Based on meta-analysis, the higher the proportion of addiction-treated patients, the higher the treatment completion rate. The rate of sustained virologic response rate in drug users is very similar to that in the general population.
Keywords: drug users, hepatitis C virus, treatment completion, SVR, meta-analysis
Abstract
Background. Hepatitis C virus (HCV)–infected drug users (DUs) have largely been excluded from HCV care. We conducted a systematic review and meta-analysis of the literature on treatment completion and sustained virologic response (SVR) rates in DUs. We assessed the effects of different treatment approaches and services to promote HCV care among DUs as well as demographic and viral characteristics.
Methods. Studies of at least 10 DUs treated with pegylated interferon/ribavirin that reported SVR were analyzed. Heterogeneity was assessed (Cochran test) and investigated (meta-regression), and pooled rates were estimated (random effects).
Results. Thirty-six studies comprising 2866 patients were retrieved. The treatment completion rate among DUs was 83.4% (95% confidence interval [CI], 77.1%–88.9%). Among studies that included addiction-treated and untreated patients during HCV therapy, the higher the proportion of addiction-treated patients, the higher the HCV treatment completion rate (P < .0001). After adjusting for human immunodeficiency virus (HIV)/HCV coinfection, sex, and treatment of addiction, support services during antiviral therapy increased treatment completion (P < .0001). The pooled SVR rate was 55.5% (95% CI, 50.6%–60.3%). Genotype 1/4 (P = .0012) and the proportion of HIV-coinfected DUs (P = .0173) influenced the SVR rate. After adjusting for HCV genotype 1/4 and HIV/HCV coinfection, the SVR rate was positively correlated with involvement of a multidisciplinary team (P < .0001).
Conclusions. Treatment of addiction during HCV therapy results in higher treatment completion. Our pooled SVR rate is similar to that obtained in registration trials in the general population. Treatment of addiction during HCV therapy will likely be important for HCV-infected DUs undergoing treatment with more complex regimens including direct-acting antivirals.
An estimated 170 million people globally and 5 million people in the United States are infected with hepatitis C virus (HCV), with injection drug use as the major transmission route [1]. Fifty percent to 80% of those exposed to HCV will develop chronic infection that can ultimately lead to hepatic fibrosis, hepatocellular carcinoma, and cirrhosis [2]. Until recently, pegylated interferon/ribavirin (PEG-IFN/RBV) had been the standard treatment for all hepatitis C genotypes, resulting in viral eradication in approximately 50% of treated patients [3, 4]. Recent approval of boceprevir [5] and telaprevir [6] for HCV genotype 1–infected patients simultaneously increased treatment efficacy and its complexity, necessitating rigorous adherence to medication administration to mitigate development of resistant variants.
In the United States, the majority of prevalent and incident HCV infections occur in drug users (DUs) [7]. Unfortunately, however, HCV treatment uptake remains low among DUs. Namely, less than one-third of patients referred to specialty clinics appear for evaluation and <20% of those evaluated initiate antiviral therapy [8–10]. DUs often cite discomfort encountered in conventional medical venues as a primary obstacle limiting pursuit of an HCV evaluation [8]. Consequently, HCV therapeutic effectiveness in DUs is an issue of treatment access, acceptance, and adherence rather than drug efficacy [11].
Understanding the factors that influence adherence of DUs to PEG-IFN/RBV has relevance to more complex treatment regimens, such as those that include boceprevir and telaprevir. While many factors that influence treatment outcome are unmodifiable, treatment approaches tailored to DUs could be pursued. To determine the influence of support services on HCV treatment completion and therapeutic success, we conducted a meta-analysis of studies on DUs treated with PEG-IFN/RBV. Because most of these studies have relatively small sample sizes, their aggregation through meta-analysis increases statistical power and facilitates generation of evidence-based conclusions.
METHODS
Search Methodology
We searched multiple electronic resources (including PubMed, ClinicalTrials.gov, EMBASE) for studies of HCV treatment in DUs using combinations of relevant keywords (hepatitis C virus, drug users, substance use, sustained virological response [SVR], pegylated interferon, ribavirin). We also reviewed references from the retrieved articles. The last search was performed in September 2011, resulting in 1144 studies screened for eligibility.
The inclusion criteria for studies were (1) a population of at least 10 DUs treated with PEG-IFN/RBV and (2) a reported treatment outcome. Successful treatment outcome was defined as achieving a sustained virologic response (SVR), that is, undetectable HCV RNA 24 weeks after treatment cessation. DUs were defined as individuals who reported exposure to illicit drugs (including injection and noninjection). Illicit drug exposure was defined as the nonmedical use of drugs prohibited by international law. We only included papers published in English except for 1 Serbian study included because of a native Serbian speaker on our team. Papers that did not satisfy the inclusion criteria were excluded.
Thirty-four publications (published 2004–2011) comprising a total of 2866 patients were included (Table 1). Because 2 publications [12, 13] contained independent arms, they were considered separately; thus, a total of 36 studies were evaluated.
Table 1.
