). Because staining intensity has been demonstrated to be reflective of the abundance of collagen (29), these observations suggest that fish oil supplementation was associated with reduced collagen deposition. These data are consistent with our gross observations at necropsy, in which the adhesions present in mice receiving the standard diet appeared to be more substantial and had more attachment points compared with those present within the fish oil groups. Interestingly, PTAH histochemistry, which stains fibrin fibers dark blue, revealed minimal differences between control tissues and tissues from mice on either of the supplemented diets (Fig. 2D–2F).
FIGURE 2.
Masson trichrome staining (A–C) or phosphotungstic acid hematoxylin staining (PTAH; D–F) of adhesions/lesions removed from control (A, D) or mice provided 10% (B, E) or 5% (E, F) fish oil–supplemented diet. Masson trichrome staining results in red-blue cell nuclei, red cytoplasm, and bright blue collagen. Notably, the intensity of the blue stain has been shown to correlate with the extent of collagen present (29). Within tissues obtained from fish oil–supplemented mice, the reduced amount and intensity of blue staining suggests that the amount of collagen is also reduced (B, C). PTAH staining of near sister sections was conducted to assess the presence of fibrin (which appears as a dark blue stain) and was similar in all groups (D–F). The asterisks denote areas containing endometrial glands and stroma, indicative of endometriotic-like lesions, and arrowheads mark sites of abnormal tissue attachment (adhesion). Photomicrographs are representative results of multiple tissues from at least four mice per group. Original magnification ×100.
Herington. Fish oil inhibition of adhesions. Fertil Steril 2013.
Immune Cell Infiltrates
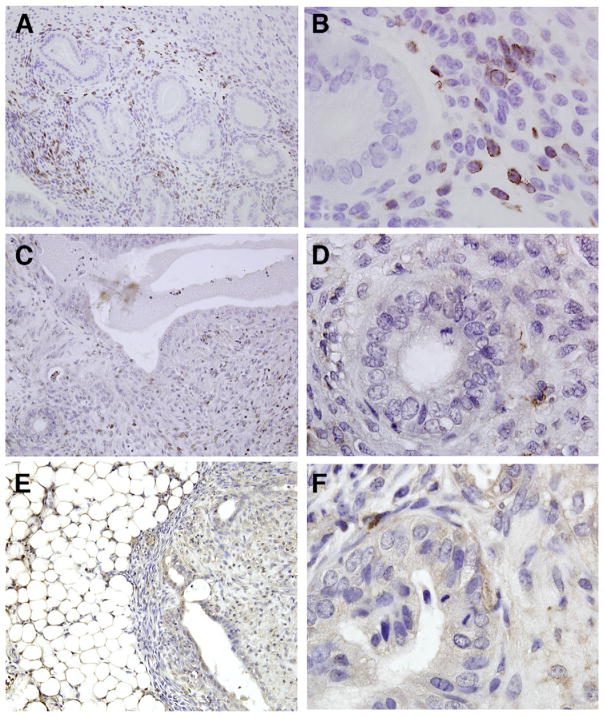
Adhesion formation has been associated with excessive immune cell influx at sites of wound healing (30); therefore, we assessed the presence of leukocytes within adhesions/lesions excised from mice provided the standard or fish oil–supplemented diet. Immunohistochemical localization of immune cells revealed a robust leukocyte infiltrate within the majority of both endometriotic-like lesions and adhesions obtained from control mice (Fig. 3A and B; 149.2 ± 18.6/×200 HPF; 91.9 ± 24.9/×400 HPF). In contrast, the presence of leukocytes within the adhesions/lesions removed from mice receiving the 10% fish oil–supplemented diet was consistently lower compared with the control group (Fig. 3C and D; 54.8 ± 13.8/×200 HPF; 30.6 ± 17.8/×400 HPF; P<.001). Although we did not have an adequate number of ×200 fields containing an appropriate amount of tissue to semiquantitatively assess the number of leukocytes present within the adhesions/lesions removed from the 5% fish oil–supplemented mice, microscopic examination and semiquantitative assessment at ×400 revealed a number of CD45+ immune cells similar to those found in the 10% fish oil–supplemented group (Fig. 3E and F; 27.3 ± 15.1/×400 HPF; P<.001). These data, taken together with our studies presented above, indicate that nutritional intervention with the omega-3 fatty acids, compounds with well known antiinflammatory actions, led to a reduction in leukocyte migration to ectopic sites which was also associated with a change in the physical nature of the adhesions.
FIGURE 3.