Characteristics of Individual Studies Included in Meta-Analysisa
| StudyID | First Author,Year of Publication | Location | Study Design | Type ofEnrollment | EnrollmentStart Date | EnrollmentEnd Date | No. |
|---|---|---|---|---|---|---|---|
| 1 | Grebely, 2007 [17] | Canada | Observational | Prospective | 1 Jan 2001 | 1 July 2003 | 28 |
| 5 | Alvarez-Uria, 2009 [18] | UK | Observational | Retrospective | 1 Nov 2003 | 1 Aug 2006 | 70 |
| 6 | Gazdik, 2009 [19] | Slovakia | Observational | Prospective | 1 Jan 2003 | 1 July 2006 | 92 |
| 10 | Ebner, 2009 [20] | Austria | Randomized control | Prospective | 1 Aug 2003 | 1 Feb 2006 | 17 |
| 11 | Waizmann, 2010 [21] | Germany | Observational | Retrospective | 1 Sept 2005 | 1 May 2008 | 49 |
| 12 | Belfiori, 2009 [22] | Italy | Observational | Prospective | 1 Sept 2003 | 1 Dec 2006 | 52 |
| 13 | Guadagnino, 2007 [23] | Italy | Observational | Prospective | 1 Dec 2002 | 1 Nov 2003 | 53 |
| 14 | Bonkovsky, 2008 [12]b,c | US | Randomized control | Prospective | 24 | ||
| 14 | Bonkovsky, 2008 [12]b,c | US | Randomized control | Prospective | |||
| 15 | Schaefer, 2007 [24] | Germany | Case control | Prospective | 1 Jan 2001 | 1 Jan 2003 | 31 |
| 16 | Litwin, 2009 [25] | US | Observational | Retrospective | 1 Jan 2003 | 15 Dec 2005 | 73 |
| 17 | Mauss, 2004 [26]b | Germany | Nonrandomizedconcurrent control trial | Prospective | 50 | ||
| 18 | Harris, 2010 [27] | US | Observational | Retrospective | 1 July 2003 | 1 July 2005 | 21 |
| 20 | Grebely, 2010 [10] | Canada | Observational | Retrospective | 1 March 2005 | 1 March 2008 | 19 |
| 35 | Krook, 2007 [28] | Norway | Nonrandomizedconcurrent control trial | Prospective | 1 Jan 2003 | 1 Jan 2004 | 17 |
| 42 | Bruggmann, 2008 [29] | Switzerland | Observational | Retrospective | 1 Sept 2000 | 31 May 2006 | 199 |
| 66 | Dimitroulopoulos, 2009 [30] | Greece | Observational | Retrospective | 1 Nov 2001 | 1 Jan 2003 | 45 |
| 69 | Fried, 2008 [31] | Switzerland | Observational | Prospective | 1 March 2002 | 1 June 2004 | 67 |
| 73 | Hallinan, 2007 [32] | Australia | Observational | Prospective | 1 Dec 2002 | 1 Nov 2005 | 11 |
| 75 | Jack, 2009 [33] | UK | Observational | Prospective | 1 Feb 2005 | 1 Jan 2008 | 21 |
| 76 | Jeffrey, 2007 [34] | Australia | Observational | Prospective | 1 Oct 2002 | 1 March 2005 | 50 |
| 85 | Schulte, 2010 [35] | Germany | Observational | Prospective | 1 Sept 2002 | 1 Dec 2007 | 26 |
| 89 | Papadopoulos, 2010 [36] | Greece | Observational | Prospective | 1 Jan 2004 | 1 Jan 2010 | 48 |
| 91 | Melin, 2010 [37] | France | Observational | Prospective | 1 Nov 2002 | 1 Jan 2005 | 822 |
| 92 | John-Baptiste, 2010 [38] | Canada | Observational | Retrospective | 1 Nov 2002 | 1 Jan 2006 | 109 |
| 98 | Sasadeusz, 2011 [39] | Australia | Observational | Prospective | 1 Feb 2004 | 1 Jan 2006 | 53 |
| 99 | Taylor, 2011 [40]b | USA | Observational | Prospective | 11 | ||
| 107 | Jovanović, 2007 [41] | Serbia | Observational | Retrospective | 1 Jan 2005 | 1 Jan 2007 | 31 |
| 129 | Wilkinson, 2009 [42] | UK | Observational | Prospective | 1 March 2005 | 1 March 2007 | 58 |
| 130 | Manolakopoulos, 2010 [43] | Greece | Observational | Retrospective | 1 Jan 2000 | 1 Dec 2007 | 175 |
| 133 | Lindenburg, 2011 [44] | Netherlands | Observational | Prospective | 1 Jan 2005 | 1 July 2009 | 58 |
| 134 | Tait, 2010 [45] | Scotland UK | Observational | Retrospective | 1 Jan 2004 | 1 Jan 2007 | 42 |
| 135 | Mauss, 2010 [46] | Germany | Observational | Retrospective | 1 Jan 2000 | 31 Dec 2007 | 407 |
| 136 | Martinez, 2012 [47] | USA | Observational | Retrospective | 1 July 2006 | 1 June 2008 | 24 |
| 137 | Curcio, 2010 [13]c | Italy | Matched control | Prospective | 1 Jan 2004 | 1 Jan 2008 | 16 |
| 137 | Curcio, 2010 [13]c | Italy | Matched control | Prospective | 1 Jan 2004 | 1 Jan 2008 | 32 |
a Of the studies that offered support services, 3 offered needle exchange, 5 counseling for risk reduction, 10 psychological counseling, 3 educational intervention, 8 directly observed therapy, and 3 case management, while some offered multiple support services simultaneously.
b Information on enrollment dates not included in manuscript.
c Study includes independent arms, which were treated as separate studies.
Data Extraction
To facilitate data coding and extraction, we designed a form (Supplementary Data). We collected information on treatment completion rates and success, study design, demographic and hepatic characteristics, treatment of addiction, support services, and methods to deliver HCV treatment. Each study was coded independently by 2 investigators (R.B.D. and M.Z.), coding results were compared, and discrepancies were resolved by discussion between the 2 reviewers.
Statistical Analysis
Units in the meta-analysis were the independent studies. Main variables of interest were the treatment completion and SVR rates. We considered HCV treatment not completed if patients were discontinued for any reason other than lack of viral response (which is a standard PEG-IFN/RBV discontinuation rule). Reasons for noncompletion included nonadherence, substance abuse, patient unwillingness to complete therapy, loss to follow-up, death, adverse events, or other reasons. The SVR rate was determined by intention to treat as the proportion of patients (DUs) who achieved an SVR among all DUs. We used as outcomes the log odds for achieving an SVR and the Freeman-Tukey double arcsine transformed [14] treatment completion rates (due to rates equal to 1). Heterogeneity of the effect sizes between the studies was investigated, tested (Cochran test) and quantified through the Q and I2 statistics (proportion of total variation due to heterogeneity between studies). If heterogeneity was present, a random effects model was used for inference and the DerSimonian-Laird estimator [15] was used for the heterogeneity parameter.
We verified the assumptions of the model and investigated for outliers using the Shapiro-Wilk normality test, as well as weighted normal plots of the elements of Q [16]. In order to determine the influence of the individual studies, we performed influential analysis. Furthermore, interstudy variability due to different study characteristics and factors, including treatment of addiction, were assessed through meta-regression analysis. Treatment of addiction was defined as participation in a pharmacological maintenance, pharmacological detoxification, or behavioral program, or a medication regimen for individuals with drug addiction disorders. We also analyzed specific support services designed to increase treatment adherence including needle exchange, counseling, educational interventions for HCV, case management, directly observed therapy, motivational interviewing, and peer support groups. We also sought to determine whether involvement of a multidisciplinary team (defined as a systematic program for treatment of HCV patients that includes specialists from 2 or more areas) affected the treatment completion and SVR rates. These teams were typically comprised of different specialists including hepatologists, addiction medicine specialists, psychologists/psychiatrists, infectious diseases specialists, and general practitioners. We investigated the potential for publication bias through funnel plots and Egger regression test of funnel plot asymmetry. The significance level in all tests was .05, and all analyses were conducted using SAS (SAS Institute, Cary, North Carolina) and R (http://www.r-project.org/).
RESULTS
Characteristics of the Studies
Thirty-six studies, ranging in size from 11 to 822 individuals, were included (Table 1). All patients had a history of illicit drug use, 77% (1606/2087) were male (10 studies did not describe sex distribution), and the median age (across 24 studies that reported age) was 38.2 years (interquartile range [IQR], 33.0–42.5 years). Active/former DU definitions varied with respect to the required duration of abstinence or were missing across the 15 studies that considered these variables. Based on the latter, 38.2% (656/1719) of the patients were active DUs. Eight studies defined former drug use as at least 6 months of abstinence prior to HCV treatment, whereas one study required at least 4 months, one 12 months, and an additional one had a median of 24 months of abstinence. Addiction treatment during HCV therapy occurred in 61.6% of patients (1303/2115 from 28 studies). Provision of support services was described in 31 studies, 15 with services and 16 without (Table 1). Twenty-two studies treated HCV using a multidisciplinary team.
Treatment Completion Rates in DUs Treated With PEG-IFN/RBV
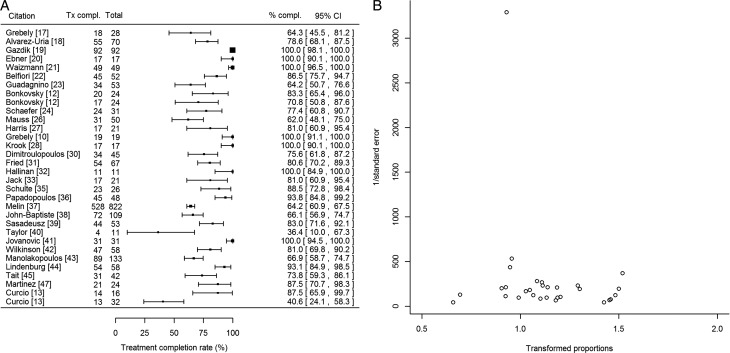
We calculated the pooled treatment completion rate as 83.4% (95% confidence interval [CI], 77.1%–88.9%) from 32 studies (Figure 1A). We identified heterogeneity in the treatment completion rates (I2 = 90.2%, P < .0001). We identified neither publication bias (P = .30; Figure 1B) nor any particular study as influential.
Figure 1.
A, Forest plot demonstrating the treatment completion rates and associated 95% confidence intervals for each of the studies included in the meta-analysis. Column labeled “Tx compl.” refers to the number of patients who completed treatment, and column labeled “% compl.” refers to the percentage of patients in each study who completed treatment. Treatment completion ranged between 36.4% and 100%. In 7 studies, all patients completed treatment including 5 studies that reported extremely high sustained virologic response rates [19–21, 28, 41] and 2 that had small sample sizes [10, 32]. B, Funnel plot assessing publication bias for studies reporting treatment completion rates for hepatitis C virus infection. Treatment completion rates were transformed using the Freeman-Tukey double arcsine transformation [14] by the following formula: 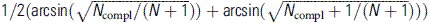 . Abbreviation: CI, confidence interval.
. Abbreviation: CI, confidence interval.
The influence of various factors on treatment completion rates, estimated coefficients, and P values are shown in Table 2. Twenty-five studies that reported treatment completion also specified the number of addiction-treated patients during HCV therapy. From these 25 studies, we identified a trend in increased treatment completion when the proportion of patients treated for addiction during HCV therapy increased (P = .105). In 19 studies, all patients were treated for addiction, and only 5 studies [20, 24, 37, 43, 44] (the control group from [13] was excluded as an outlier) included both addiction-treated and untreated patients during HCV therapy. In these studies, 1061 patients were treated for HCV, and 348 of them received addiction treatment. We found that the higher the proportion of patients treated for addiction during HCV therapy, the higher the treatment completion rate (P < .0001). Further, among these studies, only Manolakopoulos et al [43] specified the proportion of patients who used illicit drugs during HCV therapy (16.5%). Thirteen of the studies that specified the treatment completion rate also specified the number of active DUs, but this observation was not significantly associated with treatment completion (P = .93). Additionally, we identified a trend of increasing treatment completion with increasing baseline substitution therapy (P = .058).
Table 2.
Results From Univariable Meta-regression on Transformed Treatment Completion Ratesa,b
| Variable | No. ofStudies | CoefficientEstimate | 95% CI | P Valuec | PearsonCorrelationCoefficient | HeterogeneityParameter (I2)d | P Value(Heterogeneity) |
|---|---|---|---|---|---|---|---|
| Treatment of addiction during HCV therapy | 25 | 0.2773 | −.0582, .6128 | .1053 | 0.2651 | 0.0249 (0.8061) | <.0001 |
| Treatment of addiction during HCV therapy (in studies with patients who were not treated for addiction) | 5 | 0.589 | .4117, .7663 | <.0001* | 0.9664 | 0.0017 | .2059 |
| Substitution therapy at baselinee | 22 | 0.3201 | −.0113, .6516 | .0584* | 0.3503 | 0.024 (0.8084) | <.0001 |
| Drug use during HCV treatment | 15 | 0.2584 | −.0226, .5395 | .0715 | 0.4699 | 0.0172 (0.7444) | <.0001 |
| Genotype 1 or 4 | 27 | −0.4243 | −.7981, −.0506 | .0261* | −0.4462 | 0.0369 (0.8930) | <.0001 |
| Human immunodeficiency virus infection | 24 | −0.4628 | −.9134, −.0122 | .0441* | −0.4416 | 0.041 (0.9125) | <.0001 |
| Male | 22 | −0.6648 | −1.3326, .0031 | .0511* | −0.4502 | 0.0448 (0.9167) | <.0001 |
| African American from US studies | 3 | −1.8556 | −3.1240, −.5872 | .0041* | −0.9681 | 0 | .3347 |
| Caucasians from US studies | 5 | 0.0905 | −.7991, .9802 | .8419 | 0.1415 | 0.0174 (0.6058) | .0174 |
| Study design (randomized or matched control vs other) | 32 | −0.0686 | −.2843, .1470 | .5327 | −0.0979 | 0.0404 (0.9022) | <.0001 |
| Location in United States | 32 | −0.1272 | −.3446, .0902 | .2515 | −0.2438 | 0.0403 (0.9023) | <.0001 |
| Age | 23 | −0.0116 | −.0266, .0034 | .1304 | −0.3188 | 0.0414 (0.8691) | <.0001 |
| Psychiatric comorbidities | 15 | −0.2312 | −.6217, .1593 | .2459 | −0.3378 | 0.05 (0.9251) | <.0001 |
| Biopsy performed | 15 | 0.0208 | −.3646, .4062 | .9157 | 0.0218 | 0.0665 (0.9365) | <.0001 |
| Multidisciplinary team involved | 32 | 0.0313 | −.1250, .1875 | .6951 | 0.0501 | 0.0403 (0.8885) | <.0001 |
| Support services offered | 31 | 0.101 | −.0463, .2482 | .1789 | 0.1859 | 0.0353 (0.8799) | <.0001 |
| Methadone maintenance during HCV treatment | 20 | −0.1042 | −.3794, .1709 | .4578 | −0.2296 | 0.027 (0.8064) | <.0001 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus.