Immunolocalization of leukocytes with the use of an antibody directed at CD45 (leukocyte common antigen), which results in brown staining of T and B lymphocytes, granulocytes, monocytes, and macrophages. These leukocytes are abundant in adhesions/lesions obtained from control mice (A, B), but are less frequently observed in mice provided 10% (C, D) or 5% (E, F) fish oil–supplemented diet. Original magnifications ×200 (A, C, E) and ×1000 (B, D, F).
Herington. Fish oil inhibition of adhesions. Fertil Steril 2013.
DISCUSSION
Development of adhesive disease is a common postsurgical complication, with up to 50% of patients who undergo abdominal surgery developing adhesions (31). Appropriate wound healing after surgery requires both fibrin deposition to initially close the wound followed by fibrinolysis to break down the fibrin bands as healing progresses. However, if fibrinolysis is compromised, macrophages, fibroblasts, and endothelial cells may penetrate into the adhesion, leading to collagen deposition and the formation of a permanent fibrous adhesion (6). Importantly, a failure to appropriately resolve inflammation associated with wound healing can lead to chronic inflammation which further promotes postsurgical development of adhesive disease (9, 10, 32, 33).
Notably, surgical resection of endometriosis is a common procedure, placing these women at risk of developing postsurgical adhesions. Additionally, the chronic peritoneal inflammation associated with ectopic endometrial growth likely places endometriosis patients at an increased risk for the development of adhesions even in the absence of surgery (6, 9, 10, 34). Therefore, there is an urgent need to identify therapeutic regimens that will reduce the risk of endometriosis-associated adhesive disease in the presence or absence of surgery. To experimentally address the relationship between endometriosis-related inflammation and adhesion formation, we previously described an animal model in which spontaneous adhesive disease develops following the introduction of human endometrial tissue fragments subsequent to peritoneal surgery to remove the ovaries. Although ovariectomy is a surgical component of our established experimental endometriosis model (35), introduction of human endometrial tissues into the peritoneal cavity after the wound healing process is complete does not result in adhesion formation. However, a time-course study revealed that peritoneal adhesions were common if initiation of experimental endometriosis occured within 36 hours of ovariectomy. Furthermore, the most significant adhesive disease was observed when human tissues were injected at 16 hours after surgery (4). Importantly, all mice were killed 5 days after tissue injection, providing ample time for establishment of experimental endometriosis and/or the development of adhesions.
Within the peritoneal cavity, the innate immune system normally acts to promote clearance of displaced endometrial tissues following retrograde menstruation. However, under the additional influence of surgical injury, various cytokines and chemokines secreted by immune cells to promote wound healing may actually contribute to inflammation-associated establishment of both experimental endometriosis and adhesive disease (3, 4). Importantly, temporal separation of the initiation of experimental endometriosis and surgical injury by >36 hours largely eliminated the occurrence of adhesions. These data suggest that therapeutic intervention directed at reducing peritoneal inflammation related to wound healing should limit the formation of adhesions in our experimental endometriosis model. Indeed, herein we demonstrated that providing mice with an “antiinflammatory” diet containing fish oil significantly reduced experimental endometriosis-associated adhesive disease. The present study also suggests that leukocyte infiltration may play a role in promoting dense collagen-rich adhesions as well as more extensive experimental disease. These findings are consistent with human studies demonstrating that endometriosis-associated adhesions exhibit greater numbers of inflammatory cells compared with infection-related adhesions (30). Taken together, the findings described herein support a prominent role of excess inflammation, involving the migration of leukocytes, in the development of adhesions associated with establishment of experimental endometriosis. However, dietary supplementation of mice before surgery and induction of experimental disease reduced leukocyte influx and collagen deposition. In contrast, fibrin deposition, which precedes collagen deposition by 2–3 days, was similar across all groups, suggesting that fish oil supplementation did not impede normal wound healing.
Although nutritional intervention for adhesion prevention has received little attention, multiple studies suggest that an imbalance in the serum omega-6/omega-3 ratio can be associated with chronic inflammatory diseases (36, 37). This is not surprising, because studies support a role of omega-3 fatty acids in promoting the resolution of inflammation associated with wound healing (38). Specifically, the omega-3 fatty acids serve as the precursors for production of the resolvins and protectins (36, 39), endogenous molecules that have both antiinflammatory and proresolution effects (38). As described above, it is the failure of the resolution process that ultimately leads to the formation of permanent adhesions after injury. Unfortunately, humans and other mammals have only a limited capacity to synthesize the essential long-chain omega-3 fatty acids (specifically, eicosapentaenoic acid [EPA] and decosahexaenoic acid); therefore, these compounds must be obtained exogenously (40).