a Results listed in order as presented in the text.
b Results from the univariable meta-regression expressed as 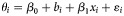 , where θi is the Freeman-Tukey double arcsine transformed treatment completion rate from study i, β0 is intercept, bi is random effect for study i, xi is the value of the covariate from study i, and εi is the within study error. The covariates are expressed either as proportions (treatment of addiction during HCV therapy, substitution therapy at baseline, drug use during HCV treatment, genotype 1 or 4, human immunodeficiency virus infection, male, African American from US studies, Caucasians from US studies, psychiatric comorbidities, biopsy performed, methadone maintenance during HCV treatment), as categorical variables (study design, location in United States, multidisciplinary team involved, support services offered) or as continuous variables (median/mean age).
, where θi is the Freeman-Tukey double arcsine transformed treatment completion rate from study i, β0 is intercept, bi is random effect for study i, xi is the value of the covariate from study i, and εi is the within study error. The covariates are expressed either as proportions (treatment of addiction during HCV therapy, substitution therapy at baseline, drug use during HCV treatment, genotype 1 or 4, human immunodeficiency virus infection, male, African American from US studies, Caucasians from US studies, psychiatric comorbidities, biopsy performed, methadone maintenance during HCV treatment), as categorical variables (study design, location in United States, multidisciplinary team involved, support services offered) or as continuous variables (median/mean age).
c Significant P values are indicated with an asterisk.
d I2 added if heterogeneity is present.
e Substitution therapy included patients on either methadone or buprenorphine.
Factors such as HCV infection with genotype 1/4 (from 12 studies, P = .026) and HIV infection (P = .044) were associated with lower treatment completion. For genotype 1/4 infection, the pooled rate was 80.0% (95% CI, 66.0%–91.3%; I2 = 85.7%). We observed the opposite association among those with genotype 2/3 infection; that is, the higher the genotype 2/3 proportion, the higher the treatment completion rate (P = .0007). For genotype 2/3, the pooled completion rate was 90.8% (95% CI, 77.3%–99.1%; I2 = 88.2%). For HCV monoinfection studies, the pooled rate was 87.0% (95% CI, 79.0%–93.3%; I2 = 89.9%), whereas the pooled rate of studies that included HCV/HIV-coinfected patients was 67.9% (95% CI, 53.0%–81.3%; I2 = 79.0%). Additionally, the higher the proportion of male DUs, the lower the treatment completion rate (P = .051). Among 3 US studies with African American patients (9/56), treatment completion rates were lower (P = .004). None of the other variables evaluated were significantly associated with treatment completion.
We also investigated the heterogeneity among the treatment completion rates through multivariable meta-regression. Seventeen studies simultaneously included data on the treatment of addiction during HCV therapy, HIV/HCV coinfection, and sex as well as the availability of support services. Based on these analyses, we found a significant negative correlation between treatment completion and HIV/HCV coinfection (P < .0001) and male sex (P < .0001), as well as a positive correlation with availability of support services (P < .0001). From this model, the heterogeneity parameter was estimated as 0 (P = .58).
Treatment Efficacy in DUs
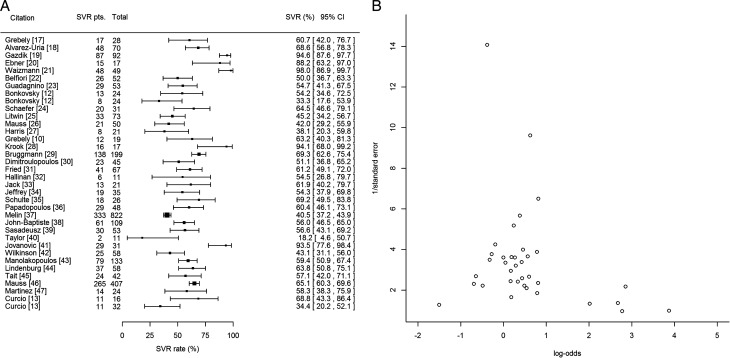
We calculated an SVR rate of 59.4% (95% CI, 54.0%–64.7%) based on all 36 studies (Figure 2A). We identified significant heterogeneity (P < .0001) and estimated I2 as 83.5% (95% CI, 78%–87.6%).
Figure 2.
A, Forest plot demonstrating the sustained virologic response (SVR) rate and associated 95% confidence interval for each of the studies included in the meta-analysis. Column labeled “SVR pts.” refers to the number of patients who achieved an SVR in the individual study. SVR was defined as absence of detectable peripheral hepatitis C virus RNA 24 weeks after treatment cessation. B, Funnel plot assessing publication bias for rate of sustained virologic response. Abbreviations: CI, confidence interval; SVR, sustained virologic response.
We initially detected potential publication bias in the SVR rates among the included studies (P = .015). After removal of 4 outlying studies [19, 21, 28, 41], publication bias resolved (P = .175; Figure 2B), I2 decreased to 78.6% (heterogeneity remained significant, P < .0001), and the pooled SVR rate became 55.5% (95% CI, 50.6%–60.3%). The outlying studies had SVR rates ≥94% [19, 21, 41] and included relatively younger patients (median/mean ages of 27.0, 30.1, and 32.9 years, respectively). These studies were excluded in all subsequent analyses.