At least one study suggests that an imbalance between serum levels of EPA and arachidonic acid correlate with the severity of endometriosis (41). However, to our knowledge, no study has examined the omega-6/omega-3 ratio regarding a patient’s susceptibility to developing adhesive disease. Despite the ample data that indicate a significant role of inflammatory processes in development of adhesive disease (4–6, 30), at present, preventive measures center on the use of various pharmacologic agents and barrier materials. Our data suggest that an antiinflammatory diet that includes fish oil supplementation may be a beneficial adjuvant to reduce development of adhesions in women undergoing surgical treatment of endometriosis.
Supplementary Material
Acknowledgments
Supported by The Eunice Kennedy Shriver Specialized Cooperative Centers Program in Reproduction and Infertility Research (NICHD U54HD052666, NCCAM AT006245, NICHD HD7043, NICHD HD055648) and the Endometriosis Association.
The authors greatly appreciate and acknowledge the women who donated endometrial biopsies for these studies as well as the physicians at the Vanderbilt Clinic for performing these critical biopsies. The authors gratefully acknowledge the donation of highly purified menhaden fish oil by Omega Protein and Dr. Carrie Schultz (Purina Test Diets) for expert assistance in formulating the fish oil diets.
Footnotes
J.L.H. has nothing to disclose. D.R.G. has nothing to disclose. J.A.L. has nothing to disclose. K.G.O. has received payment for speaking at American Society for Reproductive Medicine courses (unrelated to this work); and is a consultant with Eisai Pharmaceuticals, Inc. (unrelated to this work). K.L.B.-T. has received payment for speaking at American Society for Reproductive Medicine courses (unrelated to this work).
References
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110. [PMC free article] [PubMed] [Google Scholar]
- 3.Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611–26. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herington JL, Crispens MA, Carvalho-Macedo AC, Camargos AF, Lebovic DI, Bruner-Tran KL, et al. Development and prevention of postsurgical adhesions in a chimeric mouse model of experimental endometriosis. Fertil Steril. 2011;95:1295–301. doi: 10.1016/j.fertnstert.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imudia AN, Kumar S, Saed GM, Diamond MP. Pathogenesis of Intra-abdominal and pelvic adhesion development. Semin Reprod Med. 2008;26:289–97. doi: 10.1055/s-0028-1082387. [DOI] [PubMed] [Google Scholar]
- 6.Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26:313–21. doi: 10.1055/s-0028-1082389. [DOI] [PubMed] [Google Scholar]
- 7.Dmowski PW, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:245–63. doi: 10.1016/j.bpobgyn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Resuehr D, Glore DR, Taylor HS, Bruner-Tran KL, Osteen KG. Progesterone-dependent regulation of endometrial cannabinoid receptor type 1 (CB1-R) expression is disrupted in women with endometriosis and in isolated stromal cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Fertil Steril. 2012;98:948–56. doi: 10.1016/j.fertnstert.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulukus M, Arici A. Immunology of endometriosis. Minerva Ginecol. 2005;57:237–48. [PubMed] [Google Scholar]
- 10.Gentilini D, Perino A, Vigano P, Chiodo I, Cucinella G, Vignali M, et al. Gene expression profiling of peripheral blood mononuclear cells in endometriosis identifies genes altered in nongynaecologic chronic inflammatory diseases. Hum Reprod. 2011;26:3109–17. doi: 10.1093/humrep/der270. [DOI] [PubMed] [Google Scholar]
- 11.Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans. 2002;30:126–31. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 12.Aytan H, Caliskan AC, Yener T, Demirturk F, Aytan P, Yenisehirli A. A novel antibiotic, linezolid, reduces intraperitoneal adhesion formation in the rat uterine horn model. Acta Obstet Gynecol Scand. 2009;88:781–6. doi: 10.1080/00016340903002873. [DOI] [PubMed] [Google Scholar]
- 13.Imamoto E, Yoshida N, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, et al. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20:37–47. doi: 10.1002/biof.5520200104. [DOI] [PubMed] [Google Scholar]
- 14.Mendes JB, Campos PP, Rocha MA, Andrade SP. Cilostazol and pentoxifylline decrease angiogenesis, inflammation, and fibrosis in sponge-induced intraperitoneal adhesion in mice. Life Sci. 2009;84:537–43. doi: 10.1016/j.lfs.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Steinleitner A, Lambert H, Kazensky C, Danks P, Roy S. Pentoxifylline, a methylxanthine derivative, prevents postsurgical adhesion reformation in rabbits. Obstet Gynecol. 1990;75:926–8. [PubMed] [Google Scholar]