Differences in the proportions of patients treated for addiction during HCV therapy did not explain the heterogeneity between the SVR rates (P = .930; Table 3). The pooled SVR rate among addiction-treated patients was 53% (95% CI, 49.4%–56.6%) from 20 homogeneous (I2 = 25%, P = .15) studies that reported the respective rates. Moreover, among the studies which included addiction-treated and untreated patients, only Manolakopoulos et al [43] specified the proportion of patients using illicit drugs during HCV therapy (16.5%). Thirteen of the studies specified how many patients were active drug users, but this observation was not associated with SVR (P = .76).
Table 3.
Results From Univariable Meta-regression on Log Odds of Sustained Virologic Responsea,b
| Variable | No. ofStudies | CoefficientEstimate | 95% CI | P Valuec | PearsonCorrelationCoefficient | HeterogeneityParameter (I2)d | PValue |
|---|---|---|---|---|---|---|---|
| Treatment of addiction during HCV therapy | 26 | −0.0426 | −.9784, .8932 | .9289 | −0.0468 | 0.2014 (0.7020) | <.0001 |
| Genotype 1 or 4 | 26 | −1.7062 | −2.7413, −.6712 | .0012* | −0.7058 | 0.1349 (0.7031) | <.0001 |
| Human immunodeficiency virus | 22 | −1.5767 | −2.8753, −.2781 | .0173* | −0.5921 | 0.1418 (0.6794) | <.0001 |
| Location in United States | 32 | −0.5847 | −1.1294, −.0401 | .0354* | −0.4929 | 0.2077 (0.7777) | <.0001 |
| Support services offered | 31 | −0.0245 | −.4556, .4067 | .9114 | −0.0967 | 0.2343 (0.7909) | <.0001 |
| Multidisciplinary team involved | 32 | 0.0626 | −.3506, .4759 | .7665 | 0.0830 | 0.2265 (0.7800) | <.0001 |
| Methadone maintenance during HCV treatment | 20 | −0.4157 | −1.1916, .3602 | .2937 | −0.4871 | 0.163 (0.6029) | .0002 |
| Study design (randomized or matched control vs other) | 32 | −0.1484 | −.7701, .4733 | .6399 | 0.0747 | 0.2133 (0.7847) | <.0001 |
| Age | 20 | −0.0088 | −.0486, .0311 | .6656 | −0.3288 | 0.1191 (0.5187) | .0025 |
| Male | 22 | 0.1513 | −1.3726, 1.6752 | .8457 | 0.0123 | 0.2541 (0.7993) | <.0001 |
| Caucasians from US studies | 6 | 0.0424 | −1.1812, 1.2659 | .9459 | 0.0987 | 0.1405 | .1386 |
| African American from US studies | 4 | −4.8407 | −11.1615, 1.4801 | .1333 | −0.8544 | 0.039 | .2711 |
| Psychiatric comorbidities | 14 | −0.151 | −1.1111, .8092 | .758 | −0.3007 | 0.1704 (0.7117) | <.0001 |
| Substitution therapy at baselinee | 24 | −0.246 | −1.2266, .7345 | .6229 | −0.1652 | 0.2163 (0.7224) | <.0001 |
| Drug use during HCV treatment | 15 | 0.346 | −.1833, .8754 | .2001 | 0.3777 | 0.0462 | .0887 |
| Biopsy performed | 14 | −0.2575 | −.9557, .4407 | .4697 | −0.1952 | 0.071 (0.4498) | .0228 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus.
a Results listed in order as presented in the text.
b Results from the univariable meta-regression expressed as 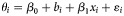 , where θi is the log odds for achieving a sustained virologic response from study i, β0 is intercept, bi is random effect for study i, xi is the value of the covariate from study i, and εi is the within study error. The covariates are expressed either as proportions (treatment of addiction during HCV therapy, substitution therapy at baseline, drug use during HCV treatment, genotype 1 or 4, human immunodeficiency virus infection, male, African American from US studies, Caucasians from US studies, psychiatric comorbidities, biopsy performed, methadone maintenance during HCV treatment), as categorical variables (study design, location in United States, multidisciplinary team involved, support services offered) or as continuous variables (median/mean age).
, where θi is the log odds for achieving a sustained virologic response from study i, β0 is intercept, bi is random effect for study i, xi is the value of the covariate from study i, and εi is the within study error. The covariates are expressed either as proportions (treatment of addiction during HCV therapy, substitution therapy at baseline, drug use during HCV treatment, genotype 1 or 4, human immunodeficiency virus infection, male, African American from US studies, Caucasians from US studies, psychiatric comorbidities, biopsy performed, methadone maintenance during HCV treatment), as categorical variables (study design, location in United States, multidisciplinary team involved, support services offered) or as continuous variables (median/mean age).
c Significant P values are indicated with an asterisk.
d I2 added if heterogeneity is present.
e Substitution therapy included patients on either methadone or buprenorphine.
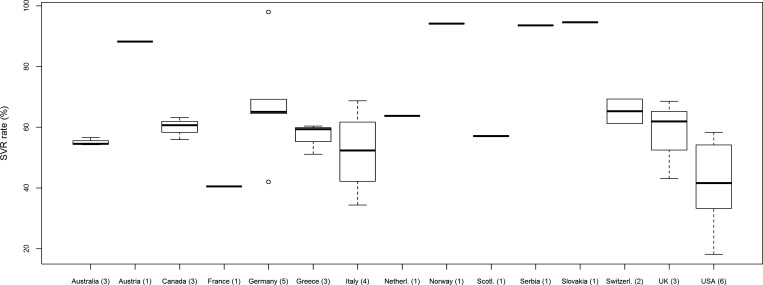
The median proportion of patients infected with HCV genotype 1/4 was 44.7% (IQR, 37.1%–57.5%), which significantly affected the SVR rate (P = .001). Moreover, when the effects of the proportions of genotypes 1 and 4 patients were assessed separately, the SVR rate was negatively correlated with the proportion of genotype 1 patients (P = .0065), but not significantly correlated with genotype 4 patients (P = .56). Similarly, higher proportions of HIV-infected patients were associated with lower SVR rates (P = .017). For HCV genotype 1/4, the SVR rate was 44.9% (95% CI, 41.0%–48.9%) from 19 homogeneous studies (I2 = 0%, P = .637). For HCV genotype 2/3, the SVR rate was 70.0% (95% CI, 62.9%–76.3%) from 18 heterogeneous studies (I2 = 57%, P = .002). Among 7 homogeneous (I2 = 44.4%, P = .095) studies with HIV-coinfected patients, the SVR rate was 41.3% (95% CI, 38.2%–44.4%). Among 15 homogeneous (I2 = 24.5%, P = .183) studies without HIV-coinfected patients, the SVR rate was 58.1% (95% CI, 54.6%–61.5%). Neither the median baseline HCV RNA level (available in 4 studies) nor the proportion of patients with advanced fibrosis (Scheuer stage ≥3; from 9 studies), were significantly associated with SVR. Additionally, in comparison with the SVR rate obtained from other countries, a significantly lower SVR rate of 44.6% (95% CI, 37.3%–52.2%, P = .035) was obtained among the 6 homogeneous (I2 = 28.1%, P = .224) studies from the United States (Figure 3). The lower SVR rate may result from inclusion of significantly more HCV genotype 1/4–infected subjects in US studies (P = .003). None of the other variables assessed were significantly associated with treatment efficacy (Table 3).
Figure 3.
Box plot illustrating sustained virologic response (SVR) rates by country of origin and includes all studies analyzed as part of meta-analysis as well as 4 outliers from Slovakia, Germany, Norway, and Serbia. The numbers in parentheses are the number of studies from each location. The SVR rate reported in US studies is significantly lower in comparison with other countries (P = .035). The box extends from the 25th to the 75th percentile. The line in the middle of the box is the median and the lines extending from either end of the box indicate the extent of the data beyond the 25th and 75th percentiles, and outliers, if any. Abbreviation: SVR, sustained virologic response.
In addition, we investigated the heterogeneity among the SVR rates through multivariable meta-regression. Nineteen studies simultaneously included data on the proportion of HCV genotype 1/4 patients, HIV infection, and treatment of HCV through a multidisciplinary team. On the basis of this analysis, we found a significant negative correlation between SVR and genotype 1/4 (P = .0003) and positive correlation with involvement of a multidisciplinary team (P < .0001). We also found a nonsignificant negative correlation with HIV/HCV coinfection (P = .19) and a heterogeneity parameter estimated as 0.0009 (P = .28).
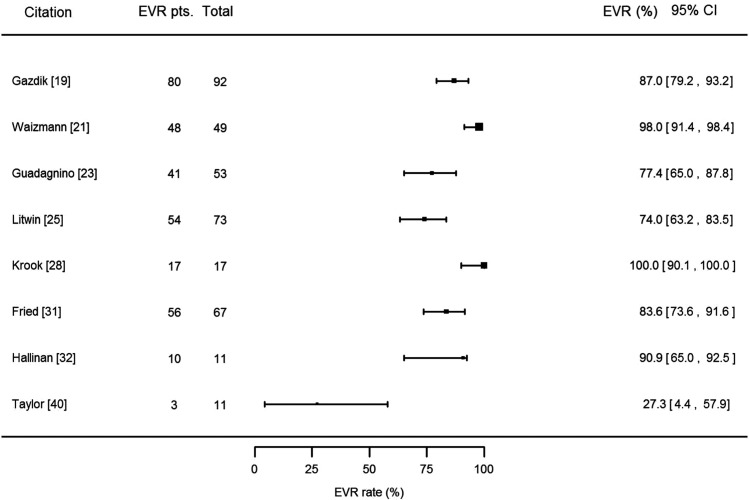
Finally, we sought to assess whether treatment of addiction affects the early virologic response (EVR) rate. EVR was defined as either undetectable HCV RNA or a 2 log10 decrease in HCV RNA by week 12. EVR rates were reported in 8 studies (Figure 4), which were heterogeneous (I2 = 82.7%, P < .0001). The pooled EVR rate across all studies was 84.4% (95% CI, 73.3%–93.2%). Seven of these 8 studies reported that all of their patients were treated for addiction during HCV therapy (1 study did not specify), which did not enable us to evaluate the effect of treatment of addiction on EVR. The higher the proportion of genotype 1/4 patients, the lower the EVR rate (P < .0001).
Figure 4.
Forest plot demonstrating the early virologic response (EVR) rate and associated 95% confidence interval for each of the included studies that specified EVR rate. EVR was defined as undetectable hepatitis C virus (HCV) RNA or a 2 log10 decrease in HCV RNA by week 12. Column labeled “EVR pts.” refers to the number of patients who achieved an EVR in the individual study. Abbreviations: CI, confidence interval; EVR, early virologic response.
DISCUSSION
Despite high HCV prevalence and incident infections, DUs may have difficulty adhering to the therapeutic regimen for HCV. Understanding whether various support services for HCV can assist DUs to complete HCV therapy and improve treatment outcome could have important clinical and public health implications. In this study, we observed that addiction-treated DUs have higher PEG-IFN/RBV completion rates than nonaddiction-treated DUs. In addition, we observed lower rates of treatment completion among DUs infected with genotype 1/4 as well as among HIV-infected individuals as compared to genotype 2/3 and HCV-monoinfected DUs, respectively. After adjusting for HIV/HCV coinfection, sex, and treatment of addiction during HCV therapy, we observed that the availability of support services during HCV treatment significantly increased the treatment completion rates among DUs. We also observed that our SVR rate of 55.5% among all PEG-IFN/RBV-treated DUs and of 53% for those treated for addiction during HCV treatment are comparable to those obtained in PEG-IFN/RBV registration trials (54% and 56%, respectively [3, 4]). After adjusting for HCV genotype 1/4 and HIV/HCV coinfection, we observed that involvement of multidisciplinary team led to higher SVR rates among DUs.
The treatment completion rate among all DUs was estimated to be 83.4% (from 32 studies), which is comparable to the 14%–22% of patients who discontinued PEG-IFN/RBV treatment in registration trials [3, 4]. Decreased treatment completion among patients with genotype 1/4 could be explained by the longer course of PEG-IFN/RBV treatment for these genotypes. Similarly, HIV infection may affect PEG-IFN/RBV completion by adding to the complexity of the treatment regimen.