- 16.Aksakal O, Yilmaz B, Gungor T, Sirvan L, Sut N, Inan I, et al. A randomised controlled trial on melatonin and rosiglitazone for prevention of adhesion formation in a rat uterine horn model. Arch Gynecol Obstet. 2010;282:55–61. doi: 10.1007/s00404-009-1240-8. [DOI] [PubMed] [Google Scholar]
- 17.Batukan C, Ozgun MT, Basbug M, Muderris M., II Sildenafil reduces postoperative adhesion formation in a rat uterine horn model. Eur J Obstet Gynecol Reprod Biol. 2007;135:183–7. doi: 10.1016/j.ejogrb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz B, Aksakal O, Gungor T, Sirvan L, Sut N, Kelekci S, et al. Metformin and atorvastatin reduce adhesion formation in a rat uterine horn model. Reprod Biomed Online. 2009;18:436–42. doi: 10.1016/s1472-6483(10)60106-x. [DOI] [PubMed] [Google Scholar]
- 19.Ustun Y, Engin-Ustun Y, Ovayolu A, Meydanli MM, Temel I, Kafkasli A. The effect of resveratrol on prevention of the development of postoperative adhesions in a rat model. Gynecol Endocrinol. 2007;23:518–22. doi: 10.1080/09513590701581648. [DOI] [PubMed] [Google Scholar]
- 20.Pierce RA, Perrone JM, Nimeri A, Sexton JA, Walcutt J, Frisella MM, et al. 120-day comparative analysis of adhesion grade and quantity, mesh contraction, and tissue response to a novel omega-3 fatty acid bioabsorbable barrier macroporous mesh after intraperitoneal placement. Surg Innov. 2009;16:46–54. doi: 10.1177/1553350608330479. [DOI] [PubMed] [Google Scholar]
- 21.Victory R, Saed GM, Diamond MP. Antiadhesion effects of docosahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88:1657–62. doi: 10.1016/j.fertnstert.2007.01.123. [DOI] [PubMed] [Google Scholar]
- 22.Massiera FP, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, et al. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010;51:2352–61. doi: 10.1194/jlr.M006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83(6 Suppl):1483S–93S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 24.Krikun G, Hu Z, Osteen K, Bruner-Tran KL, Schatz F, Taylor HS, et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am J Pathol. 2010;176:1050–6. doi: 10.2353/ajpath.2010.090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruner-Tran KL, Zhang Z, Eisenberg E, Winneker RC, Osteen KG. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J Clin Endocrinol Metab. 2006;91:1554–60. doi: 10.1210/jc.2005-2024. [DOI] [PubMed] [Google Scholar]
- 26.Lauder CI, Garcea G, Strickland A, Maddern GJ. Use of a modified chitosandextran gel to prevent peritoneal adhesions in a rat model. J Surg Res. 2011;171:877–82. doi: 10.1016/j.jss.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson G, Gullberg B, Hafstrom L. Estimation of liver tumor volume using different formulas—an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–3. doi: 10.1007/BF00391826. [DOI] [PubMed] [Google Scholar]
- 28.Walter A, Barysch MJ, Behnke S, Dziunycz P, Schmid B, Ritter E, et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16:3562–70. doi: 10.1158/1078-0432.CCR-09-3136. [DOI] [PubMed] [Google Scholar]
- 29.Meinen S, Barzaghi P, Lin S, Lochmuller H, Ruegg MA. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2–deficient muscular dystrophy at all disease stages. J Cell Biol. 2007;176:979–93. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tulandi T, Chen MF, Al-Took S, Watkin K. A study of nerve fibers and histopathology of postsurgical, postinfectious, and endometriosis-related adhesions. Obstet Gynecol. 1998;92:766–8. doi: 10.1016/s0029-7844(98)00298-1. [DOI] [PubMed] [Google Scholar]
- 31.Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl. 2000;232:52–9. [PubMed] [Google Scholar]
- 32.Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–80. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 33.Wilmore DW. Metabolic response to severe surgical illness: overview. World J Surg. 2000;24:705–11. doi: 10.1007/s002689910113. [DOI] [PubMed] [Google Scholar]
- 34.Barcz E, Milewski L, Dziunycz P, Kaminski P, Ploski R, Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil Steril. 2012;97:1380–6.e1. doi: 10.1016/j.fertnstert.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 35.Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–7. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2012.04374.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 39.Zhang MJ, Spite M. Resolvins: antiinflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 40.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–79. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 41.Khanaki K, Nouri M, Ardekani AM, Ghassemzadeh A, Shahnazi V, Sadeghi MR, et al. Evaluation of the relationship between endometriosis and omega-3 and omega-6 polyunsaturated fatty acids. Iran Biomed J. 2012;16:38–43. doi: 10.6091/IBJ.1025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.