We observed a trend between treatment completion and the proportion of male DUs: the higher the proportion of males, the lower the treatment completion. We previously reported that male DUs were more likely to pursue HCV evaluation after at least 3 years of substitution therapy [47]. These, combined with similar results [48, 49], suggest that sex-based interventions may increase pursuit of and adherence to HCV care. In addition, regional variations in HCV disease characteristics may influence intervention design and execution. For example, US studies showed that an increased prevalence of genotype 1 infection potentially contributed to a significantly lower SVR rate than that observed in other countries.
Morbidity due to HCV continues to increase. The number of individuals with cirrhosis in the United States is expected to reach 1 million by 2020, and the number of HCV-attributable deaths is predicted to increase 5-fold between 2030 and 2050 [50, 51]. We recently reported that 84% (n = 54) of methadone-maintained patients had at least moderate hepatic fibrosis (Scheuer stage ≥2) [47]. Although we demonstrated the importance of addiction treatment during HCV therapy and the availability of support services in general, we were unable to demonstrate the effectiveness of any specific intervention. Studies included in our meta-analysis were largely designed as efficacy studies, which varied widely in the description of services offered and complicated cross-study comparisons. Furthermore, these studies may have excluded patients not on addiction treatment or who were current DUs. To that end, we found a significant result of the role of addiction treatment on treatment completion among 5 European studies with addiction-treated and untreated patients. However, patients not treated for addiction during HCV therapy were mostly former drug users.
While publication bias was not a factor in the treatment completion rate, it was detected in the assessment of SVR likely due to few studies with large samples, studies with high SVR rates (>88%), and no studies with SVR rates in the interval of 70%–88%. Elimination of 4 outlier studies mitigated the publication bias. Although we demonstrated that treatment of addiction is associated with higher HCV treatment completion rates, our study was likely underpowered to demonstrate that treatment of addiction increases the SVR rate. Additional limitations include the paucity of papers on DUs not in drug treatment and the small number of African Americans in these studies.
In conclusion, published data suggest that the overall rates for treatment completion and SVR for PEG-IFN/RBV–treated DUs are comparable to registration trials. Further work should evaluate care models in DUs. On the basis of our results, we recommend that DUs treated for addiction should be considered for HCV treatment under the same circumstances as the non-DUs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge the assistance of Ray Peterson and Julio Quintero for helpful discussions.
Financial support. This work was supported as an investigator-initiated project by Merck, Inc, and by the National Institutes of Health (DA 003574 to D. C. D.). The authors wrote the protocol, conducted the study, performed the statistical analysis, and wrote the manuscript. Although Merck reviewed the final manuscript prior to submission, the authors take full responsibility for the data and the conclusions reported.
Potential conflicts of interest. R. B. D., M. Z., D. C. D., and H. H. received research support by Merck for the conduct of this study. A. H. T. has been a consultant/advisor for Merck, Genentech, Vertex, Boerhinger-Ingelheim, Pfizer, and Bayer/Onyx; has received research support from Merck, Genentech, Vertex, Boehringer-Ingelheim, Gilead, Tibotec, Abbott, and BMS; and is a member of the speakers’ bureaus for Vertex and Genentech. I. M. J. has received grant/research support from Schering/Merck, Tibotec/Janssen, Roche/Genentech, Pharmasset, Achillion, Anadys, Boehringer Ingelheim, Novartis, Gilead, Vertex, GlobeImmune, Pfizer, Bristol-Myers Squibb, and Zymogenetics; has been a consultant/advisor for Abbott, Achillion, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, GlobeImmune, Inhibitex, Kadmon, Novartis, Pharmasset, Presidio, Roche/Genentech, Schering/Merck, Tibotec/Janssen, and Vertex; and is on the speakers’ bureaus for Schering/Merck, Gilead, Bristol-Myers Squibb, Roche/Genentech, and Vertex.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24(suppl 2):3–8. doi: 10.1055/s-2004-832922. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Eng J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 7.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141–7. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Grebely J, Petoumenos K, Matthews GV, et al. Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: the ATAHC Study. Drug Alcohol Depend. 2010;107:244–9. doi: 10.1016/j.drugalcdep.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broers B, Helbling B, Francois A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–8. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Bonkovsky HL, Tice AD, Yapp RG, et al. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol. 2008;103:2757–65. doi: 10.1111/j.1572-0241.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- 13.Curcio F, Di Martino F, Capraro C, et al. Together … to take care: multidisciplinary management of hepatitis C virus treatment in randomly selected drug users with chronic hepatitis. J Addict Med. 2010;4:223–32. doi: 10.1097/ADM.0b013e3181cae4d0. [DOI] [PubMed] [Google Scholar]
- 14.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11. [Google Scholar]
- 15.Hartung J, Knapp G, Sinha BK. Statistical meta-analysis with applications. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 16.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–56. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Grebely J, Genoway K, Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–43. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Uria G, Day JN, Nasir AJ, Russell SK, Vilar FJ. Factors associated with treatment failure of patients with psychiatric diseases and injecting drug users in the treatment of genotype 2 or 3 hepatitis C chronic infection. Liver Int. 2009;29:1051–5. doi: 10.1111/j.1478-3231.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 19.Gazdik F, Gazdikova K, Laktis K, et al. High virologic sustained response for former young intravenous drug users with chronic hepatitis C treated by pegylated interferon-alpha plus ribavirin. Bratisl Lek Listy. 2009;110:77–84. [PubMed] [Google Scholar]
- 20.Ebner N, Wanner C, Winklbaur B, et al. Retention rate and side effects in a prospective trial on hepatitis C treatment with pegylated interferon alpha-2a and ribavirin in opioid-dependent patients. Addict Biol. 2009;14:227–37. doi: 10.1111/j.1369-1600.2009.00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Waizmann M, Ackermann G. High rates of sustained virological response in hepatitis C virus-infected injection drug users receiving directly observed therapy with peginterferon alpha-2a (40KD) (PEGASYS) and once-daily ribavirin. J Subst Abuse Treat. 2010;38:338–45. doi: 10.1016/j.jsat.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Belfiori B, Ciliegi P, Chiodera A, et al. Peginterferon plus ribavirin for chronic hepatitis C in opiate addicts on methadone/buprenorphine maintenance therapy. Dig Liver Dis. 2009;41:303–7. doi: 10.1016/j.dld.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Guadagnino V, Trotta MP, Montesano F, et al. Effectiveness of a multi-disciplinary standardized management model in the treatment of chronic hepatitis C in drug addicts engaged in detoxification programmes. Addiction. 2007;102:423–31. doi: 10.1111/j.1360-0443.2006.01698.x. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991–8. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 25.Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–4. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 27.Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4:20–6. doi: 10.1097/ADM.0b013e3181add3de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krook AL, Stokka D, Heger B, Nygaard E. Hepatitis C treatment of opioid dependants receiving maintenance treatment: results of a Norwegian pilot study. Eur Addict Res. 2007;13:216–21. doi: 10.1159/000104884. [DOI] [PubMed] [Google Scholar]
- 29.Bruggmann P, Falcato L, Dober S, et al. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15:747–52. doi: 10.1111/j.1365-2893.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 30.Dimitroulopoulos D, Petroulaki E, Manolakopoulos S, et al. Peginterferon/ribavirin treatment achieves a higher compliance rate than interferon/ribavirin combination in patients chronically infected with HCV on methadone maintenance. Eur J Gastroenterol Hepatol. 2009;21:1407–12. doi: 10.1097/meg.0b013e3283110198. [DOI] [PubMed] [Google Scholar]
- 31.Fried R, Monnat M, Seidenberg A, et al. Swiss multicenter study evaluating the efficacy, feasibility and safety of peginterferon-alfa-2a and ribavirin in patients with chronic hepatitis C in official opiate substitution programs. Digestion. 2008;78:123–30. doi: 10.1159/000173733. [DOI] [PubMed] [Google Scholar]
- 32.Hallinan R, Byrne A, Agho K, Dore GJ. Referral for chronic hepatitis C treatment from a drug dependency treatment setting. Drug Alcohol Depend. 2007;88:49–53. doi: 10.1016/j.drugalcdep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Jack K, Willott S, Manners J, Varnam MA, Thomson BJ. Clinical trial: a primary-care-based model for the delivery of anti-viral treatment to injecting drug users infected with hepatitis C. Aliment Pharmacol Ther. 2009;29:38–45. doi: 10.1111/j.1365-2036.2008.03872.x. [DOI] [PubMed] [Google Scholar]
- 34.Jeffrey GP, MacQuillan G, Chua F, et al. Hepatitis C virus eradication in intravenous drug users maintained with subcutaneous naltrexone implants. Hepatology. 2007;45:111–7. doi: 10.1002/hep.21470. [DOI] [PubMed] [Google Scholar]
- 35.Schulte B, Schutt S, Brack J, et al. Successful treatment of chronic hepatitis C virus infection in severely opioid-dependent patients under heroin maintenance. Drug Alcohol Depend. 2010;109:248–51. doi: 10.1016/j.drugalcdep.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos V, Gogou A, Mylopoulou T, Mimidis K. Should active injecting drug users receive treatment for chronic hepatitis C? Arq Gastroenterol. 2010;47:238–41. doi: 10.1590/s0004-28032010000300005. [DOI] [PubMed] [Google Scholar]
- 37.Melin P, Chousterman M, Fontanges T, et al. Effectiveness of chronic hepatitis C treatment in drug users in routine clinical practice: results of a prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:1050–7. doi: 10.1097/MEG.0b013e328338d9aa. [DOI] [PubMed] [Google Scholar]
- 38.John-Baptiste A, Krahn M, Heathcote J, Laporte A, Tomlinson G. The natural history of hepatitis C infection acquired through injection drug use: meta-analysis and meta-regression. J Hepatol. 2010;53:245–51. doi: 10.1016/j.jhep.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Sasadeusz JJ, Dore G, Kronborg I, Barton D, Yoshihara M, Weltman M. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106:977–84. doi: 10.1111/j.1360-0443.2010.03347.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor LE, Bowman SE, Chapman S, et al. Treatment for hepatitis C virus genotype 1 infection in HIV-infected individuals on methadone maintenance therapy. Drug Alcohol Depend. 2011;116:233–7. doi: 10.1016/j.drugalcdep.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jovanović M, Konstantinović L, Kostić V, Vrbić M, Popović L. Efficiency of a combined peginterferon alpha-2a and ribavarin therapy in intravenous opiate substances abusers with chronic hepatitis C [in Serbian] Vojnosanit Pregl. 2009;66:791–5. doi: 10.2298/vsp0910791j. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson M, Crawford V, Tippet A, et al. Community-based treatment for chronic hepatitis C in drug users: high rates of compliance with therapy despite ongoing drug use. Aliment Pharmacol Ther. 2009;29:29–37. doi: 10.1111/j.1365-2036.2008.03834.x. [DOI] [PubMed] [Google Scholar]
- 43.Manolakopoulos S, Deutsch MJ, Anagnostou O, et al. Substitution treatment or active intravenous drug use should not be contraindications for antiviral treatment in drug users with chronic hepatitis C. Liver Int. 2010;30:1454–60. doi: 10.1111/j.1478-3231.2010.02341.x. [DOI] [PubMed] [Google Scholar]
- 44.Lindenburg CE, Lambers FA, Urbanus AT, et al. Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH-C project. Eur J Gastroenterol Hepatol. 2011;23:23–31. doi: 10.1097/MEG.0b013e328340c451. [DOI] [PubMed] [Google Scholar]
- 45.Tait JM, McIntyre PG, McLeod S, Nathwani D, Dillon JF. The impact of a managed care network on attendance, follow-up and treatment at a hepatitis C specialist centre. J Viral Hepat. 2010;17:698–704. doi: 10.1111/j.1365-2893.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 46.Mauss S, Hueppe D, John C, et al. Estimating the likelihood of sustained virological response in chronic hepatitis C therapy. J Viral Hepat. 2010;18:e81–90. doi: 10.1111/j.1365-2893.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 47.Martinez AD, Dimova R, Marks KM, et al. Integrated internist-addiction medicine-hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19:47–54. doi: 10.1111/j.1365-2893.2010.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senn O, Seidenberg A, Rosemann T. Determinants of successful chronic hepatitis C case finding among patients receiving opioid maintenance treatment in a primary care setting. Addiction. 2009;104:2033–8. doi: 10.1111/j.1360-0443.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 49.del Rio M, Mino A, Perneger TV. Predictors of patient retention in a newly established methadone maintenance treatment programme. Addiction. 1997;92:1353–60. [PubMed] [Google Scholar]
- 50.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138 doi: 10.1053/j.gastro.2009.09.067. 513–521, 521 e511–516. [DOI] [PubMed] [Google Scholar]
- 51.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2010;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.